The Impact of Klotho in Cancer: From Development and Progression to Therapeutic Potential
Abstract
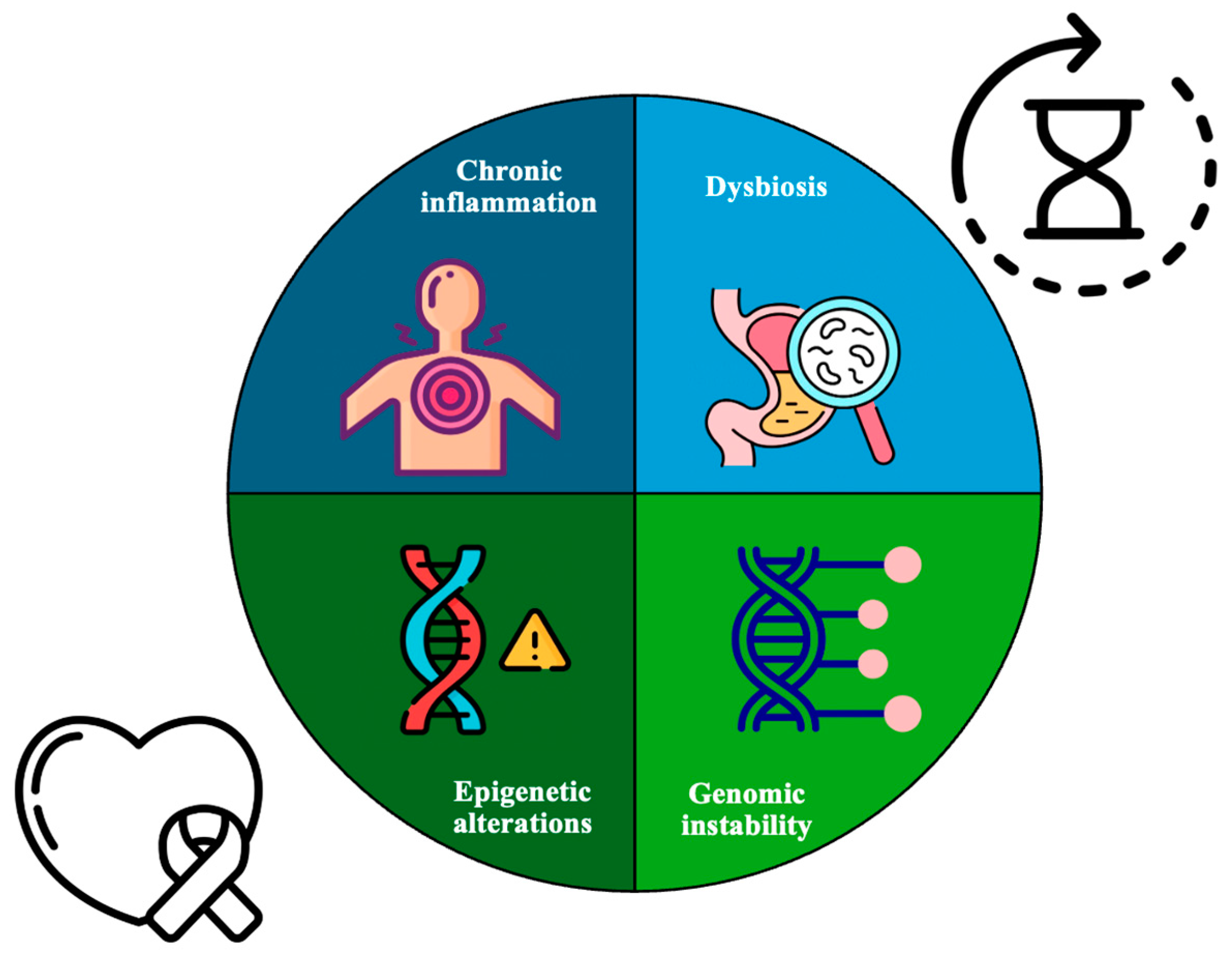
1. Introduction
2. Klotho Isoforms
3. Pleiotropic Functions of Klotho in Cancer
3.1. Acute Myeloid Leukemia
3.2. Bladder Cancer
3.3. Breast Cancer
3.4. Colorectal Cancer
3.5. Esophageal Cancer
3.6. Gastric Cancer
3.7. Hepatocellular Carcinoma
3.8. Lung Cancer
3.9. Ovarian Cancer
3.10. Pancreatic Cancer
3.11. Prostate Cancer
3.12. Renal Cell Carcinoma
3.13. Thyroid Cancer
| Type of Cancer | In Vivo | In Vitro | Isoform | Pathways/Mechanism Involved | Reference(s) |
|---|---|---|---|---|---|
| Acute myeloid leukemia | x | α | In AML, miR-126-5p suppressed the expression of α-Klotho, resulting in elevated phosphorylation of Akt | [91] | |
| Bladder cancer | x | x | γ | γ-Klotho was linked to cell proliferation, apoptosis, EMT, and growth of human UCB | [93] |
| Breast cancer | x | α, β, γ | γ-Klotho helped cancer cells manage oxidative stress Overexpression of Klotho or the KL1 domain inhibited tumor formation in the breast cancer cell lines MCF-7 and MBA-MB-231 | [98,99,100,101] | |
| Colorectal cancer | x | x | α | It has been demonstrated that Klotho inhibits the activation of NF-κB. Remarkably, overexpressing Klotho in Caco-2 cells sensitized the TRAIL death receptor DR4 and impeded cell proliferation by promoting apoptosis. Furthermore, an increase in KL expression suppressed tumor growth and invasion, primarily through the inhibition of the IGF1R-mediated PI3K/Akt pathway in colon cancer cells | [103,104,105,106,107,108] |
| Esophageal cancer | x | α | An inverse correlation was found between Klotho and β-catenin expression levels. Klotho was identified as a significant factor for a good prognosis | [110] | |
| Gastric cancer | x | α | SOX17 promoted the expression of the Klotho gene in gastric cancer cells circ-ITCH suppressed gastric cancer metastasis by acting as a sponge for miR-199a-5p, thereby increasing Klotho expression | [112,113,114] | |
| Hepatocellular cancer | x | x | α, β | The overexpression of Klotho curtailed the proliferation of liver cancer cells. Also, both KL gene expression and the methylation of its promoter DNA emerged as strong indicators of poor prognosis with HCC | [88,117,118] |
| Lung cancer | x | x | α | The inhibition of the PI3K/Akt pathway using the inhibitor LY294002 diminished the enhanced cancer growth observed with Klotho knockdown. Furthermore, transfecting Klotho into SQ5 lung cancer cells demonstrated its ability to suppress the mesenchymal marker N-cadherin | [120,121,122] |
| Ovarian cancer | x | x | α | Restoring Klotho expression slowed EOC cell growth and inhibited key signaling pathways. Klotho functions as a tumor inhibitor in human ovarian cancer cells | [124,125] |
| Pancreatic cancer | x | α, β | Klotho functions as a tumor suppressor in PDAC. Treatment with miR-504 inhibitor and a demethylation agent upregulated Klotho gene expression, while concurrently inhibiting the invasion and migration of BxPC-3 and Panc-1 cells. | [128,129] | |
| Prostate cancer | x | α, β, γ | Prostate cancer cells exhibit the expression of both α-Klotho and β-Klotho, in vitro and in vivo, indicating that other endocrine FGFs might also influence the biological processes in this cancer. | [89,131] | |
| Renal cell carcinoma | - | - | α | Klotho acted as a tumor suppressor by inhibiting the PI3K/Akt/GDK3β/Snail pathway | [133] |
| Thyroid cancer | x | α | High Klotho levels were associated with low stanniocalcin 1 (STC1) levels in FTC133 and FTC238 cell lines | [135] |
4. Conclusions of Exploring the Tumor-Suppressive Role of Klotho in Cancer Progression
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the Mouse Klotho Gene Leads to a Syndrome Resembling Ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Guimond, M.; Leonard, W.J.; Spolski, R.; Rossi, S.W.; Veenstra, R.G.; Hollander, G.A.; Mackall, C.L.; Blazar, B.R. Thymic Stromal Lymphopoietin Is Not Necessary or Sufficient to Mediate the Thymopoietic Effects of Keratinocyte Growth Factor. Blood 2008, 111, 969. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuro-o, M. Klotho. Pflug. Arch. 2010, 459, 333–343. [Google Scholar] [CrossRef]
- Kinoshita, S.; Kawai, M. The FGF23/KLOTHO Regulatory Network and Its Roles in Human Disorders. Vitam. Horm. 2016, 101, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Erben, R.G.; Andrukhova, O. FGF23-Klotho Signaling Axis in the Kidney. Bone 2017, 100, 62–68. [Google Scholar] [CrossRef]
- Kurosu, H.; Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Nandi, A.; Gurnani, P.; McGuinness, O.P.; Chikuda, H.; Yamaguchi, M.; Kawaguchi, H.; et al. Suppression of Aging in Mice by the Hormone Klotho. Science 2005, 309, 1829–1833. [Google Scholar] [CrossRef]
- Gilbert, S.F. Aging: The Biology of Senescence. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Jane Henley, S. Age and Cancer Risk: A Potentially Modifiable Relationship. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef]
- Hashim, D.; Carioli, G.; Malvezzi, M.; Bertuccio, P.; Waxman, S.; Negri, E.; La Vecchia, C.; Boffetta, P. Cancer Mortality in the Oldest Old: A Global Overview. Aging 2020, 12, 16744–16758. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Suppression of Klotho Expression by Protein-Bound Uremic Toxins Is Associated with Increased DNA Methyltransferase Expression and DNA Hypermethylation. Kidney Int. 2012, 81, 640–650. [Google Scholar] [CrossRef]
- Xu, X.; Tan, X.; Tampe, B.; Wilhelmi, T.; Hulshoff, M.S.; Saito, S.; Moser, T.; Kalluri, R.; Hasenfuss, G.; Zeisberg, E.M.; et al. High-Fidelity CRISPR/Cas9- Based Gene-Specific Hydroxymethylation Rescues Gene Expression and Attenuates Renal Fibrosis. Nat. Commun. 2018, 9, 3509. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Flores, B.; Gillings, N.; Bian, A.; Cho, H.J.; Yan, S.; Liu, Y.; Levine, B.; Moe, O.W.; Hu, M.C. AKlotho Mitigates Progression of AKI to CKD through Activation of Autophagy. J. Am. Soc. Nephrol. 2016, 27, 2331–2345. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef]
- Aunan, J.R.; Cho, W.C.; Søreide, K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis. 2017, 8, 628–642. [Google Scholar] [CrossRef]
- Van Beneden, K.; Mannaerts, I.; Pauwels, M.; Van den Branden, C.; van Grunsven, L.A. HDAC Inhibitors in Experimental Liver and Kidney Fibrosis. Fibrogenes. Tissue Repair. 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.J.; Li, X.; Chou, C.J. Advances and Challenges of HDAC Inhibitors in Cancer Therapeutics. Adv. Cancer Res. 2018, 138, 183–211. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Izquierdo, M.C.; Sanchez-Niño, M.D.; Suárez-Alvarez, B.; Lopez-Larrea, C.; Jakubowski, A.; Blanco, J.; Ramirez, R.; Selgas, R.; Ruiz-Ortega, M.; et al. The Inflammatory Cytokines TWEAK and TNFα Reduce Renal Klotho Expression through NFκB. J. Am. Soc. Nephrol. 2011, 22, 1315–1325. [Google Scholar] [CrossRef]
- Sato, Y.; Yanagita, M. Immune Cells and Inflammation in AKI to CKD Progression. Am. J. Physiol. Ren. Physiol. 2018, 315, F1501–F1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gao, Q.; Wei, A.; Chen, X.; Shi, Y.; Wang, H.; Cao, W. Histone Deacetylase 3 Aberration Inhibits Klotho Transcription and Promotes Renal Fibrosis. Cell Death Differ. 2021, 28, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, J.; Tolbert, E.; Zhao, T.C.; Bayliss, G.; Zhuang, S. Identification of Histone Deacetylase 8 as a Novel Therapeutic Target for Renal Fibrosis. FASEB J. 2020, 34, 7295–7310. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Cheng, P.; Yan, M.; Gu, Y.; Xue, J. Aldosterone Induces Renal Fibrosis by Promoting HDAC1 Expression, Deacetylating H3K9 and Inhibiting Klotho Transcription. Mol. Med. Rep. 2019, 19, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, Y.; Zhang, H.; Song, N.; Gu, Y.; Shi, Y.; Cai, J.; Ding, X.; Zhang, X. METTL14-Dependent M6A Regulates Vascular Calcification Induced by Indoxyl Sulfate. Life Sci. 2019, 239, 117034. [Google Scholar] [CrossRef]
- Liu, L.; Zou, J.; Guan, Y.; Zhang, Y.; Zhang, W.; Zhou, X.; Xiong, C.; Tolbert, E.; Zhao, T.C.; Bayliss, G.; et al. Blocking the Histone Lysine 79 Methyltransferase DOT1L Alleviates Renal Fibrosis through Inhibition of Renal Fibroblast Activation and Epithelial-Mesenchymal Transition. FASEB J. 2019, 33, 11941–11958. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, D.; Xu, G. Long Noncoding RNA MALAT1 Mediates High Glucose-Induced Glomerular Endothelial Cell Injury by Epigenetically Inhibiting Klotho via Methyltransferase G9a. IUBMB Life 2019, 71, 873–881. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic Instability—An Evolving Hallmark of Cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Wang, J.J.; Lei, K.F.; Han, F. Tumor Microenvironment: Recent Advances in Various Cancer Treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal Production, Uptake, and Handling of Circulating AKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef]
- Lindberg, K.; Amin, R.; Moe, O.W.; Hu, M.C.; Erben, R.G.; Wernerson, A.Ö.; Lanske, B.; Olauson, H.; Larsson, T.E. The Kidney Is the Principal Organ Mediating Klotho Effects. J. Am. Soc. Nephrol. 2014, 25, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, T.; Inoue, T.; Miyazaki, T.; Kobori, H.; Nishiyama, A.; Ishii, N.; Hayashi, M.; Suzuki, H. Klotho Suppresses the Renin-Angiotensin System in Adriamycin Nephropathy. Nephrol. Dial. Transpl. 2017, 32, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Groen, A.; Molostvov, G.; Lu, T.; Lilley, K.S.; Snead, D.; James, S.; Wilkinson, I.B.; Ting, S.; Hsiao, L.L.; et al. α-Klotho Expression in Human Tissues. J. Clin. Endocrinol. Metab. 2015, 100, E1308–E1318. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, E.; Pérez-Castro, A.; Tagua, V.G.; Hernández-Carballo, C.; Ferri, C.; Pérez-Delgado, N.; Rodríguez-Ramos, S.; Cerro-López, P.; López-Castillo, Á.; Delgado-Molinos, A.; et al. Klotho Expression in Peripheral Blood Circulating Cells Is Associated with Vascular and Systemic Inflammation in Atherosclerotic Vascular Disease. Sci. Rep. 2022, 12, 8422. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shiizaki, K.; Kuro-o, M.; Moe, O.W. Fibroblast Growth Factor 23 and Klotho: Physiology and Pathophysiology of an Endocrine Network of Mineral Metabolism. Annu. Rev. Physiol. 2013, 75, 503–533. [Google Scholar] [CrossRef]
- Kuro-o, M. The Klotho Proteins in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 27–44. [Google Scholar] [CrossRef]
- Matsumura, Y.; Aizawa, H.; Shiraki-Iida, T.; Nagai, R.; Kuro-o, M.; Nabeshima, Y.I. Identification of the Human Klotho Gene and Its Two Transcripts Encoding Membrane and Secreted Klotho Protein. Biochem. Biophys. Res. Commun. 1998, 242, 626–630. [Google Scholar] [CrossRef]
- Zou, D.; Wu, W.; He, Y.; Ma, S.; Gao, J. The Role of Klotho in Chronic Kidney Disease. BMC Nephrol. 2018, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Yoshizawa, H.; Watanabe, Y.; Numata, A.; Yamazaki, T.; Takeshima, E.; Iwazu, K.; Komada, T.; Otani, N.; Morishita, Y.; et al. Characteristics of Urinary and Serum Soluble Klotho Protein in Patients with Different Degrees of Chronic Kidney Disease. BMC Nephrol. 2012, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Moghekar, A.R.; Hu, J.; Sun, K.; Turner, R.; Ferrucci, L.; O’Brien, R. Klotho in the Cerebrospinal Fluid of Adults with and without Alzheimer’s Disease. Neurosci. Lett. 2014, 558, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Rotondi, S.; Pasquali, M.; Tartaglione, L.; Muci, M.L.; Mandanici, G.; Leonangeli, C.; Sales, S.; Farcomeni, A.; Mazzaferro, S. Soluble α -Klotho Serum Levels in Chronic Kidney Disease. Int. J. Endocrinol. 2015, 2015, 872193. [Google Scholar] [CrossRef]
- Buendia-Roldan, I.; Machuca, N.; Mejía, M.; Maldonado, M.; Pardo, A.; Selman, M. Lower Levels of α-Klotho in Serum Are Associated with Decreased Lung Function in Individuals with Interstitial Lung Abnormalities. Sci. Rep. 2019, 9, 10801. [Google Scholar] [CrossRef] [PubMed]
- Memmos, E.; Sarafidis, P.; Pateinakis, P.; Tsiantoulas, A.; Faitatzidou, D.; Giamalis, P.; Vasilikos, V.; Papagianni, A. Soluble Klotho Is Associated with Mortality and Cardiovascular Events in Hemodialysis. BMC Nephrol. 2019, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Nam, B.Y.; Lee, M.J.; Kim, C.H.; Koo, H.M.; Doh, F.M.; Han, J.H.; Kim, E.J.; Han, J.S.; Park, J.T.; et al. Decreased Circulating Klotho Levels in Patients Undergoing Dialysis and Relationship to Oxidative Stress and Inflammation. Perit. Dial. Int. 2015, 35, 43. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Ohishi, M.; Ikushima, M.; Yamamoto, K.; Yasuda, O.; Oguro, R.; Yamamoto-Hanasaki, H.; Tatara, Y.; Takeya, Y.; Rakugi, H. Klotho Protein Diminishes Endothelial Apoptosis and Senescence via a Mitogen-Activated Kinase Pathway. Geriatr. Gerontol. Int. 2011, 11, 510–516. [Google Scholar] [CrossRef]
- Rakugi, H.; Matsukawa, N.; Ishikawa, K.; Yang, J.; Imai, M.; Ikushima, M.; Maekawa, Y.; Kida, I.; Miyazaki, J.I.; Ogihara, T. Anti-Oxidative Effect of Klotho on Endothelial Cells through CAMP Activation. Endocrine 2007, 31, 82–87. [Google Scholar] [CrossRef]
- Lee, J.; Ju, K.D.; Kim, H.J.; Tsogbadrakh, B.; Ryu, H.; Kang, E.; Kang, M.; Yang, J.; Kang, H.G.; Ahn, C.; et al. Soluble α-Klotho Anchors TRPV5 to the Distal Tubular Cell Membrane Independent of FGFR1 by Binding TRPV5 and Galectin-1 Simultaneously. Am. J. Physiol. Ren. Physiol. 2021, 320, F559–F568. [Google Scholar] [CrossRef] [PubMed]
- Di Bona, D.; Accardi, G.; Virruso, C.; Candore, G.; Caruso, C. Association of Klotho Polymorphisms with Healthy Aging: A Systematic Review and Meta-Analysis. Rejuvenation Res. 2014, 17, 212–216. [Google Scholar] [CrossRef]
- Lim, K.; Lu, T.S.; Molostvov, G.; Lee, C.; Lam, F.T.; Zehnder, D.; Hsiao, L.L. Vascular Klotho Deficiency Potentiates the Development of Human Artery Calcification and Mediates Resistance to Fibroblast Growth Factor 23. Circulation 2012, 125, 2243–2255. [Google Scholar] [CrossRef]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of Fibroblast Growth Factor-23 Signaling by Klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Goetz, R.; Fu, L.; Jayaraman, S.; Hu, M.C.; Moe, O.W.; Liang, G.; Li, X.; Mohammadi, M. α-Klotho Is a Non-Enzymatic Molecular Scaffold for FGF23 Hormone Signalling. Nature 2018, 553, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho Inhibits Transforming Growth Factor-Beta1 (TGF-Beta1) Signaling and Suppresses Renal Fibrosis and Cancer Metastasis in Mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fergusson, M.M.; Castilho, R.M.; Liu, J.; Cao, L.; Chen, J.; Malide, D.; Rovira, I.I.; Schimel, D.; Kuo, C.J.; et al. Augmented Wnt Signaling in a Mammalian Model of Accelerated Aging. Science 2007, 317, 803–806. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of Oxidative Stress by the Anti-Aging Hormone Klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Mencke, R.; Olauson, H.; Hillebrands, J.L. Effects of Klotho on Fibrosis and Cancer: A Renal Focus on Mechanisms and Therapeutic Strategies. Adv. Drug Deliv. Rev. 2017, 121, 85–100. [Google Scholar] [CrossRef]
- Rubinek, T.; Wolf, I. The Role of Alpha-Klotho as a Universal Tumor Suppressor. Vitam. Horm. 2016, 101, 197–214. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z. Current Understanding of Klotho. Ageing Res. Rev. 2009, 8, 43–51. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular Basis of Klotho: From Gene to Function in Aging. Endocr. Rev. 2015, 36, 174. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kinoshita, S.; Shiraishi, N.; Nakagawa, S.; Sekine, S.; Fujimori, T.; Nabeshima, Y.I. Molecular Cloning and Expression Analyses of Mouse Βklotho, Which Encodes a Novel Klotho Family Protein. Mech. Dev. 2000, 98, 115–119. [Google Scholar] [CrossRef]
- Hua, S.; Liu, Q.; Li, J.; Fan, M.; Yan, K.; Ye, D. Beta-Klotho in Type 2 Diabetes Mellitus: From Pathophysiology to Therapeutic Strategies. Rev. Endocr. Metab. Disord. 2021, 22, 1091–1109. [Google Scholar] [CrossRef] [PubMed]
- Aaldijk, A.S.; Verzijl, C.R.C.; Jonker, J.W.; Struik, D. Biological and Pharmacological Functions of the FGF19- and FGF21-Coreceptor Beta Klotho. Front. Endocrinol. 2023, 14, 1150222. [Google Scholar] [CrossRef]
- Kuzina, E.S.; Ung, P.M.U.; Mohanty, J.; Tome, F.; Choi, J.; Pardon, E.; Steyaert, J.; Lax, I.; Schlessinger, A.; Schlessinger, J.; et al. Structures of Ligand-Occupied β-Klotho Complexes Reveal a Molecular Mechanism Underlying Endocrine FGF Specificity and Activity. Proc. Natl. Acad. Sci. USA 2019, 116, 7819–7824. [Google Scholar] [CrossRef]
- Dolegowska, K.; Marchelek-Mysliwiec, M.; Nowosiad-Magda, M.; Slawinski, M.; Dolegowska, B. FGF19 Subfamily Members: FGF19 and FGF21. J. Physiol. Biochem. 2019, 75, 229–240. [Google Scholar] [CrossRef]
- Tacer, K.F.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-o, M.; Mangelsdorf, D.J.; et al. Research Resource: Comprehensive Expression Atlas of the Fibroblast Growth Factor System in Adult Mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Fujimori, T.; Hayashizaki, Y.; Nabeshima, Y. Identification of a Novel Mouse Membrane-Bound Family 1 Glycosidase-like Protein, Which Carries an Atypical Active Site Structure. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 2002, 1576, 341–345. [Google Scholar] [CrossRef]
- Ligumsky, H.; Merenbakh-Lamin, K.; Keren-Khadmy, N.; Wolf, I.; Rubinek, T. The Role of α-Klotho in Human Cancer: Molecular and Clinical Aspects. Oncogene 2022, 41, 4487–4497. [Google Scholar] [CrossRef]
- Mikuła-Pietrasik, J.; Rutecki, S.; Książek, K. The Functional Multipotency of Transforming Growth Factor β Signaling at the Intersection of Senescence and Cancer. Cell. Mol. Life Sci. 2022, 79, 196. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Kurt, M.; Wang, Q. Pathobiology of the Klotho Antiaging Protein and Therapeutic Considerations. Front. Aging 2022, 3, 931331. [Google Scholar] [CrossRef]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Ren, Q.; Li, L.; Tan, H.; Lu, M.; Tian, Y.; Huang, L.; Zhao, B.; Fu, H.; Hou, F.F.; et al. A Klotho-Derived Peptide Protects against Kidney Fibrosis by Targeting TGF-β Signaling. Nat. Commun. 2022, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Aashaq, S.; Batool, A.; Mir, S.A.; Beigh, M.A.; Andrabi, K.I.; Shah, Z.A. TGF-β Signaling: A Recap of SMAD-Independent and SMAD-Dependent Pathways. J. Cell. Physiol. 2022, 237, 59–85. [Google Scholar] [CrossRef]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Budi, E.H. Specificity, Versatility, and Control of TGF-β Family Signaling. Sci. Signal 2019, 12, eaav5183. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt Signaling Pathway: A Comprehensive Review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Bian, A.; Neyra, J.A.; Zhan, M.; Hu, M.C. Klotho, Stem Cells, and Aging. Clin. Interv. Aging 2015, 10, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Glinka, Y.; Matkar, P.N.; Leong-Poi, H. The Role of Neuropilins in TGF-β Signaling and Cancer Biology. In The Neuropilins: Role and Function in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2017; pp. 187–212. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt Signaling during Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Qiao, L.-Y.; Huang, F.-J.; Zhao, M.; Xie, J.-H.; Shi, J.; Wang, J.; Lin, X.-Z.; Zuo, H.; Wang, Y.-L.; Geng, T.-C. A Two-Year Follow-up Study of Cotransplantation with Neural Stem/Progenitor Cells and Mesenchymal Stromal Cells in Ischemic Stroke Patients. Cell Transpl. 2014, 23 (Suppl. S1), 65–72. [Google Scholar] [CrossRef]
- Abraham, C.R.; Li, A. Aging-Suppressor Klotho: Prospects in Diagnostics and Therapeutics. Ageing Res. Rev. 2022, 82, 101766. [Google Scholar] [CrossRef]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like Growth Factors and Neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.Y.; Iyaswamy, A.; Sreenivasmurthy, S.G.; Krishnamoorthi, S.; Guan, X.J.; Zhu, Z.; Su, C.F.; Liu, J.; Kan, Y.; Zhang, Y.; et al. Klotho an Autophagy Stimulator as a Potential Therapeutic Target for Alzheimer’s Disease: A Review. Biomedicines 2022, 10, 705. [Google Scholar] [CrossRef]
- von Frieling, J.; Roeder, T. Factors That Affect the Translation of Dietary Restriction into a Longer Life. IUBMB Life 2020, 72, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.N.; Kilgour, E.; Smith, P.D. Molecular Pathways: Fibroblast Growth Factor Signaling: A New Therapeutic Opportunity in Cancer. Clin. Cancer Res. 2012, 18, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Greulich, H.; Pollock, P.M. Targeting Mutant Fibroblast Growth Factor Receptors in Cancer. Trends Mol. Med. 2011, 17, 283–292. [Google Scholar] [CrossRef]
- Lin, B.C.; Wang, M.; Blackmore, C.; Desnoyers, L.R. Liver-Specific Activities of FGF19 Require Klotho Beta. J. Biol. Chem. 2007, 282, 27277–27284. [Google Scholar] [CrossRef] [PubMed]
- Abramovitz, L.; Rubinek, T.; Ligumsky, H.; Bose, S.; Barshack, I.; Avivi, C.; Kaufman, B.; Wolf, I. KL1 Internal Repeat Mediates Klotho Tumor Suppressor Activities and Inhibits BFGF and IGF-I Signaling in Pancreatic Cancer. Clin. Cancer Res. 2011, 17, 4254–4266. [Google Scholar] [CrossRef]
- Poh, W.; Wong, W.; Ong, H.; Aung, M.O.; Lim, S.G.; Chua, B.T.; Ho, H.K. Klotho-Beta Overexpression as a Novel Target for Suppressing Proliferation and Fibroblast Growth Factor Receptor-4 Signaling in Hepatocellular Carcinoma. Mol. Cancer 2012, 11, 14. [Google Scholar] [CrossRef]
- Feng, S.; Dakhova, O.; Creighton, C.J.; Ittmann, M. Endocrine Fibroblast Growth Factor FGF19 Promotes Prostate Cancer Progression. Cancer Res. 2013, 73, 2551–2562. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Gibson, B.; Smith, F.O. Acute Myeloid Leukemia. Hematol. Oncol. Clin. N. Am. 2010, 24, 35–63. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, Y.; Kondo, T.; Ohya, H.; Fujisawa, S.I.; Teshima, T.; Iseki, K. Upregulation of MicroRNA-126-5p Is Associated with Drug Resistance to Cytarabine and Poor Prognosis in AML Patients. Oncol. Rep. 2015, 33, 2176. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Hori, S.; Miyake, M.; Tatsumi, Y.; Morizawa, Y.; Nakai, Y.; Onishi, S.; Onishi, K.; Iida, K.; Gotoh, D.; Tanaka, N.; et al. Gamma-Klotho Exhibits Multiple Roles in Tumor Growth of Human Bladder Cancer. Oncotarget 2018, 9, 19508–19524. [Google Scholar] [CrossRef]
- Nagarajan, D.; McArdle, S.E.B. Immune Landscape of Breast Cancers. Biomedicines 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, I.; Atiq, M.; Alwbari, A.; Kieber-Emmons, T. Breast Cancer Immunotherapy: An Update. Breast Cancer 2018, 12, 1178223418774802. [Google Scholar] [CrossRef] [PubMed]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.N.; Saha, S. Breast Cancer: Presentation, Investigation and Management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Trošt, N.; Peña-Llopis, S.; Koirala, S.; Stojan, J.; Potts, P.R.; Tacer, K.F.; Martinez, E.D. ΓKlotho Is a Novel Marker and Cell Survival Factor in a Subset of Triple Negative Breast Cancers. Oncotarget 2016, 7, 2611–2628. [Google Scholar] [CrossRef] [PubMed]
- Rubinek, T.; Shulman, M.; Israeli, S.; Bose, S.; Avraham, A.; Zundelevich, A.; Evron, E.; Gal-Yam, E.N.; Kaufman, B.; Wolf, I. Epigenetic Silencing of the Tumor Suppressor Klotho in Human Breast Cancer. Breast Cancer Res. Treat. 2012, 133, 649–657. [Google Scholar] [CrossRef]
- Ligumsky, H.; Rubinek, T.; Merenbakh-Lamin, K.; Yeheskel, A.; Sertchook, R.; Shahmoon, S.; Aviel-Ronen, S.; Wolf, I. Tumor Suppressor Activity of Klotho in Breast Cancer Is Revealed by Structure-Function Analysis. Mol. Cancer Res. 2015, 13, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.; Levanon-Cohen, S.; Bose, S.; Ligumsky, H.; Sredni, B.; Kanety, H.; Kuro-o, M.; Karlan, B.; Kaufman, B.; Koeffler, H.P.; et al. Klotho: A Tumor Suppressor and a Modulator of the IGF-1 and FGF Pathways in Human Breast Cancer. Oncogene 2008, 27, 7094–7105. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, J.; Pan, X.; Wu, L.; Bian, J.; Lin, Z.; Xue, M.; Su, T.; Lai, S.; Chen, F.; et al. Klotho-Mediated Targeting of CCL2 Suppresses the Induction of Colorectal Cancer Progression by Stromal Cell Senescent Microenvironments. Mol. Oncol. 2019, 13, 2460–2475. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Hu, F.; Li, M.; Mo, L.; Xu, C.; Xiao, Y.; Wang, X.; Nie, J.; Yang, L.; He, Y. FLI-1 Mediates Tumor Suppressor Function via Klotho Signaling in Regulating CRC. Cell Biol. Int. 2020, 44, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Soykan, M.N.; Sariboyaci, A.E.; Uysal, O.; Sevimli, T.S. Enhancement of Apo2L/TRAIL Signaling Pathway Receptors by the Activation of Klotho Gene with CRISPR/Cas9 in Caco-2 Colon Cancer Cells. Med. Oncol. 2021, 38, 146. [Google Scholar] [CrossRef] [PubMed]
- Sariboyaci, A.E.; Uysal, O.; Soykan, M.N.; Gunes, S. The Potential Therapeutic Effect of Klotho on Cell Viability in Human Colorectal Adenocarcinoma HT-29 Cells. Med. Oncol. 2022, 39, 191. [Google Scholar] [CrossRef]
- Arbel Rubinstein, T.; Shahmoon, S.; Zigmond, E.; Etan, T.; Merenbakh-Lamin, K.; Pasmanik-Chor, M.; Har-Zahav, G.; Barshack, I.; Vainer, G.W.; Skalka, N.; et al. Klotho Suppresses Colorectal Cancer through Modulation of the Unfolded Protein Response. Oncogene 2019, 38, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Huang, L.Y.; Peng, J.J.; Liang, L.; Shi, D.B.; Zheng, H.T.; Cai, S.J. Klotho Suppresses Growth and Invasion of Colon Cancer Cells through Inhibition of IGF1R-Mediated PI3K/AKT Pathway. Int. J. Oncol. 2014, 45, 611–618. [Google Scholar] [CrossRef]
- Arnal, M.J.D.; Arenas, Á.F.; Arbeloa, Á.L. Esophageal Cancer: Risk Factors, Screening and Endoscopic Treatment in Western and Eastern Countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef]
- Tang, X.; Fan, Z.; Wang, Y.; Ji, G.; Wang, M.; Lin, J.; Huang, S. Expression of Klotho and β-Catenin in Esophageal Squamous Cell Carcinoma, and Their Clinicopathological and Prognostic Significance. Dis. Esophagus 2016, 29, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; He, H.; Hu, F.; Li, M.; Mo, L.; Xiao, Y.; Wang, X.; Xie, B. Delivery of BR2-SOX17 Fusion Protein Can Inhibit Cell Survival, Proliferation, and Invasion in Gastric Cancer Cells through Regulating Klotho Gene Expression. Cell Biol. Int. 2020, 44, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zheng, R.; Wu, P.; Sun, Z.; Chen, J.; Zhang, L.; Zhang, C.; Qian, H.; Jiang, J.; et al. Circular RNA ITCH Suppresses Metastasis of Gastric Cancer via Regulating MiR-199a-5p/Klotho Axis. Cell Cycle 2021, 20, 522–536. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, X.; Jie, P.; Lu, H.; Zhang, S.; Lin, X.; Emily, K.Y.; Cui, Y.; Yu, J.; et al. Klotho Is Silenced through Promoter Hypermethylation in Gastric Cancer. Am. J. Cancer Res. 2011, 1, 111. [Google Scholar] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular Carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Ganesan, P.; Kulik, L.M. Hepatocellular Carcinoma: New Developments. Clin. Liver Dis. 2023, 27, 85–102. [Google Scholar] [CrossRef]
- Sun, H.; Gao, Y.; Lu, K.; Zhao, G.; Li, X.; Li, Z.; Chang, H. Overexpression of Klotho Suppresses Liver Cancer Progression and Induces Cell Apoptosis by Negatively Regulating Wnt/β-Catenin Signaling Pathway. World J. Surg. Oncol. 2015, 13, 307. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, J.; Yuan, L.; Ren, F.; Liu, D.C.; Li, Q.; Shu, G. Epigenetic Silencing of Klotho Expression Correlates with Poor Prognosis of Human Hepatocellular Carcinoma. Hum. Pathol. 2013, 44, 795–801. [Google Scholar] [CrossRef]
- Cagle, P.T.; Allen, T.C.; Olsen, R.J. Lung Cancer Biomarkers: Present Status and Future Developments. Arch. Pathol. Lab. Med. 2013, 137, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Huang, G.; He, D.; He, J.; Xu, W.; Zou, C.; Zong, F.; Li, Y.; Chen, B.; et al. Klotho Sensitizes Human Lung Cancer Cell Line to Cisplatin via PI3k/Akt Pathway. PLoS ONE 2013, 8, e57391. [Google Scholar] [CrossRef]
- Ibi, T.; Usuda, J.; Inoue, T.; Sato, A.; Takegahara, K. Klotho Expression Is Correlated to Molecules Associated with Epithelial-Mesenchymal Transition in Lung Squamous Cell Carcinoma. Oncol. Lett. 2017, 14, 5526–5532. [Google Scholar] [CrossRef] [PubMed]
- Brominska, B.; Gabryel, P.; Jarmołowska-Jurczyszyn, D.; Janicka-Jedyńska, M.; Kluk, A.; Trojanowski, M.; Brajer-Luftmann, B.; Woliński, K.; Czepczyński, R.; Gut, P.; et al. Klotho Expression and Nodal Involvement as Predictive Factors for Large Cell Lung Carcinoma. Arch. Med. Sci. 2019, 15, 1010–1016. [Google Scholar] [CrossRef]
- Warren, J.L.; Harlan, L.C.; Trimble, E.L.; Stevens, J.; Grimes, M.; Cronin, K.A. Trends in the Receipt of Guideline Care and Survival for Women with Ovarian Cancer: A Population-Based Study. Gynecol. Oncol. 2017, 145, 486–492. [Google Scholar] [CrossRef]
- Lojkin, I.; Rubinek, T.; Orsulic, S.; Schwarzmann, O.; Karlan, B.Y.; Bose, S.; Wolf, I. Reduced Expression and Growth Inhibitory Activity of the Aging Suppressor Klotho in Epithelial Ovarian Cancer. Cancer Lett. 2015, 362, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, Y.; Xiong, Y.; Lin, X.; Zhou, P.; Chen, Z. Reduced Klotho Expression Contributes to Poor Survival Rates in Human Patients with Ovarian Cancer, and Overexpression of Klotho Inhibits the Progression of Ovarian Cancer Partly via the Inhibition of Systemic Inflammation in Nude Mice. Mol. Med. Rep. 2017, 15, 1777–1785. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P. Pancreatic Cancer Epidemiology: Understanding the Role of Lifestyle and Inherited Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Rubinstein, T.A.; Reuveni, I.; Hesin, A.; Klein-Goldberg, A.; Olauson, H.; Larsson, T.E.; Abraham, C.R.; Zeldich, E.; Bosch, A.; Chillón, M.; et al. A Transgenic Model Reveals the Role of Klotho in Pancreatic Cancer Development and Paves the Way for New Klotho-Based Therapy. Cancers 2021, 13, 6297. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Gu, Y.; Chen, Y. Identification of Novel Predictive Markers for the Prognosis of Pancreatic Ductal Adenocarcinoma. Cancer Investig. 2014, 32, 218–225. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Spring, D.J.; Depinho, R.A. Genetics and Biology of Prostate Cancer. Genes. Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Miyake, M.; Hori, S.; Onishi, S.; Iida, K.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Tanaka, N.; Fujimoto, K. γ–Klotho Is Correlated with Resistance to Docetaxel in Castration–Resistant Prostate Cancer. Oncol. Lett. 2020, 19, 2306–2316. [Google Scholar] [CrossRef]
- Drucker, B.J. Renal Cell Carcinoma: Current Status and Future Prospects. Cancer Treat. Rev. 2005, 31, 536–545. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, L.; Zhang, J.; Xu, W.; Liu, Y.; Yin, H.; Lv, T.; An, H.; Liu, L.; He, H.; et al. Klotho Suppresses Tumor Progression via Inhibiting PI3K/Akt/GSK3β/Snail Signaling in Renal Cell Carcinoma. Cancer Sci. 2013, 104, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid Cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Dai, D.; Wang, Q.; Li, X.; Liu, J.; Ma, X.; Xu, W. Klotho Inhibits Human Follicular Thyroid Cancer Cell Growth and Promotes Apoptosis through Regulation of the Expression of Stanniocalcin-1. Oncol. Rep. 2016, 35, 552–558. [Google Scholar] [CrossRef][Green Version]
- Cesari, M.; Prince, M.; Thiyagarajan, J.A.; De Carvalho, I.A.; Bernabei, R.; Chan, P.; Gutierrez-Robledo, L.M.; Michel, J.P.; Morley, J.E.; Ong, P.; et al. Frailty: An Emerging Public Health Priority. J. Am. Med. Dir. Assoc. 2016, 17, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R. Biomarkers of Frailty: Moving the Field Forward. Exp. Gerontol. 2020, 133, 110868. [Google Scholar] [CrossRef]
- Pan, Y.; Ji, T.; Li, Y.; Ma, L. Omics Biomarkers for Frailty in Older Adults. Clin. Chim. Acta 2020, 510, 363–372. [Google Scholar] [CrossRef]
- Shardell, M.; Semba, R.D.; Kalyani, R.R.; Bandinelli, S.; Prather, A.A.; Chia, C.W.; Ferrucci, L. Plasma Klotho and Frailty in Older Adults: Findings From the InCHIANTI Study. J. Gerontol. Ser. A 2019, 74, 1052–1057. [Google Scholar] [CrossRef]
- He, S.; Bhatt, R.; Brown, C.; Brown, E.A.; Buhr, D.L.; Chantranuvatana, K.; Danaher, P.; Dunaway, D.; Garrison, R.G.; Geiss, G.; et al. High-Plex Imaging of RNA and Proteins at Subcellular Resolution in Fixed Tissue by Spatial Molecular Imaging. Nat. Biotechnol. 2022, 40, 1794–1806. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Xia, C.; Close, J.L.; Zhang, M.; He, J.; Huang, Z.; Halpern, A.R.; Long, B.; Miller, J.A.; Lein, E.S.; et al. Conservation and Divergence of Cortical Cell Organization in Human and Mouse Revealed by MERFISH. Science 2022, 377, 56–62. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, X.; Li, Z.; Zhang, T.; Yang, B.; Su, J.; Song, Q. SpaRx: Elucidate Single-Cell Spatial Heterogeneity of Drug Responses for Personalized Treatment. Brief. Bioinform. 2023, 24, bbad338. [Google Scholar] [CrossRef]
- Lin, B.; Ma, Y.; Wu, S. Multi-Omics and Artificial Intelligence-Guided Data Integration in Chronic Liver Disease: Prospects and Challenges for Precision Medicine. OMICS 2022, 26, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of Liver Diseases in the World. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, H.; Chen, D.; Lu, Y.; Li, Y.; Qiao, J. Multi-Omic Analysis Tools for Microbial Metabolites Prediction. Brief. Bioinform. 2024, 25, bbae264. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The Microbiome and Human Cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef] [PubMed]
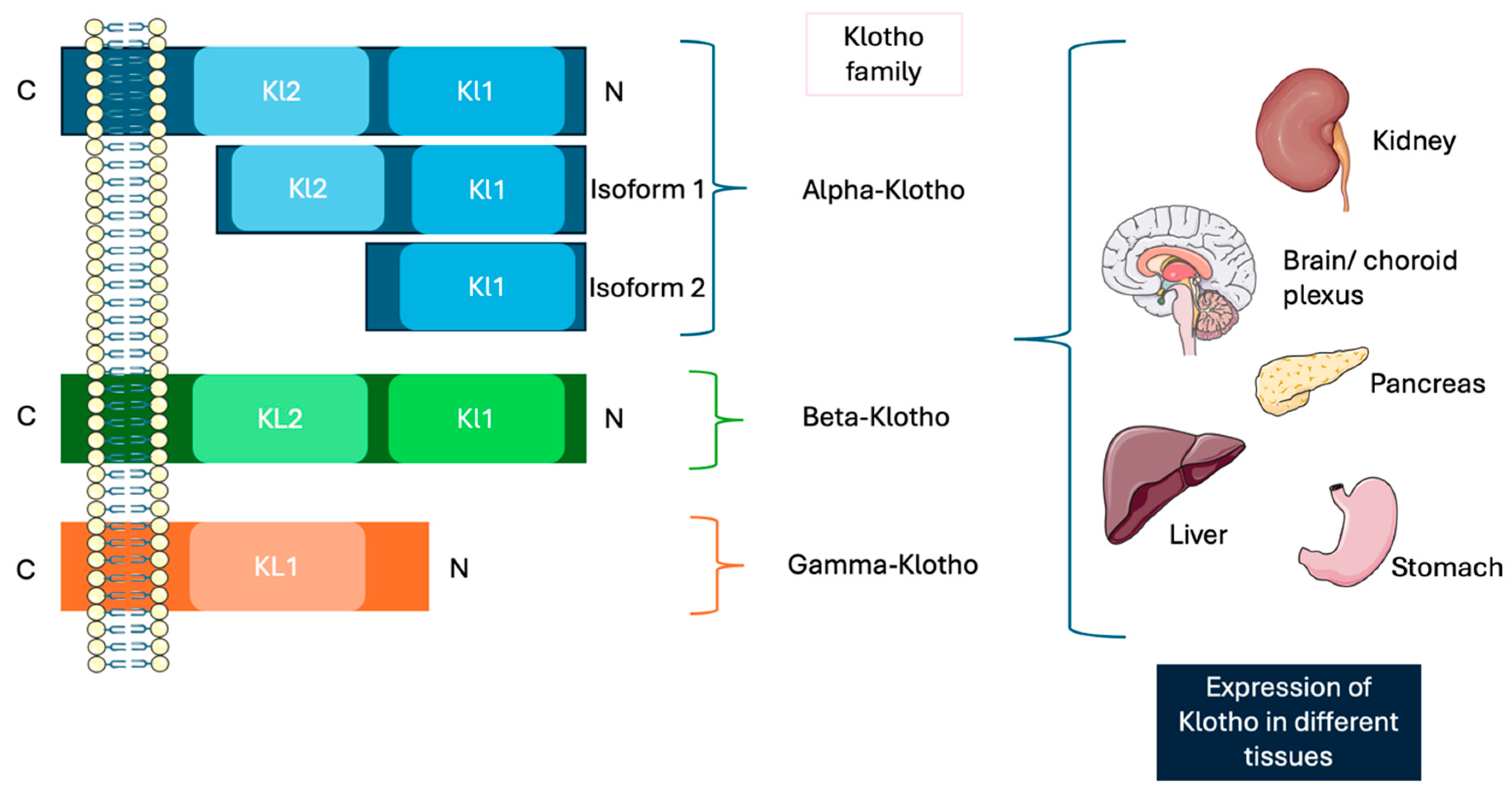
| α-Klotho | β-Klotho | γ-Klotho | |
|---|---|---|---|
| Human chromosome location | Chromosome 13 | Chromosome 4 | - |
| Full-length protein size | 130 KDa/1020 aa | 130 KDa/1044 aa | - |
| Receptor(s) | FGF23 | FGF19 and FGF21 | FGFR1b, FGFR1c, FGFR2c, FGFR4, and FGF19 |
| Expression pattern | Kidneys and brain | Adipocytes, liver, and brain | Ocular, adipose, and renal tissues |
| Functions | Protection against oxidative stress, inhibition of apoptosis and fibrogenesis, promotion of angiogenesis and vascularization, and vasculoprotective functions | Regulation of several metabolic pathways, energy balance, and glucose and lipid homeostasis | Metabolic regulation and cellular protection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega, M.A.; Boaru, D.L.; De Leon-Oliva, D.; De Castro-Martinez, P.; Minaya-Bravo, A.M.; Casanova-Martín, C.; Barrena-Blázquez, S.; Garcia-Montero, C.; Fraile-Martinez, O.; Lopez-Gonzalez, L.; et al. The Impact of Klotho in Cancer: From Development and Progression to Therapeutic Potential. Genes 2025, 16, 128. https://doi.org/10.3390/genes16020128
Ortega MA, Boaru DL, De Leon-Oliva D, De Castro-Martinez P, Minaya-Bravo AM, Casanova-Martín C, Barrena-Blázquez S, Garcia-Montero C, Fraile-Martinez O, Lopez-Gonzalez L, et al. The Impact of Klotho in Cancer: From Development and Progression to Therapeutic Potential. Genes. 2025; 16(2):128. https://doi.org/10.3390/genes16020128
Chicago/Turabian StyleOrtega, Miguel A., Diego Liviu Boaru, Diego De Leon-Oliva, Patricia De Castro-Martinez, Ana M. Minaya-Bravo, Carlos Casanova-Martín, Silvestra Barrena-Blázquez, Cielo Garcia-Montero, Oscar Fraile-Martinez, Laura Lopez-Gonzalez, and et al. 2025. "The Impact of Klotho in Cancer: From Development and Progression to Therapeutic Potential" Genes 16, no. 2: 128. https://doi.org/10.3390/genes16020128
APA StyleOrtega, M. A., Boaru, D. L., De Leon-Oliva, D., De Castro-Martinez, P., Minaya-Bravo, A. M., Casanova-Martín, C., Barrena-Blázquez, S., Garcia-Montero, C., Fraile-Martinez, O., Lopez-Gonzalez, L., Saez, M. A., Alvarez-Mon, M., & Diaz-Pedrero, R. (2025). The Impact of Klotho in Cancer: From Development and Progression to Therapeutic Potential. Genes, 16(2), 128. https://doi.org/10.3390/genes16020128







