The Role of the MTUS1 Gene in the Development of Left Ventricular Noncompaction Cardiomyopathy—A Case Report
Abstract
1. Introduction
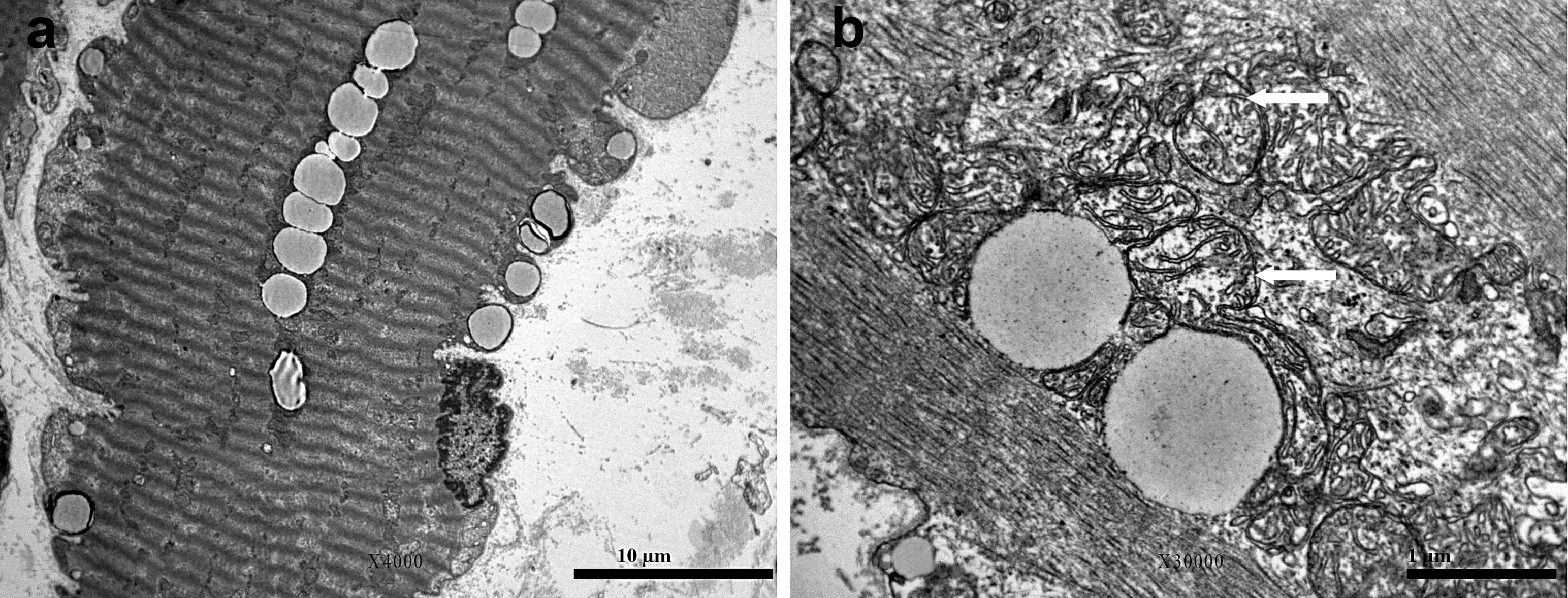
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Benedetto, M.; Bièche, I.; Deshayes, F.; Vacher, S.; Nouet, S.; Collura, V.; Seitz, I.; Louis, S.; Pineau, P.; Amsellem-Ouazana, D.; et al. Structural Organization and Expression of Human MTUS1, a Candidate 8p22 Tumor Suppressor Gene Encoding a Family of Angiotensin II AT2 Receptor-Interacting Proteins, ATIP. Gene 2006, 380, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Asakura, M.; Liao, Y.; Min, K.; Takahashi, A.; Shindo, K.; Yamazaki, S.; Tsukamoto, O.; Asanuma, H.; Mogi, M.; et al. Identification of the Mtus1 Splice Variant as a Novel Inhibitory Factor Against Cardiac Hypertrophy. J. Am. Heart Assoc. 2016, 5, e003521. [Google Scholar] [CrossRef] [PubMed]
- Caporizzo, M.A.; Prosser, B.L. The Microtubule Cytoskeleton in Cardiac Mechanics and Heart Failure. Nat. Rev. Cardiol. 2022, 19, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, B.; Guo, A.; Zhu, Y.; Miller, J.D.; Gao, S.; Yuan, C.; Kutschke, W.; Zimmerman, K.; Weiss, R.M.; et al. Microtubule-Mediated Defects in Junctophilin-2 Trafficking Contribute to Myocyte Transverse-Tubule Remodeling and Ca2+ Handling Dysfunction in Heart Failure. Circulation 2014, 129, 1742–1750. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, Y.; Ouyang, N.; Liu, L.; Huang, X.; Tian, J.; Lv, T. A de Novo Mutation in the MTUS1 Gene Decreases the Risk of Non-Compaction of Ventricular Myocardium via the Rac1/Cdc42 Pathway. Front. Pediatr. 2019, 7, 247. [Google Scholar] [CrossRef]
- Ajima, R.; Bisson, J.A.; Helt, J.-C.; Nakaya, M.-A.; Habas, R.; Tessarollo, L.; He, X.; Morrisey, E.E.; Yamaguchi, T.P.; Cohen, E.D. DAAM1 and DAAM2 Are Co-Required for Myocardial Maturation and Sarcomere Assembly. Dev. Biol. 2015, 408, 126–139. [Google Scholar] [CrossRef]
- Zuern, C.; Krenacs, L.; Starke, S.; Heimrich, J.; Palmetshofer, A.; Holtmann, B.; Sendtner, M.; Fischer, T.; Galle, J.; Wanner, C.; et al. Microtubule Associated Tumor Suppressor 1 Deficient Mice Develop Spontaneous Heart Hypertrophy and SLE-like Lymphoproliferative Disease. Int. J. Oncol. 2012, 40, 1079–1088. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Di Tommaso, A.; Dimitrov, A.; Cazaubon, S.; Gruel, N.; Colasson, H.; Nicolas, A.; Chaverot, N.; Molinié, V.; Reyal, F.; et al. 8p22 MTUS1 Gene Product ATIP3 Is a Novel Anti-Mitotic Protein Underexpressed in Invasive Breast Carcinoma of Poor Prognosis. PLoS ONE 2009, 4, e7239. [Google Scholar] [CrossRef]
- Cheng, L.-Y.; Huang, M.; Zhong, H.-G.; Ru, H.-M.; Mo, S.-S.; Wei, C.-Y.; Su, Z.-J.; Mo, X.-W.; Yan, L.-H.; Tang, W.-Z. MTUS1 Is a Promising Diagnostic and Prognostic Biomarker for Colorectal Cancer. World J. Surg. Oncol. 2022, 20, 257. [Google Scholar] [CrossRef]
- Luxán, G.; Casanova, J.C.; Martínez-Poveda, B.; Prados, B.; D’Amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F.; et al. Mutations in the NOTCH Pathway Regulator MIB1 Cause Left Ventricular Noncompaction Cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef]
- Shi, W.Y.; Moreno-Betancur, M.; Nugent, A.W.; Cheung, M.; Colan, S.; Turner, C.; Sholler, G.F.; Robertson, T.; Justo, R.; Bullock, A.; et al. Long-Term Outcomes of Childhood Left Ventricular Noncompaction Cardiomyopathy: Results From a National Population-Based Study. Circulation 2018, 138, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Price, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and Sudden Death in Pediatric Left Ventricular Noncompaction in a Tertiary Referral Center. Circulation 2013, 127, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, E.J.; Ezeh, E.; Sebro, N.; Olanipekun, O.; Rueda Rios, C. A Novel Case of Acquired Isolated Left Ventricular Non-Compaction in a Primigravida: Revisiting the Diagnostic Criteria of Left Ventricular Non-Compaction. Cureus 2023, 15, e33823. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left Ventricular Non-Compaction Cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P. Can Left Ventricular Noncompaction Be Acquired, and Can It Disappear? Tex. Heart Inst. J. 2017, 44, 264–265. [Google Scholar] [CrossRef]
- Srivastava, S.; Yavari, M.; Al-abcha, A.; Banga, S.; Abela, G. Ventricular Non-Compaction Review. Heart Fail. Rev. 2022, 27, 1063–1076. [Google Scholar] [CrossRef]
- dLib.Si—Spongiformna Kardiomiopatija—Redek Vzrok Srčnega Popuščanja. Available online: https://www.dlib.si/details/URN:NBN:SI:doc-FJSFAAGM?&language=eng (accessed on 19 October 2023).
- Isolated Noncompaction of the Left Ventricular Myocardium in Adults: A Systematic Overview—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21872148/ (accessed on 19 October 2023).
- Varghayee, N.; Krezel, M.A.; Rezmann, L.; Chow, L.; Frauman, A.G.; Louis, W.J.; Louis, S.N. Function and Expression of ATIP and Its Variants in Cardiomyoblast Cell Line H9c2. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 79–91. [Google Scholar] [CrossRef]
- Nouet, S.; Amzallag, N.; Li, J.-M.; Louis, S.; Seitz, I.; Cui, T.-X.; Alleaume, A.-M.; Benedetto, M.D.; Boden, C.; Masson, M.; et al. Trans-Inactivation of Receptor Tyrosine Kinases by Novel Angiotensin II AT2 Receptor-Interacting Protein, ATIP *. J. Biol. Chem. 2004, 279, 28989–28997. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The Role of Reactive Oxygen Species in the Pathophysiology of Cardiovascular Diseases and the Clinical Significance of Myocardial Redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Sandireddy, R.; Cibi, D.M.; Gupta, P.; Singh, A.; Tee, N.; Uemura, A.; Epstein, J.A.; Singh, M.K. Semaphorin 3E/PlexinD1 Signaling Is Required for Cardiac Ventricular Compaction. JCI Insight 2019, 4, e125908. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Qu, X.; Chang, C.-P.; Shou, W. Molecular Mechanism of Ventricular Trabeculation/Compaction and the Pathogenesis of the Left Ventricular Noncompaction Cardiomyopathy (LVNC). Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, J.; Zhu, Z.; Zhang, Z.; Xian, J.; Yang, Z.; Qin, T.; Chen, L.; Huang, J.; Huang, Y.; et al. Overlap Phenotypes of the Left Ventricular Noncompaction and Hypertrophic Cardiomyopathy with Complex Arrhythmias and Heart Failure Induced by the Novel Truncated DSC2 Mutation. Orphanet J. Rare Dis. 2021, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, A.W.; Berman, D.; Moens, C.B. Microtubules Are Required for the Maintenance of Planar Cell Polarity in Monociliated Floorplate Cells. Dev. Biol. 2019, 452, 21–33. [Google Scholar] [CrossRef]
- In Cell Polarity, Microtubules Are Important after All. Available online: https://www.fredhutch.org/en/news/spotlight/2019/06/bs_mathewson_devbiol.html (accessed on 13 January 2025).
- Nakayama, S.; Yano, T.; Namba, T.; Konishi, S.; Takagishi, M.; Herawati, E.; Nishida, T.; Imoto, Y.; Ishihara, S.; Takahashi, M.; et al. Planar Cell Polarity Induces Local Microtubule Bundling for Coordinated Ciliary Beating. J. Cell Biol. 2021, 220, e202010034. [Google Scholar] [CrossRef]
- Taylan Şekeroğlu, H.; Utine, G.E. Congenital Cataract and Its Genetics: The Era of Next-Generation Sequencing. Turk. J. Ophthalmol. 2021, 51, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rahi, J.S.; Dezateux, C. Congenital and Infantile Cataract in the United Kingdom: Underlying or Associated Factors. British Congenital Cataract Interest Group. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2108–2114. [Google Scholar]
- Bundschu, K.; Schuh, K. Cardiovascular ATIP (Angiotensin Receptor Type 2 Interacting Protein) Expression in Mouse Development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2014, 243, 699–711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorjanc, T.; Šikonja, J.; Drole Torkar, A.; Žerjav Tanšek, M.; Kovač, J.; Bertok, S.; Debeljak, M.; Dolenc-Stražar, Z.; Meznarič, M.; Mlakar, J.; et al. The Role of the MTUS1 Gene in the Development of Left Ventricular Noncompaction Cardiomyopathy—A Case Report. Genes 2025, 16, 110. https://doi.org/10.3390/genes16020110
Gorjanc T, Šikonja J, Drole Torkar A, Žerjav Tanšek M, Kovač J, Bertok S, Debeljak M, Dolenc-Stražar Z, Meznarič M, Mlakar J, et al. The Role of the MTUS1 Gene in the Development of Left Ventricular Noncompaction Cardiomyopathy—A Case Report. Genes. 2025; 16(2):110. https://doi.org/10.3390/genes16020110
Chicago/Turabian StyleGorjanc, Tevž, Jaka Šikonja, Ana Drole Torkar, Mojca Žerjav Tanšek, Jernej Kovač, Sara Bertok, Maruša Debeljak, Zvezdana Dolenc-Stražar, Marija Meznarič, Jernej Mlakar, and et al. 2025. "The Role of the MTUS1 Gene in the Development of Left Ventricular Noncompaction Cardiomyopathy—A Case Report" Genes 16, no. 2: 110. https://doi.org/10.3390/genes16020110
APA StyleGorjanc, T., Šikonja, J., Drole Torkar, A., Žerjav Tanšek, M., Kovač, J., Bertok, S., Debeljak, M., Dolenc-Stražar, Z., Meznarič, M., Mlakar, J., Topalović, M., Mlakar, G., Battelino, T., & Grošelj, U. (2025). The Role of the MTUS1 Gene in the Development of Left Ventricular Noncompaction Cardiomyopathy—A Case Report. Genes, 16(2), 110. https://doi.org/10.3390/genes16020110







