Abstract
Background/Objectives: The microtubule-associated scaffold protein 1 (MTUS1) gene affects the microtubule stability and cell polarity in the heart and could thus lead to the development of left ventricular noncompaction (LVNC). Pathological gene variants in MTUS1 are associated with pathological phenotypes in both cell cultures and animal models. However, the literature lacks human studies on the specific effects of the MTUS1 gene in heart disease, particularly in congenital LVNC. Methods: We present a case of a male infant, diagnosed with LVNC, who passed away at the age of 8 months due to end-stage heart failure. In the investigation process of the etiology of LVNC, whole-genome sequencing using next-generation sequencing was performed in the patient and his first-degree family members. Results: Genetic analysis identified two heterozygous variants in the MTUS1 gene (NM_001363059.2:c.87C>G and NM_001363059.2:c.2449+421_2288-425del) in the presented patient. The first variant introduced an early stop codon, while the second caused the deletion of an entire exon, both of which significantly altered the protein structure. The older brother of the patient, at the age of 5 years, was a carrier of both variants; however, he was asymptomatic and without signs of heart disease on cardiac ultrasonography. Conclusions: Although, in theory, defects in the MTUS1 gene may contribute to the development of LVNC, our observations indicate that MTUS1 variants alone are not sufficient to cause LVNC or lead to any significant developmental disorder. Additional factors, whether genetic or environmental, are likely necessary for the clinical manifestation of LVNC.
1. Introduction
The microtubule-associated scaffold protein 1 (MTUS1) gene, located on chromosome 8p22, encodes multiple isoforms of angiotensin II receptor-interacting proteins (ATIP1/3/4) and plays a role in cardiovascular system development and function [,]. The protein ATIP3 influences microtubule stability, thereby regulating the structure and function of the cytoskeleton. Sustained microtubule stabilization negatively impacts cardiac function in cardiomyopathies by altering calcium handling and the distribution of cell organelles, while, conversely, microtubule destabilization is associated with maintained cardiac function in heart pathologies and improved heart failure outcomes in animal models [,]. Microtubule stability also serves a crucial role in influencing cell polarization, a fundamental process that is vital for myocardial compaction during cardiac embryogenesis []. Given its role in cytoskeleton regulation, MTUS1 can impact cardiac embryogenesis and function throughout life [,].
Defects in the MTUS1 gene have been associated with numerous diseases []. It exhibits antiproliferative effects and is considered a tumor suppressor gene; its reduced expression is associated with various types of malignancies, including breast, colorectal, pancreatic, lung, ovarian, and oral cancers [,]. Furthermore, its antiproliferative function is also significant in the cardiovascular system during both embryogenesis and in adulthood, as MTUS1 knockout mice displayed a higher body mass throughout their lifespans, elevated blood pressure, spontaneous heart hypertrophy, and a shorter lifespan than wild-type mice. On the other hand, the overexpression of certain MTUS1 gene transcripts reduced cardiac hypertrophic remodeling in mouse models subjected to pressure overload or phenylephrine treatment, preventing both a beneficial adaptive phase and a maladaptive phase of cardiac hypertrophy [,]. Additionally, the MTUS1 gene decreases the microtubule stability in cell lines and may therefore have a role in advanced heart failure where microtubule overstabilization is seen [,,]. The literature lacks human studies on the specific effects of the MTUS1 gene in heart disease.
Left ventricular noncompaction cardiomyopathy (LVNC) is a distinct congenital cardiomyopathy characterized by abnormally prominent trabeculations and deep recesses within the wall of the LV []. The clinical features are heterogeneous, as patients may present with signs and symptoms of acute or chronic heart failure early in life or in adulthood; however, in the majority of cases, the condition remains asymptomatic []. While many genes have already been linked to LVNC [], the role of MTUS1 remains unclear. In a recent study, it was shown that, in cell lines, a genetic variant in the MTUS1 gene reduces the extent of noncompaction by decreasing the microtubule stability and enhancing cell polarization; consequently, this effect could confer a protective role against LVNC, given the significance of cell polarity in the normal densification of the myocardium [,,].
In this article, we aim to further explain the role of MTUS1 in LVCN by presenting two brothers with identical heterozygous genetic variants in the MTUS1 gene and a diametrical phenotype. While the first developed LVNC that led to lethal heart failure, the other remained asymptomatic without detectable heart disease.
2. Case Presentation
A male infant was referred to the University Children’s Hospital Ljubljana at seven months of age due to acute decompensation of dilated noncompaction cardiomyopathy. The patient was born to a Caucasian mother at 37 weeks of gestation. The pregnancy was uneventful, and he had a birth weight of 2440 g (3rd percentile), birth length of 45 cm (3rd percentile), and head circumference of 34.5 cm (25th percentile). His Apgar score was 9/9.
At the age of 3 months, he was diagnosed with bilateral cataracts that were surgically corrected. While suffering from a respiratory infection, a chest radiograph was performed at the age of 6 months, which revealed mild interstitial thickening together with cardiomegaly. In the following month, the boy became unusually irritable and was referred to the University Medical Centre Ljubljana due to the acute decompensation of heart failure with respiratory insufficiency. Initial laboratory tests revealed significantly elevated troponin (10.6 μg/I; reference range (RR): <0.1 μg/I), NT-pro-BNP (111011 ng/L; RR: <125.0 ng/L), and liver transaminases (ALT 11.51 µkat/L, RR: <0.58 µkat/L; AST 15.55 µkat/L, RR: <0.58 µkat/L). Parenteral diuretic therapy was immediately initiated; however, the condition progressed to cardiogenic shock.
Echocardiography revealed noncompaction dilated cardiomyopathy characterized by a dilated (LV diameter: 35 mm) and globally hypokinetic LV. The left ventricular ejection fraction, assessed using the M-Mode and BiPlane methods, was significantly reduced (27% and 14%, respectively). Hypertrabeculation of the apicolateral wall was observed on segmental analysis. The coexisting conditions included atrioventricular and ventriculoarterial concordance, mitral and tricuspid insufficiency, and an atrial septal defect with a patent foramen ovale. Cardiac MRI demonstrated marked left ventricular enlargement with severely compromised systolic function (ejection fraction < 10%). Pronounced trabeculations were observed in the lateral, inferior, and anterior walls. Diminished function and enlargement of the right ventricle were also observed. Despite aggressive intensive-care treatment with diuretics, multiple inotropic agents, and vasopressors, his condition continued to deteriorate, and he needed intubation and mechanical ventilation. Over the next few days, he was successfully resuscitated twice; however, in the following days, the patient passed away due to heart failure at the age of 8 months and 11 days.
The postmortem findings showed distinctive macroscopic and histological abnormalities consistent with noncompaction cardiomyopathy and focal dystrophic calcifications within the heart tissue. Heart examination also revealed characteristics of chronic hypoxic–ischemic injury. Furthermore, there were indications of fibrointimal hyperplasia within the coronary arteries, accompanied by the accumulation of a mucoid substance, which was also observed in the aortic valve.
An ultrastructural analysis of the vastus lateralis muscle biopsy revealed normal sarcomeres along with lipid and glycogen accumulation, pointing to a metabolic disorder. Additionally, there were alterations in the mitochondrial structure, including variations in size, a reduced number of cristae, and the presence of giant mitochondria, which lacked paracrystalline inclusions (Figure 1). The enzyme activity of the respiratory chain complexes, determined at the Neurological Institute “Carlo Besta” I.R.C.C.S. Foundation in Milan, was normal.
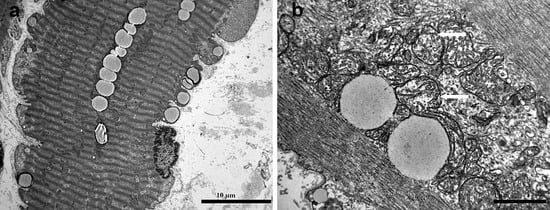
Figure 1.
Electron microscopy of vastus lateralis muscle biopsy: (a) increased number of lipid droplets and subsarcolemmal glycogen in a hypercontracted muscle fiber; (b) arrows point to mitochondria with reduced number of cristae.
Due to the deviations in the mitochondrial structure, suggestive of a mitochondrial disease, we performed next-generation sequencing (NGS) of the mitochondrial genome. We achieved 569-fold average horizontal coverage, allowing for the accurate detection of heteroplasmy levels as low as 10%, and it did not reveal any mitochondrial DNA pathological variants associated with the patient’s clinical phenotype.
Furthermore, we performed a whole-genome sequencing (WGS) trio analysis using NGS in the patient and both of his parents and analyzed genes included in the reference Human Genome 19 (HG19; also known as GRCh37), which revealed that the patient carried two heterozygous variants within the MTUS1 gene. Both variants are classified as variants of unknown significance due to the lack of data. The first variant, NM_001363059.2:c.87C>G, was inherited from his mother and resulted in a significant molecular change, substituting tyrosine at position 29 with a termination codon (p.Tyr29Ter). Predictive algorithms (CADD) indicate a high likelihood of pathogenicity for this variant. The second variant, NM_001363059.2:c.2449+421_2288-425del, spans over the entire exon 4 within the MTUS1 gene and results in a 1-kilobase deletion. This specific genetic change was inherited from his father. The clinical significance of this deletion remains unclear; however, it may be considered pathogenic due to a large-scale deletion. No relevant variants were identified in the analyzed genes in any of the three family members that could explain the clinical presentation, including those associated with cataract development, cardiomyopathies, and inborn errors of metabolism.
The patient’s father, aged 45, has an ascending aortic aneurysm that extends into the common carotid artery, which is currently being monitored. His echocardiography revealed dilation of the aortic bulbus and left ventricular wall hypertrophy. The mother, aged 39 years, has no known chronic comorbidities and her physical examination was normal.
The older brother, who is now 5 years old, showed no apparent signs of disease and was in good general health. Moreover, cardiac ultrasonography performed at the age of 3 did not reveal any cardiac anomalies. On the other hand, no eye disease or vision impairment was detected, including cataracts. This is particularly intriguing as he shared identical genetic variants in the MTUS1 gene (c.87C>G and c.2449+421_2288-425del) with his younger brother. However, as siblings, they had a similar environmental background and shared approximately half of their genetic material.
3. Discussion
This study presents a case of an infant with LVNC and with heterozygous genetic variants in the MTUS1 gene, who passed away at the age of 8 months due to end-stage heart failure.
Considered a congenital anomaly, LVNC has also been reported in acquired forms, occurring in response to physiological stressors such as exercise, dilated cardiomyopathy, and pregnancy [,]. In these cases, the presence of LVNC is likely a result of transient left ventricular dilatation, making milder preexisting forms of LVNC detectable on echocardiography []. During the first few weeks of uterine cardiac development, trabeculations and recesses in the ventricular wall increase the gas exchange area. By the eighth gestational week, these structures compress, and coronary circulation forms, resulting in a smooth ventricular wall. It is believed that any alterations in this compaction process can be causative for LVNC [].
Among all patients who undergo cardiac ultrasonography, LVNC is observed in 0.014% []. Although LVNC can occur sporadically, approximately 30% of cases present as a familial condition among first-degree relatives, most commonly with autosomal dominant inheritance []. Several genes have been implicated in LVNC, including those responsible for encoding sarcomeric proteins (TTN and MYH7), cytoskeletal proteins (P121L), genes related to nuclear membrane components, chaperones, and the TAFAZZIN gene, causing Barth syndrome [].
In pediatric cohorts, LVNC often presents with abnormal ECG, arrhythmias, chest pain, syncope, and sudden cardiac death. Pathological ECG findings were present in 87% of children with LVNC. A particularly poor prognosis was observed in cases where arrhythmia coexisted with morphological abnormalities and ventricular insufficiency [].
The gene MTUS1 encodes multiple ATIP protein isoforms that are widely expressed in normal human tissue. Owing to alternative splicing, three major ATIP isoforms have been identified in humans: ATIP1, ATIP3, and ATIP4 []. In normal and cancer cell lines, research has shown that ATIP3 and ATIP1 effectively suppress cell proliferation by inhibiting the growth factor-induced autophosphorylation of receptor tyrosine kinases and extracellular-regulated kinase and by lowering the reactive oxygen species levels in the mitochondria, which is an independent factor for cardiac hypertrophy [,,].
The exact role of MTUS1 in cardiac embryogenesis and later in life remains unclear in the current literature, and most of the studies were conducted on animals and cell models. The overexpression of MTUS1 can lead to defects, primarily due to its influence on the cytoskeleton. Firstly, specific MTUS1 isoform overexpression in adult mice causes left ventricular thinning, LV dysfunction, and reduced hypertrophic adaptation to artificially induced myocardial overload [,]. On the other hand, adult MTUS1 knockout mice have elevated blood pressure and develop spontaneous heart hypertrophy compared to wild-type mice []. It is supposed that these observations are mainly due to MTUS1’s inhibitory effect on ERK signaling [].
The pathogenesis of LVNC involves two key processes: (i) hypertrabeculation, characterized by excessive trabeculae formation, and (ii) noncompaction, the failure of trabeculae to remodel into a compact myocardial wall. Enhanced Notch signaling, through pathways involving Neuregulin1, EphrinB2, BMP10, TBX20, and Sema3E/PlexinD1, drives abnormal cardiomyocyte proliferation and these structural abnormalities [,]. Planar cell polarity (PCP), regulated by noncanonical Wnt signaling, plays a vital role in cardiomyocyte alignment and myocardial organization, with disruptions contributing to LVNC. Additionally, desmosomal proteins such as desmocollin 2 (DSC2) and desmoglein 2 (DSG2) are critical for cell–cell adhesion and polarization. Mutations in desmosomal genes and PCP pathways disturb cardiomyocyte polarity, mechanical coupling, and electrical signaling, further driving LVNC development [].
Only one study has described the potential role of MTUS1 in LVNC, where it was shown that the MTUS1 genetic variant c.2617A>C (p.Asn873His) in HEK-293 cell lines could reduce the extent of noncompaction, since it decreases the microtubule stability and enhances the cell polarity through the decreased expression of α-tubulin and increased expression of α/β-tubulin heterodimer and PAR6 protein, and it also decreases the level of phosphorylated Rac1/Cdc42 []. In summary, MTUS1 could influence LVNC through its impact on cell polarity and PCP signaling. Microtubules, which MTUS1 helps to regulate, are critical for proper cell–cell junction functioning, receptor localization (e.g., Frizzled receptors), and the orientation of polarity proteins (e.g., Prickle). These processes are essential in maintaining the cytoskeletal arrangement, which in turn supports proper cellular organization and polarization during cardiac morphogenesis. Disruptions in microtubule dynamics and PCP signaling could lead to abnormal trabeculation and the failure of myocardial compaction, key features of LVNC [,,].
During cardiac embryogenesis, the MTUS1 gene affects the microtubule stability and other proteins involved in adequate cell polarization, an essential process for myocardial compaction, and could therefore be associated with congenital cardiomyopathies, including LVNC [,]. Furthermore, it was shown that the MTUS1 genetic variant c.2617A>C (p. Asn873His) in HEK-293 cell lines reduces the extent of noncompaction through enhanced polarization and exerts a protective effect in the development of LVNC; since microtubule overstabilization is also observed in advanced heart failure, variations in MTUS1 might have not yet described roles in cardiac functioning [,].
In addition to LVNC, our patient also had congenital cataracts, which are associated with numerous diseases and genetic abnormalities []. In our case, the cataract was bilateral, and it has been shown that 25% of congenital bilateral cataracts are associated with systemic disease []. However, none of the close relatives had any eye problems, and the genetic analysis of the whole genome did not reveal any pathological variants in genes associated with the development of cataracts. The role of the MTUS1 variant in cataracts is not known, although, in mouse embryogenesis, multiple isoforms of MTUS1 are expressed in different parts of the developing eye and in the vasculature around the eye, indicating a possible role of MTUS1 in eye pathologies [].
The pathological findings in the postmortem autopsy, including an abnormal mitochondrial morphology and muscle biopsy results, along with bilateral congenital cataracts, in the presented case report suggest the possibility of an underlying systemic disease as the cause of LVNC. However, despite extensive diagnostic efforts, including the comprehensive genetic analysis of both the nuclear and mitochondrial genomes, we were unable to fully explain the clinical presentation in our patient.
The association between the identified MTUS1 variant and the underlying heart condition (ascending aortic aneurysm) in the patient’s father is unclear. However, since the pathophysiological mechanisms of aortic aneurysms and LVNC differ, the heart condition in the father may also be an incidental finding. Further research is needed in this area.
Importantly, the patient’s brother also carried the same two genetic variants in the MTUS1 gene and remained in good health at the age of five. Since both variants have a substantial impact on the protein structure (an early stop codon and the deletion of an entire exon), our observations suggest that MTUS1 variants alone do not cause LVNC or manifest in any major developmental disorder, and we suggest that additional factors, possibly genetic or environmental, may be required for the clinical manifestation of LVNC.
4. Conclusions
In conclusion, we analyzed two variants in the MTUS1 gene that significantly affect the protein structure, but these variants alone may not be sufficient to cause LVNC or any other major developmental defect observable in childhood. Given the limited understanding derived from the literature and our own findings, the precise role of MTUS1 in our patient’s unique phenotype remains largely unknown, and additional investigations are required to fully understand the potential clinical effects of MTUS1 mutations later in life.
Author Contributions
Conceptualization, T.G., J.Š. and U.G.; methodology, J.K., S.B., M.D., Z.D.-S., M.M., J.M., M.T. and U.G.; software, J.K., M.D., Z.D.-S., M.T., M.M. and J.M.; validation, M.Ž.T., G.M. and T.B.; formal analysis, T.G. and J.Š.; investigation, all authors; resources, T.B.; data curation, T.G.; writing—original draft preparation, T.G., J.Š., A.D.T. and M.M; writing—review and editing, all authors; visualization, M.M.; supervision, T.B.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by the Slovenian Research and Innovation Agency (grant P3-0343).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki. The study and this paper were approved by the Scientific Committee of the Department of Endocrinology, Diabetes, and Metabolic Diseases, University Children’s Hospital, University Medical Centre Ljubljana.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) or/and their parents to participate in this study, to publish this paper, and to publish pictures and results from genetic studies in an anonymized form. Informed consent forms are available for inspection upon request.
Data Availability Statement
Data are available upon request.
Acknowledgments
We would like to thank the patient’s family for their contribution and support of this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Di Benedetto, M.; Bièche, I.; Deshayes, F.; Vacher, S.; Nouet, S.; Collura, V.; Seitz, I.; Louis, S.; Pineau, P.; Amsellem-Ouazana, D.; et al. Structural Organization and Expression of Human MTUS1, a Candidate 8p22 Tumor Suppressor Gene Encoding a Family of Angiotensin II AT2 Receptor-Interacting Proteins, ATIP. Gene 2006, 380, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Asakura, M.; Liao, Y.; Min, K.; Takahashi, A.; Shindo, K.; Yamazaki, S.; Tsukamoto, O.; Asanuma, H.; Mogi, M.; et al. Identification of the Mtus1 Splice Variant as a Novel Inhibitory Factor Against Cardiac Hypertrophy. J. Am. Heart Assoc. 2016, 5, e003521. [Google Scholar] [CrossRef] [PubMed]
- Caporizzo, M.A.; Prosser, B.L. The Microtubule Cytoskeleton in Cardiac Mechanics and Heart Failure. Nat. Rev. Cardiol. 2022, 19, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, B.; Guo, A.; Zhu, Y.; Miller, J.D.; Gao, S.; Yuan, C.; Kutschke, W.; Zimmerman, K.; Weiss, R.M.; et al. Microtubule-Mediated Defects in Junctophilin-2 Trafficking Contribute to Myocyte Transverse-Tubule Remodeling and Ca2+ Handling Dysfunction in Heart Failure. Circulation 2014, 129, 1742–1750. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, Y.; Ouyang, N.; Liu, L.; Huang, X.; Tian, J.; Lv, T. A de Novo Mutation in the MTUS1 Gene Decreases the Risk of Non-Compaction of Ventricular Myocardium via the Rac1/Cdc42 Pathway. Front. Pediatr. 2019, 7, 247. [Google Scholar] [CrossRef]
- Ajima, R.; Bisson, J.A.; Helt, J.-C.; Nakaya, M.-A.; Habas, R.; Tessarollo, L.; He, X.; Morrisey, E.E.; Yamaguchi, T.P.; Cohen, E.D. DAAM1 and DAAM2 Are Co-Required for Myocardial Maturation and Sarcomere Assembly. Dev. Biol. 2015, 408, 126–139. [Google Scholar] [CrossRef]
- Zuern, C.; Krenacs, L.; Starke, S.; Heimrich, J.; Palmetshofer, A.; Holtmann, B.; Sendtner, M.; Fischer, T.; Galle, J.; Wanner, C.; et al. Microtubule Associated Tumor Suppressor 1 Deficient Mice Develop Spontaneous Heart Hypertrophy and SLE-like Lymphoproliferative Disease. Int. J. Oncol. 2012, 40, 1079–1088. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Di Tommaso, A.; Dimitrov, A.; Cazaubon, S.; Gruel, N.; Colasson, H.; Nicolas, A.; Chaverot, N.; Molinié, V.; Reyal, F.; et al. 8p22 MTUS1 Gene Product ATIP3 Is a Novel Anti-Mitotic Protein Underexpressed in Invasive Breast Carcinoma of Poor Prognosis. PLoS ONE 2009, 4, e7239. [Google Scholar] [CrossRef]
- Cheng, L.-Y.; Huang, M.; Zhong, H.-G.; Ru, H.-M.; Mo, S.-S.; Wei, C.-Y.; Su, Z.-J.; Mo, X.-W.; Yan, L.-H.; Tang, W.-Z. MTUS1 Is a Promising Diagnostic and Prognostic Biomarker for Colorectal Cancer. World J. Surg. Oncol. 2022, 20, 257. [Google Scholar] [CrossRef]
- Luxán, G.; Casanova, J.C.; Martínez-Poveda, B.; Prados, B.; D’Amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F.; et al. Mutations in the NOTCH Pathway Regulator MIB1 Cause Left Ventricular Noncompaction Cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef]
- Shi, W.Y.; Moreno-Betancur, M.; Nugent, A.W.; Cheung, M.; Colan, S.; Turner, C.; Sholler, G.F.; Robertson, T.; Justo, R.; Bullock, A.; et al. Long-Term Outcomes of Childhood Left Ventricular Noncompaction Cardiomyopathy: Results From a National Population-Based Study. Circulation 2018, 138, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Price, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and Sudden Death in Pediatric Left Ventricular Noncompaction in a Tertiary Referral Center. Circulation 2013, 127, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Akhigbe, E.J.; Ezeh, E.; Sebro, N.; Olanipekun, O.; Rueda Rios, C. A Novel Case of Acquired Isolated Left Ventricular Non-Compaction in a Primigravida: Revisiting the Diagnostic Criteria of Left Ventricular Non-Compaction. Cureus 2023, 15, e33823. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left Ventricular Non-Compaction Cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Angelini, P. Can Left Ventricular Noncompaction Be Acquired, and Can It Disappear? Tex. Heart Inst. J. 2017, 44, 264–265. [Google Scholar] [CrossRef]
- Srivastava, S.; Yavari, M.; Al-abcha, A.; Banga, S.; Abela, G. Ventricular Non-Compaction Review. Heart Fail. Rev. 2022, 27, 1063–1076. [Google Scholar] [CrossRef]
- dLib.Si—Spongiformna Kardiomiopatija—Redek Vzrok Srčnega Popuščanja. Available online: https://www.dlib.si/details/URN:NBN:SI:doc-FJSFAAGM?&language=eng (accessed on 19 October 2023).
- Isolated Noncompaction of the Left Ventricular Myocardium in Adults: A Systematic Overview—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21872148/ (accessed on 19 October 2023).
- Varghayee, N.; Krezel, M.A.; Rezmann, L.; Chow, L.; Frauman, A.G.; Louis, W.J.; Louis, S.N. Function and Expression of ATIP and Its Variants in Cardiomyoblast Cell Line H9c2. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 79–91. [Google Scholar] [CrossRef]
- Nouet, S.; Amzallag, N.; Li, J.-M.; Louis, S.; Seitz, I.; Cui, T.-X.; Alleaume, A.-M.; Benedetto, M.D.; Boden, C.; Masson, M.; et al. Trans-Inactivation of Receptor Tyrosine Kinases by Novel Angiotensin II AT2 Receptor-Interacting Protein, ATIP *. J. Biol. Chem. 2004, 279, 28989–28997. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The Role of Reactive Oxygen Species in the Pathophysiology of Cardiovascular Diseases and the Clinical Significance of Myocardial Redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Sandireddy, R.; Cibi, D.M.; Gupta, P.; Singh, A.; Tee, N.; Uemura, A.; Epstein, J.A.; Singh, M.K. Semaphorin 3E/PlexinD1 Signaling Is Required for Cardiac Ventricular Compaction. JCI Insight 2019, 4, e125908. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Qu, X.; Chang, C.-P.; Shou, W. Molecular Mechanism of Ventricular Trabeculation/Compaction and the Pathogenesis of the Left Ventricular Noncompaction Cardiomyopathy (LVNC). Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, J.; Zhu, Z.; Zhang, Z.; Xian, J.; Yang, Z.; Qin, T.; Chen, L.; Huang, J.; Huang, Y.; et al. Overlap Phenotypes of the Left Ventricular Noncompaction and Hypertrophic Cardiomyopathy with Complex Arrhythmias and Heart Failure Induced by the Novel Truncated DSC2 Mutation. Orphanet J. Rare Dis. 2021, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, A.W.; Berman, D.; Moens, C.B. Microtubules Are Required for the Maintenance of Planar Cell Polarity in Monociliated Floorplate Cells. Dev. Biol. 2019, 452, 21–33. [Google Scholar] [CrossRef]
- In Cell Polarity, Microtubules Are Important after All. Available online: https://www.fredhutch.org/en/news/spotlight/2019/06/bs_mathewson_devbiol.html (accessed on 13 January 2025).
- Nakayama, S.; Yano, T.; Namba, T.; Konishi, S.; Takagishi, M.; Herawati, E.; Nishida, T.; Imoto, Y.; Ishihara, S.; Takahashi, M.; et al. Planar Cell Polarity Induces Local Microtubule Bundling for Coordinated Ciliary Beating. J. Cell Biol. 2021, 220, e202010034. [Google Scholar] [CrossRef]
- Taylan Şekeroğlu, H.; Utine, G.E. Congenital Cataract and Its Genetics: The Era of Next-Generation Sequencing. Turk. J. Ophthalmol. 2021, 51, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Rahi, J.S.; Dezateux, C. Congenital and Infantile Cataract in the United Kingdom: Underlying or Associated Factors. British Congenital Cataract Interest Group. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2108–2114. [Google Scholar]
- Bundschu, K.; Schuh, K. Cardiovascular ATIP (Angiotensin Receptor Type 2 Interacting Protein) Expression in Mouse Development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2014, 243, 699–711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).