Metabolic and Proteomic Reveals of 7Li (Lithium-7) Ion Beam Radiation in Capsicum annuum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenotypic Identification and Analysis
2.3. Identification of Different Resistance Levels of Pepper Seedlings
2.4. Proteomics and Data Analysis
2.5. Untargeted Metabolomics and Data Analysis
2.6. Quantitative Real-Time PCR
3. Results
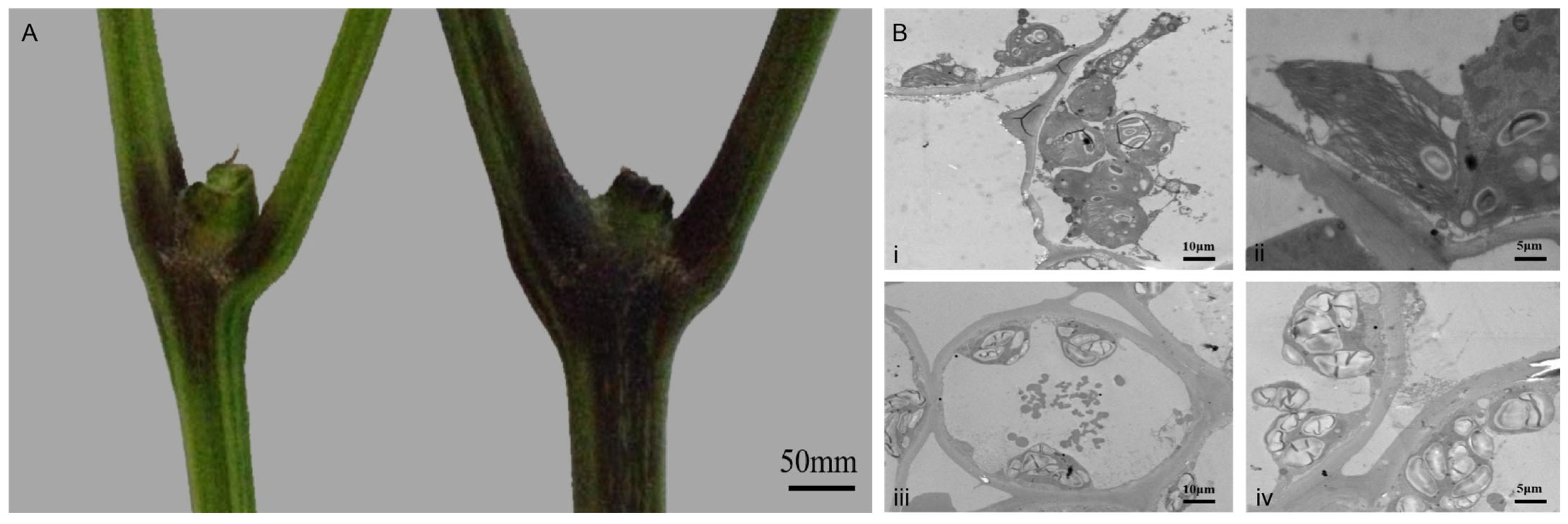
3.1. Plant Growth Phenotypes Under Distinct Stress Regimes
3.2. TEM Analyses of Pepper Stem Cross-Sections
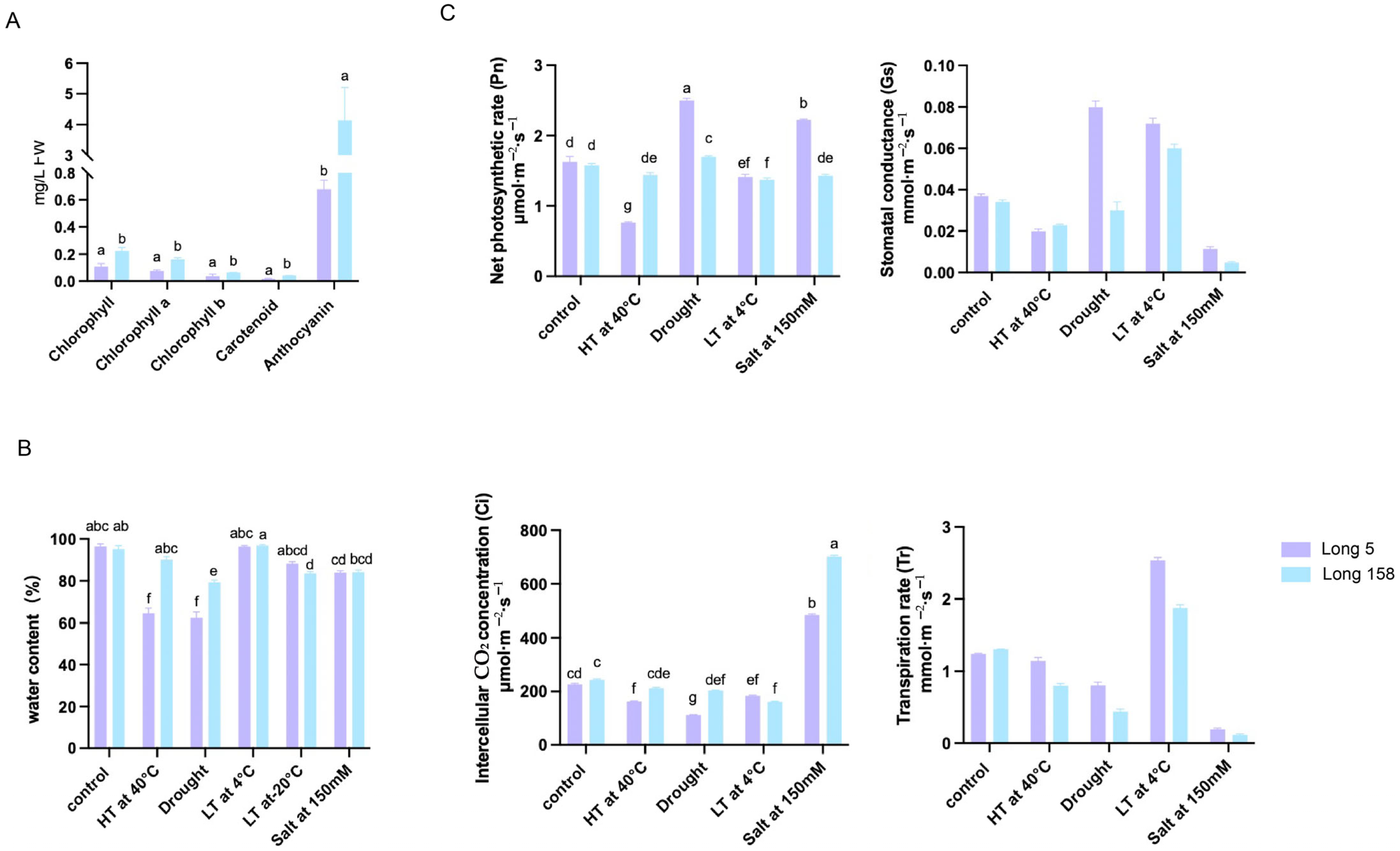
3.3. Contents of Physiological Assays and Enzyme Activity Determination
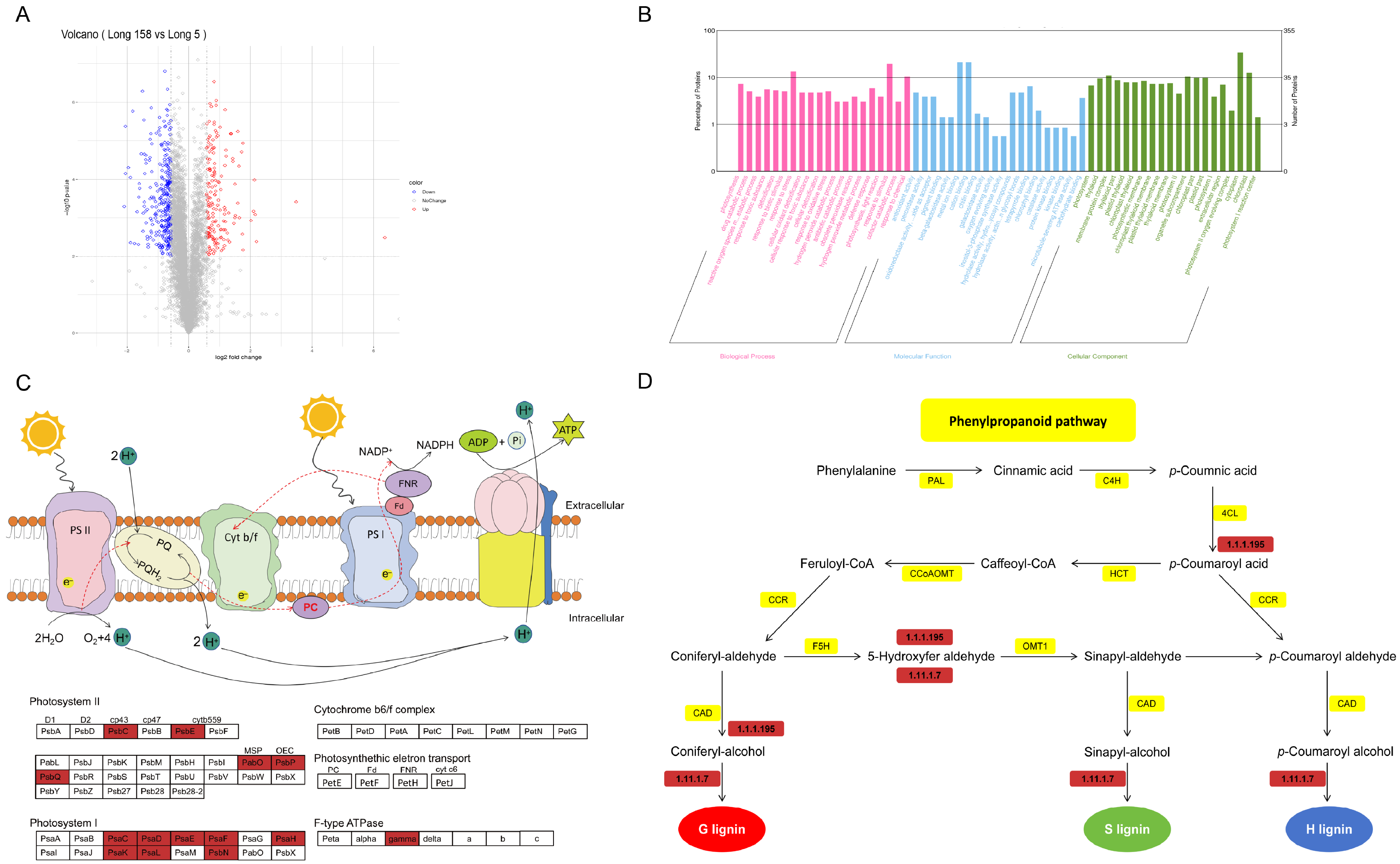
3.4. GO Enrichment and KEGG Pathway Analyses of DEPs Response to Long 5 and Long 158
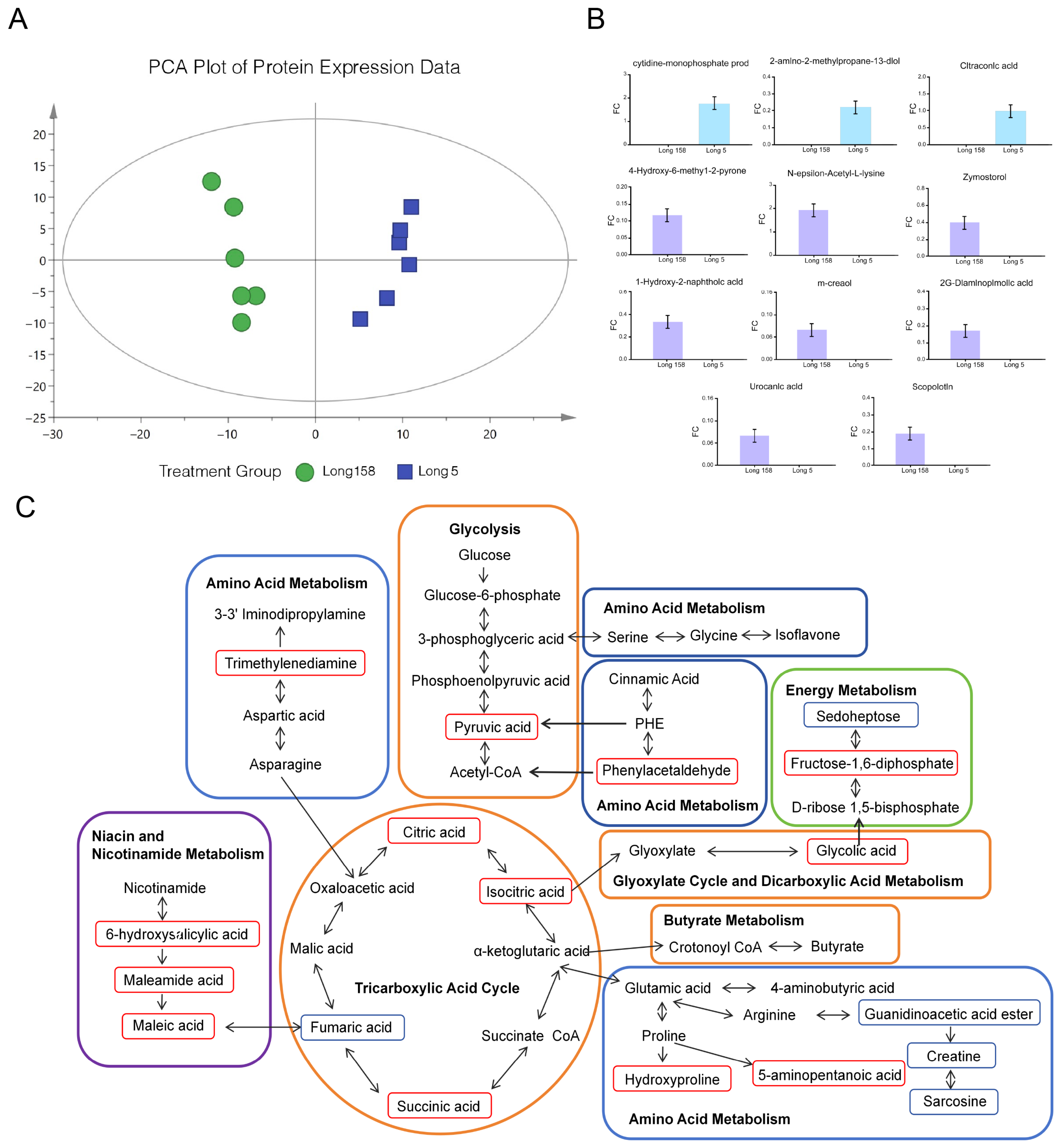
3.5. Differentially Accumulated Metabolites in Capsicum annuum L.
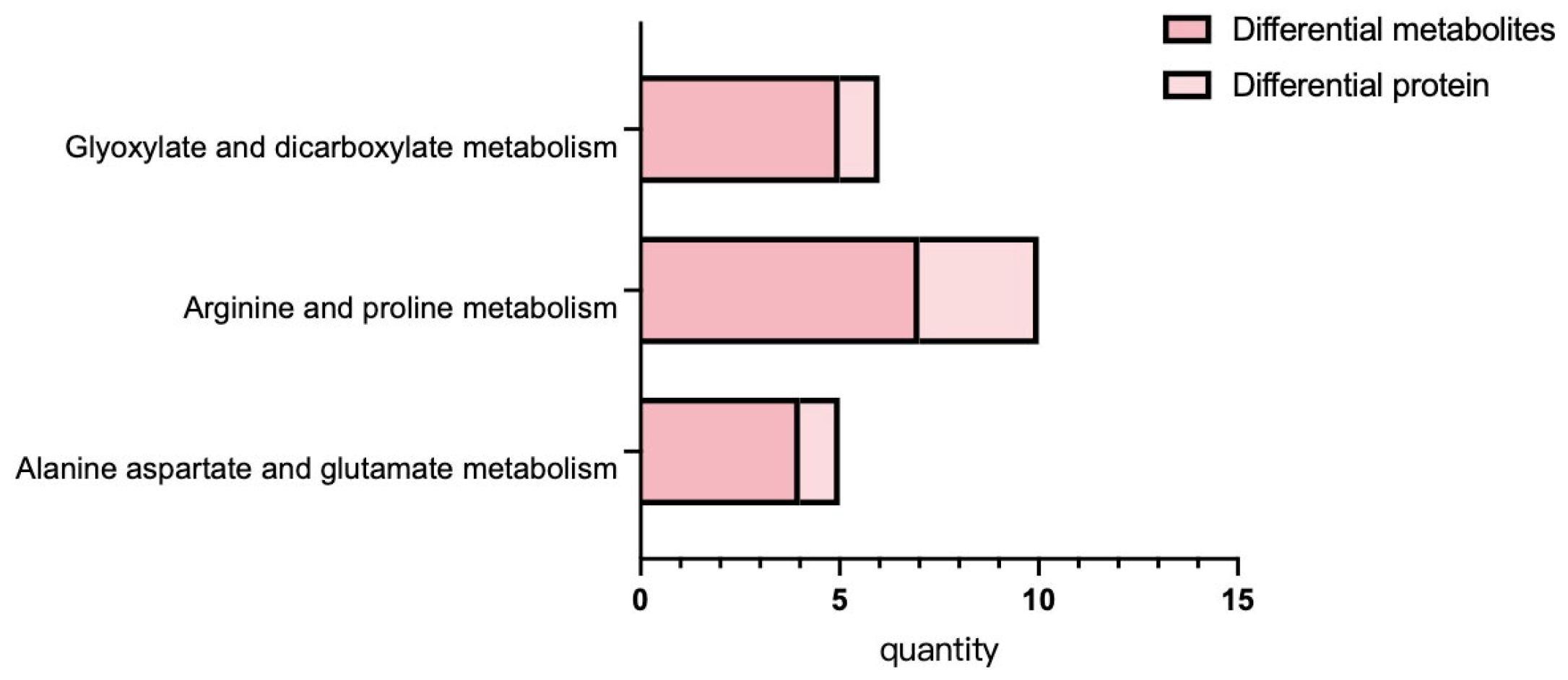
3.6. Correlation Analysis of Proteomic and Metabolome
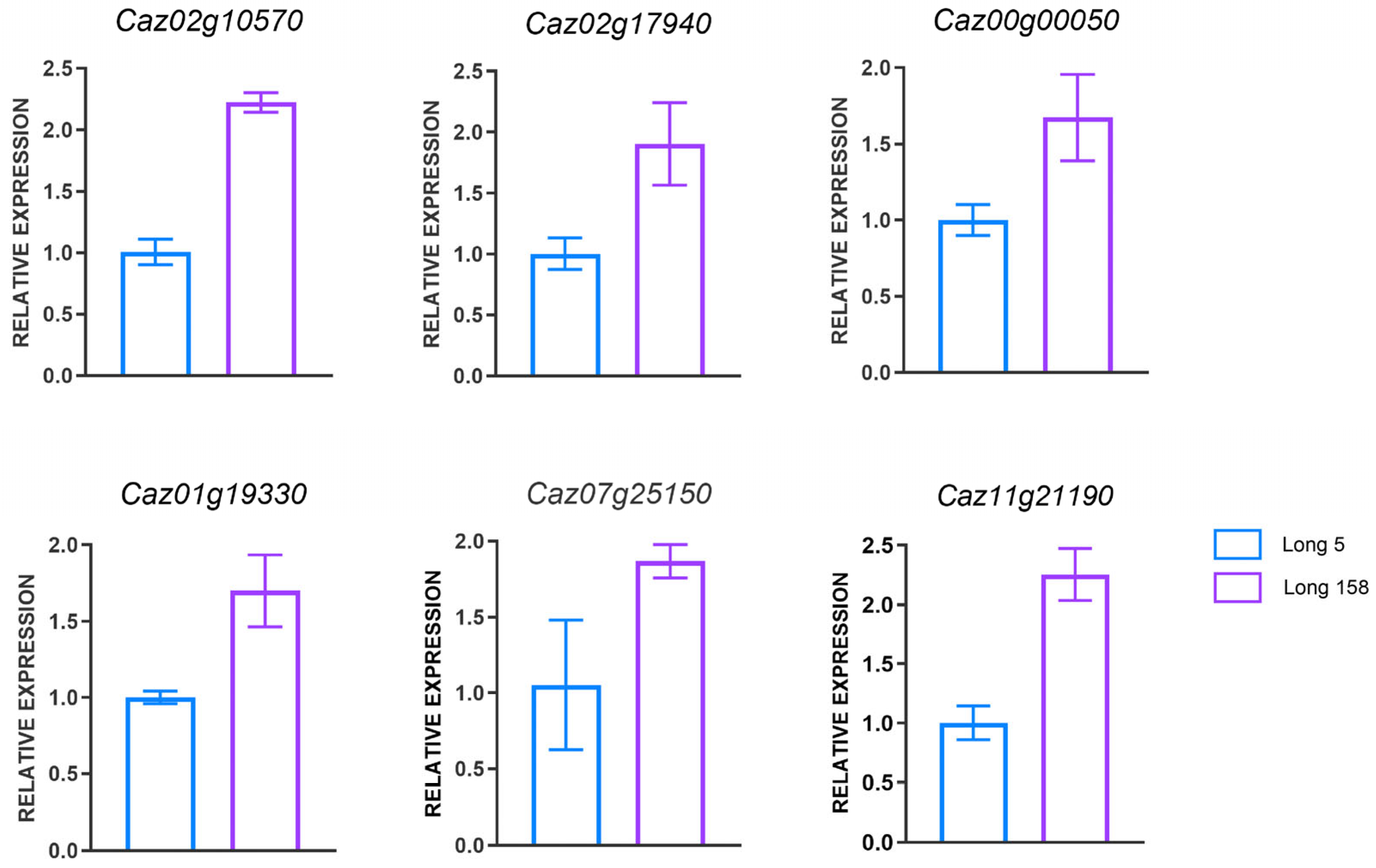
3.7. Real-Time PCR Validation of DEGs in Capsicum annuum L.
4. Discussion
4.1. Physiological Traits of Pepper Plants to 7Li Ion Beam Radiation
4.2. Differential Protein and Metabolite Analysis
4.3. Analysis of Differentially Expressed Genes in Phenylpropanoid and Photosynthetic Pathways Following 7Li Ion Mutagenesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hervert-Hernandez, D.; Sayago-Ayerdi, S.G.; Goni, I. Bioactive compounds of four hot pepper varieties (Capsicum annuum L.), antioxidant capacity, and intestinal bioaccessibility. J. Agric. Food Chem. 2010, 58, 3399–3406. [Google Scholar] [CrossRef] [PubMed]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Li, L.; Hu, Z.; Chen, Y.; Tan, T.; Jia, Y.; Xie, Q.; Chen, G. Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in purple pepper. J. Agric. Food Chem. 2020, 68, 12152–12163. [Google Scholar] [CrossRef] [PubMed]
- Suprasanna, P.; Mirajkar, S.; Bhagwat, S. Induced mutations and crop improvement. In Plant Biology and Biotechnology: Volume I: Plant Diversity, Organization, Function and Improvement; Springer: New York, NY, USA, 2015; pp. 593–617. [Google Scholar]
- Xiong, H.; Guo, H.; Xie, Y.; Gu, J.; Zhao, L.; Zhao, S.; Ding, Y.; Kong, F.; Sui, L.; Liu, L. Comparative transcriptome analysis of two common wheat varieties induced by 7Li-ion beam irradiation reveals mutation hotspot regions and associated pathways. Radiat. Phys. Chem. 2020, 170, 108650. [Google Scholar] [CrossRef]
- Shikazono, N.; Tanaka, A.; Kitayama, S.; Watanabe, H.; Tano, S. LET dependence of lethality in Arabidopsis thaliana irradiated by heavy ions. Radiat. Environ. Biophys. 2002, 41, 159–162. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, L.; Li, W.; Du, Y.; Yu, L.; Feng, H.; Mu, J.; Chen, Y. Mutagenic effects of carbon ion beam irradiations on dry Lotus japonicus seeds. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 383, 123–128. [Google Scholar] [CrossRef]
- Du, Y.; Luo, S.; Yu, L.; Cui, T.; Chen, X.; Yang, J.; Li, X.; Li, W.; Wang, J.; Zhou, L. Strategies for identification of mutations induced by carbon-ion beam irradiation in Arabidopsis thaliana by whole genome re-sequencing. Mutat. Res. 2018, 807, 21–30. [Google Scholar] [CrossRef]
- Li, C.; Tang, J.; Hu, Z.; Wang, J.; Yu, T.; Yi, H.; Cao, M. A novel maize dwarf mutant generated by Ty1-copia LTR-retrotransposon insertion in Brachytic2 after spaceflight. Plant Cell Rep. 2020, 39, 393–408. [Google Scholar] [CrossRef]
- Zeng, D.; Cui, J.; Yin, Y.; Dai, C.; Zhao, H.; Song, C.; Guan, S.; Cheng, D.; Sun, Y.; Lu, W. Combining proteomics and metabolomics to analyze the effects of spaceflight on rice progeny. Front. Plant Sci. 2022, 13, 900143. [Google Scholar] [CrossRef]
- Wang, C.L.; Chen, Q.F.; Mei, S.; Jin, W. Mutagtnic Effect of Some New Mutagens on Rice. J. Nucl. Agric. Sci. 2000, 14, 268–273. [Google Scholar] [CrossRef]
- Hirano, T.; Kazama, Y.; Ishii, K.; Ohbu, S.; Shirakawa, Y.; Abe, T. Comprehensive identification of mutations induced by heavy-ion beam irradiation in Arabidopsis thaliana. Plant J. 2015, 82, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kazama, Y.; Saito, H.; Yamamoto, Y.Y.; Hayashi, Y.; Ichida, H.; Ryuto, H.; Fukunishi, N.; Abe, T. LET-dependent effects of heavy-ion beam irradiation in Arabidopsis thaliana. Plant Biotechnol. 2008, 25, 113–117. [Google Scholar] [CrossRef]
- Du, Y.; Luo, S.; Li, X.; Yang, J.; Cui, T.; Li, W.; Yu, L.; Feng, H.; Chen, Y.; Mu, J. Identification of substitutions and small insertion-deletions induced by carbon-ion beam irradiation in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1851. [Google Scholar] [CrossRef] [PubMed]
- Magori, S.; Tanaka, A.; Kawaguchi, M. Physically induced mutation: Ion beam mutagenesis. In The Handbook of Plant Mutation Screening; WILEY-VCH Verlag: Weinheim, Germany, 2010; pp. 3–16. [Google Scholar]
- Wang, X.; Xie, L.; Liu, L.; Chen, L.; Zhang, H. Biological effects of 7lithium (7Li) ion beam radiation on mutation induction in Capsicum annuum L. Pak. J. Bot 2020, 52, 861–864. [Google Scholar] [CrossRef]
- Dwiranti, A.; Takata, H.; Uchiyama, S.; Fukui, K. The effect of magnesium ions on chromosome structure as observed by scanning electron microscopy (SEM) and scanning transmission electron microscope (STEM) tomography. Chromosome Sci. 2016, 19, 19–23. [Google Scholar] [CrossRef]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficients of chlorophyll a and b in N, N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef]
- Larbi, A.; Abadía, A.; Abadía, J.; Morales, F. Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynth. Res. 2006, 89, 113–126. [Google Scholar] [CrossRef]
- Gao, M.; Qi, Y.; Song, W.; Xu, H. Effects of di-n-butyl phthalate and di (2-ethylhexyl) phthalate on the growth, photosynthesis, and chlorophyll fluorescence of wheat seedlings. Chemosphere 2016, 151, 76–83. [Google Scholar] [CrossRef]
- Sax, K. The stimulation of plant growth by ionizing radiation. Radiat. Bot. 1963, 3, 179–186. [Google Scholar] [CrossRef]
- Ling, A.P.K.; Ung, Y.C.; Hussein, S.; Harun, A.R.; Tanaka, A.; Yoshihiro, H. Morphological and biochemical responses of Oryza sativa L. (cultivar MR219) to ion beam irradiation. J. Zhejiang Univ. Sci. B 2013, 14, 1132–1143. [Google Scholar] [CrossRef]
- Jia, C.; Li, A. Effect of gamma radiation on mutant induction of Fagopyrum dibotrys Hara. Photosynthetica 2008, 46, 363–369. [Google Scholar] [CrossRef]
- Jie, H.; Shaoning, R.; Sizu, L.; Mingyu, X.; Tao, Z.; Hengxian, D. A comparative study on chlorophyll fluorescence and chlorophyll content characteristics effects of Ti+ and Fe+ ion implantation in Chinese fir seedling. J. Radiat. Res. Radiat. Process. 2015, 33, 010402. [Google Scholar] [CrossRef]
- Shi, Q.; Du, J.; Zhu, D.; Li, X.; Li, X. Metabolomic and transcriptomic analyses of anthocyanin biosynthesis mechanisms in the color mutant Ziziphus jujuba cv. Tailihong. J. Agric. Food Chem. 2020, 68, 15186–15198. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.A.; Van Velzen, E.J.; Hoefsloot, H.C.; Smilde, A.K. Multivariate paired data analysis: Multilevel PLSDA versus OPLSDA. Metabolomics 2010, 6, 119–128. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Cass, C.L.; Peraldi, A.; Dowd, P.F.; Mottiar, Y.; Santoro, N.; Karlen, S.D.; Bukhman, Y.V.; Foster, C.E.; Thrower, N.; Bruno, L.C. Effects of PHENYLALANINE AMMONIA LYASE (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachypodium. J. Exp. Bot. 2015, 66, 4317–4335. [Google Scholar] [CrossRef]
- La Camera, S.; Gouzerh, G.; Dhondt, S.; Hoffmann, L.; Fritig, B.; Legrand, M.; Heitz, T. Metabolic reprogramming in plant innate immunity: The contributions of phenylpropanoid and oxylipin pathways. Immunol. Rev. 2004, 198, 267–284. [Google Scholar] [CrossRef]
- Humphreys, J.M.; Chapple, C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002, 5, 224–229. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Zhang, F.; Yu, J.; Li, C.; Zhao, T.; Zhang, Y.; Xie, Q.; Su, B.; Mei, L. Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. 2019, 100, 784–800. [Google Scholar] [CrossRef]
- Mant, A.; Woolhead, C.A.; Moore, M.; Henry, R.; Robinson, C. Insertion of PsaK into the thylakoid membrane in a “Horseshoe” conformation occurs in the absence of signal recognition particle, nucleoside triphosphates, or functional albino3. J. Biol. Chem. 2001, 276, 36200–36206. [Google Scholar] [CrossRef]
- Naver, H.; Haldrup, A.; Scheller, H.V. Cosuppression of Photosystem I Subunit PSI-H in Arabidopsis thaliana: Efficient electron transfer and stability of photosystem I is dependent upon the PSI-H subunit. J. Biol. Chem. 1999, 274, 10784–10789. [Google Scholar] [CrossRef]
- Pan, X.; Ma, J.; Su, X.; Cao, P.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 2018, 360, 1109–1113. [Google Scholar] [CrossRef]

| protein | Accession | Name | Flod Change | p-Value |
| A0A2G2YUX8 | carbamoyl-phosphate synthase | −1.63 | 0.0001 | |
| metabolin | Name | Mutation Weight Value | Flod Change | p-Value |
| Citrate | 1.23 | 1.81 | 0.046 | |
| Fumarate | 1.12 | 1.24 | 0.0247 | |
| Succinate | 1.09 | 1.48 | 0.0239 | |
| Pyruvate | 1.20 | 2.05 | 0.0130 |
| protein | Accession | Name | Flod Change | p-Value |
| A0A2G2YAT8 | catalase (CAT1; CAT2) | 1.78 | 0.0001 | |
| metabolin | Name | Mutation Weight Value | Flod Change | p-Value |
| Citrate | 1.23 | 1.81 | 0.046 | |
| Isocitrate | 1.36 | 1.60 | 0.003 | |
| Succinate | 1.09 | 1.48 | 0.0239 | |
| Pyruvate | 1.20 | 2.05 | 0.0138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, M.; Li, Y.; Wang, X.; Gu, C.; Wu, J.; Wang, X. Metabolic and Proteomic Reveals of 7Li (Lithium-7) Ion Beam Radiation in Capsicum annuum L. Genes 2025, 16, 1486. https://doi.org/10.3390/genes16121486
Huang Y, Li M, Li Y, Wang X, Gu C, Wu J, Wang X. Metabolic and Proteomic Reveals of 7Li (Lithium-7) Ion Beam Radiation in Capsicum annuum L. Genes. 2025; 16(12):1486. https://doi.org/10.3390/genes16121486
Chicago/Turabian StyleHuang, Yue, Maojingkai Li, Yan Li, Xingliang Wang, Chongyu Gu, Jianzhong Wu, and Xue Wang. 2025. "Metabolic and Proteomic Reveals of 7Li (Lithium-7) Ion Beam Radiation in Capsicum annuum L." Genes 16, no. 12: 1486. https://doi.org/10.3390/genes16121486
APA StyleHuang, Y., Li, M., Li, Y., Wang, X., Gu, C., Wu, J., & Wang, X. (2025). Metabolic and Proteomic Reveals of 7Li (Lithium-7) Ion Beam Radiation in Capsicum annuum L. Genes, 16(12), 1486. https://doi.org/10.3390/genes16121486







