Exploring the Genetic Causes of Nonsyndromic Retinal Dystrophies in Qatar
Abstract
1. Introduction
2. Materials and Methods
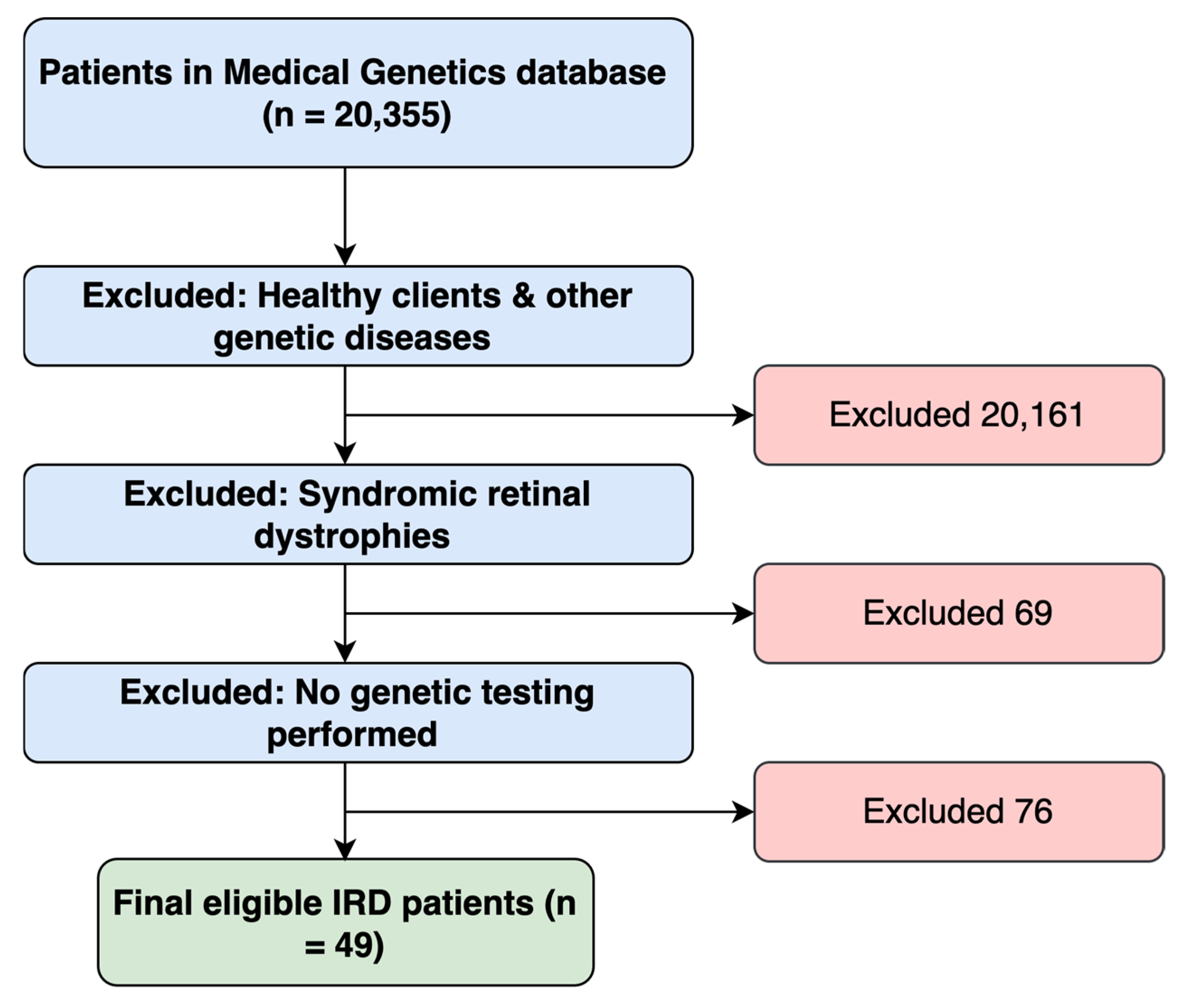
2.1. Study Design and Participants
2.2. Genetic Testing
2.3. Patient Classification
2.4. Investigating Identified Variants
2.5. Investigations on the Variants’ Novelty and PyMol Analysis
2.6. Statistical Analysis
3. Results
3.1. Patients’ Demographics and Clinical Characteristics
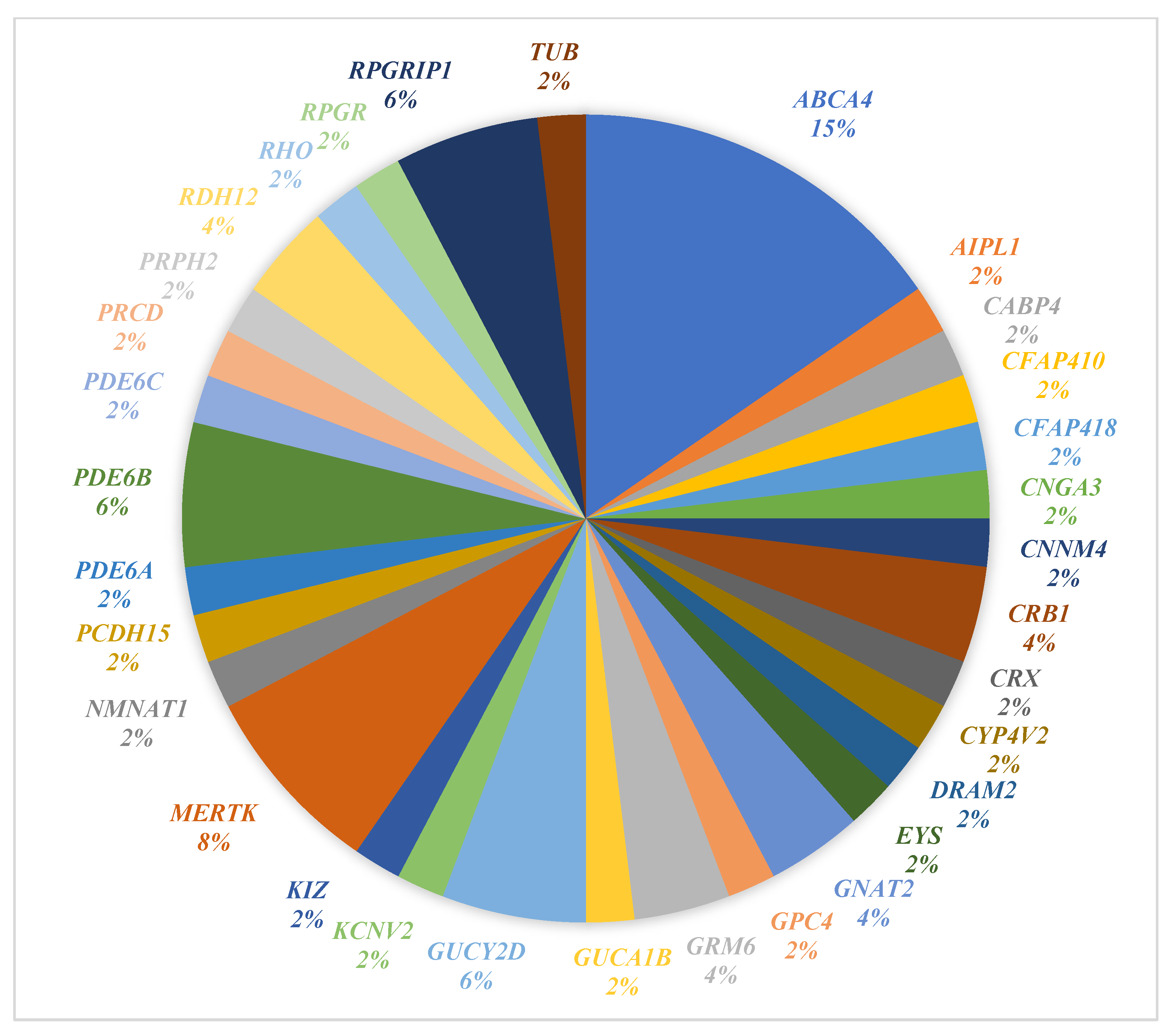
3.2. Genetic Findings
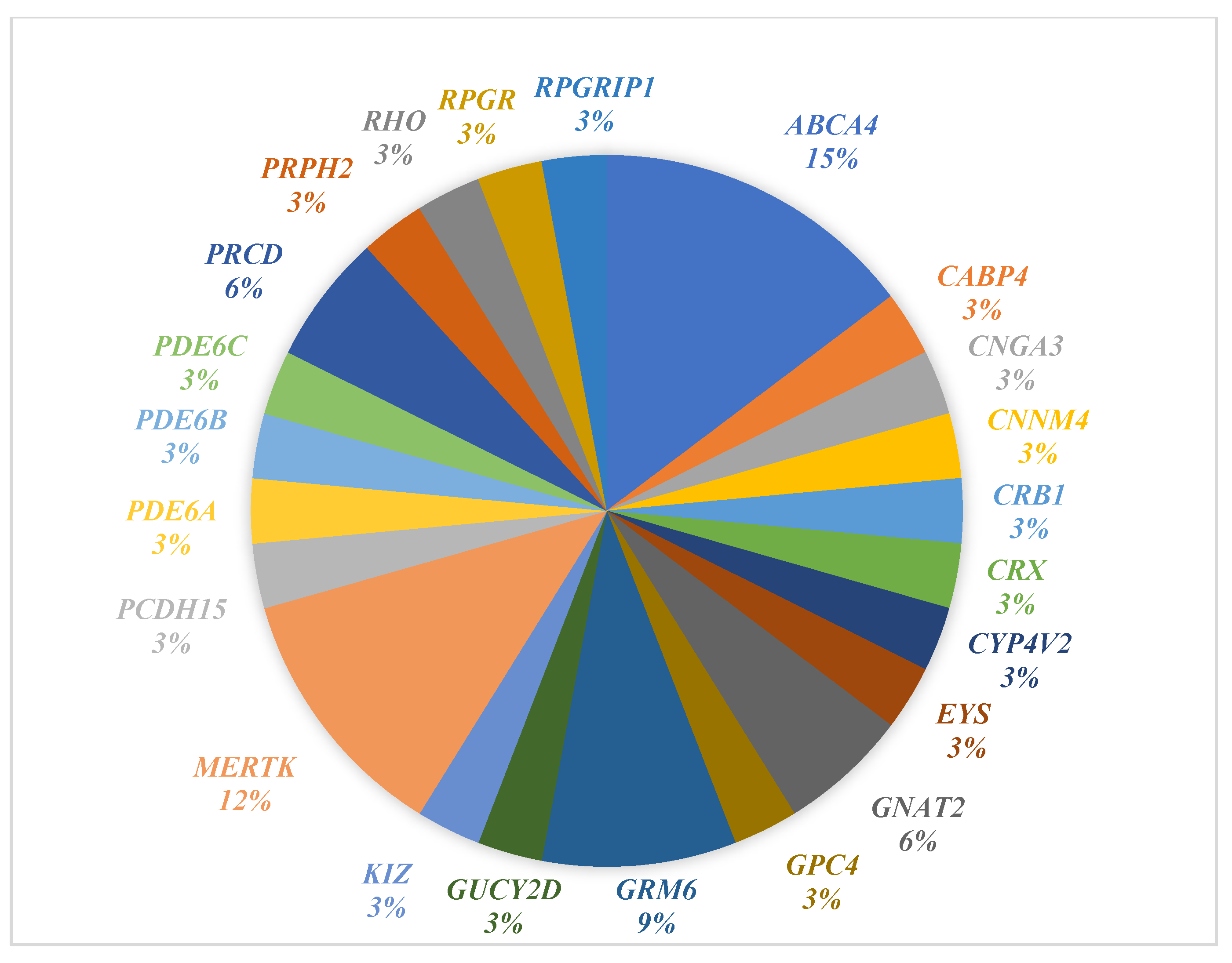
3.3. Solved Cases
3.4. Unsolved Cases
3.5. Uncertain Cases
3.6. Shared vs. Novel Variants
3.7. Molecular Visualization Analysis
3.8. Test Frequency and Diagnostic Yield
4. Discussion
4.1. Genetic Testing Options
4.2. Genetic Test Results
4.3. Solved Cases
4.4. Unsolved Cases
4.5. Uncertain Cases
4.6. Molecular Visualization of the Six Novel Variants
4.7. Genotype-Phenotype Correlation
4.8. Therapeutic Options
4.9. Qatar’s IRD Genetic Landscape in the Context of Arab Populations
4.10. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IRD | Inherited Retinal Dystrophies |
| LCA | Leber Congenital Amaurosis |
| RP | Retinitis Pigmentosa |
| HMC | Hamad Medical Corporation |
| WES | Whole-exome sequencing |
| WES Plus | Whole-exome sequencing and mitochondrial genome testing |
| WGS | Whole genome sequencing |
| NGS | Next-generation sequencing |
| ACMG | American College of Medical Genetics and Genomics |
| VUS | Variant of uncertain significance |
| AD | Autosomal dominant |
| AR | Autosomal recessive |
| AD/AR | Autosomal dominant & Autosomal recessive |
| XL | X-linked |
| AVMD | Adult-onset vitelliform macular dystrophy |
| AAV | Adeno-associated virus |
References
- Georgiou, M.; Robson, A.G.; Fujinami, K.; de Guimarães, T.A.C.; Fujinami-Yokokawa, Y.; Varela, M.D.; Pontikos, N.; Kalitzeos, A.; Mahroo, O.A.; Webster, A.R.; et al. Phenotyping and genotyping inherited retinal diseases: Molecular genetics, clinical and imaging features, and therapeutics of macular dystrophies, cone and cone-rod dystrophies, rod-cone dystrophies, Leber congenital amaurosis, and cone dysfunction syndromes. Prog. Retin. Eye Res. 2024, 100, 101244. [Google Scholar]
- Werdich, X.Q.; Place, E.M.; Pierce, E.A. Systemic diseases associated with retinal dystrophies. Semin. Ophthalmol. 2014, 29, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.O. Phenotype-Guided Genetic Testing of Pediatric Inherited Retinal Disease in the United Arab Emirates. Retina 2020, 40, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.P.W.; Lamey, T.; McLaren, T.; Thompson, J.A.; Montgomery, H.; De Roach, J. Progress and prospects of next-generation sequencing testing for inherited retinal dystrophy. Expert Rev. Mol. Diagn. 2015, 15, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.; Cordeddu, V.; Gaddini, L.; Matteucci, A.; Parravano, M.; Malchiodi-Albedi, F.; Varano, M. Gene therapy in retinal dystrophies. Int. J. Mol. Sci. 2019, 20, 5722. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.H.; Chuang, J.Z. The cell biology of vision. J. Cell Biol. 2010, 190, 953–963. [Google Scholar] [CrossRef]
- Nash, B.M.; Wright, D.C.; Grigg, J.R.; Bennetts, B.; Jamieson, R.V. Retinal dystrophies, genomic applications in diagnosis and prospects for therapy. Transl. Pediatr. 2015, 4, 139. [Google Scholar]
- Wang, F.; Wang, H.; Tuan, H.F.; Nguyen, D.H.; Sun, V.; Keser, V.; Bowne, S.J.; Sullivan, L.S.; Luo, H.; Zhao, L.; et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: Identification of a novel genotype-phenotype correlation and clinical refinements. Hum. Genet. 2014, 133, 331–345. [Google Scholar] [CrossRef]
- Lam, B.L.; Leroy, B.P.; Black, G.; Ong, T.; Yoon, D.; Trzupek, K. Genetic testing and diagnosis of inherited retinal diseases. Orphanet J. Rare Dis. 2021, 16, 514. [Google Scholar] [CrossRef]
- Audo, I.; Bujakowska, K.M.; Léveillard, T.; Mohand-Saïd, S.; Lancelot, M.-E.; Germain, A.; Antonio, A.; Michiels, C.; Saraiva, J.-P.; Letexier, M.; et al. Development and application of a next-generation-sequencing (NGS) approach to detect known and novel gene defects underlying retinal diseases. Orphanet J. Rare Dis. 2012, 7, 8. [Google Scholar] [CrossRef]
- Gonzàlez-Duarte, R.; de Castro-Miró, M.; Tuson, M.; Ramírez-Castañeda, V.; Gils, R.V.; Marfany, G. Scaling New Heights in the Genetic Diagnosis of Inherited Retinal Dystrophies. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Al Mansouri, F.; Al Laftah, F.A.M. Causes of blindness among children at Al Noor Institute for the Visually handicapped in Qatar. Qatar Med. J. 2003, 12, 14. [Google Scholar] [CrossRef]
- Jaffal, L.; Joumaa, H.; Mrad, Z.; Zeitz, C.; Audo, I.; El Shamieh, S. The genetics of rod-cone dystrophy in Arab countries: A systematic review. Eur. J. Hum. Genet. 2021, 29, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Strong, S.; Liew, G.; Michaelides, M. Retinitis pigmentosa-associated cystoid macular oedema: Pathogenesis and avenues of intervention. Br. J. Ophthalmol. 2017, 101, 31–37. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA approves hereditary blindness gene therapy. Nat. Biotechnol. 2018, 36, 6. [Google Scholar] [CrossRef]
- Ellard, S.; Baple, E.L.; Callaway, A.; Berry, I.; Forrester, N.; Turnbull, C.; Owens, M.; Eccles, D.M.; Abbs, S.; Scott, R.; et al. ACGS Best Practice Guidelines for Variant Classification in Rare Disease (Version 4.01). Association for Clinical Genomic Science. 2020. Available online: https://www.acgs.uk.com/media/11631/uk-practice-guidelines-for-variant-classification-v4-01-2020.pdf (accessed on 23 June 2024).
- Sharon, D.; Ben-Yosef, T.; Goldenberg-Cohen, N.; Pras, E.; Gradstein, L.; Soudry, S.; Mezer, E.; Zur, D.; Abbasi, A.H.; Zeitz, C.; et al. A nationwide genetic analysis of inherited retinal diseases in Israel as assessed by the Israeli inherited retinal disease consortium (IIRDC). Hum. Mutat. 2020, 41, 140–149. [Google Scholar] [CrossRef]
- Jespersgaard, C.; Fang, M.; Bertelsen, M.; Dang, X.; Jensen, H.; Chen, Y.; Bech, N.; Dai, L.; Rosenberg, T.; Zhang, J.; et al. Molecular genetic analysis using targeted NGS analysis of 677 individuals with retinal dystrophy. Sci. Rep. 2019, 9, 1219. [Google Scholar] [CrossRef]
- Maltese, P.E.; Colombo, L.; Martella, S.; Rossetti, L.; El Shamieh, S.; Sinibaldi, L.; Passarelli, C.; Coppè, A.M.; Buzzonetti, L.; Falsini, B.; et al. Genetics of Inherited Retinal Diseases in Understudied Ethnic Groups in Italian Hospitals. Front. Genet. 2022, 13, 914345. [Google Scholar] [CrossRef]
- Patel, N.; Alkuraya, H.; Alzahrani, S.; Nowailaty, S.; Seidahmed, M.; Alhemidan, A.; Ben-Omran, T.; Ghazi, N.; Al-Aqeel, A.; Al-Owain, M.; et al. Mutations in known disease genes account for the majority of autosomal recessive retinal dystrophies. Clin. Genet. 2018, 94, 554–563. [Google Scholar] [CrossRef]
- Huang, X.-F.; Huang, F.; Wu, K.-C.; Wu, J.; Chen, J.; Pang, C.-P.; Lu, F.; Qu, J.; Jin, Z.-B. Genotype-phenotype correlation and mutation spectrum in a large cohort of patients with inherited retinal dystrophy revealed by next-generation sequencing. Genet. Med. 2015, 17, 271–278. [Google Scholar] [CrossRef]
- Khan, A.O.; Alrashed, M.; Alkuraya, F.S. Clinical characterisation of the CABP4-related retinal phenotype. Br. J. Ophthalmol. 2013, 97, 262–265. [Google Scholar] [CrossRef]
- El Mouzan, M.I.; Al Salloum, A.A.; Al Herbish, A.S.; Qurachi, M.M.; Al Omar, A.A. Consanguinity and major genetic disorders in Saudi children: A community-based cross-sectional study. Ann. Saudi Med. 2008, 28, 169–173. [Google Scholar]
- Shahid, H.; Khan, J.C.; Cipriani, V.; Sepp, T.; Matharu, B.K.; Bunce, C.; Harding, S.P.; Clayton, D.G.; Moore, A.T.; Yates, J.R.W.; et al. Age-related macular degeneration: The importance of family history as a risk factor. Br. J. Ophthalmol. 2012, 96, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Abu-Safieh, L.; Alrashed, M.; Anazi, S.; Alkuraya, H.; Khan, A.O.; Al-Owain, M.; Al-Zahrani, J.; Al-Abdi, L.; Hashem, M.; Al-Tarimi, S.; et al. Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res. 2013, 23, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Carelli, V.; Ross-Cisneros, F.N.; Sadun, A.A. Mitochondrial dysfunction as a cause of optic neuropathies. Prog. Retin. Eye Res. 2004, 23, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Areblom, M.; Kjellström, S.; Andréasson, S.; Öhberg, A.; Gränse, L.; Kjellström, U. A Description of the Yield of Genetic Reinvestigation in Patients with Inherited Retinal Dystrophies and Previous Inconclusive Genetic Testing. Genes 2023, 14, 1413. [Google Scholar] [CrossRef]
- Pontikos, N.; Arno, G.; Jurkute, N.; Schiff, E.; Ba-Abbad, R.; Malka, S.; Gimenez, A.; Georgiou, M.; Wright, G.; Armengol, M.; et al. Genetic Basis of Inherited Retinal Disease in a Molecularly Characterized Cohort of More Than 3000 Families from the United Kingdom. Ophthalmology 2020, 127, 1384–1394. [Google Scholar] [CrossRef]
- Maugeri, A.; Klevering, B.J.; Rohrschneider, K.; Blankenagel, A.; Brunner, H.G.; Deutman, A.F.; Hoyng, C.B.; Cremers, F.P. Mutations in the ABCA4 (ABCR) Gene Are the Major Cause of Autosomal Recessive Cone-Rod Dystrophy. Am. J. Hum. Genet. 2000, 67, 960–966. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Yu, S.; Gong, Y.; Zhang, M.; Wu, Y.; Liu, W.; Sun, J.; Chen, J.; Sun, X.; et al. The central retinal thickness and its related genotype in ABCA4-related retinopathy. Eye 2024, 38, 2718–2733. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Chen, N.; Wang, L.; Zhang, F.; Ma, Z.; Li, G.; Yang, L. Application of Whole Exome and Targeted Panel Sequencing in the Clinical Molecular Diagnosis of 319 Chinese Families with Inherited Retinal Dystrophy and Comparison Study. Genes 2018, 9, 360. [Google Scholar] [CrossRef]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Strick, D.J.; Vollrath, D. Focus on Molecules: MERTK. Exp. Eye Res. 2010, 91, 786. [Google Scholar] [CrossRef]
- Tada, A.; Wada, Y.; Sato, H.; Itabashi, T.; Kawamura, M.; Tamai, M.; Nishida, K. Screening of the MERTK gene for mutations in Japanese patients with autosomal recessive retinitis pigmentosa. Mol. Vis. 2006, 12, 441–444. Available online: https://europepmc.org/article/med/16710167 (accessed on 8 April 2023).
- Pagnamenta, A.T.; Camps, C.; Giacopuzzi, E.; Taylor, J.M.; Hashim, M.; Calpena, E.; Kaisaki, P.J.; Hashimoto, A.; Yu, J.; Sanders, E.; et al. Structural and non-coding variants increase the diagnostic yield of clinical whole genome sequencing for rare diseases. Genome Med. 2023, 15, 94. [Google Scholar] [CrossRef]
- Crincoli, E.; Zhao, Z.; Querques, G.; Sacconi, R.; Carlà, M.M.; Giannuzzi, F.; Ferrara, S.; Ribarich, N.; L’aBbate, G.; Rizzo, S.; et al. Deep learning to distinguish Best vitelliform macular dystrophy (BVMD) from adult-onset vitelliform macular degeneration (AVMD). Sci. Rep. 2022, 12, 12745. [Google Scholar] [CrossRef]
- Méjécase, C.; Malka, S.; Guan, Z.; Slater, A.; Arno, G.; Moosajee, M. Practical guide to genetic screening for inherited eye diseases. Ther. Adv. Ophthalmol. 2020, 12, 251584142095459. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.; Parens, E.; Chung, W.K.; Berger, S.M.; Appelbaum, P.S. The Challenge of Genetic Variants of Uncertain Clinical Significance: A Narrative Review. Ann. Intern. Med. 2022, 175, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Tzekov, R.; Stein, L.; Kausha, S. Protein Misfolding and Retinal Degeneration. Cold Spring Harb. Perspect. Biol. 2011, 3, a007492. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.A.; Shroyer, N.F.; Singh, N.; Allikmets, R.; Hutchinson, A.; Li, Y.; Lupski, J.R.; Leppert, M.; Dean, M. Genotype/phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am. J. Hum. Genet. 1999, 64, 422–434. [Google Scholar]
- Patel, N.; Aldahmesh, M.A.; Alkuraya, H.; Anazi, S.; Alsharif, H.; Khan, A.O.; Sunker, A.; Al-Mohsen, S.; Abboud, E.B.; Nowilaty, S.R.; et al. Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet. Med. 2016, 18, 554–562. [Google Scholar]
- Khan, A.O. Ocular genetic disease in the Middle East. Curr. Opin. Ophthalmol. 2013, 24, 369–378. [Google Scholar] [CrossRef]
- Khan, A.O. Homozygosity for a Novel Double Mutant Allele (G1961E/L857P) Underlies Childhood-Onset Abca4-Related Retinopathy in The United Arab Emirates. Retina 2020, 40, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.A.; Pennesi, M.E. The new landscape of retinal gene therapy. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Piotter, E.; McClements, M.E.; MacLaren, R.E. Therapy approaches for stargardt disease. Biomolecules 2021, 11, 1179. [Google Scholar] [CrossRef] [PubMed]
- John, S.W.; Smith, R.S.; Savinova, O.V.; Hawes, N.L.; Chang, B.; Turnbull, D.; Davisson, M.; Roderick, T.H.; Heckenlively, J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 1998, 39, 951–962. [Google Scholar]
- Lorget, F.; Marie, M.; Khabou, H.; Simon, C.; Nuno, D.; Vanlandingham, P.; Quiambao, A.; Farjo, R.; Dalkara, D.; Sahel, J.A.; et al. SPVN06, a novel mutation-independent AAV-based gene therapy, dramatically reduces vision loss in the rd10 mouse model of rod-cone dystrophy. Investig. Ophthalmol. Vis. Sci. 2022, 63, 56-A0029. [Google Scholar]
- Jaffal, L.; Joumaa, H.; Noureldine, J.; Banjak, M.; Ibrahim, M.; Mrad, Z.; Salami, A.; El Shamieh, S. The genetic landscape of inherited retinal dystrophies in Arabs. BMC Med. Genom. 2023, 16, 89. [Google Scholar] [CrossRef]
- El Goundali, K.; Chebabe, M.; Laamiri, F.Z.; Hilali, A. The Determinants of Consanguineous Marriages among the Arab Population: A Systematic Review. Iran. J. Public Health 2022, 51, 253–265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abiib, S.; Khodjet-El-Khil, H.; El-Akouri, K.; Bux, R.I.; Rezoug, Z.; Abualainin, W.; Alkowari, M.; Musa, S.O.; Al Mulla, M.; Al Saleh, R.; et al. Qatar’s genetic counseling landscape: Current insights and future prospects. Genet. Med. Open 2024, 2 (Suppl. 2), 101866. [Google Scholar] [CrossRef]
- Alkaf, B.; Jha, A.; Saad, A.; Al Awadhi, A.; El-Khani, A.; Henschel, A.; Milano, A.; Al Mannaei, A.; Al Ali, A.; Khan, A.; et al. The Emirati Genome Program enables population-wide penetrance estimation and novel discovery for inherited retinal disease. medRxiv 2025. [Google Scholar] [CrossRef]
- Saudi Genomics Organisation. About Us. Available online: https://saudigenomics.org/about/ (accessed on 16 November 2025).
- Schulz, H.L.; Grassmann, F.; Kellner, U.; Spital, G.; Rüther, K.; Jägle, H.; Hufendiek, K.; Rating, P.; Huchzermeyer, C.; Baier, M.J.; et al. Mutation Spectrum of the ABCA4 Gene in 335 Stargardt Disease Patients From a Multicenter German Cohort-Impact of Selected Deep Intronic Variants and Common SNPs. Investig. Ophthalmol. Vis. Sci. 2017, 58, 394–403. [Google Scholar] [CrossRef]
| Variable/Diagnosis | Count/Mean | Percentage/SD |
|---|---|---|
| Age | ||
| Age at Data Collection (years) | 23.35 | ±18.73 |
| Age at Genetic Diagnosis (years) | 20.28 | ±18.69 |
| Gender | ||
| Female | 27 | 55.1% |
| Male | 22 | 44.9% |
| Nationality | ||
| Total Arabs | 44 | 89.7% |
| Qatar | 30 | 61.2% |
| United Arab Emirates | 1 | 2.0% |
| Yemen | 2 | 4.1% |
| Lebanon | 2 | 4.1% |
| Palestine | 4 | 8.2% |
| Syria | 1 | 2.0% |
| Egypt | 4 | 8.2% |
| Total non-Arab | 5 | 10.2% |
| Pakistan | 4 | 8.2% |
| Croatia | 1 | 2.0% |
| Consanguinity | ||
| Yes | 39 | 79.6% |
| No | 6 | 12.2% |
| Not Available | 4 | 8.2% |
| Family History | ||
| Positive | 33 | 67.3% |
| Negative | 13 | 26.5% |
| Not Available | 3 | 6.1% |
| Diagnosis | Count | Percentage |
|---|---|---|
| Cone-related Disorders | 8 | 16.3% |
| Cone dystrophy | 3 | 6.1% |
| Cone-Rod dystrophy | 4 | 8.2% |
| Achromatopsia | 1 | 2.0% |
| Macular Disorders | 8 | 16.3% |
| Stargardt disease | 3 | 6.1% |
| Macular dystrophy | 5 | 10.2% |
| Rod-related Disorders | 22 | 44.9 |
| Retinitis pigmentosa | 21 | 42.9% |
| Rod-Cone dystrophy (non-RP) | 1 | 2.0% |
| Early-Onset/Severe Congenital Disorders | 7 | 14.3% |
| Congenital stationary night blindness | 3 | 6.1% |
| Leber congenital amaurosis | 4 | 8.2% |
| Unspecified retinal dystrophy | 4 | 8.2% |
| Patient ID | Country of Origin | Age (Years) * | Age of Diagnosis (Years) | Patient Phenotype | Gene | Gene (NM Number) | rsID | Variant (cDNA) | Variant (Protein) | Variant Type | Variant Impact | Zygosity | Pattern of Inheritance | Test Performed | ACMG | ACMG Highest Pathogenicity Evidence | VUS Subclassification | Mutation Taster | Reports from Other Populations/Ethnicities | Reported Phenotype | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRD-1 | Qatar | 2 | 3 months | RP | GUCY2D | NM_000180.4 | rs763890649 | c.1040_1041delTT | p.Phe347TrpfsX5 | Deletion | Frameshift | Homozygous | AR | WES | Pathogenic | PVS1 | _ | Disease causing | |||
| MERTK | NM_006343.3 | rs886039422 | c.2214delT | p.Cys738TrpfsX32 | Deletion | Frameshift | Homozygous | AR | Pathogenic | PVS1 | _ | Disease causing | Saudi Arabia, UAE | RP & Rod cone dystrophy | [3,17] | ||||||
| IRD-21 | Qatar | 34 | 28 | RP | MERTK | NM_006343.3 | rs886039422 | c.2214delT | p.Cys738TrpfsX32 | Deletion | Frameshift | Homozygous | AR | Familial targeted testing | Pathogenic | PVS1 | _ | Disease causing | Saudi Arabia, UAE | RP & Rod cone dystrophy | [3,17] |
| IRD-2 | Qatar | 3 | 3 | Congenital stationary night blindness | GRM6 | NM_000843.4 | rs752205220 | c.1478G>A | p.Trp493Ter | Substitution | Nonsense | Homozygous | AR | WES | Pathogenic | PVS1 | _ | _ | _ | _ | |
| IRD-6 | Qatar | 6 | 6 | LCA | GRM6 | NM_000843.4 | rs752205220 | c.1478G>A | p.Trp493Ter | Substitution | Nonsense | Homozygous | AR | WES Plus | Pathogenic | PVS1 | _ | _ | _ | _ | |
| IRD-4 | Qatar | 4 | 6 months | Uncategorized Retinal Dystrophy | CABP4 | NM_145200.5 | rs786205852 | c.81_82insA | p.Pro28ThrfsX4 | Substitution | Frameshift | Homozygous | AR | WES Plus | Pathogenic | PVS1 | _ | Disease causing | Saudi Arabia | Segregated with congenital retinal dysfunction in 11 affected individuals (aged 2–26 years) from four consanguineous families | [18] |
| IRD-8 | Qatar | 26 | 23 | Stargardt Disease | ABCA4 | NM_000350.3 | _ | c.5584G>C | p.Gly1862Arg | Substitution | Missense | Homozygous | AR | WES Plus | Pathogenic | PS4 | _ | Disease causing | China | Stargardt disease | [19] |
| IRD-9 | Qatar | 14 | 11 | Macular Dystrophy | ABCA4 | NM_000350.3 | rs61748556 | c.1609C>T | p.Arg537Cys | Substitution | Missense | Heterozygous | AR | WES Plus | Likely Pathogenic | PM1 | _ | _ | Germany | Stargardt disease | [20] |
| rs1800553 | c.5882G>A | p.Gly1961Glu | Substitution | Missense | Heterozygous | AR | Pathogenic | PS3 | _ | _ | China, Spain, UAE, Italy | Stargardt disease | [3,19,21] | ||||||||
| CRX | NM_000554.6 | rs771736389 | c.128G>A | p.Arg43His | Substitution | Missense | Heterozygous | AD | Pathogenic | PS4 | _ | Disease causing | |||||||||
| IRD-25 | Yemen | 20 | 18 | Stargardt Disease | ABCA4 | NM_000350.3 | rs61750155 | c.4793C>A | p.Ala1598Asp | Substitution | Missense | Homozygous | AR | WES Plus | Pathogenic | PM3 | _ | _ | Germany | Stargardt disease | [22] |
| IRD-22 | Qatar | 56 | 53 | RP | ABCA4 | NM_000350.3 | rs1800553 | c.5882G>A | p.Gly1961Glu | Substitution | Missense | Heterozygous | AR | WES | Pathogenic | PS3 | _ | _ | China, Spain, UAE, Italy | Stargardt disease | [3,19,21,23] |
| IRD-47 | Qatar | 69 | 64 | RP | ABCA4 | NM_000350.3 | rs1800553 | c.5882G>A | p.Gly1961Glu | Substitution | Missense | Heterozygous | AR | WES Plus | Pathogenic | PS3 | _ | _ | China, Spain, UAE, Italy | Stargardt disease | [3,19,21,23] |
| CYP4V2 | NM_207352.4 | rs199476204 | c.1348C>T | p.Gln450Ter | Substitution | Nonsense | Homozygous | AR | Pathogenic | PVS1 | _ | _ | _ | _ | _ | ||||||
| IRD-15 | Croatia | 41 | 38 | RP | PDE6B | NM_000283.4 | rs370898371 | c.1107+3A>G | IVS8+3A>G | Substitution | Intron Variant | Compound Het | AR | Gene Panel Testing | Likely Pathogenic | PS4 | _ | _ | _ | ||
| rs1737315492 | c.1859A>G | p.His620Arg | Substitution | Missense | AR | Likely Pathogenic | PS4 | _ | Disease causing | _ | _ | ||||||||||
| IRD-18 | Qatar | 25 | 24 | RP | PDE6B | NM_000283.4 | rs751859807 | c.1655G>A | p.Arg552Gln | Substitution | Missense | Homozygous | AR | Gene Panel testing | Pathogenic | PS4 | _ | _ | |||
| IRD-10 | Qatar | 15 | 9 | RP | PDE6C | NM_006204.4 | rs1057518244 | c.724-1G>T | IVS3-1G>T (in intron3) | Substitution | Splice Acceptor | Homozygous | AR | WES Plus | Pathogenic | PVS1 | _ | _ | |||
| IRD-19 | Palestine | 21 | 17 | RP | RDH12 | NM_152443.3 | rs1594867597 | c.821T>C | p.Leu274Pro | Substitution | Missense | Homozygous | AR | WES Plus | Pathogenic | PS4 | _ | _ | Israel | RP, LCA | [17] |
| IRD-31 | Palestine | 6 | 2.5 | Uncategorized Retinal Dystrophy | RDH12 | NM_152443.3 | rs1594867597 | c.821T>C | p.Leu274Pro | Substitution | Missense | Homozygous | AR | WES Plus | Pathogenic | PS4 | _ | _ | Israel | RP, LCA | [17] |
| IRD-24 | Qatar | 50 | 49 | RP | KIZ | NM_018474.6 | rs775124094 | c.247C>T | p.Arg83Ter | Substitution | Nonsense | Homozygous | AR | Gene Panel testing | Pathogenic | PM3 | _ | _ | _ | _ | _ |
| IRD-29 | Qatar | 37 | 37 | RP | RPGR | NM_001034853.2 | rs1186795749 | c.3092del | p.Glu1031Glyfs*58 | Deletion | Frameshift | Hemizygous | XLR | Gene Panel testing | Pathogenic | PM3 | _ | Disease causing | Denmark | RP | [18] |
| IRD-30 | Syria | 14 | 12 | Macular dystrophy | AIPL1 | NM_014336.5 | rs62637014 | c.834G>A | p.Trp278Ter | Substitution | Nonsense | Homozygous | AR | WES Plus | Pathogenic | PM3 | _ | Disease causing | Romania | LCA | [19] |
| IRD-32 | Qatar | 44 | 41 | RP | GNAT2 | NM_001377295.2 | rs1553226581 | c.720+5G>C | IVS6+5G>C | Substitution | Splicing site | Homozygous | AR | WES Plus | Likely Pathogenic | PM3 | _ | _ | _ | _ | _ |
| IRD-44 | Qatar | 16 | 11 | Achromatopsia | GNAT2 | NM_001377295.2 | rs1553226581 | c.720+5G>C | IVS6+5G>C | Substitution | Splicing site | Homozygous | AR | WES Plus | Likely Pathogenic | PM3 | _ | _ | _ | _ | _ |
| IRD-33 | Pakistan | 9 | 6 | LCA | NMNAT1 | NM_022787.4 | rs201994921 | c.634G>A | p.Val212Met | Substitution | Missense | Compound Heterozygous | AR | Familial Targeted Testing | Likely Pathogenic | PM1 | _ | _ | _ | LCA | _ |
| _ | chr1: 10035650_10035833 | _ | Deletion | Deletion | AR | Likely Pathogenic | _ | _ | _ | _ | _ | _ | |||||||||
| IRD-35 | Lebanon | 7 | 6 | Rod & Rod-Cone dystrophy | RPGRIP1 | NM_020366.4 | _ | c.3278dupC | p.Gln1094Thrfs*6 | Duplication | Frameshift | Compound Heterozygous | AR | WES Plus | Likely Pathogenic | PVS1 | _ | Disease causing | _ | _ | _ |
| rs1371805993 | c.2935C>T | p.Gln979Ter | substitution | Missense | AR | Pathogenic | PVS1 | _ | _ | Israel | RP | [17] | |||||||||
| IRD-14 | Qatar | 5 | 2 | Uncategorized Retinal Dystrophy | RPGRIP1 | NM_020366.4 | rs61751266 | c.1107delA | p.Glu370AsnfsX5 | Deletion | Frameshift | Homozygous | AR | WES | Pathogenic | PM3 | _ | _ | Saudi Arabia | Cone-Rod Dystrophy, LCA | [20] |
| IRD-42 | Lebanon | 27 | 27 | RP | CFAP418 | NM_177965.4 | _ | c.478dupA | p.Met160Asnfs*25 | Duplication | Frameshift | Homozygous | AR | WES Plus | Likely Pathogenic | PVS1 | _ | Disease causing | _ | _ | _ |
| IRD-46 | Qatar | 61 | 61 | Macular dystrophy | PRPH2 | NM_000322.5 | rs1799986489 | c.936del | p.Pro313Argfs*11 | Deletion | Frameshift | Heterozygous | AD | Familial Targeted Testing | Likely Pathogenic | PVS1 | _ | Disease causing | _ | _ | _ |
| IRD-12 | Qatar | 7 | 7 | RP | CNGA3 | NM_001298.3 | rs104893613 | c.847C>T | p.Arg283Trp | Substitution | Missense | Homozygous | AR | WES | Pathogenic | PM3 | _ | _ | _ | _ | _ |
| IRD-41 | Egypt | 13 | 9 | Cone-Rod dystrophy | CFAP410 | NM_004928.3 | rs771024688 | c.209G>A | p.Arg70Gln | Substitution | Missense | Homozygous | AR | WES Plus Trio | Likely Pathogenic | PM2 | _ | Disease causing | _ | _ | _ |
| IRDs-45 | Qatar | 54 years | 50 years | RP | ABCA4 | NM_000350.3 | rs752850266 | c.6218G>C | p.Gly2073Ala | Substitution | Missense | Homozygous | AR | WES Plus | Likely Pathogenic | PM1 | _ | Disease causing | _ | _ | _ |
| IRDs-28 | United Arab Emirates | 10 years | 6 years | Macular dystrophy | CRB1 | NM_201253.3 | rs1571522690 | c.1313G>A | p.Cys438Tyr | Substitution | Missense | Homozygous | AR | Gene Panel testing | Likely Pathogenic | PM1 | _ | Disease causing | _ | _ | _ |
| IRDs-49 | Egypt | 12 years | 3 years | LCA | RPGRIP1 | NM_020366.4 | _ | c.105dupA | p.Pro36Thrfs*35 | Duplication | Frameshift | Homozygous | AR | Gene Panel testing | Likely Pathogenic | PVS1 | _ | Disease causing | _ | _ | _ |
| IRD-23 | Qatar | 48 | 46 | RP | PRCD | NM_001077620.3 | rs757471313 | c.74C>T | p.Pro25Leu | Substitution | Missense | Homozygous | AR | WES Plus Trio | Likely Pathogenic | PM3 | _ | Disease causing | _ | _ | _ |
| IRD-11 | Qatar | 21 | 20 | RP | MERTK | NM_006343.3 | _ | c.2020A>G | p.Met674Val | Substitution | Missense | Homozygous | AR | WES Trio | Likely Pathogenic | PM3 | _ | Disease causing | _ | _ | _ |
| IRD-39 | Yemen | 3 | 1.5 | LCA | GUCY2D | NM_000180.4 | _ | c.2213_2215del | p.Glu738del | Deletion | Frameshift | Homozygous | AR | WES Trio | Likely Pathogenic | PS4 | _ | Disease causing | _ | _ | _ |
| IRD-13 | Qatar | 48 | 40 | RP | MERTK | NM_006343.3 | _ | c.2020A>G | p.Met674Val | Substitution | Missense | Homozygous | AR | WES | Likely Pathogenic | PM3 | _ | Disease causing | _ | _ | _ |
| rs141361084 | c.2435A>C | p.Tyr812Ser | Substitution | Missense | Homozygous | AR | ** Variant of uncertain significance | PM2 | Warm | Disease causing | _ | _ | _ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abiib, S.; Khodjet-El-khil, H.; Bux, R.I.; El-Akouri, K.; Okashah, S.; Ben Omran, T.; Al Saleh, R.; Al-Shafai, M. Exploring the Genetic Causes of Nonsyndromic Retinal Dystrophies in Qatar. Genes 2025, 16, 1415. https://doi.org/10.3390/genes16121415
Abiib S, Khodjet-El-khil H, Bux RI, El-Akouri K, Okashah S, Ben Omran T, Al Saleh R, Al-Shafai M. Exploring the Genetic Causes of Nonsyndromic Retinal Dystrophies in Qatar. Genes. 2025; 16(12):1415. https://doi.org/10.3390/genes16121415
Chicago/Turabian StyleAbiib, Sumaya, Houssein Khodjet-El-khil, Reem Ibrahim Bux, Karen El-Akouri, Sarah Okashah, Tawfeg Ben Omran, Rehab Al Saleh, and Mashael Al-Shafai. 2025. "Exploring the Genetic Causes of Nonsyndromic Retinal Dystrophies in Qatar" Genes 16, no. 12: 1415. https://doi.org/10.3390/genes16121415
APA StyleAbiib, S., Khodjet-El-khil, H., Bux, R. I., El-Akouri, K., Okashah, S., Ben Omran, T., Al Saleh, R., & Al-Shafai, M. (2025). Exploring the Genetic Causes of Nonsyndromic Retinal Dystrophies in Qatar. Genes, 16(12), 1415. https://doi.org/10.3390/genes16121415






