Transcriptome Analysis Suggests Dietary Tributyrin Enhances Feeding Intensity via Modulating Steroid Biosynthesis in Mandarin Fish (Siniperca chuatsi)
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Experimental Procedures
2.3. Histological Analysis
2.4. RNA Extraction, cDNA Library Construction, and RNA-Seq
2.5. Screening of DEGs
2.6. Co-Expression Network Construction and Module Identification
2.7. Construction and Analysis of Bayesian Networks
2.8. Confirmation of RNA-Seq Data by qRT-PCR
2.9. Statistical Analysis
3. Results
3.1. Histological Comparison
3.2. Intestinal RNA-Seq Analysis
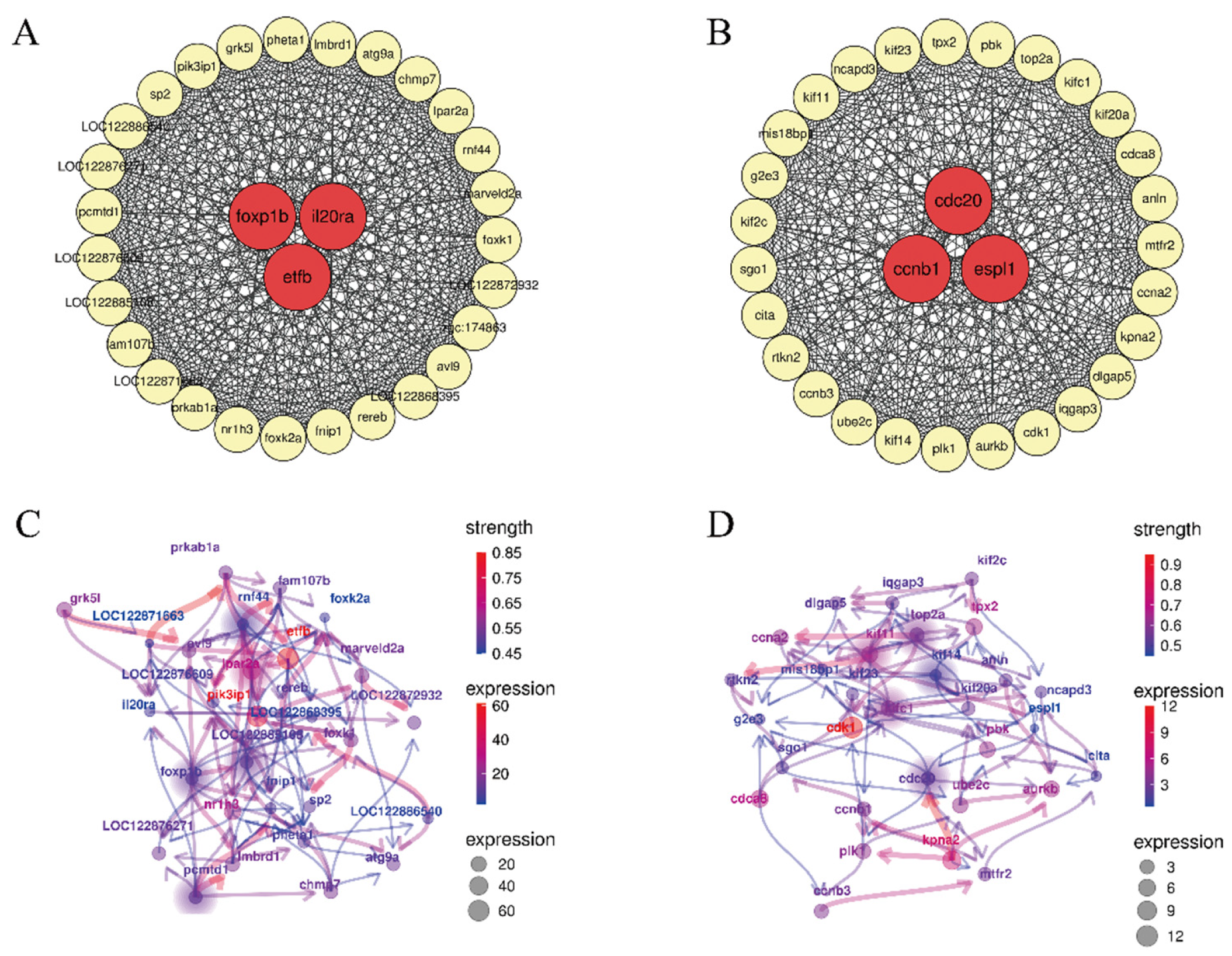
3.3. WGCNA and Bayesian Analysis
3.4. qRT-PCR Validation of Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, P. Behavior Genetics and the Domestication of Animals. Annu. Rev. Anim. Biosci. 2014, 2, 85–104. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.Z.; Lu, J.T.; Li, Z.; Shi, S.C.; Liu, Z.J. Effects of Live and Artificial Feeds on the Growth, Digestion, Immunity and Intestinal Microflora of Mandarin Fish Hybrid (Siniperca chuatsi♀ × Siniperca scherzeri♂). Aquac. Res. 2017, 48, 4479–4485. [Google Scholar] [CrossRef]
- Lorenzen, K.; Beveridge, M.C.M.; Mangel, M. Cultured Fish: Integrative Biology and Management of Domestication and Interactions with Wild Fish. Biol. Rev. 2012, 87, 639–660. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Z.; Li, J.; Xie, S.; Shi, L.-J.; He, Y.-H.; Liang, X.-F.; Zhu, Q.-S.; He, S. Different Regulation of Branched-Chain Amino Acid on Food Intake by TOR Signaling in Chinese Perch (Siniperca chuatsi). Aquaculture 2021, 530, 735792. [Google Scholar] [CrossRef]
- Li, L.; Fang, J.; Liang, X.; Alam, M.S.; Liu, L.; Yuan, X. Effect of Feeding Stimulants on Growth Performance, Feed Intake and Appetite Regulation of Mandarin Fish, Siniperca Chuatsi. Aquac. Res. 2019, 50, 3684–3691. [Google Scholar] [CrossRef]
- Wu, D.L.; Peng, D.; Liang, X.F.; Xie, R.P.; Zeng, M.; Chen, J.L.; Lan, J.; Yang, R.; Hu, J.C.; Lu, P.S. Dietary Soybean Lecithin Promoted Growth Performance and Feeding in Juvenile Chinese Perch (Siniperca chuatsi) Could Be by Optimizing Glucolipid Metabolism. Fish Physiol. Biochem. 2023, 49, 1097–1114. [Google Scholar] [CrossRef]
- Chen, X.L.; Yi, H.D.; Liu, S.; Zhang, Y.; Su, Y.Q.; Liu, X.G.; Bi, S.; Lai, H.; Zeng, Z.Y.; Li, G.F. Promotion of Pellet-Feed Feeding in Mandarin Fish (Siniperca chuatsi) by Bdellovibrio bacteriovorus Is Influenced by Immune and Intestinal Flora. Aquaculture 2021, 542, 736864. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Shang, Q.; Long, S.; Liu, S.; Mahfuz, S. Mixed Organic Acids as an Alternative to Antibiotics Improve Serum Biochemical Parameters and Intestinal Health of Weaned Piglets. Anim. Nutr. 2021, 7, 737–749. [Google Scholar] [CrossRef]
- Pham, V.H.; Kan, L.; Huang, J.; Geng, Y.; Zhen, W.; Guo, Y.; Abbas, W.; Wang, Z. Dietary Encapsulated Essential Oils and Organic Acids Mixture Improves Gut Health in Broiler Chickens Challenged with Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, K.; Wang, G.; Mo, W.; Huang, Y.; Cao, J. Metabolic Changes, Antioxidant Status, Immune Response and Resistance to Ammonia Stress in Juvenile Yellow Catfish (Pelteobagrus fulvidraco) Fed Diet Supplemented with Sodium Butyrate. Aquaculture 2021, 536, 736441. [Google Scholar] [CrossRef]
- Lum, J.; Sygall, R.; Ros Felip, J.M. Comparison of Tributyrin and Coated Sodium Butyrate as Sources of Butyric Acid for Improvement of Growth Performance in Ross 308 Broilers. Int. J. Poult. Sci. 2018, 17, 290–294. [Google Scholar] [CrossRef]
- Liang, H.L.; Ji, K.; Ge, X.P.; Xi, B.W.; Ren, M.C.; Chen, X.R. Tributyrin Plays an Important Role in Regulating the Growth and Health Status of Juvenile Blunt Snout Bream (Megalobrama amblycephala), as Evidenced by Pathological Examination. Front. Immunol. 2021, 12, 652294. [Google Scholar] [CrossRef]
- Sakdee, J.; Poeikhamph, T.; Rakangthon, C.; Poungpong, K.; Bunchasak, C. Effect of Tributyrin Supplementation in Diet on Production Performance and Gastrointestinal Tract of Healthy Nursery Pigs. Pak. J. Nutr. 2016, 15, 954–962. [Google Scholar] [CrossRef]
- Dong, L.; Zhong, X.; He, J.T.; Zhang, L.L.; Bai, K.W.; Xu, W.; Wang, T.; Huang, X.X. Supplementation of Tributyrin Improves the Growth and Intestinal Digestive and Barrier Functions in Intrauterine Growth-Restricted Piglets. Clin. Nutr. 2016, 35, 399–407. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Liu, Y.L.; Hu, J.; Shen, W.H. Effects of Lactitol and Tributyrin on Growth Performance, Small Intestinal Morphology and Enzyme Activity in Weaned Pigs. Asian-Australas. J. Anim. Sci. 2006, 19, 1470–1477. [Google Scholar] [CrossRef]
- Ren, Q.C.; Xuan, J.J.; Wang, L.K.; Zhan, Q.W.; Yin, D.Z.; Hu, Z.Z.; Yang, H.J.; Zhang, W.; Jiang, L.S. Effects of Tributyrin Supplementation on Ruminal Microbial Protein Yield, Fermentation Characteristics and Nutrients Degradability in Adult Small Tail Ewes. Anim. Sci. J. 2018, 89, 1271–1279. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Shi, S.; Liu, Z.; Li, Z.; Li, J. Effect of Live, Frozen and Artificial Feeds on Digestive Enzymes, Aminotransferase, Histology of Liver and Intestine in Mandarin Fish Hybrid (Siniperca chuatsi♀ × Siniperca scherzeri♂). Isr. J. Aquac.-Bamidgeh 2015, 67, 41–IJA_67.2015. [Google Scholar]
- Xie, D.Z.; Dai, Q.Y.; Xu, C.; Li, Y.Y. Dietary Tributyrin Modifies Intestinal Function by Altering Morphology, Gene Expression and Microbiota Profile in Common Carp (Cyprinus carpio) Fed All-Plant Diets. Aquac. Nutr. 2021, 27, 439–453. [Google Scholar] [CrossRef]
- Tan, P.; Wu, X.; Zhu, W.L.; Lou, B.; Chen, R.Y.; Wang, L.G. Effect of Tributyrin Supplementation in High-Soya Bean Meal Diet on Growth Performance, Body Composition, Intestine Morphology and Microbiota of Juvenile Yellow Drum (Nibea albiflora). Aquac. Res. 2020, 51, 2004–2019. [Google Scholar] [CrossRef]
- Kondo, H.; Hirono, I.; Aoki, T. Application of High-Throughput Transcriptome Analyses in Aquaculture. Nippon Suisan Gakkaishi 2012, 78, 267. [Google Scholar] [CrossRef][Green Version]
- Ortí, G.; Lovejoy, N.R.; Shi, Q. Special Issue on Fish Transcriptomics. Genomics 2019, 111, 213–214. [Google Scholar] [CrossRef]
- Guan, W.Z.; Qiu, G.F.; Feng, L. Transcriptome Analysis of the Growth Performance of Hybrid Mandarin Fish after Food Conversion. PLoS ONE 2020, 15, e0240308. [Google Scholar] [CrossRef]
- GB/T 35892-2018; Laboratory Animal—Guideline for Ethical Review of Animal Welfare. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- Wu, S.J.; Huang, J.Q.; Li, Y.J.; Zhao, L.; Liu, Z.; Kang, Y.J.; Wang, J.F. Integrative mRNA-miRNA Interaction Analysis Reveals the Molecular Mechanism of Skin Color Variation Between Wild-Type and Yellow Mutant Rainbow Trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part D-Genom. Proteom. 2021, 40, 100914. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.K.; Wang, R.Q.; Chen, S.L. Transcriptomics Analysis Revealing Candidate Networks and Genes for the Body Size Sexual Dimorphism of Chinese Tongue Sole (Cynoglossus semilaevis). Funct. Integr. Genom. 2018, 18, 327–339. [Google Scholar] [CrossRef]
- Li, H.X.; Qiang, J.; Song, C.Y.; Xu, P. Transcriptome Profiling Reveal Acanthopanax senticosus Improves Growth Performance, Immunity and Antioxidant Capacity by Regulating Lipid Metabolism in GIFT (Oreochromis niloticus). Comp. Biochem. Physiol. Part D-Genom. Proteom. 2021, 37, 100784. [Google Scholar] [CrossRef]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Lu, J.F.; Luo, S.; Tang, H.; Liang, J.H.; Zhao, Y.F.; Hu, Y.; Yang, G.J.; Chen, J. Micropterus salmoides Rhabdovirus Enters Cells via Clathrin-Mediated Endocytosis Pathway in a pH-, Dynamin-, Microtubule-, Rab5-, and Rab7-Dependent Manner. J. Virol. 2023, 97, e00714-23. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Pan, L.; Shen, J.F.; Tan, B.P.; Dong, X.H.; Yang, Q.H.; Chi, S.Y.; Zhang, S. Effects of Carbohydrase Supplementation on Growth Performance, Intestinal Digestive Enzymes and Flora, Glucose Metabolism Enzymes, and Glut2 Gene Expression of Hybrid Grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂) Fed Different CHO/L Ratio Diets. Metabolites 2023, 13, 98. [Google Scholar] [CrossRef]
- Suzuki, T.; Mayanagi, Y.; Keta, A.; Kasahara, A.; Sato, A.; Takahashi, T. Oral Administration of Fructose Improves Jejunal Villous Morphology and Nutrient Digestion and Absorption Capabilities in a Rat Model of Total Parenteral Nutrition. Biomed. Res. Clin. Pract. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Rysman, K.; Eeckhaut, V.; Ducatelle, R.; Goossens, E.; Van Immerseel, F. Broiler Performance Correlates with Gut Morphology and Intestinal Inflammation Under Field Conditions. Avian Pathol. 2023, 52, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.L.; Wang, Y.Y.; Li, M.L.; Zhang, C.Y.; Lahaye, L.; Chowdhury, M.A.K.; Li, X.Q.; Leng, X.J. Supplementation of a Multienzyme Complex, an Organic Acid-Essential Oil Complex, and Prebiotic Alone or in Combination Affects Growth, Nutrient Utilization, and Immune Function of Rainbow Trout (Oncorhynchus mykiss). Aquac. Nutr. 2022, 2022, 068537. [Google Scholar] [CrossRef]
- Buttle, L.G.; Burrells, A.C.; Good, J.E.; Williams, P.D.; Southgate, P.J.; Burrells, C. The Binding of Soybean Agglutinin (SBA) to the Intestinal Epithelium of Atlantic Salmon, Salmo salar and Rainbow Trout, Oncorhynchus mykiss, Fed High Levels of Soybean Meal. Vet. Immunol. Immunopathol. 2001, 80, 237–244. [Google Scholar] [CrossRef]
- Zhao, H.X.; Hou, D.Q.; Li, P.J.; Li, M.; Chen, B.; Zhu, X.F.; Cao, J.M. The Effects of Dietary Tributyrin on Growth, Intestinal Health, Inflammatory Response and Antioxidant Status in Juvenile Yellow Catfish (Pelteobagrus fulvidraco). Aquac. Rep. 2022, 27, 101370. [Google Scholar] [CrossRef]
- Luo, J.X.; Gao, X.T.; Rong, Z.; Zhang, L.H.; Sun, Y.F.; Qi, Z.L.; Yu, Q.; Waiho, K.; Zhao, W.X.; Xu, Y.H.; et al. Transcriptome Sequencing Reveals Effects of Artificial Feed Domestication on Intestinal Performance and Gene Expression of Carnivorous Mandarin Fish (Siniperca chuatsi) and Related Mechanisms. Mar. Biotechnol. 2025, 27, 41. [Google Scholar] [CrossRef]
- Ferreira, I.M.; Quesñay, J.E.N.; Bastos, A.C.S.; Rodrigues, C.T.; Vollmar, M.; Krojer, T.; Strain-Damerell, C.; Burgess-Brown, N.A.; von Delft, F.; Yue, W.W.; et al. Structure and Activation Mechanism of the Human Liver-Type Glutaminase GLS2. Biochimie 2021, 185, 96–104. [Google Scholar] [CrossRef]
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and Functions of L-Glutamate in the Epithelial Cells of the Small and Large Intestines. Am. J. Clin. Nutr. 2009, 90, 814S–821S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Fan, M.; Candas, D.; Zhang, T.Q.; Qin, L.L.; Eldridge, A.; Wachsmann-Hogiu, S.; Ahmed, K.M.; Chromy, B.A.; Nantajit, D.; et al. Cyclin B1/Cdk1 Coordinates Mitochondrial Respiration for Cell-Cycle G2/M Progression. Dev. Cell 2014, 29, 217–232. [Google Scholar] [CrossRef]
- Zheng, J.L.; Luo, Z.; Zhu, Q.L.; Tan, X.Y.; Chen, Q.L.; Sun, L.D.; Hu, W. Molecular Cloning and Expression Pattern of 11 Genes Involved in Lipid Metabolism in Yellow Catfish Pelteobagrus Fulvidraco. Gene 2013, 531, 53–63. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Chen, H.M.; Li, Y.Y.; Liu, Q.; Lu, K.Y.; Zhu, X.S.; Wang, Y. Transcriptome and Biochemical Analyses of Rainbow Trout (Oncorhynchus mykiss) RTG-2 Gonadal Cells in Response to BDE-47 Stress Indicates Effects on Cell Proliferation. Aquat. Toxicol. 2022, 245, 106108. [Google Scholar] [CrossRef]
- Boukouvala, E.; Leaver, M.J.; Favre-Krey, L.; Theodoridou, M.; Krey, G. Molecular Characterization of a Gilthead Sea Bream (Sparus aurata) Muscle Tissue cDNA for Carnitine Palmitoyltransferase 1B (CPT1B). Comp. Biochem. Physiol. Part B-Biochem. Mol. Biol. 2010, 157, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Marti-Masso, J.F.; Ruiz-Martínez, J.; Makarov, V.; de Munain, A.L.; Gorostidi, A.; Bergareche, A.; Yoon, S.; Buxbaum, J.D.; Paisán-Ruiz, C. Exome Sequencing Identifies GCDH (Glutaryl-CoA Dehydrogenase) Mutations as a Cause of a Progressive Form of Early-Onset Generalized Dystonia. Hum. Genet. 2012, 131, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Y.; Ye, H.Z.; Xiao, J.; Hogstrand, C.; Green, I.; Guo, Z.Q.; Han, D. Dietary Trivalent Chromium Exposure Up-Regulates Lipid Metabolism in Coral Trout: The Evidence From Transcriptome Analysis. Front. Physiol. 2021, 12, 640898. [Google Scholar] [CrossRef]
- Shi, M.J.; Zhang, Q.X.; Li, Y.M.; Zhang, W.T.; Liao, L.J.; Cheng, Y.Y.; Jiang, Y.X.; Huang, X.L.; Duan, Y.; Xia, L.; et al. Global Gene Expression Profile Under Low-Temperature Conditions in the Brain of the Grass Carp (Ctenopharyngodon idellus). PLoS ONE 2020, 15, e0239730. [Google Scholar] [CrossRef]
- Kaymak, T.; Kaya, B.; Wuggenig, P.; Nuciforo, S.; Göldi, A.; Oswald, F.; Roux, J.; Noti, M.; Melhem, H.; Hruz, P.; et al. IL-20 Subfamily Cytokines Impair the Oesophageal Epithelial Barrier by Diminishing Filaggrin in Eosinophilic Oesophagitis. Gut 2023, 72, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Sunyer, J.O. Fishing for Mammalian Paradigms in the Teleost Immune System. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef]
- Zhang, M.L.; Li, M.; Sheng, Y.; Tan, F.; Chen, L.Q.; Cann, I.; Du, Z.Y. Citrobacter Species Increase Energy Harvest by Modulating Intestinal Microbiota in Fish: Nondominant Species Play Important Functions. mSystems 2020, 5, e00303-20. [Google Scholar] [CrossRef]



| Item | Content (%) |
|---|---|
| Crude protein | ≥50% |
| Crude fat | ≥9% |
| Crude fiber | ≤5% |
| Coarse ash | ≤12% |
| Lysine | ≥3% |
| Calcium | ≥1.8% |
| Total phosphorus | ≥1.8% |
| Moisture | ≤12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, E.-X.; Li, H.-Y.; Hou, Z.-G.; Xu, Y.-H.; Wu, J.; Bao, T.-F.; Wu, C.-B.; Gao, X.-W.; Tan, Y.-M. Transcriptome Analysis Suggests Dietary Tributyrin Enhances Feeding Intensity via Modulating Steroid Biosynthesis in Mandarin Fish (Siniperca chuatsi). Genes 2025, 16, 1395. https://doi.org/10.3390/genes16121395
Xu E-X, Li H-Y, Hou Z-G, Xu Y-H, Wu J, Bao T-F, Wu C-B, Gao X-W, Tan Y-M. Transcriptome Analysis Suggests Dietary Tributyrin Enhances Feeding Intensity via Modulating Steroid Biosynthesis in Mandarin Fish (Siniperca chuatsi). Genes. 2025; 16(12):1395. https://doi.org/10.3390/genes16121395
Chicago/Turabian StyleXu, Er-Xue, Hao-Yu Li, Zhi-Guang Hou, Yi-Huan Xu, Jun Wu, Teng-Fei Bao, Cheng-Bin Wu, Xiao-Wei Gao, and Yan-Miao Tan. 2025. "Transcriptome Analysis Suggests Dietary Tributyrin Enhances Feeding Intensity via Modulating Steroid Biosynthesis in Mandarin Fish (Siniperca chuatsi)" Genes 16, no. 12: 1395. https://doi.org/10.3390/genes16121395
APA StyleXu, E.-X., Li, H.-Y., Hou, Z.-G., Xu, Y.-H., Wu, J., Bao, T.-F., Wu, C.-B., Gao, X.-W., & Tan, Y.-M. (2025). Transcriptome Analysis Suggests Dietary Tributyrin Enhances Feeding Intensity via Modulating Steroid Biosynthesis in Mandarin Fish (Siniperca chuatsi). Genes, 16(12), 1395. https://doi.org/10.3390/genes16121395






