Mountains, Lakes, and Ancient Drainage Networks Sculpt the Phylogeographic Architecture of the Stream Headwater Fish Acrossocheilus kreyenbergii in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. DNA Sequencing and Alignment
2.3. Phylogeny and Divergence-Time Estimation
2.4. Genetic Structure and Population History
2.5. Ancestral Area Reconstruction
3. Results
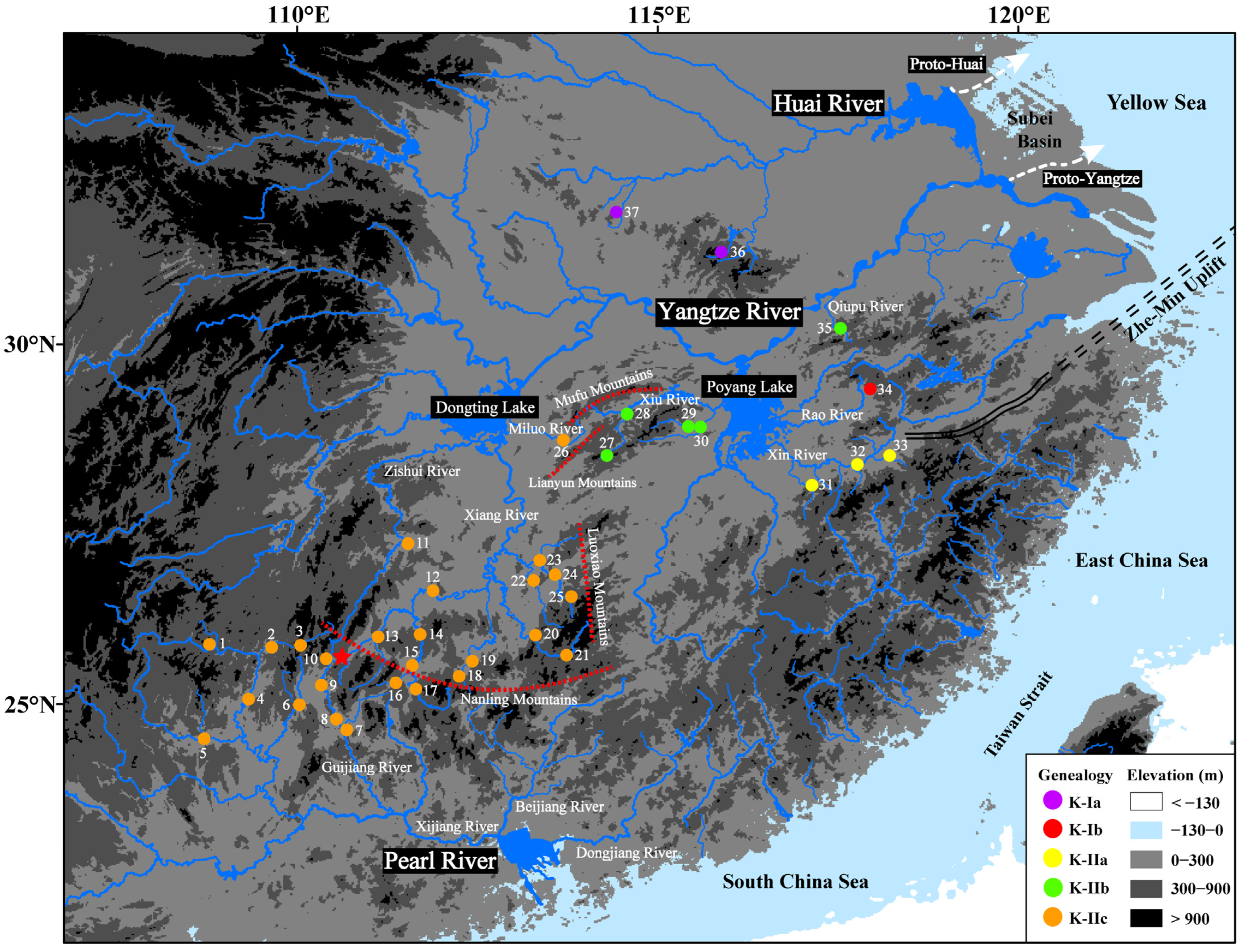
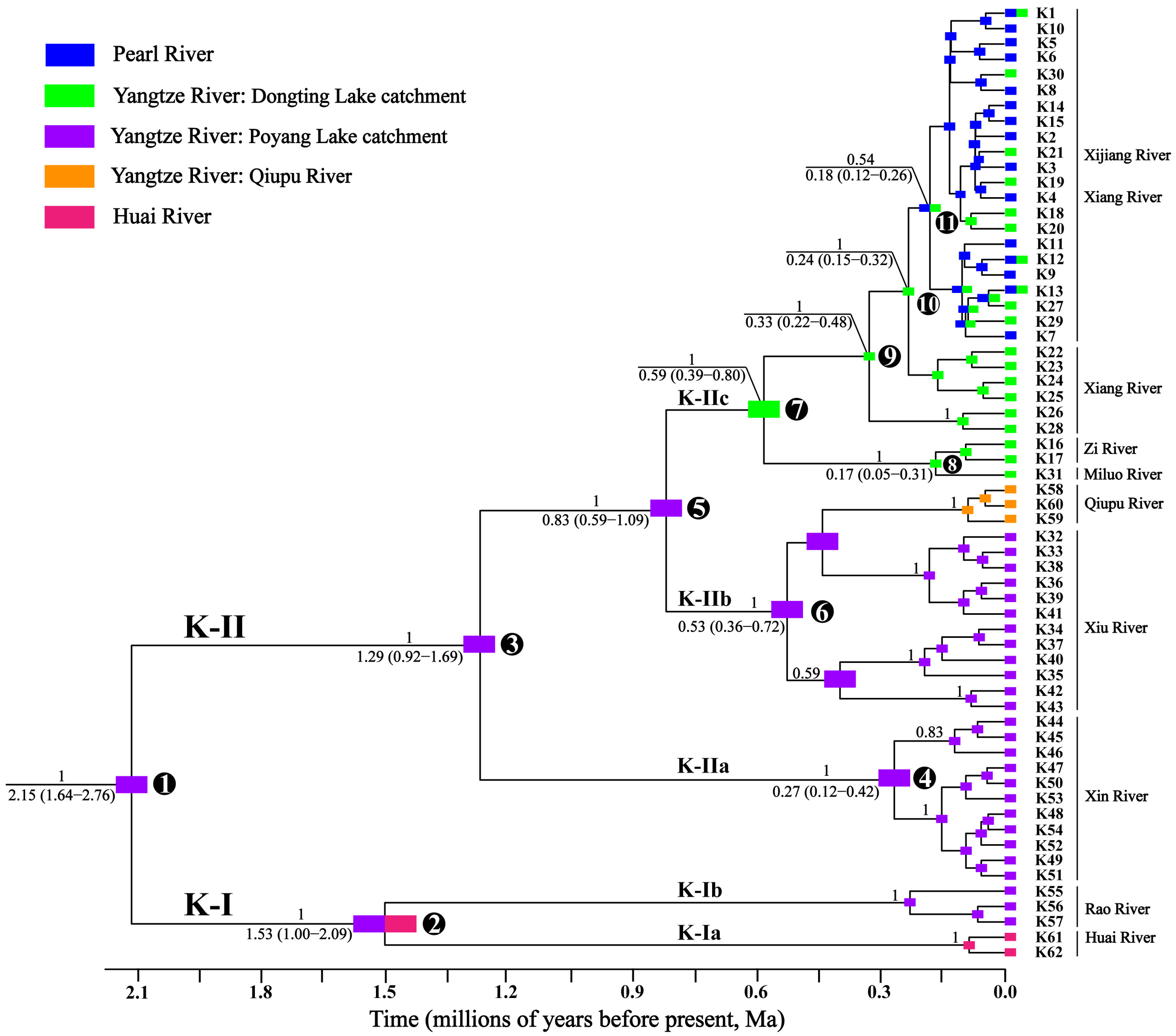
3.1. Phylogeny, Divergence Time, and Geographic Distribution
3.2. Ancestral-Area Reconstruction
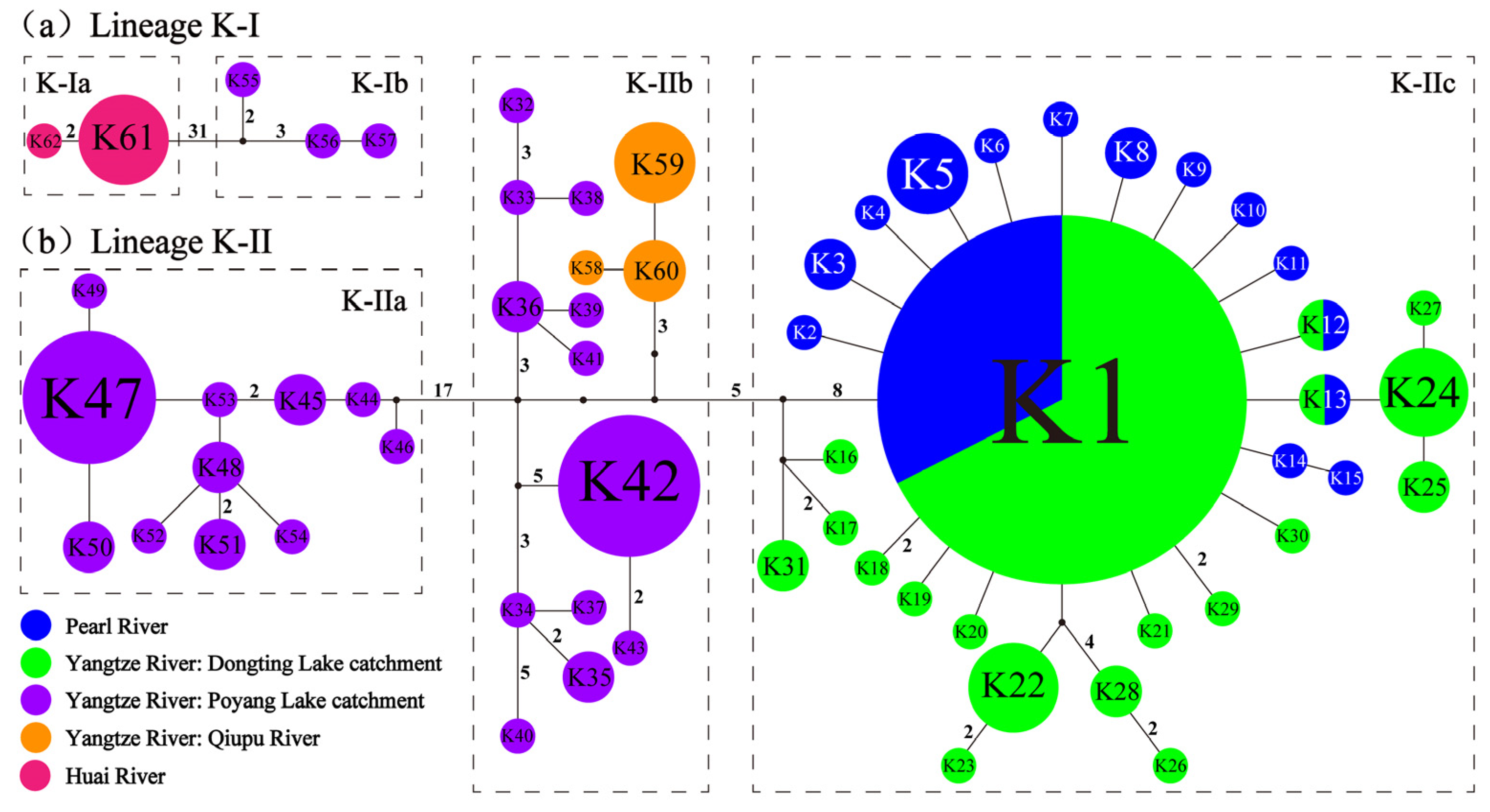
3.3. Genetic Diversity and Genetic Structure
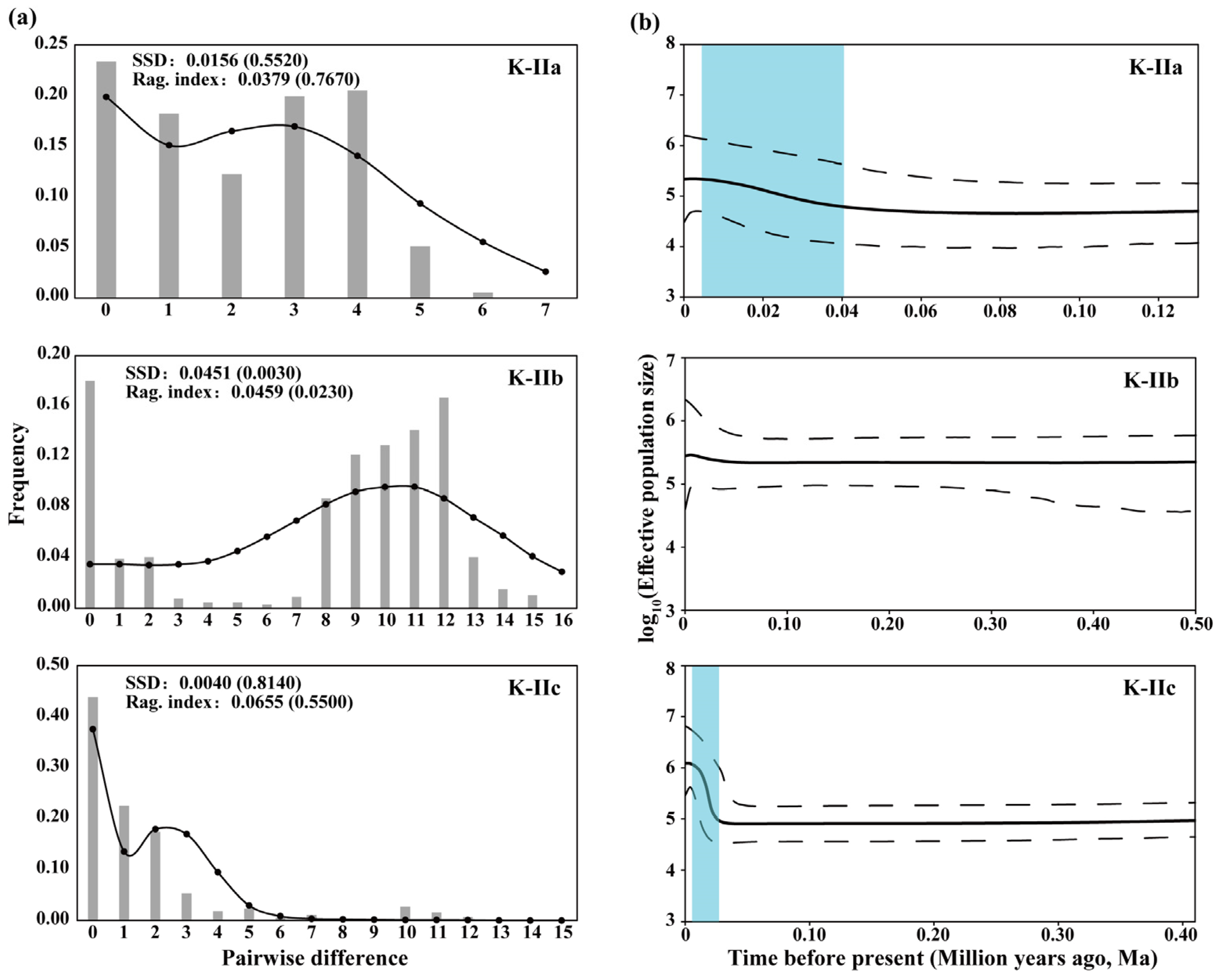
3.4. Population History
4. Discussion
4.1. Main Causes of Lineage Splitting
4.2. Genetic Diversity and Demographic History
4.3. Conservation Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waters, J.M.; Craw, D.; Youngson, J.H.; Wallis, G.P. Genes meet geology: Fish phylogeographic pattern reflects ancient, rather than modern, drainage connections. Evolution 2001, 55, 1844–1851. [Google Scholar] [CrossRef]
- Hughes, J.M.; Daniel, J.S.; Debra, S.F. Genes in streams: Using DNA to understand the movement of freshwater fauna and their riverine habitat. BioScience 2009, 59, 573–583. [Google Scholar] [CrossRef]
- Craw, D.; Upton, P.; Burridge, C.P.; Wallis, G.P.; Waters, J.M. Rapid biological speciation driven by tectonic evolution in New Zealand. Nat. Geosci. 2016, 9, 140–144. [Google Scholar] [CrossRef]
- Burridge, C.P.; Craw, D.; Waters, J.M. An empirical test of freshwater vicariance via river capture. Mol. Ecol. 2007, 16, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.B.; Knowles, L.L.; Mascarenhas, R.; Affonso, P.R.A.D.; Batalha-Filho, H. Drainage rearrangements and in situ diversification of an endemic freshwater fish genus from north-eastern Brazilian rivers. Freshw. Biol. 2022, 67, 759–773. [Google Scholar] [CrossRef]
- Ni, X.M.; Chen, Y.; Deng, G.M.; Fu, C.Z. Pleistocene landscape dynamics drives lineage divergence of a temperate freshwater fish Gobio rivuloides in coastal drainages of Northern China. Genes 2023, 14, 2146. [Google Scholar] [CrossRef] [PubMed]
- Vance, G.; Kirschner, D.; Willett, S.D.; Pellissier, L. Drainage reorganization and intraspecific genetic diversity of riverine fish in the Ligurian Alps and northern Apennines. J. Geophys. Res. Earth Surf. 2025, 130, e2024JF008028. [Google Scholar] [CrossRef]
- Bishop, P. Drainage rearrangement by river capture, beheading and diversion. Prog. Phys. Geogr. 1995, 19, 449–473. [Google Scholar] [CrossRef]
- He, C.Q.; Braun, J.; Tang, H.; Yuan, X.P.; Acevedo-Trejos, E.; Ott, R.F.; Quay, G.S.D. Drainage divide migration and implications for climate and biodiversity. Nat. Rev. Earth Environ. 2024, 5, 177–192. [Google Scholar] [CrossRef]
- Dias, M.S.; Oberdorff, T.; Hugueny, B.; Leprieur, F.; Jezequel, C.; Cornu, J.F.; Brosse, S.; Grenouillet, G.; Tedesco, P.A. Global imprint of historical connectivity on freshwater fish biodiversity. Ecol. Lett. 2014, 17, 1130–1140. [Google Scholar] [CrossRef]
- Thomaz, A.T.; Malabarba, L.R.; Bonatto, S.L.; Knowles, L.L. Testing the effect of palaeodrainages versus habitat stability on genetic divergence in riverine systems: Study of a Neotropical fish of the Brazilian coastal Atlantic Forest. J. Biogeogr. 2015, 42, 2389–2401. [Google Scholar] [CrossRef]
- Xu, W.; Yin, W.; Chen, A.H.; Li, J.; Lei, G.C.; Fu, C.Z. Phylogeographical analysis of a cold-temperate freshwater fish, the Amur sleeper (Perccottus glenii) in the Amur and Liaohe River Basins of Northeast Asia. Zool. Sci. 2014, 31, 671–679. [Google Scholar] [CrossRef]
- Yang, J.Q.; Hsu, K.C.; Liu, Z.Z.; Su, L.W.; Kuo, P.H.; Tang, W.Q.; Zhou, Z.C.; Liu, D.; Bao, B.L.; Lin, H.D. The population history of Garra orientalis (Teleostei: Cyprinidae) using mitochondrial DNA and microsatellite data with approximate Bayesian computation. BMC Evol. Biol. 2016, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, A.T.; Malabarba, L.R.; Knowles, L.L. Genomic signatures of paleodrainages in a freshwater fish along the southeastern coast of Brazil: Genetic structure reflects past riverine properties. Heredity 2017, 119, 287–294. [Google Scholar] [CrossRef]
- Chen, W.T.; Li, C.; Chen, F.C.; Li, Y.F.; Yang, J.P.; Li, J.; Li, X.H. Phylogeographic analyses of a migratory freshwater fish (Megalobrama terminalis) reveal a shallow genetic structure and pronounced effects of sea-level changes. Gene 2020, 737, 144580. [Google Scholar] [CrossRef]
- Waters, J.M.; Burridge, C.P.; Craw, D. River capture and freshwater biological evolution: A review of galaxiid fish vicariance. Diversity 2020, 12, 216. [Google Scholar] [CrossRef]
- Masusa, T.; Shimono, Y.; Kishi, D.; Koizumi, I. Systematic headwater sampling of white-spotted charr reveals stream capture events across dynamic topography. J. Biogeogr. 2022, 50, 453–466. [Google Scholar] [CrossRef]
- Li, M.Y.; Yang, X.S.; Ni, X.M.; Fu, C.Z. The role of landscape evolution in the genetic diversification of a stream fish Sarcocheilichthys parvus from Southern China. Front. Genet. 2023, 13, 1075617. [Google Scholar] [CrossRef]
- Loureiro, M.; Stareczek, S.; D’anatro, A.; Thompson, A.W.; Ortí, G. River drainage rearrangements and the phylogeographic pattern of the annual fish Austrolebias arachan (Cyprinodontiformes, Rivulidae). Zool. Scr. 2023, 53, 142–156. [Google Scholar] [CrossRef]
- Ramirez, J.L.; Machado, C.B.; Affonso, P.; Galetti, P.M. Speciation in coastal basins driven by staggered headwater captures: Dispersal of a species complex, Leporinus bahiensis, as revealed by genome-wide SNP data. Syst. Biol. 2023, 72, 973–983. [Google Scholar] [CrossRef]
- Souto-Santos, I.C.A.; Jennings, W.B.; Buckup, P.A. Testing palaeodrainage hypotheses in south-eastern Brazil: Phylogeography of the sinistral livebearer fish of the genus Phalloceros (Cyprinodontiformes: Poeciliidae). Zool. J. Linn. Soc. 2023, 197, 514–531. [Google Scholar] [CrossRef]
- Wang, J.J.; Wu, J.X.; Yang, J.Q.; Chen, J.B.; Yang, J.M.; Li, C.; Lin, H.D.; Zhao, J. Phylogeography and demographic history of the cyprinid fish Barbodes semifasciolatus: Implications for the history of landform changes in south mainland China, Hainan and Taiwan. Front. Ecol. Evol. 2023, 11, 1193619. [Google Scholar] [CrossRef]
- Argolo, L.A.; Campos, I.P.A.; López-Fernández, H.; Batalha-Filho, H.; Affonso, P.R.A.D.M.A. Effects of past riverine connectivity on the population structure and species distribution of ‘Geophagus’ brasiliensis (Cichlidae) complex in a Neotropical hotspot. Freshw. Biol. 2024, 69, 907–916. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.Y.; Luo, J.; Murphy, R.W.; Du, R.; Wu, S.F.; Zhu, C.L.; Li, Y.; Poyarkov, A.D.; Nguyen, S.N.; et al. Quaternary palaeoenvironmental oscillations drove the evolution of the Eurasian Carassius auratus complex (Cypriniformes, Cyprinidae). J. Biogeogr. 2012, 39, 2264–2278. [Google Scholar] [CrossRef]
- Yu, D.; Chen, M.; Tang, Q.Y.; Li, X.J.; Liu, H.Z. Geological events and Pliocene climate fluctuations explain the phylogeographical pattern of the cold water fish Rhynchocypris oxycephalus (Cypriniformes: Cyprinidae) in China. BMC Evol. Biol. 2014, 14, 225. [Google Scholar] [CrossRef]
- Thomaz, A.T.; Christie, M.R.; Knowles, L.L. The architecture of river networks can drive the evolutionary dynamics of aquatic populations. Evolution 2016, 70, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Li, Y.F.; Cai, X.W.; Xiang, D.G.; Gao, S.; Li, C.; Lan, C.; Zhu, S.L.; Yang, J.P.; Li, X.H.; et al. Genetic structure of an East Asian minnow (Toxabramis houdemeri) in southern China, with implications for conservation. Biology 2022, 11, 1641. [Google Scholar] [CrossRef]
- Yang, X.S.; Ni, X.M.; Fu, C.Z. Phylogeographical analysis of the freshwater gudgeon Huigobio chenhsienensis (Cypriniformes: Gobionidae) in southern China. Life 2022, 12, 1024. [Google Scholar] [CrossRef]
- Gao, J.X.; Yu, D.; Liu, H.Z. Phylogeographic analysis revealed allopatric distribution pattern and biogeographic processes of the widespread pale chub Opsariichthys acutipinnis-evolans complex (Teleostei: Cyprinidae) in southeastern China. Front. Ecol. Evol. 2023, 11, 1142810. [Google Scholar] [CrossRef]
- Stokes, M.F.; Kim, D.; Gallen, S.F.; Benavides, E.; Keck, B.P.; Wood, J.; Goldberg, S.L.; Larsen, J.L.; Mollish, J.M.; Simmons, J.W.; et al. Erosion of heterogeneous rock drives diversification of Appalachian fishes. Science 2023, 380, 855–859. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Wang, J.J.; Ren, J.F.; Li, F.; Wu, J.X.; Zhou, J.J.; Li, J.L.; Yang, J.Q.; Lin, H.D. Historical landscape evolution shaped the phylogeography and population history of the cyprinid fishes of Acrossocheilus (Cypriniformes: Cyprinidae) according to mitochondrial DNA in Zhejiang province, China. Diversity 2023, 15, 425. [Google Scholar] [CrossRef]
- Waters, J.M.; King, T.M.; Craw, D. Gorges partition diversity within New Zealand flathead Galaxias populations. J. Fish Biol. 2024, 104, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ye, H.; Yue, H.M.; Li, J.Y.; Huang, L.; Qu, Z.L.; Ruan, R.; Lin, D.Q.; Liang, Z.Q.; Xie, Y.; et al. Genetic diversity and population structure of largefin longbarbel catfish (Hemibagrus macropterus) Inferred by mtDNA and microsatellite DNA markers. Animals 2025, 15, 770. [Google Scholar] [CrossRef]
- dos Reis, R.B.; Stabile, B.H.M.; Frota, A.; Ferrer, J.; Oliveira, A.V.D.; Graça, W.J.D. Recent dispersion routes between freshwater ecoregions evidence headwater captures in southern Brazil: A case study using cryptic species of the neotropical freshwater fish genus Cambeva (Siluriformes: Trichomycteridae). Hydrobiologia 2025, 852, 873–890. [Google Scholar] [CrossRef]
- Huang, R.R.; Chen, J.M.; Zhang, R.Y.; Chen, W.T. Phylogeography and demography of Pterocryptis cochinchinensis (Siluriformes: Siluridae) in Southern China. Glob. Ecol. Conserv. 2025, 62, e03728. [Google Scholar] [CrossRef]
- Maldonado-Sánchez, D.; Rios-Cardenas, O.; Morris, M.R.; Gutiérrez-Rodríguez, C. Phylogeography and demographic history of the swordtail fish Xiphophorus multilineatus. Environ. Biol. Fishes 2025, 108, 969–985. [Google Scholar] [CrossRef]
- Emery, K.O.; Hayashi, Y.; Hilde, T.W.C.; Kobayashi, K.; Koo, J.H.; Meng, C.Y.; Niino, H.; Osterhagen, J.H.; Reynolds, L.M.; Wageman, J.M.; et al. Geological structure and some water characteristics of the East China Sea and the Yellow Sea. CCOP Tech. Bull. 1969, 2, 3–43. [Google Scholar]
- Jin, X.; Yu, P. Tectonics of the Yellow Sea and the East China Sea. In The Geology of the Yellow Sea and the East China Sea; Science Press: Beijing, China, 1982; pp. 1–22. (In Chinese) [Google Scholar]
- Wageman, J.M.; Hilde, T.W.C.; Emery, K.O. Structural framework of East China sea and yellow sea. Aapg. Bull. 1970, 54, 1611–1643. [Google Scholar] [CrossRef]
- Juan, V.C. Thermal-tectonic evolution of the Yellow Sea and East China Sea—Implication for transformation of continental to oceanic crust and marginal basin formation. Tectonophysics 1986, 125, 231–244. [Google Scholar] [CrossRef]
- Yi, L.; Ye, X.Y.; Chen, J.B.; Li, Y.; Long, H.; Wang, X.L.; Du, J.H.; Zhao, S.L.; Deng, C.L. Magnetostratigraphy and luminescence dating on a sedimentary sequence from northern East China sea: Constraints on evolutionary history of eastern marginal seas of China since the early Pleistocene. Quatern. Int. 2014, 349, 316–326. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.K.; Zhu, D.K.; Yang, J.H.; Mao, L.J.; Li, S.H. River-Sea interaction and the North Jiangsu Plain Formation. Quat. Sci. 2006, 26, 301–320. (In Chinese) [Google Scholar]
- Liu, J.X.; Liu, Q.S.; Zhang, X.H.; Liu, J.; Wu, Z.Q.; Mei, X.; Shi, X.F.; Zhao, Q.H. Magnetostratigraphy of a long Quaternary sediment core in the South Yellow Sea. Quat. Sci. Rev. 2016, 144, 1–15. [Google Scholar] [CrossRef]
- Liao, X.; Wang, P.; Jiang, R.; Yin, Y.; Hu, Z.J.; Zhang, Z.P.; Zhu, L.C. Progress of research on the Quaternary sedimentary strata and environment in the southern Huang-Huai-Hai. J. Earth Environ. 2024, 15, 173–192. (In Chinese) [Google Scholar]
- Liu, J.; Zhang, X.H.; Mei, X.; Zhao, Q.H.; Guo, X.W.; Zhao, W.N.; Liu, J.X.; Saito, Y.; Wu, Z.Q.; Li, J.; et al. The sedimentary succession of the last ~3.50Myr in the western South Yellow Sea: Paleoenvironmental and tectonic implications. Mar. Geol. 2018, 399, 47–65. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.Q.; Shu, J.W.; Bai, S.B.; Zhao, Z.Y.; Zhang, X.Y.; Guo, G.; Zhang, P.; Lin, J.X. Sedimentary evolution and transgressions of the western Subei Basin in eastern China since the Late Pliocene. Acta Geol. Sin. 2019, 93, 155–166. [Google Scholar] [CrossRef]
- Zhang, J.; Wan, S.M.; Clift, P.D.; Huang, J.; Yu, Z.J.; Zhang, K.D.; Mei, X.; Liu, J.; Han, Z.Y.; Nan, Q.Y.; et al. History of Yellow River and Yangtze River delivering sediment to the Yellow Sea since 3.5 Ma: Tectonic or climate forcing? Quat. Sci. Rev. 2019, 216, 74–88. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Stanley, D.J. Quaternary subsidence and river channel migration in the Yangtze Delta Plain, eastern China. J. Coast. Res. 1995, 11, 927–945. [Google Scholar]
- Shu, Q.; Zhao, Z.J.; Zhao, Y.F.; Chen, Y.; Zhang, M.P. Magnetic properties of Late Cenozoic sediments in the Subei Basin: Implications for the Yangtze River run-through time. J. Coast. Res. 2021, 37, 122–131. [Google Scholar] [CrossRef]
- Liu, J.H.; Yue, W.; Chen, J.; Yue, X.Y.; Zhang, L.M.; Li, Y.L.; Liu, X.B. Provenance of Plio-Pleistocene sediments of the Subei Basin, East China with implication for proto-Yangtze channelization. J. Asian Earth Sci. 2025, 280, 106466. [Google Scholar] [CrossRef]
- Wu, M. Study on Formation and Evolution of the Huaihe Water System. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2013. (In Chinese). [Google Scholar]
- Liu, Y.; Liu, X.B.; Wang, S.J.; Xu, S.; Ellam, R.M.; Fabel, D.; Chen, J. Late Cenozoic channel migration of the proto-Yangtze River in the delta region: Insights from cosmogenic nuclide burial dating of onshore boreholes. Geomorphology 2022, 407, 108228. [Google Scholar] [CrossRef]
- Wang, Z.R.; Xie, F.; Ling, F.; Du, Y. Monitoring surface water inundation of Poyang Lake and Dongting Lake in China using Sentinel-1 SAR images. Remote Sens. 2022, 14, 3473. [Google Scholar] [CrossRef]
- Jing, C.Y. The formation and evolution of Dongting Lake. J. Nanjing Norm. Coll. (Nat. Sci. Ed.) 1982, 2, 52–60. (In Chinese) [Google Scholar]
- Yang, D.Y. The evolution of the Poyang Lake in Quaternary. Oceanol. Limnol. Sin. 1986, 17, 429–435. (In Chinese) [Google Scholar]
- Su, S.D. Historical Documents on the Origin and Evolution of Poyang Lake. J. Lake Sci. 1992, 4, 40–47. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Wang, C.L. Formation and evolution of the Dongting Basin. Geotecton. Metallog. 1992, 16, 98–99. (In Chinese) [Google Scholar]
- Cai, X.F.; Zhang, Z.J.; Lu, L.; Tang, Z.C. Filled sequence and evolution characteristics of Mesozoic Basin in old Poyanghu. Resour. Surv. Environ. 2003, 24, 167–176. (In Chinese) [Google Scholar]
- Yang, X.D.; Wu, Z.H.; Zhang, H.J. Geological evolution, neotectonics and genetic mechanism of the Poyang Lake Basin. J. Geomech. 2016, 22, 667–684. (In Chinese) [Google Scholar]
- Zhao, J.X.; Li, C.A.; Zhang, Y.F.; Qiang, X.K.; Xiong, D.Q. Quaternary Chronostratigraphy of borehole S3-7 in Dongting Basin. Earth Sci. 2016, 41, 633–643. (In Chinese) [Google Scholar]
- Qin, J.; Liu, X.; Xu, Y.; Wu, X.; Ouyang, S. Beta diversity patterns of fish and conservation implications in the Luoxiao Mountains, China. ZooKeys 2019, 817, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Qin, J.J.; Ao, X.F.; Guo, X.; Xiao, W.L.; Wu, X.P.; Ouyang, S. Species diversity of fish in the Luoxiao Mountains region. Biodivers. Sci. 2020, 28, 889–895. (In Chinese) [Google Scholar] [CrossRef]
- Monaghan, M.T.; Spaak, P.; Robinson, C.T.; Ward, J.V. Genetic differentiation of Baetis alpinus Pictet (Ephemeroptera: Baetidae) in fragmented alpine streams. Heredity 2001, 86, 395–403. [Google Scholar] [CrossRef]
- Pelicice, M.P.; Pompeu, P.S.; Agostinho, A.A. Large reservoirs as ecological barriers to downstream movements of Neotropical migratory fish. Fish Fish. 2015, 16, 697–715. [Google Scholar] [CrossRef]
- Chen, X.L.; Chiang, T.Y.; Lin, H.D.; Zheng, H.S.; Shao, K.T.; Zhang, Q.; Hsu, K.C. Mitochondrial DNA phylogeography of Glyptothorax fokiensis and Glyptothorax hainanensis in Asia. J. Fish Biol. 2007, 70, 75–93. [Google Scholar] [CrossRef]
- Cao, L.; Liang, X.F.; Tang, W.Q.; Zhao, J. Phylogeography of Coreoperca whiteheadi (Perciformes: Coreoperca) in China based on mitochondrial and nuclear gene sequences. Biochem. Syst. Ecol. 2013, 50, 223–231. [Google Scholar] [CrossRef]
- Berrebi, P.; Retif, X.; Fang, F.; Zhang, C.G. Population structure and systematics of Opsariichthys bidens (Osteichthyes: Cyprinidae) in south-east China using a new nuclear marker: The introns (EPIC-PCR). Biol. J. Linn. Soc. 2006, 87, 155–166. [Google Scholar] [CrossRef]
- Yang, L.; Mayden, R.L.; He, S.P. Population genetic structure and geographical differentiation of the Chinese catfish Hemibagrus macropterus (Siluriformes, Bagridae): Evidence for altered drainage patterns. Mol. Phylogenet. Evol. 2009, 51, 405–411. [Google Scholar] [CrossRef]
- Yang, J.Q.; Tang, W.Q.; Liao, T.Y.; Sun, Y.; Zhou, Z.C.; Han, C.C.; Liu, D.; Lin, H.D. Phylogeographical analysis on Squalidus argentatus recapitulates historical landscapes and drainage evolution on the island of Taiwan and mainland China. Int. J. Mol. Sci. 2012, 13, 1405–1425. [Google Scholar] [CrossRef]
- Tsao, Y.F.; Lin, W.W.; Chang, C.H.; Ueda, T.; Jang-Liaw, N.H.; Zhao, Y.H.; Kao, H.W. Phylogeography, historical demography, and genetic Structure of the rose bitterling, Rhodeus ocellatus (Kner, 1866) (Cypriniformes: Acheilognathidae), in East Asia. Zool. Stud. 2016, 55, e49. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhong, Z.X.; Dai, W.; Fan, Q.; He, S.P. Phylogeographic structure, cryptic speciation and demographic history of the sharpbelly (Hemiculter leucisculus), a freshwater habitat generalist from southern China. BMC Evol. Biol. 2017, 17, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yi, S.K.; Ma, L.Y.; Wang, W.M. Evolution and phylogeography analysis of diploid and polyploid Misgurnus anguillicaudatus populations across China. Proc. R. Soc. B 2019, 286, 20190076. [Google Scholar] [CrossRef]
- Zuo, F.Y. A new discover of rebuilding Lingqu Canal during the Ming Dynasty. Guizhou Cult. Hist. 2024, 3, 42–51. (In Chinese) [Google Scholar]
- Li, D.A.; Zhao, B.Q. Research on function changes of the Lingqu Canal water conservancy project. China Three Gorges Trib. 2012, 2, 14–19. (In Chinese) [Google Scholar]
- Chen, Y. Studies on Phylogeny and Biogeography of Subfamily Acrossocheilinae Fishes. Ph.D. Thesis, Fudan University, Shanghai, China, 2025. (In Chinese). [Google Scholar]
- Yuan, L.Y. Monophyly, Affinity and Taxonomic Revision of the Cyprinid Genus Acrossocheilus Oshima, 1919. Ph.D. Thesis, Institute of Hydrobiology, The Chinese Academy of Sciences, Wuhan, China, 2009. (In Chinese). [Google Scholar]
- Zhou, C.J.; Zhao, H.P.; Li, H.T. The complete mitogenome of Acrossocheilus kreyenbergii (Cypriniformes; Cyprinidae). Mitochondrial DNA Part A 2016, 27, 1068–1069. [Google Scholar] [CrossRef]
- Zhao, H.P.; Yang, H.; Zhang, S.Q.; Shi, Y.Z.; Jing, G.W.; Yao, P.; Lu, J.Q. Primary investigation of fish species diversity in Henan Province Liankangshan National Nature Reserve. Fish Fish. 2018, 48, 75–79. (In Chinese) [Google Scholar]
- Chen, W.J.; Fu, H.Y. The Fishes of Jiangxi; Science Press: Beijing, China, 2024; pp. 75–76. (In Chinese) [Google Scholar]
- GB/T 35892-2018; Laboratory Animal—Guideline for Ethical Review of Animal Welfare. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Xia, X.H. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchene, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kuhnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.R.; Drummond, A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.4. Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018; Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 14 October 2016).
- Hoang, H.D.; Jang-Liaw, N.H.; Pham, H.M.; Tran, N.T.; Durand, J.D.; Nguyen, T.D.; Pfeiffer, J.; Page, L.M. Generic revision of the Southeast and East Asian torrent carp subfamily Acrossocheilinae (Pisces: Teleostei) with description of three new genera and a new species from Vietnam. J. Zool. Syst. Evol. Res. 2025, 2025, 8895501. [Google Scholar] [CrossRef]
- Bandelt, H.J.; Forster, P.; Rohl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Pons, O.; Petit, R.J. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 1996, 144, 1237–1245. [Google Scholar] [CrossRef]
- Dupanloup, I.; Schneider, S.; Excoffier, L. A simulated annealing approach to define the genetic structure of populations. Mol. Ecol. 2002, 11, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Matzke, N.J. BioGeoBEARS: Biogeography with Bayesian (and Likelihood) Evolutionary Analysis in R Scripts. R Package, Version 021 ed; University of California Berkeley: Berkeley, CA, USA, 2013. [Google Scholar]
- Avise, J.C. Phylogeography: The History and Formation of Species; Harvard University Press: London, UK, 2000; pp. 135–147. [Google Scholar]
- Xu, Y.T.; Lai, Z.P.; Li, C.A. Sea-level change as the driver for lake formation in the Yangtze Plain—A review. Glob. Planet. Change 2019, 181, 102980. [Google Scholar] [CrossRef]
- Lai, Z.P.; Liu, Y.X.; Wu, Z.Y.; Xu, Y.T.; Zhang, Z.B.; Montgomery, D.R. Headward incision of large rivers in response to glacial sea level fall. Sci. Adv. 2025, 11, eadr5446. [Google Scholar] [CrossRef]
- Wang, K.X.; Lu, H.Y.; Lei, F.; Lyu, H.Z.; Wang, H.L.; Wang, Y.C. East Asian monsoon precipitation decrease during Plio-Pleistocene transition revealed by changes in the chemical weathering intensity of Red Clay and loess-paleosol. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2022, 601, 111080. [Google Scholar] [CrossRef]
- An, Z.; Zhou, W.; Zhang, Z.; Zhang, X.; Liu, Z.H.; Sun, Y.B.; Clemens, S.C.; Wu, L.X.; Zhao, J.J.; Shi, Z.G.; et al. Mid-Pleistocene climate transition triggered by Antarctic Ice Sheet growth. Science 2024, 385, 560–565. [Google Scholar] [CrossRef]
- Qu, X.; Guo, C.B.; Xiong, F.Y.; Xin, W.; Chen, Y.S.; Sui, W. Characterization of the fish community and environment driving factors during development of cascaded dams in the lower Jinsha River. J. Hydroecol. 2020, 41, 46–56. (In Chinese) [Google Scholar]
- Xiong, F.; Guo, Q.; Zhang, W.; Liu, H.Y.; Zhai, D.D.; Duan, X.B.; Tian, H.W.; Chen, D.Q. Spatial pattern of fish community structure in Xiangjiaba Reservoir on the lower Jinsha River. J. Hydroecol. 2024, 45, 82–91. (In Chinese) [Google Scholar]
- Ma, Q.; Li, M.; Liu, H. Population genetics of the endemic Hemiculterella wui (Wang, 1935) in the Poyang Lake Basin (China). Fishes 2024, 9, 260. [Google Scholar] [CrossRef]
- Grant, K.M.; Rohling, E.J.; Ramsey, C.B.; Cheng, H.; Edwards, R.L.; Florindo, F.; Heslop, D.; Marra, F.; Roberts, A.P.; Tamisiea, M.E.; et al. Sea-level variability over five glacial cycles. Nat. Commun. 2014, 5, 5076. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Rohling, E.J.; Stringer, C.; Roberts, A.P.; Dekkers, M.J.; Dupont-Nivet, G.; Yu, J.M.; Liu, Q.S.; Zhang, P.; Liu, Z.H.; et al. Two-stage mid-Brunhes climate transition and mid-Pleistocene human diversification. Earth Sci. Rev. 2020, 210, 103354. [Google Scholar] [CrossRef]
- Grant, W.S.; Bowen, B.W. Shallow population histories in deep evolutionary lineages of marine fishes: Insights from sardines and anchovies and lessons for conservation. J. Hered. 1998, 89, 415–426. [Google Scholar] [CrossRef]
- Yang, L.; He, S. Phylogeography of the freshwater catfish Hemibagrus guttatus (Siluriformes, Bagridae): Implications for south China biogeography and influence of sea-level changes. Mol. Phylogenet. Evol. 2008, 49, 393–398. [Google Scholar] [CrossRef]
- Huang, X.X.; Hsu, K.C.; Kang, B.; Kuo, P.H.; Tsai, W.H.; Liang, C.M.; Lin, H.D.; Wang, W.K. Population structure of Aphyocypris normalis: Phylogeography and systematics. ZooKeys 2019, 872, 77–90. [Google Scholar] [CrossRef]
- Sun, Y.B.; Kutzbach, J.; An, Z.S.; Clemens, S.; Liu, Z.Y.; Liu, W.G.; Liu, X.D.; Shi, Z.G.; Zhang, W.P.; Liang, L.J.; et al. Astronomical and glacial forcing of East Asian summer monsoon variability. Quat. Sci. Rev. 2015, 115, 132–142. [Google Scholar] [CrossRef]
- Cheng, H.; Edwards, R.L.; Sinha, A.; Spötl, C.; Yi, L.; Chen, S.T.; Kelly, M.; Kathayat, G.; Wang, X.F.; Li, X.L.; et al. Corrigendum: The Asian monsoon over the past 640,000 years and ice age terminations. Nature 2016, 541, 122. [Google Scholar] [CrossRef]
- Zhou, W.J.; Kong, X.H.; Paterson, G.A.; Sun, Y.B.; Wu, Y.B.; Ao, H.; Feng, H.; Du, Y.J.; Tang, L.; Zhou, J.; et al. Eccentricity-paced geomagnetic field and monsoon rainfall variations over the last 870 kyr. Proc. Natl. Acad. Sci. USA 2023, 120, e2211495120. [Google Scholar] [CrossRef]
- Li, Y.P.; Ludwig, A.; Peng, Z.G. Geographical differentiation of the Euchiloglanis fish complex (Teleostei: Siluriformes) in the Hengduan Mountain Region, China: Phylogeographic evidence of altered drainage patterns. Ecol. Evol. 2017, 7, 928–940. [Google Scholar] [CrossRef]
- Liang, Y.Y.; He, D.K.; Jia, Y.T.; Sun, H.Y.; Chen, Y.F. Phylogeographic studies of Schizothoracine fishes on the central Qinghai-Tibet Plateau reveal the highest known glacial microrefugia. Sci. Rep. 2016, 7, 10983. [Google Scholar] [CrossRef]
- Hu, J.X.; Liu, M.D.; He, D.K. Phylogeography of Triplophysa stenura (Nemacheilidae): Responded to the mid-Pleistocene climate transition in the Qinghai-Tibetan Plateau. Zool. Stud. 2020, 59, 67. [Google Scholar] [CrossRef]
- O’Brien, D.; Bader, E.; Hall, J.; Hoban, S.; Segelbacher, G.; Vilaça, S.T.; Leigh, D.M. Genetic diversity is key to a nature-positive future. People Nat. 2025, 7, 2578–2584. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Rahbek, C. Conservation strategies aided by assessment of global genetic diversity. Nature 2025, 638, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Hoban, S.; Hvilsom, C.; Aissi, A.; Aleixo, A.; Bélanger, J.; Biala, K.; Ekblom, R.; Fedorca, A.; Funk, W.C.; Gonzalez, A.; et al. How can biodiversity strategy and action plans incorporate genetic diversity and align with global commitments? BioScience 2025, 75, 47–60. [Google Scholar] [CrossRef]
- Henry, R.C. Future projections of biodiversity under global change need to include genetic diversity. Glob. Change Biol. 2025, 31, e70477. [Google Scholar] [CrossRef]
- Exposito-Alonso, M.; Booker, T.R.; Czech, L.; Gillespie, L.; Hateley, S.; Kyriazis, C.C.; Lang, P.L.M.; Leventhal, L.; Nogues-Bravo, D.; Pagowski, V.; et al. Genetic diversity loss in the Anthropocene. Science 2022, 377, 1431–1435. [Google Scholar] [CrossRef]
- Mastretta-Yanes, A.; Silva, J.M.; Grueber, C.E.; Castillo-Reina, L.; Köppä, V.; Forester, B.R.; Funk, W.C.; Heuertz, M.; Ishihama, F.; Jordan, R.; et al. Multinational evaluation of genetic diversity indicators for the Kunming-Montreal Global Biodiversity Framework. Ecol. Lett. 2024, 27, e14461. [Google Scholar] [CrossRef]
- Hu, Y.S.; Zhou, W.L.; Hu, Y.B.; Wei, F.W. Conservation evolutionary biology: A unified framework connecting the past, present, and future of biodiversity conservation. Mol. Biol. Evol. 2025, 42, msaf122. [Google Scholar] [CrossRef]
- Paz-Vinas, I.; Vandergast, A.G.; Schmidt, C.; Leigh, D.M.; Blanchet, S.; Clark, R.D.; Crandall, E.D.; Kort, H.D.; Falgout, J.; Garroway, C.J.; et al. Sparse genetic data limit biodiversity assessments in protected areas globally. Front. Ecol. Environ. 2025, 23, e2867. [Google Scholar] [CrossRef]
- Shaw, R.E.; Farquharson, K.A.; Bruford, M.W.; Coates, D.J.; Elliott, C.P.; Mergeay, J.; Ottewell, K.M.; Segelbacher, G.; Hoban, S.; Hvilsom, C.; et al. Global meta-analysis shows action is needed to halt genetic diversity loss. Nature 2025, 638, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C. Defining ‘Evolutionarily Significant Units’ for conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef]
- Avise, J.C. Phylogenetic units and currencies above and below the species level. In Phylogeny and Conservation; Purvis, A., Gittleman, J.L., Brooks, T., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 76–100. [Google Scholar]
- Coates, D.J.; Byrne, M.; Moritz, C. Genetic diversity and conservation units: Dealing with the species-population continuum in the age of genomics. Front. Ecol. Evol. 2018, 6, 13. [Google Scholar] [CrossRef]
- Davis, C.D.; Epps, C.W.; Flitcroft, R.L.; Banks, M.A. Refining and defining riverscape genetics: How rivers influence population genetic structure. Wires Water 2018, 5, e1269. [Google Scholar] [CrossRef]
- Terui, A.; Kim, S.; Dolph, C.L.; Kadoya, T.; Miyazaki, Y. Emergent dual scaling of riverine biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2105574118. [Google Scholar] [CrossRef]
| River Basin or Catchment | No. of Individuals | No. of Haplotypes | No. of Private Haplotypes | Haplotype Diversity | Nucleotide Diversity |
|---|---|---|---|---|---|
| Pearl River | 93 | 15 | 12 | 0.383 ± 0.065 | 0.0004 ± 0.0004 |
| Huai River | 7 | 2 | 2 | 0.286 ± 0.196 | 0.0005 ± 0.0005 |
| Yangtze River | 124 | 48 | 45 | 0.927 ± 0.014 | 0.0160 ± 0.0079 |
| Dongting Lake catchment | 57 | 19 | 16 | 0.776 ± 0.054 | 0.0029 ± 0.0017 |
| Poyang Lake catchment | 58 | 26 | 26 | 0.886 ± 0.030 | 0.0159 ± 0.0080 |
| Qiupu River | 9 | 3 | 3 | 0.639 ± 0.126 | 0.0007 ± 0.0006 |
| Overall | 224 | 62 | 59 | 0.795 ± 0.028 | 0.0135 ± 0.0067 |
| Yangtze River | |||||
|---|---|---|---|---|---|
| Pearl River | DLC | PLC | Qiupu River | Huai River | |
| Pearl River | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| Dongting Lake catchment (DLC) | 0.1182 | 0.0000 | 0.0000 | 0.0000 | |
| Poyang Lake catchment (PLC) | 0.6990 | 0.5774 | 0.0000 | 0.0000 | |
| Qiupu River | 0.9737 | 0.8456 | 0.3931 | 0.0000 | |
| Huai River | 0.9911 | 0.9415 | 0.6890 | 0.9872 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Deng, G.; Le, Z.; Fu, C. Mountains, Lakes, and Ancient Drainage Networks Sculpt the Phylogeographic Architecture of the Stream Headwater Fish Acrossocheilus kreyenbergii in China. Genes 2025, 16, 1393. https://doi.org/10.3390/genes16121393
Chen Y, Deng G, Le Z, Fu C. Mountains, Lakes, and Ancient Drainage Networks Sculpt the Phylogeographic Architecture of the Stream Headwater Fish Acrossocheilus kreyenbergii in China. Genes. 2025; 16(12):1393. https://doi.org/10.3390/genes16121393
Chicago/Turabian StyleChen, Yun, Guangmin Deng, Ziyu Le, and Cuizhang Fu. 2025. "Mountains, Lakes, and Ancient Drainage Networks Sculpt the Phylogeographic Architecture of the Stream Headwater Fish Acrossocheilus kreyenbergii in China" Genes 16, no. 12: 1393. https://doi.org/10.3390/genes16121393
APA StyleChen, Y., Deng, G., Le, Z., & Fu, C. (2025). Mountains, Lakes, and Ancient Drainage Networks Sculpt the Phylogeographic Architecture of the Stream Headwater Fish Acrossocheilus kreyenbergii in China. Genes, 16(12), 1393. https://doi.org/10.3390/genes16121393






