SLC30A3 as a Zinc Transporter-Related Biomarker and Potential Therapeutic Target in Alzheimer’s Disease
Abstract
1. Introduction
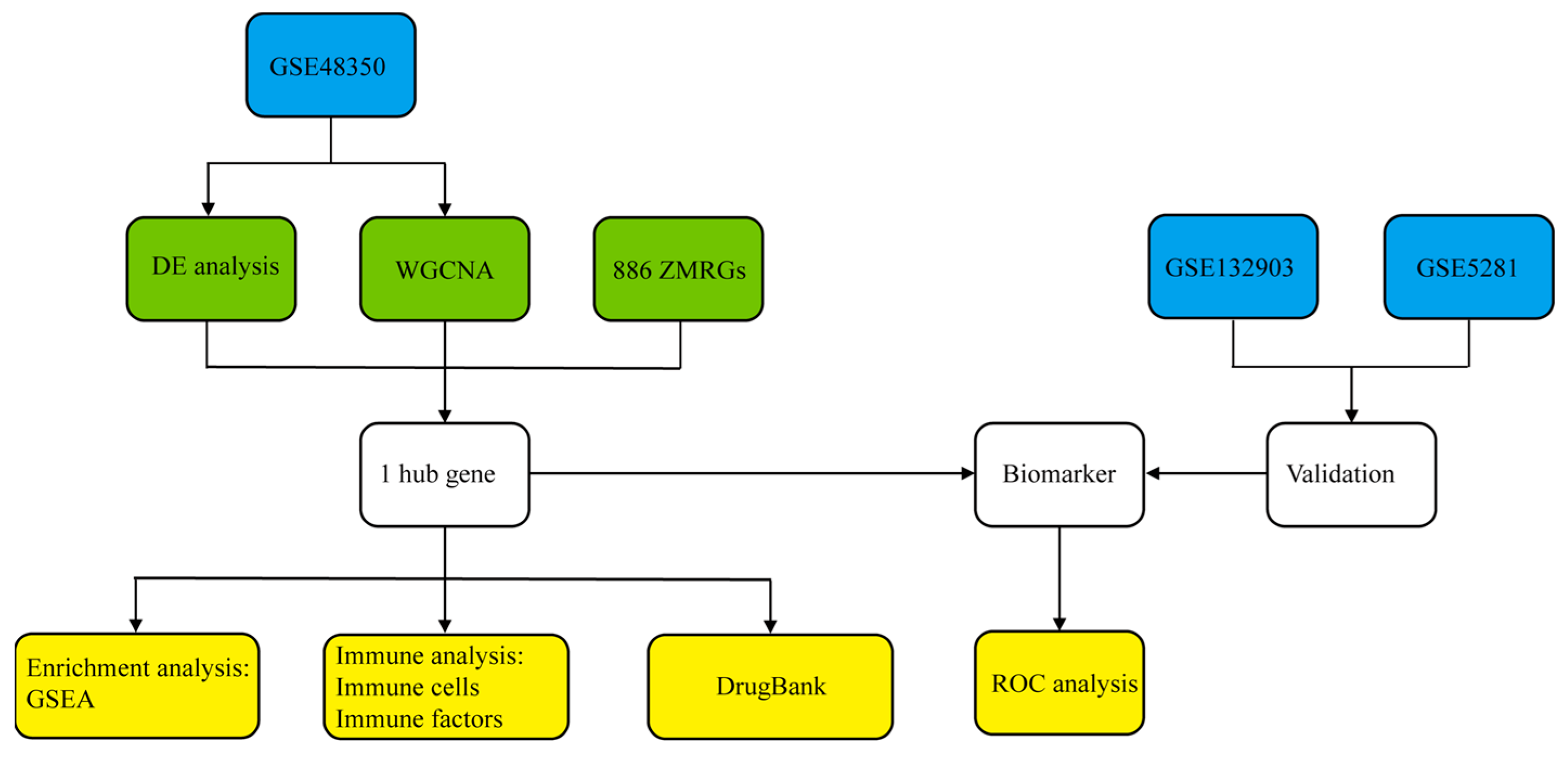
2. Materials and Methods
2.1. Data Acquisition
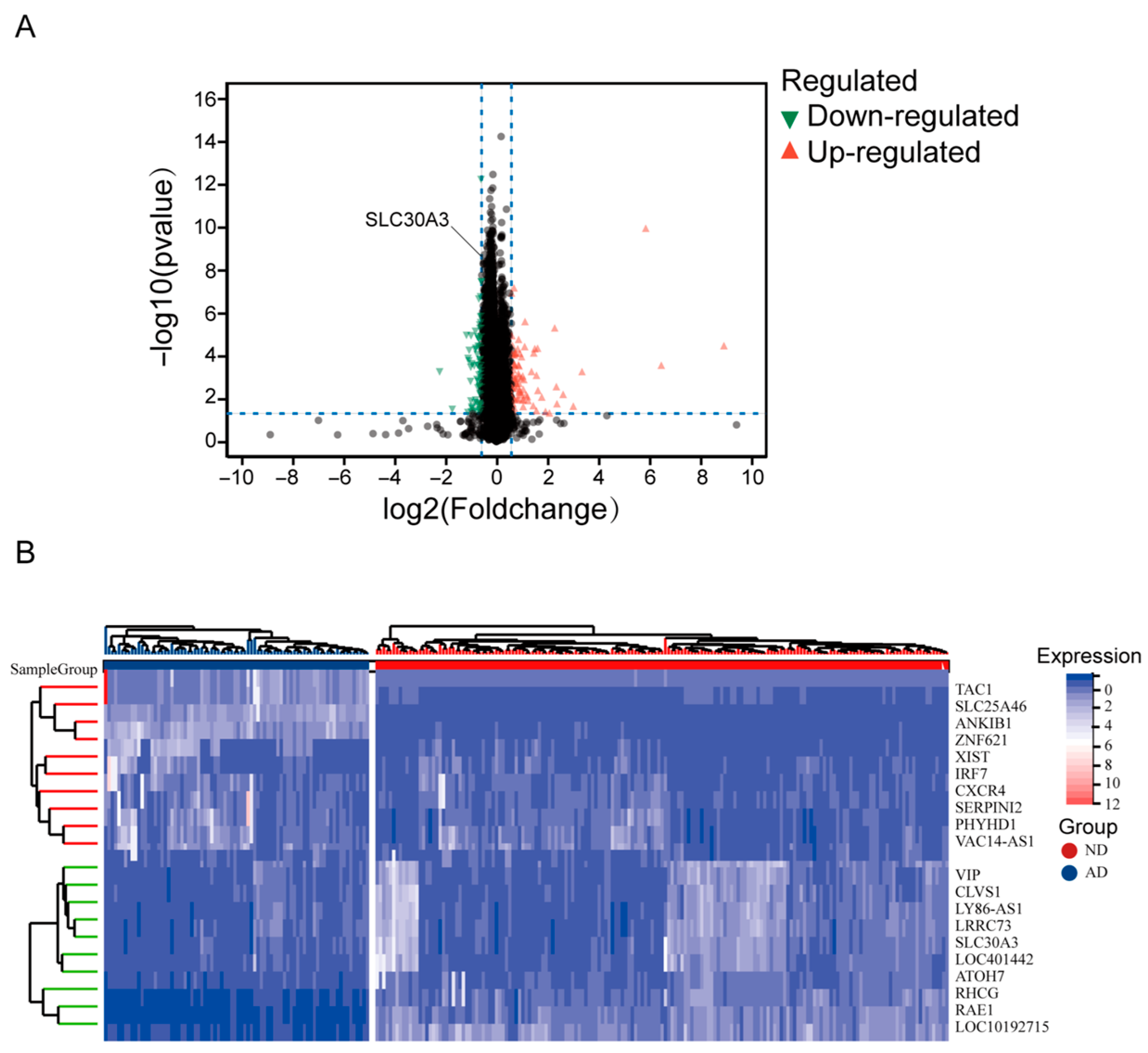
2.2. Differential Expression Analysis
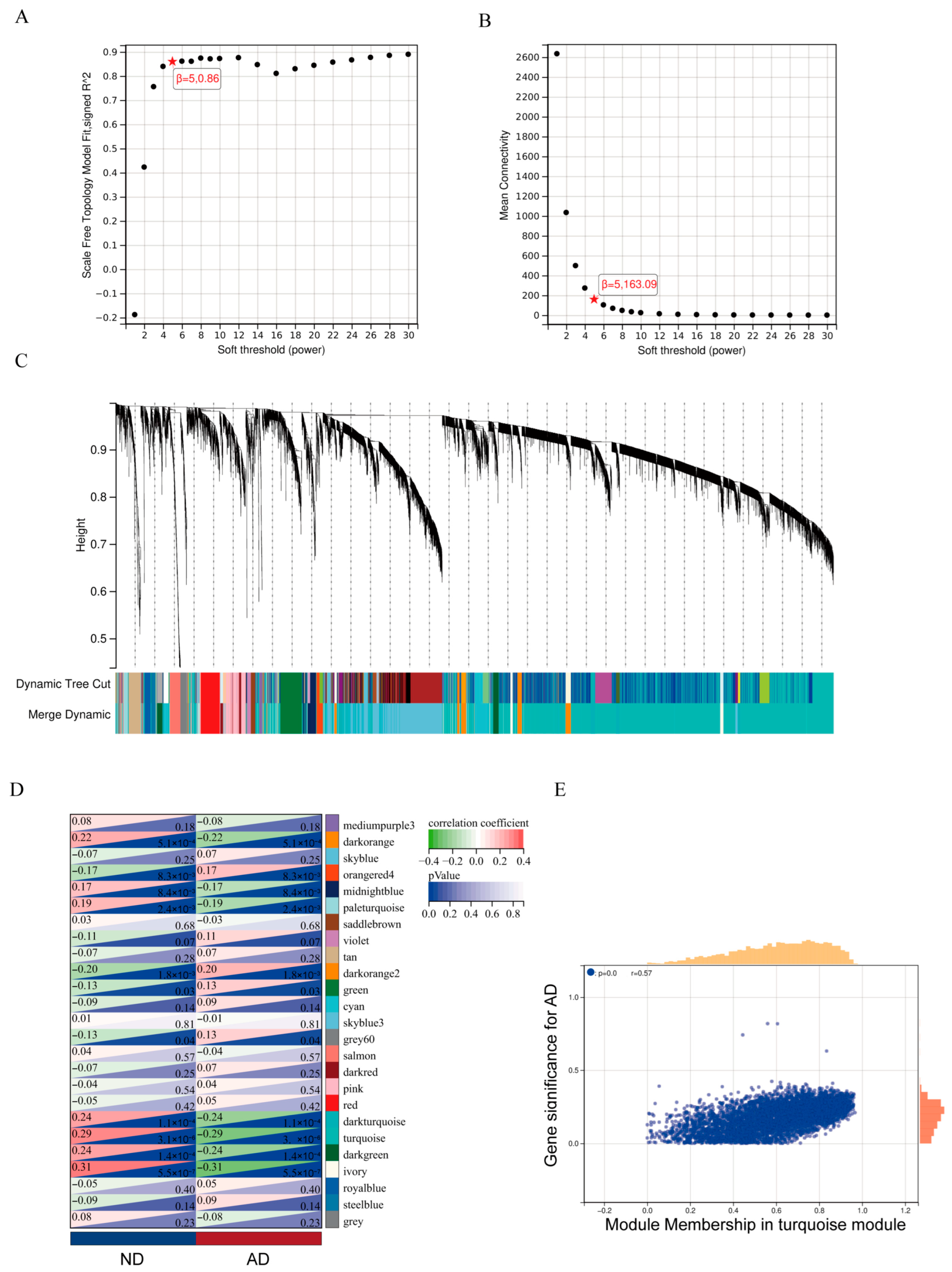
2.3. Weighted Gene Co-Expression Network Analysis (WGCNA)
2.4. Identification of Hub Genes
2.5. Gene Set Enrichment Analysis (GSEA)
2.6. Receiver Operating Characteristic (ROC) Analysis
2.7. Immune Cell Infiltration and Immune-Related Factor Analysis
2.8. Drug Prediction from DrugBank
2.9. Western Blot
3. Results
3.1. SLC30A3 Was the Hub Gene Associated with Zinc Metabolism and AD
3.2. Pathways Enriched for SLC30A3
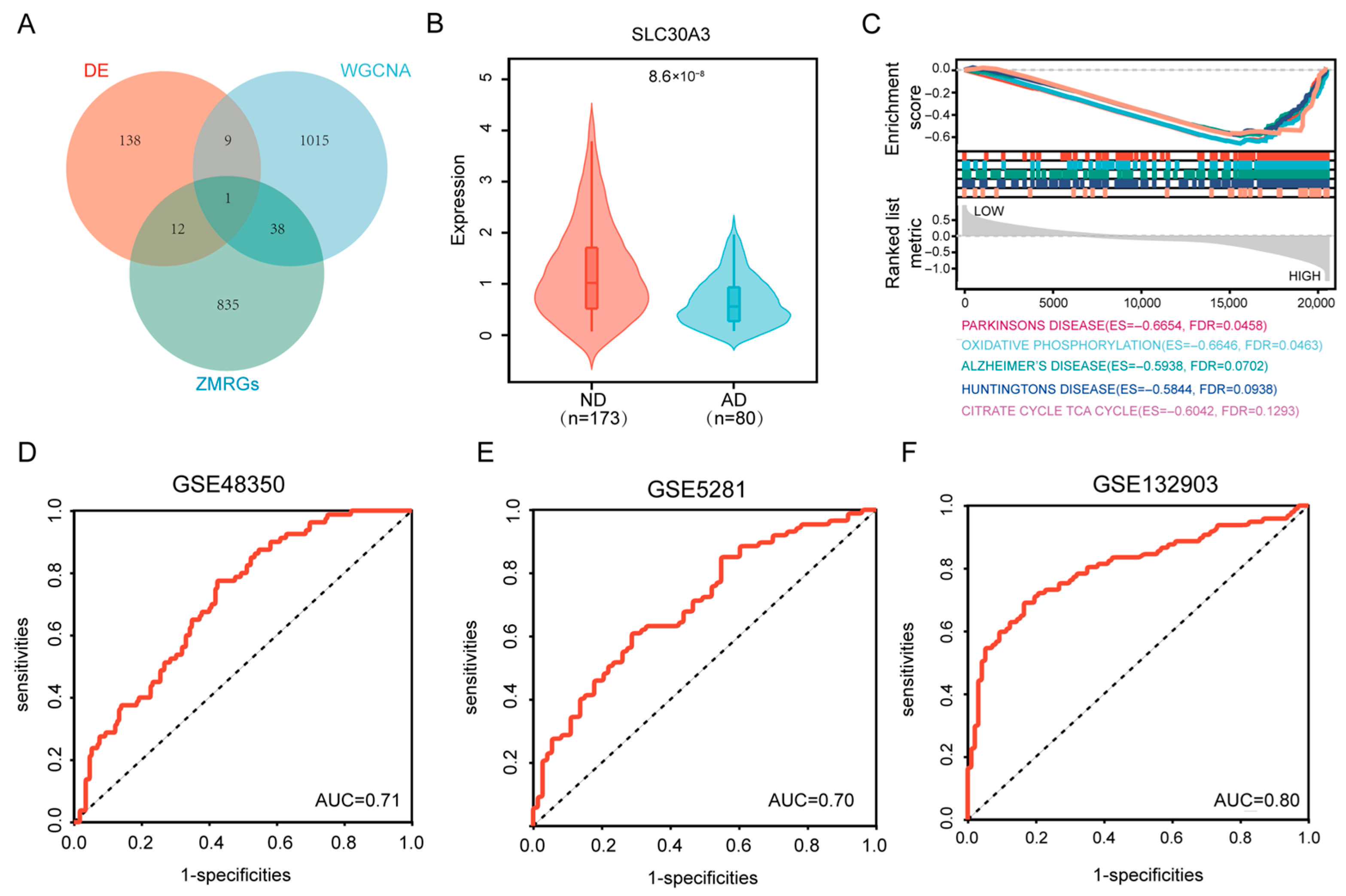
3.3. Validation of the Diagnostic Model
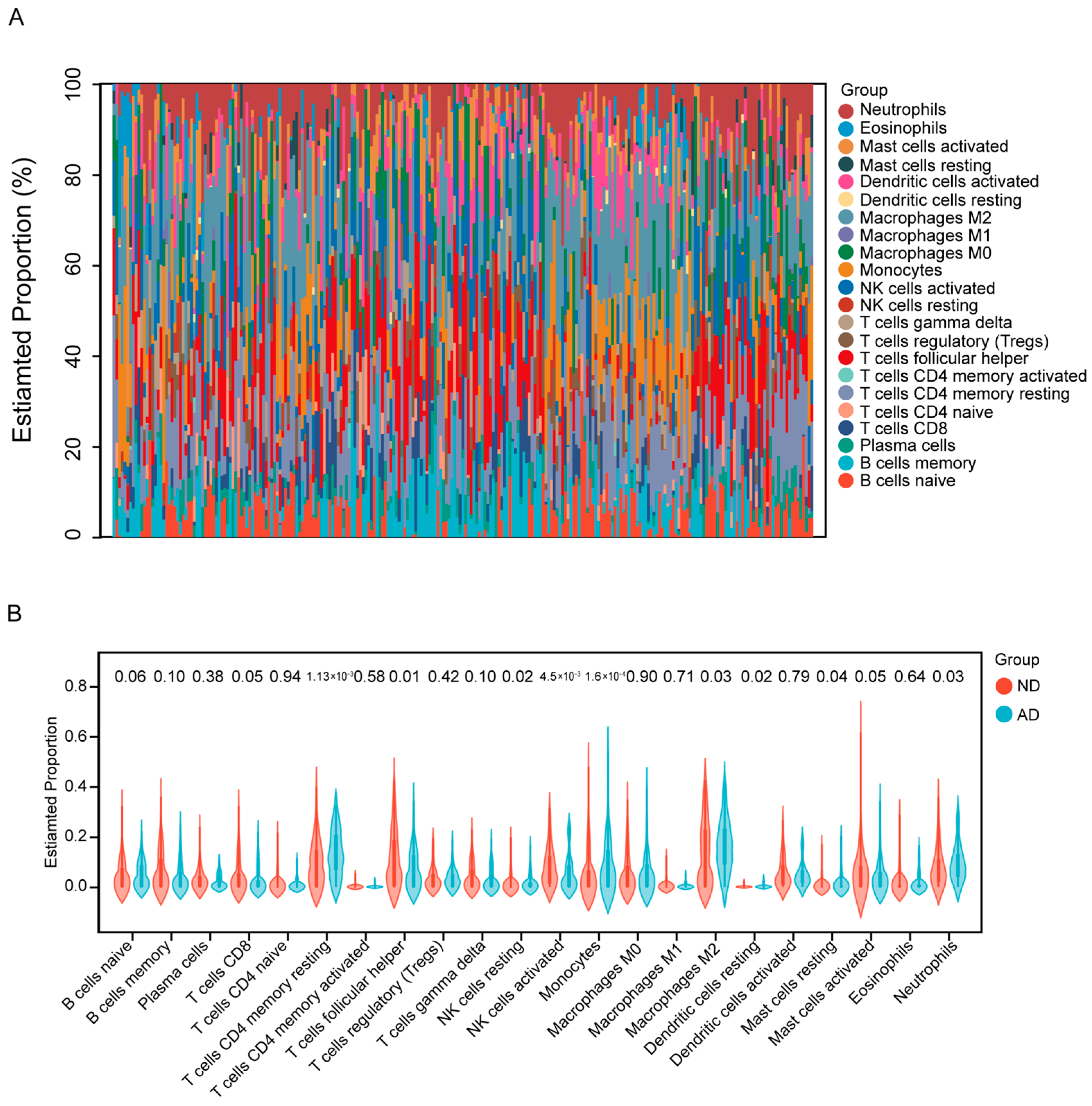
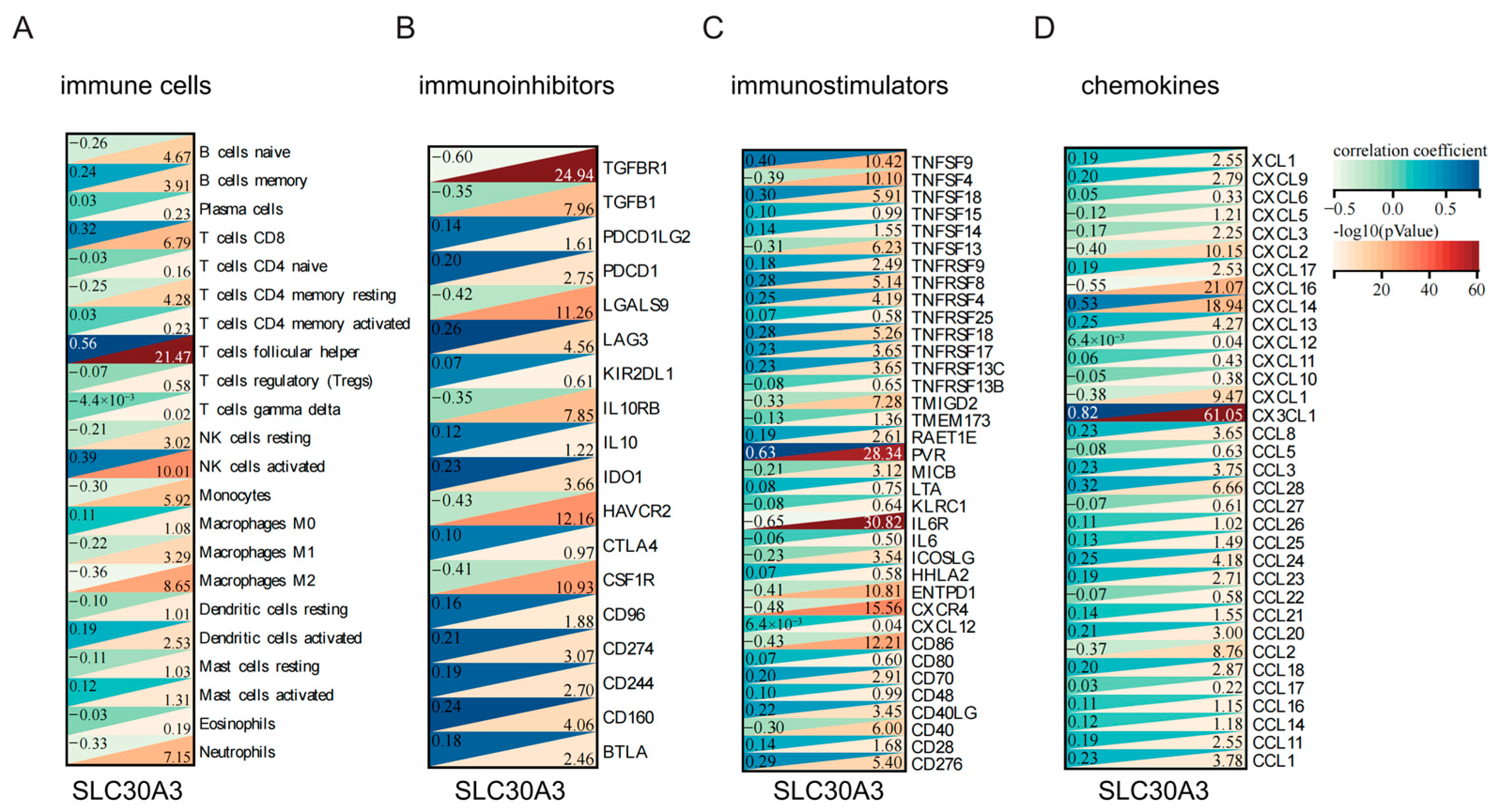
3.4. SLC30A3 Affects the Immune Microenvironment of AD
3.5. Drugs Identified from DrugBank
3.6. Downregulation of SLC30A3 Expression in an AD Cell Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Jia, J.; Xu, J.; Liu, J.; Wang, Y.; Wang, Y.; Cao, Y.; Guo, Q.; Qu, Q.; Wei, C.; Wei, W.; et al. Comprehensive Management of Daily Living Activities, behavioral and Psychological Symptoms, and Cognitive Function in Patients with Alzheimer’s Disease: A Chinese Consensus on the Comprehensive Management of Alzheimer’s Disease. Neurosci. Bull. 2021, 37, 1025–1038. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- De Ture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement. Transl. Res. Clin. 2023, 9, e12385. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Tcw, J.; Goate, A.M. Genetics of β-Amyloid Precursor Protein in Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2017, 7, a024539. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Abelein, A. Metal Binding of Alzheimer’s Amyloid-β and Its Effect on Peptide Self-Assembly. Acc. Chem. Res. 2023, 56, 2653–2663. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, C.; Xia, X.; Wang, Y.; Yan, C.; Wang, X.; Lei, T.; Yang, X.; Yang, X.; Cheng, G.; et al. Pathological BBB Crossing Melanin-Like Nanoparticles as Metal-Ion Chelators and Neuroinflammation Regulators against Alzheimer’s Disease. Research 2023, 6, 0180. [Google Scholar] [CrossRef]
- Bush, A.I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Watt, N.T.; Whitehouse, I.J.; Hooper, N.M. The role of zinc in Alzheimer’s disease. Int. J. Alzheimers Dis. 2010, 2011, 971021. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Byun, M.S.; Yi, D.; Lee, J.H.; Lee, J.H.; Jung, G.; Lee, J.Y.; Kang, K.M.; Sohn, C.H.; Lee, Y.S.; et al. Serum zinc levels and in vivo beta-amyloid deposition in the human brain. Alzheimers Res. Ther. 2021, 13, 190. [Google Scholar] [CrossRef]
- Li, X.; Le, L.; Shi, Q.; Xu, H.; Wang, C.; Xiong, Y.; Wang, X.; Wu, G.; Liu, Q.; Du, X. Zinc exacerbates tau-induced Alzheimer-like pathology in C57BL/6J mice. Int. J. Biol. Macromol. 2023, 242, 124652. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Chen, Z.; Ding, Y.; Chen, Q.; Su, S.; Liu, H.; Ni, R.; Tang, Z. Rapamycin Attenuated Zinc-Induced Tau Phosphorylation and Oxidative Stress in Rats: Involvement of Dual mTOR/p70S6K and Nrf2/HO-1 Pathways. Front. Immunol. 2022, 13, 782434. [Google Scholar] [CrossRef] [PubMed]
- Niyangoda, S.; Kearney, J.; Hettiarachchi, P.; Theismann, J.; Shigemoto, A.; Hickey, E.E.; Burdette, S.C.; Johnson, M.A. Quantitative Control of Zn2+ Photorelease: A Step toward Decoding Mechanisms of Subsecond Metal Signaling in the Brain. Anal. Chem. 2025, 97, 20887–20896. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, X.; Lin, S.; Yang, W.; Wang, T.; Nie, X.; Shi, Z.; Liu, Z.; Zhang, R.; Li, D. Zinc gluconate improves atopic dermatitis by modulating CXCL10 release of keratinocytes via PPARα activation. Biomed. Pharmacother. 2024, 177, 117129. [Google Scholar] [CrossRef]
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52. [Google Scholar] [CrossRef]
- You, X.; Ye, Y.; Lin, S.; Zhang, Z.; Guo, H.; Ye, H. Identification of key genes and immune infiltration in osteoarthritis through analysis of zinc metabolism-related genes. BMC Musculoskelet. Disord. 2024, 25, 227. [Google Scholar] [CrossRef]
- Gu, X.; Lai, D.; Liu, S.; Chen, K.; Zhang, P.; Chen, B.; Huang, G.; Cheng, X.; Lu, C. Hub Genes, Diagnostic Model, and Predicted Drugs Related to Iron Metabolism in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 949083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, H.; Zheng, W.; Xu, H. Current understanding of the interactions between metal ions and Apolipoprotein E in Alzheimer’s disease. Neurobiol. Dis. 2022, 172, 105824. [Google Scholar] [CrossRef]
- Feng, J.; She, Y.; Li, C.; Shen, L. Metal ion mediated aggregation of Alzheimer’s disease peptides and proteins in solutions and at surfaces. Adv. Colloid Interface Sci. 2023, 320, 103009. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, J.; Ahn, B.; Han, J.; Lim, M.H. Multi-target-directed therapeutic strategies for Alzheimer’s disease: Controlling amyloid-β aggregation, metal ion homeostasis, and enzyme inhibition. Chem. Sci. 2025, 16, 2105–2135. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Zhong, T.; Wang, L.Q.; Zhang, N.; Zeng, Y.; Hu, J.Y.; Dang, H.B.; Chen, J.; Liang, Y. Zinc enhances liquid-liquid phase separation of Tau protein and aggravates mitochondrial damages in cells. Int. J. Biol. Macromol. 2022, 209, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Cole, T.B.; Quaife, C.J.; Findley, S.D. ZnT3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. USA 1996, 93, 14934–14939. [Google Scholar] [CrossRef]
- Adlard, P.A.; Parncutt, J.M.; Finkelstein, D.I.; Bush, A.I. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010, 30, 1631–1636. [Google Scholar] [CrossRef]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef]
- Vergnano, A.M.; Rebola, N.; Savtchenko, L.P.; Pinheiro, P.S.; Casado, M.; Kieffer, B.L.; Rusakov, D.A.; Mulle, C.; Paoletti, P. Zinc dynamics and action at excitatory synapses. Neuron 2014, 82, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Morabito, A.; Zerlaut, Y.; Serraz, B.; Sala, R.; Paoletti, P.; Rebola, N. Activity-dependent modulation of NMDA receptors by endogenous zinc shapes dendritic function in cortical neurons. Cell Rep. 2022, 38, 110415. [Google Scholar] [CrossRef]
- Bender, P.T.R.; McCollum, M.; Boyd-Pratt, H.; Mendelson, B.Z.; Anderson, C.T. Synaptic zinc potentiates AMPA receptor function in mouse auditory cortex. Cell Rep. 2023, 42, 112932. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Bonaventura, J.; Keighron, J.; Wright, K.M.; Marable, D.L.; Rodriguez, L.A.; Lam, S.; Carlton, M.L.; Ellis, R.J.; Jordan, C.J.; et al. Synaptic Zn2+ potentiates the effects of cocaine on striatal dopamine neurotransmission and behavior. Transl. Psychiatry 2021, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- McCollum, M.; Manning, A.; Bender, P.T.R.; Mendelson, B.Z.; Anderson, C.T. Cell-type-specific enhancement of deviance detection by synaptic zinc in the mouse auditory cortex. Proc. Natl. Acad. Sci. USA 2024, 121, e2405615121. [Google Scholar] [CrossRef]
- Chrusch, M.J.; Fu, S.; Spanswick, S.C.; Vecchiarelli, H.A.; Patel, P.P.; Hill, M.N.; Dyck, R.H. Environmental Enrichment Engages Vesicular Zinc Signaling to Enhance Hippocampal Neurogenesis. Cells 2023, 12, 883. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Si, X.; Li, J.; Wu, G.; Wang, M. The role of mitochondrial dysfunction in the pathogenesis of Alzheimer’s disease and future strategies for targeted therapy. Eur. J. Med. Res. 2025, 30, 434. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Huang, L.; Gao, W.; He, X.; Yuan, T.; Zhang, H.; Zhang, X.; Zheng, W.; Wu, Q.; Liu, J.; Wang, W.; et al. Maternal zinc alleviates tert-butyl hydroperoxide-induced mitochondrial oxidative stress on embryonic development involving the activation of Nrf2/PGC-1α pathway. J. Anim. Sci. Biotechnol. 2023, 14, 45. [Google Scholar] [CrossRef]
- Fukada, T.; Kambe, T. Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 2011, 3, 662–674. [Google Scholar] [CrossRef]
- Lai, X.L.; Xiong, W.J.; Li, L.S.; Lan, M.F.; Zhang, J.X.; Zhou, Y.T.; Niu, D.; Duan, X. Zinc deficiency compromises the maturational competence of porcine oocyte by inducing mitophagy and apoptosis. Ecotoxicol. Environ. Saf. 2023, 252, 114593. [Google Scholar] [CrossRef]
- Takeda, A. Zinc homeostasis and functions of zinc in the brain. Biometals 2001, 14, 343–351. [Google Scholar] [CrossRef]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef]
- Li, F.; Gong, B.; Yang, T.; Long, S.; Zhang, J.; Jiang, Y.; Yu, Y.; Yang, Y.; Li, D. Estrogen improves sevoflurane-induced cognitive dysfunction by regulating synaptic zinc homeostasis. Mol. Med. 2025, 31, 312. [Google Scholar] [CrossRef]
- Yi, Y.; Kim, B.; Kim, M.; Ko, Y.H.; Kim, J.H.; Lim, M.H. Zn(ii)-driven impact of monomeric transthyretin on amyloid-β amyloidogenesis. Chem. Sci. 2025, 16, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gu, W.; Fu, S.; Zou, G.; Jiang, Z. Zinc homeostasis regulates caspase activity and inflammasome activation. PLoS Pathog. 2024, 20, e1012805. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, L.; Li, Q.; Feng, L.; Wang, B.; Chen, M.; Wang, M.; Wang, J.; Feng, W. Isotope dilution LA-ICP-MS for quantitative imaging of trace elements in mouse brain sections. Anal. Chim. Acta 2023, 1273, 341524. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Gao, Y.; Ma, Q.; Li, W. Zinc Transporters in diseases, including diabetes and related conditions. Physiology 2025. epub ahead of print. [Google Scholar] [CrossRef]
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [CrossRef]
- Fan, Y.G.; Wu, T.Y.; Zhao, L.X.; Jia, R.J.; Ren, H.; Hou, W.J.; Wang, Z.Y. From zinc homeostasis to disease progression: Unveiling the neurodegenerative puzzle. Pharmacol. Res. 2024, 199, 107039. [Google Scholar] [CrossRef]
- Gong, M.; Fang, Y.; Yang, K.; Yuan, F.; Hu, R.; Su, Y.; Yang, Y.; Xu, W.; Ma, Q.; Cha, J.; et al. The WFS1-ZnT3-Zn2+ Axis Regulates the Vicious Cycle of Obesity and Depression. Adv. Sci. 2024, 11, e2403405. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, X.; Wang, T.; Song, Z.; Ni, P.; Zhong, M.; Su, Y.; Xie, E.; Sun, S.; Lin, Y.; et al. SLC39A8-mediated zinc dyshomeostasis potentiates kidney disease. Proc. Natl. Acad. Sci. USA 2025, 122, e2426352122. [Google Scholar] [CrossRef]
- Gate, D.; Saligrama, N.; Leventhal, O.; Yang, A.C.; Unger, M.S.; Middeldorp, J.; Chen, K.; Lehallier, B.; Channappa, D.; De Los Santos, M.B.; et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 2020, 577, 399–404. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [Google Scholar] [CrossRef] [PubMed]
- Basilico, B.; Ferrucci, L.; Ratano, P.; Golia, M.T.; Grimaldi, A.; Rosito, M.; Ferretti, V.; Reverte, I.; Sanchini, C.; Marrone, M.C.; et al. Microglia control glutamatergic synapses in the adult mouse hippocampus. Glia 2022, 70, 173–195. [Google Scholar] [CrossRef]
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Schulz, M.T.; Rink, L. Zinc deficiency as possible link between immunosenescence and age-related diseases. Immun. Ageing 2025, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Kiouri, D.P.; Chasapis, C.T.; Mavromoustakos, T.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and its binding proteins: Essential roles and therapeutic potential. Arch. Toxicol. 2025, 99, 23–41. [Google Scholar] [CrossRef]
- Narayan, S.; Dalal, R.; Rizvi, Z.A.; Awasthi, A. Zinc dampens antitumor immunity by promoting Foxp3+ regulatory T cells. Front. Immunol. 2024, 15, 1389387. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Cortina Chen, H.J.; Barry, T.L.; Bourgognon, J.M.; Steinert, J.R. Redox stress and metal dys-homeostasis appear as hallmarks of early prion disease pathogenesis in mice. Free Radic. Biol. Med. 2022, 192, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Radiom, M.; Zhou, J.; Yan, H.; Zhang, J.; Wu, D.; Sun, Q.; Xuan, Q.; Li, Y.; Mezzenga, R. Cation Triggered Self-Assembly of α-Lactalbumin Nanotubes. Nano Lett. 2024, 24, 4951–4958. [Google Scholar] [CrossRef]
- Yatoui, D.; Tsvetkov, P.O.; La Rocca, R.; Baksheeva, V.E.; Allegro, D.; Breuzard, G.; Ferracci, G.; Byrne, D.; Devred, F. Binding of two zinc ions promotes liquid-liquid phase separation of Tau. Int. J. Biol. Macromol. 2022, 223, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, M.; Zu, C.; Su, X.; Wei, Y.; Gan, X.; Zhang, Y.; He, P.; Zhou, C.; Ye, Z.; et al. L-shaped association between dietary zinc intake and cognitive decline in Chinese older people. Age Ageing 2024, 53, afae008. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cole, T.B.; Palmiter, R.D.; Suh, S.W.; Koh, J.Y. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. USA 2002, 99, 7705–7710. [Google Scholar] [CrossRef]
- Yu, F.; Hou, Z.S.; Luo, H.R.; Li, H.X.; Cui, X.F.; Li, J.L.; Feng, W.R.; Tang, Y.K.; Su, S.Y.; Gao, Q.F.; et al. Neurobehavioral disorders induced by environmental zinc in female zebrafish (Danio rerio): Insights from brain and intestine transcriptional and metabolic signatures. Chemosphere 2023, 335, 138962. [Google Scholar] [CrossRef]

| Gene Symbol | Protein | UniProt ID | Name | Drug Group | Actions |
|---|---|---|---|---|---|
| SLC30A3 | Zinc transporter 3 (ZNT3) | Q99726 | Zinc chloride | approved | substrate |
| Zinc sulfate, unspecified form | approved | substrate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Cheng, Z.; Diao, Y. SLC30A3 as a Zinc Transporter-Related Biomarker and Potential Therapeutic Target in Alzheimer’s Disease. Genes 2025, 16, 1380. https://doi.org/10.3390/genes16111380
Bai R, Cheng Z, Diao Y. SLC30A3 as a Zinc Transporter-Related Biomarker and Potential Therapeutic Target in Alzheimer’s Disease. Genes. 2025; 16(11):1380. https://doi.org/10.3390/genes16111380
Chicago/Turabian StyleBai, Ruyu, Zhiyun Cheng, and Yong Diao. 2025. "SLC30A3 as a Zinc Transporter-Related Biomarker and Potential Therapeutic Target in Alzheimer’s Disease" Genes 16, no. 11: 1380. https://doi.org/10.3390/genes16111380
APA StyleBai, R., Cheng, Z., & Diao, Y. (2025). SLC30A3 as a Zinc Transporter-Related Biomarker and Potential Therapeutic Target in Alzheimer’s Disease. Genes, 16(11), 1380. https://doi.org/10.3390/genes16111380





