Pharmacogenetic Analysis of TPMT and NUDT15 in a European Pediatric Cohort with IBD and Autoimmune Diseases: Frequency Data and Clinical Relevance
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Sample Collection
2.2. DNA Extraction
2.3. TPMT and NUDT15 HRM-PCR Genotyping
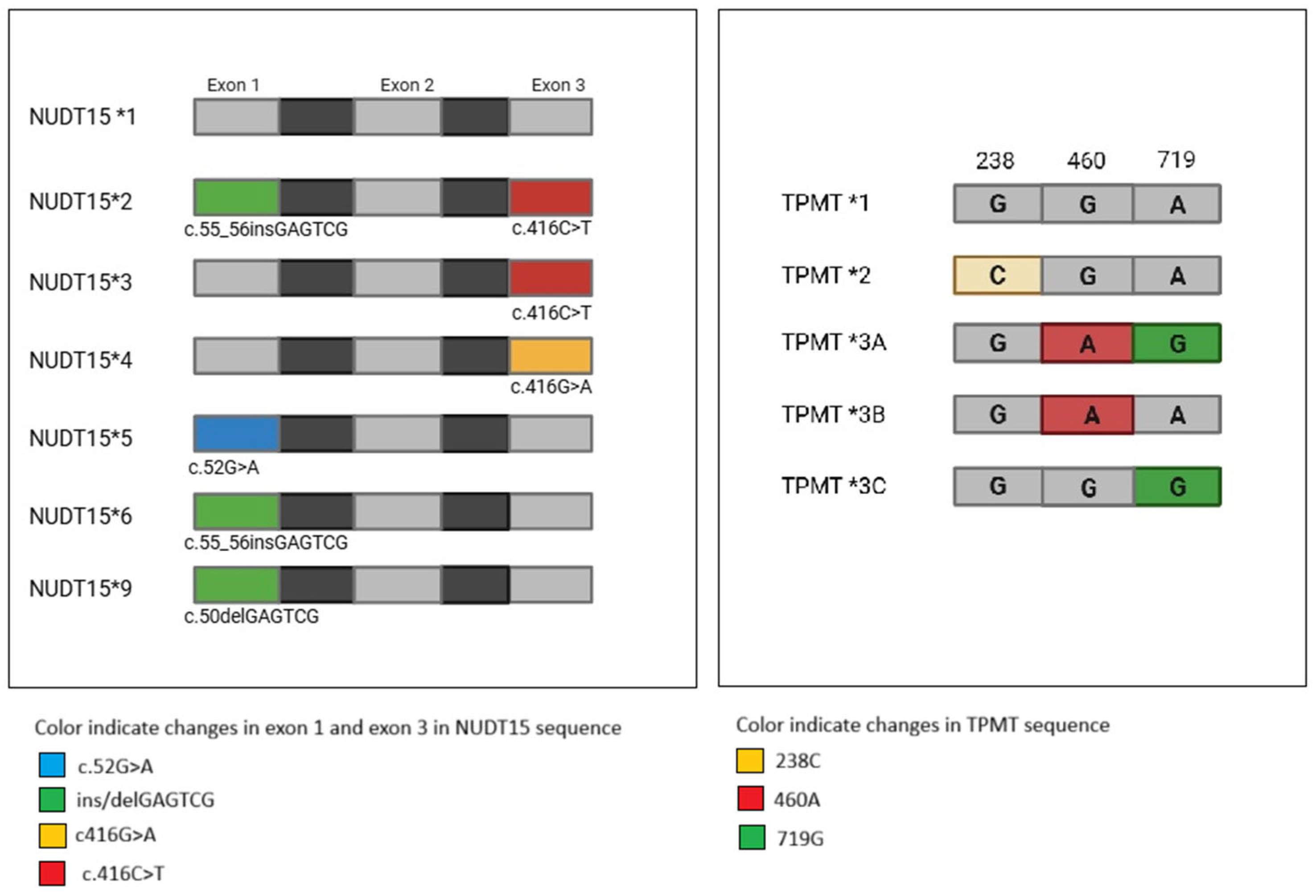
2.4. Sequencing
2.5. Statistical Analysis
3. Results
3.1. Patients’ Clinical Characteristics
3.2. TPMT and NUDT15 Genetic Analysis and Variant Frequencies
3.3. Clinical Response to Azathioprine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quaranta, S.; Thomas, F. Pharmacogenetics of anti-cancer drugs: State of the art and implementation– recommendations of the French National Network of Pharmacogenetics. Therapies 2017, 72, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Schwab, M.; Whirl-Carrillo, M.; Suarez-Kurtz, G.; Pui, C.H.; Stein, C.M.; Moyer, A.M.; Evans, W.E.; Klein, T.E.; Antillon-Klussmann, F.G.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clin. Pharmacol. Ther. 2019, 105, 1095–1105. [Google Scholar] [CrossRef]
- Marinaki, A.M.; Arenas-Hernandez, M. Reducing risk in thiopurine therapy. Xenobiotica 2020, 50, 101–109. [Google Scholar] [CrossRef]
- Khanna, R.; Bressler, B.; Levesque, B.G.; Zou, G.; Stitt, L.W.; Greenberg, G.R.; Panaccione, R.; Bitton, A.; Paré, P.; Vermeire, S.; et al. REACT Study Investigators: Early combined immunosuppression for the management of Crohn’s disease (REACT): A cluster randomised controlled trial. Lancet 2015, 386, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Derijks, L.J.; Gilissen, L.P.; Hooymans, P.M.; Hommes, D.W. Review article: Thiopurines in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 24, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weinshilboum, R. Thiopurine S-methyltransferase pharmacogenetics: Insights, challenges and future directions. Oncogene 2006, 25, 1629–1638. [Google Scholar] [CrossRef]
- Colleoni, L.; Kapetis, D.; Maggi, L.; Camera, G.; Canioni, E.; Cavalcante, P.; de Rosbo, N.K.; Baggi, F.; Antozzi, C.; Confalonieri, P.; et al. A new thiopurine s-methyltransferase haplotype associated with intolerance to azathioprine. J. Clin. Pharmacol. 2013, 53, 67–74. [Google Scholar] [CrossRef]
- Sparrow, M.P.; Hande, S.A.; Friedman, S.; Lim, W.C.; Reddy, S.I.; Cao, D.; Hanauer, S.B. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment. Pharmacol. Ther. 2005, 22, 441–446. [Google Scholar] [CrossRef]
- Sparrow, M.P.; Hande, S.A.; Friedman, S.; Cao, D.; Hanauer, S.B. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin. Gastroenterol. Hepatol. 2007, 5, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Stamoulos, P.; Abdulrehman, A.; Kerr, P.; Bull, C.; Duley, J.; Ansari, A. Long-term safety and efficacy of low-dose azathioprine and allopurinol cotherapy in inflammatory bowel disease: A large observational study. Inflamm. Bowel Dis. 2016, 22, 1639–1646. [Google Scholar] [CrossRef]
- Tominaga, K.; Sugaya, T.; Tanaka, T.; Kanazawa, M.; Iijima, M.; Irisawa, A. Thiopurines: Recent Topics and Their Role in the Treatment of Inflammatory Bowel Diseases. Front. Pharmacol. 2021, 11, 582291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.clinpgx.org/ampAllelesToTest (accessed on 9 October 2025).
- Available online: https://www.nccn.org/patients/guidelines/content/pdf/ped_all_patient.pdf (accessed on 10 October 2025).
- Andersen, J.B.; Szumlanski, C.; Weinshilboum, R.M.; Schmiegelow, K. Pharmacokinetics, dose adjustments, and 6-mercaptopurine/ methotrexate drug interactions in two patients with thiopurine methyltransferase deficiency. Acta Paediatr. 1998, 87, 108–111. [Google Scholar]
- Krynetski, E.Y.; Schuetz, J.D.; Galpin, A.J.; Pui, C.H.; Relling, M.V.; Evans, W.E. A single point mutation leading to loss of catalytic activity in human thiopurine S-methyltransferase. Proc. Natl. Acad. Sci. USA 1995, 92, 949–953. [Google Scholar] [CrossRef]
- Mackay, I.M. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 2004, 10, 190–212. [Google Scholar] [CrossRef]
- Rucci, F.; Cigoli, M.S.; Marini, V.; Fucile, C.; Mattioli, F.; Robbiano, L.; Cavallari, U.; Scaglione, F.; Perno, C.F.; Penco, S.; et al. Combined evaluation of genotype and phenotype of thiopurine S-methyltransferase (TPMT) in the clinical management of patients in chronic therapy with azathioprine. Drug Metab. Pers. Ther. 2019, 6, 34. [Google Scholar] [CrossRef]
- Yates, C.R.; Krynetski, E.Y.; Loennechen, T.; Fessing, M.Y.; Tai, H.L.; Pui, C.H.; Relling, M.V.; Evans, W.E. Molecular diagnosis of thiopurine Smethyltransferase deficiency: Genetic basis for azathioprine and mercaptopurine intolerance. Ann. Intern. Med. 1997, 126, 08–14. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Ferrari, N.; Debuysere, H.; Marteau, P.; Gendre, J.P.; Bonaz, B.; Soulé, J.C.; Modigliani, R.; Touze, Y.; Catala, P.; et al. Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn’s disease and severe myelosuppression during azathioprine therapy. Gastroenterology 2000, 118, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Takatsu, N.; Matsui, T.; Murakami, Y.; Ishihara, H.; Hisabe, T.; Nagahama, T.; Maki, S.; Beppu, T.; Takaki, Y.; Hirai, F.; et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2009, 24, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Landier, W.; Yang, W.; Liu, C.; Hageman, L.; Cheng, C.; Pei, D.; Chen, Y.; Crews, K.R.; Kornegay, N.; et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. Journal of Clinical Oncology. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1235–1242. [Google Scholar] [CrossRef]
- Takagi, Y.; Setoyama, D.; Ito, R.; Kamiya, H.; Yamagata, Y.; Sekiguchi, M. Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and Accepted Manuscript deoxyguanosine diphosphates: Comparison with MTH1 and MTH2. J. Biol. Chem. 2012, 287, 21541–21549. [Google Scholar] [CrossRef]
- Moriyama, T.; Nishii, R.; Perez-Andreu, V.; Yang, W.; Klussmann, F.A.; Zhao, X.; Lin, T.-N.; Hoshitsuki, K.; Nersting, J.; Kihira, K.; et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat. Genet. 2016, 48, 367–373. [Google Scholar] [CrossRef]
- Tanaka, Y.; Saito, Y. Importance of NUDT15 Polymorphisms in Thiopurine Treatments. J. Pers. Med. 2021, 11, 778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.K.; Hong, M.; Baek, J.; Choi, H.; Zhao, W.; Jung, Y.; Haritunians, T.; Ye, B.D.; Kim, K.-J.; Park, S.H.; et al. A Common Missense Variant in NUDT15 Confers Susceptibility to Thiopurine-Induced Leukopenia. Nat. Genet. 2014, 46, 1017–1020. [Google Scholar] [CrossRef]
- Sandritter, T.; Chevalier, R.; Abt, R.; Shakhnovich, V. Pharmacogenetic Testing for the Pediatric Gastroenterologist: Actionable Drug-Gene Pairs to Know. Pharmaceuticals 2023, 16, 889. [Google Scholar] [CrossRef]
- Clinical Pharmacogenetics Implementation Consortium (CPIC). Guideline for Thiopurines and TPMT and NUDT15; CPIC: Memphis, TN, USA, 2023; Available online: https://cpicpgx.org/guidelines/guideline-for-thiopurines-and-tpmt/ (accessed on 13 October 2025).
- Guariso, G.; Gasparetto, M. Treating children with inflammatory bowel disease: Current and new perspectives. World J. Gastroenterol. 2017, 23, 5469–5485. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004, Erratum in J. Hepatol. 2015, 63, 1543–1544. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.; Grancher, K.; Kohn, N.; Lesser, M.; Daum, F.A. Multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology 2000, 119, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Chatu, S.; Subramanian, V.; Saxena, S.; Pollok, R.C. The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: A systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.; Loftus, E.V., Jr. Review article: Recent advances in pharmacogenetics and pharmacokinetics for safe and effective thiopurine therapy in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2016, 43, 863–883. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl 3), S1–S106, Erratum in Gut 2021, 70, 1. [Google Scholar] [CrossRef]

| NUDT15 exon 1 primer forward | 5′-CAAAGCACAACTGTAAGCGACT-3′ |
| NUDT15 exon 1 primer reverse | 5′-GCAAAGACCTCGCCTGACCCA-3′ |
| NUDT15 exon 3 primer forward | 5′-AGCCAAGCAAATGCAAAGCA-3′ |
| NUDT15 exon 3 primer reverse | 5′-TGGCTGAAAGAGTGGGGGATA-3′ |
| TPMT 460 Primer forward | 5′-GGACGCTGCTCATCTTCTTAAAG-3′ |
| TPMT 460 primer reverse | 5′-AGCCTTATAGCCTTACACCCAG-3′ |
| TPMT 238 primer forward | 5′-CTTTGAAACCCTATGAACCT-3′ |
| TPMT 238 primer reverse | 5′-CAATTATTTACCCAAATCAAAACAAACC-3′ |
| TPMT 719 primer forward | 5′-GAATCCCTGATGTCATTCTTCA-3′ |
| TPMT 719 primer reverse | 5′-CCATTACATTTTCAGGCTTTAG-3′ |
| PATIENT | N (%) |
|---|---|
| Total | 83 |
| Age | 3–18 [Mean 12] |
| Males | 47 [56.6%] |
| Females | 36 [43.4%] |
| Underlying pathology | |
| Ulcerative colitis | 25 [30.1%] |
| Chron’s disease | 32 [38.6%] |
| Autoimmune hepatitis | 10 [12%] |
| Very early onset of inflammatory bowel disease | 6 [7.2%] |
| Indeterminate inflammatory bowel disease | 2 [2.4%] |
| Lymphocytic colitis | 1 [1.2%] |
| Patients with comorbidities | 6 [5%] |
| Ulcerative colitis + sclerosing cholangitis | 5 [6%] |
| Chron’s disease + sclerosing cholangitis | 1 [1.2%] |
| Ulcerative colitis + sclerosing cholangitis + autoimmune hepatitis | 1 [1.2%] |
| Azathioprine clinical response data | |
| Available | 71 |
| Not available | 14 |
| TPMT Allele | European CPIC Frequency % | Frequency in the Study Cohort % |
|---|---|---|
| *1/*1 | 90.9 | 92.8 |
| *1/*2 | 0.4 | |
| *1/*3A | 6.5 | 7.2 |
| *1/*3B | 0.5 | |
| *1/*3C | 0.9 | |
| *2/*2 | 0.0 | |
| *2/*3A | 0.0 | |
| *2/*3B | 0.0 | |
| *2/*3C | 0.0 | |
| *3A/*3A | 0.1 | |
| *3A/*3B | 0.0 | |
| *3A/*3C | 0.0 | |
| *3B/*3B | 0.0 | |
| *3B/*3C | 0.0 | |
| *3C/*3C | 0.0 |
| NUDT15 Allele | European CPIC Frequency % | Frequency in the Study Cohort % |
|---|---|---|
| *1/*1 | 98.6 | 97.6 |
| *1/*2 | 0.0 | |
| *1/*3 | 0.4 | |
| *1/*4 | 0.0 | |
| *1/*5 | 0.0 | |
| *1/*6 | 0.6 | |
| *1/*9 | 0.4 | 2.4 |
| Genotype | Phenotype | Azathioprine Dose | n | Any Toxicity n (%) | Hematological Toxicity n | GI Toxicity n (%) | Other Toxicity n (%) |
|---|---|---|---|---|---|---|---|
| TPMT *1/*1; NUDT15 *1/*1 | Normal metabolizer | Standard dose 1.5–2.27 mg/kg/day | 64 | 4 (6.25%) | 0 | 3 (4.6%) | 1 (1.5%) |
| TPMT *1/*3A | Intermediate metabolizer | Lower dose 0.6–1 mg/kg/day | 4 | 2 (50%) | 0 | 0 | 2 (50%) |
| NUDT15 *1/*9 | Possible Intermediate metabolizer | Standard dose 1.5–2.27 mg/kg/day | 2 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pau, A.; Galliano, I.; Ponte, A.; Clemente, A.; Dini, M.; Calvi, C.; Montanari, P.; Pizzol, A.; Gambarino, S.; Calvo, P.L.; et al. Pharmacogenetic Analysis of TPMT and NUDT15 in a European Pediatric Cohort with IBD and Autoimmune Diseases: Frequency Data and Clinical Relevance. Genes 2025, 16, 1372. https://doi.org/10.3390/genes16111372
Pau A, Galliano I, Ponte A, Clemente A, Dini M, Calvi C, Montanari P, Pizzol A, Gambarino S, Calvo PL, et al. Pharmacogenetic Analysis of TPMT and NUDT15 in a European Pediatric Cohort with IBD and Autoimmune Diseases: Frequency Data and Clinical Relevance. Genes. 2025; 16(11):1372. https://doi.org/10.3390/genes16111372
Chicago/Turabian StylePau, Anna, Ilaria Galliano, Alice Ponte, Anna Clemente, Maddalena Dini, Cristina Calvi, Paola Montanari, Antonio Pizzol, Stefano Gambarino, Pier Luigi Calvo, and et al. 2025. "Pharmacogenetic Analysis of TPMT and NUDT15 in a European Pediatric Cohort with IBD and Autoimmune Diseases: Frequency Data and Clinical Relevance" Genes 16, no. 11: 1372. https://doi.org/10.3390/genes16111372
APA StylePau, A., Galliano, I., Ponte, A., Clemente, A., Dini, M., Calvi, C., Montanari, P., Pizzol, A., Gambarino, S., Calvo, P. L., & Bergallo, M. (2025). Pharmacogenetic Analysis of TPMT and NUDT15 in a European Pediatric Cohort with IBD and Autoimmune Diseases: Frequency Data and Clinical Relevance. Genes, 16(11), 1372. https://doi.org/10.3390/genes16111372





