Abstract
Background: Stress-related disorders, including PTSD, acute stress disorders, adjustment disorder, and attachment disorders, arise from complex interactions between genetic susceptibility and environmental stressors. While early environmental factors play a central role in the development of these disorders, there is growing evidence that genetic predisposition also contributes to individual differences in vulnerability and resilience. This narrative review examines current evidence on genetic predisposition and resilience mechanisms in stress-related psychopathology during developmental age. Methods: A literature search was performed using PubMed, Cochrane, MedRxiv, and Medline databases, focusing on studies published between 2010 and 2025, written in English, in the pediatric and adolescent population. Priority was given to original research articles and high-impact reviews. Studies were selected based on relevance to the genetic mechanisms underlying vulnerability and resilience to stress. 71 of 317 were selected. Two hundred forty-six articles were excluded due to a lack of relevance to the topic or because they included an adult population. Results: Polymorphisms and epigenetic modifications in genes involved in hypothalamus–pituitary–adrenal axis (FKBP5, NR3C1, ADCYAP1R1 and ACE), serotoninergic (SLC6A4 and HTR2A), noradrenergic and dopaminergic system (COMT and MAOA), BDNF, estrogen receptor and excitatory amino acid transporters are associated with increased risk of psychopathology following early trauma, but are also implicated in the development of resilience. Conclusions: Genetic factors influence both vulnerability and resilience to stress-related disorders. However, further studies based on the role of genetics are needed to advance precision and personalized medicine, which is still largely underexplored to this day in the field of stress-induced disorders.
1. Introduction
Stress-related disorders (SRDs) are heterogeneous conditions and can manifest with both mental and physical symptoms. The pathophysiology of SRDs involves multiple factors, including the nature of the stressor (observable or perceived) and an individual’s dynamically evolving vulnerability and maladaptation leading to imbalance. Additional variables, such as environmental context, culture, gender, and developmental stage, play a significant role [1].
According to DSM-5, Trauma- and Stress-Related Disorders include Reactive Attachment Disorder, Disinhibited Social Engagement Disorder, Post-Traumatic Stress Disorder (PTSD), Acute Stress Disorder, Adjustment Disorders, and Prolonged Grief Disorders [2,3,4]. These conditions are closely linked to other psychiatric disorders that may be associated with exposure to trauma, such as Borderline Personality Disorder [5], Major Depressive Disorder [3,6], suicidal behavior [6], and alcohol and substance use disorders [3,7]. It is well established that early life stressors and trauma significantly increase the risk of developing these mental disorders, not only in adulthood, but also during childhood and adolescence [6]. Existing evidence suggests that there are commonalities and differences in epigenetic and genetic profiling between adolescents and adults, with important individual variability [6].
According to several studies, the onset of mental SRDs depends on the interaction between genetic and environmental factors (GxE), especially the combination of biological vulnerabilities and the exposure to traumatic experiences during childhood [5,6,8]. GxE effects are statistical interactions that model the way in which genetic variants may influence the effect of environmental factors on a phenotype, including individual differences in response to trauma [8,9].
More recently, research has focused on the biological and neurobiological consequences of early life stressors to determine the molecular and cellular mechanisms underlying the vulnerability to mental disorders [3,7]. Emerging evidence suggests that psychosocial trauma can lead to epigenetic modifications, resulting in downstream effects on gene transcriptional regulation [3,9]. Notably, these epigenetic changes are passed down somatically from cell to cell and in some cases can be transmitted across generations, persisting even in the absence of the stressor [3,6,10,11]. In parallel, growing evidence suggests the role of genetic predisposition in shaping individual vulnerability to SRDs, especially during sensitive periods of neurodevelopment [7]. In this regard, key genes are involved in hypothalamus–pituitary–adrenal (HPA) axis regulation [12], monoaminergic signaling [13,14], and neuroplasticity [15] and contribute to these differences.
Genetic factors and epigenetic differences play a significant role in shaping resilient responses to trauma and stress [16]. Resilience—the ability to adapt successfully in the face of stress and adversity—emerges from the complex interplay of multiple factors, including genetics, epigenetics, developmental environment, psychosocial factors, neurochemicals, and functional neural circuitry. These systems play a critical role in the development and modulation of resilience [16]. While research has identified various contributors to resilience in response to child maltreatments—such as higher cognitive ability, self-regulation, supportive family and community relationships [17]—there is currently limited evidence on whether, or how, these factors directly influence genomic regulation or epigenetic process [2].
Neural circuits are central to both resilience and vulnerability to SRD, with altered connectivity between key brain regions associated with low-resilience phenotypes. Two core networks, reward and fear circuits, are critical for adaptive responses to stress and social challenges [16].
The aim of this review is to summarize current findings on genetic and epigenetic alterations associated with early life stress in adolescents, explore their potential role in the development of psychiatric SRDs, and investigate how these mechanisms may also underlie resilience.
2. Materials and Methods
This narrative review focuses on existing evidence about genetic predisposition and resilience mechanisms in stress-related psychopathology during developmental age. The methodology follows established guidelines for conducting narrative reviews, focusing on a comprehensive exploration and critical analysis of the literature without imposing systematic limitations.
2.1. Search Strategy
The relevant literature was obtained using the PubMed (USA), Cochrane (UK), MedRxiv (USA), and Medline (USA) databases, focusing on studies published between 2010 and 2025 that complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
The search was carried out until 30 September 2025. The search terms comprised combinations of “trauma-related disorder”, ”stress-related disorder”, “resilience”, “genetic”, “genes”, “genomic”, “epigenetic”, and “gene environment interaction”. Various forms of SRD were considered, including “post-traumatic stress disorder” (“PTSD”), “acute post-traumatic stress disorder”, “acute stress reaction”, “attachment disorder”, and “adjustment disorder”. Boolean operators (AND/OR) were applied to refine the search results.
Studies were included in the review if they involved pediatric and adolescent subjects (<18 years of age) experiencing traumatic/adverse experiences during childhood and were selected based on relevance to the genetic mechanisms underlying vulnerability and resilience to stress.
English-written full-text original research articles and high-impact reviews were included. Exclusion criteria included textbooks, editorials, letters to the editor, quantitative meta-analysis, and articles not connected to the topic of our review, concentrated on adult populations, and not providing sufficient detail on the genetics of risk or prevention factors.
2.2. Data Extraction and Synthesis
The results of the search process are summarized in Figure 1. Out of a total of 412 papers selected, 95 duplicates were removed. A total of 317 records were screened, and 71 full-text articles were assessed for eligibility and were included in the narrative review.
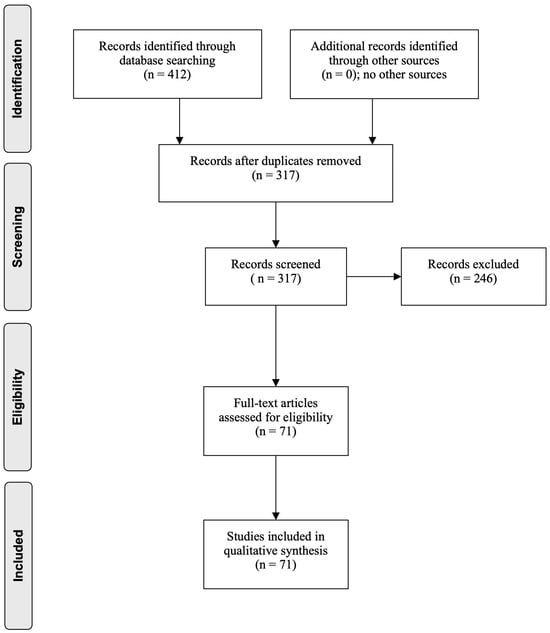
Figure 1.
Flow diagram through the different phases of the review (PRISMA flowchart).
3. Genetic Predisposition and Epigenetics of SRDs During Developmental Age
The main results of the selected studies have been summarized in Table 1.

Table 1.
Genetic and epigenetic predisposition to SRDs in Children and Adolescents.
3.1. Genetic Predisposition to SRDs
An increasing number of studies highlight the influence of genetic predisposition on susceptibility to SRD during childhood and adolescence. Genetic factors contribute to individual differences in susceptibility to environmental stressors, particularly those occurring during critical periods of neurodevelopment. Key candidate genes identified thus far are involved in the regulation of the HPA axis, monoaminergic neurotransmission, and neuroplasticity, reflecting the multifaceted neurobiological mechanisms underpinning stress response and psychopathology.
3.1.1. HPA Axis
The HPA axis is a neuroendocrine system that plays a pivotal role in regulating the body’s response to stress.
When a stressor is perceived, corticotropin-releasing hormone (CRH) is secreted by the hypothalamus, stimulating the pituitary gland to release adrenocorticotropic hormone (ACTH), which in turn triggers the adrenal cortex to produce cortisol and dehydroepiandrosterone (DHEA). Cortisol regulates the system through negative feedback on the hypothalamus and pituitary, while DHEA appears to counterbalance cortisol’s effects, exerting a protective, anti-glucocorticoid role [36,37].
The stress response system plays a crucial role in maintaining physiological balance (homeostasis) in the face of threat or change. However, when this system is chronically activated, it can have detrimental effects on the body, increasing the risk of developing psychiatric SRDs. Dysregulation of the HPA axis has been particularly implicated in various psychopathologies: elevated cortisol levels are frequently observed in major depressive disorder, whereas PTSD is often associated with reduced cortisol levels, which may act either as a predisposing factor or as a consequence of trauma exposure [7]. A growing body of research also indicates that early-life stress, such as childhood trauma, can lead to long-lasting alterations in HPA axis functioning, thereby affecting cortisol regulation and increasing vulnerability to stress-related phenotypes [38].
A key regulator of the HPA axis is FKBP5, a gene encoding a co-chaperone protein that modulates glucocorticoid receptor sensitivity and thus regulates the HPA axis negative feedback loop [18]. Several studies have consistently linked specific polymorphisms in FKBP5, such as rs1360780, to increased vulnerability to early life stress, including childhood maltreatment, neglect, and maternal trauma exposure [3,18]. FKBP5 alleles interact with early life stress to increase the risk for PTSD [3,7,18].
The NR3C1 gene, which encodes the glucocorticoid receptor, represents another central genetic target within the stress response system. Variants in NR3C1 have been associated with altered HPA axis feedback sensitivity and deficits in emotion regulation [10]. Single-nucleotide polymorphisms (SNPs) in NR3C1—such as rs41423247, rs10482605, and rs10052957—are commonly included in genetic profile scores used to predict HPA axis function. These variants are suggested to be linked to an increased risk of volume alteration in regions such as the amygdala and hippocampus in children, as well as an increased risk for developing PTSD [10].
The Angiotensin I-converting enzyme (ACE) gene, which encodes a cell surface enzyme involved in the hydrolysis of circulating peptides, plays a key role in fluid and electrolyte balance, blood pressure regulation, and vascular remodeling. In addition to these physiological functions, ACE also contributes to the modulation of stress and fear responses by influencing HPA axis activity [26]. ACE D allele of rs4311 may be linked to increased vulnerability to stress-related psychopathologies such as depression, PTSD, and anxiety disorders, potentially via enhanced HPA axis reactivity, but the results are controversial [26].
Several other genetic polymorphisms interacting with the HPA axis have been implicated in the modulation of stress responsivity and the emergence of psychopathology during development.
Polymorphism in ADCYAP1R1 (adenylate cyclase activating polypeptide 1 receptor type I)—such as rs2267735—has been identified as a sex-specific genetic risk factor for PTSD, particularly in trauma-exposed children [4]. This gene is involved in the regulation of stress signaling pathways and neurodevelopmental processes [4]. A study on preadolescent children found that certain risk alleles of ADCYAP1R1 were associated with increased PTSD symptoms in girls but not boys, suggesting a potential interaction between genetic risk, developmental stage, and sex hormones [4].
CRHR1 (corticotropin-releasing hormone receptor 1) plays a central role in initiating the stress response by regulating the release of ACTH, and its dysregulation has been linked to heightened risk for anxiety, depressive symptoms, and PTSD in youth, as well as differential sensitivity to early life stress [10,39]. This may influence the threshold for activation of the HPA axis in response to threat [10,39].
3.1.2. Serotoninergic System
Genetic variations in several serotonin receptor genes have been shown to interact with environmental stress in shaping vulnerability to psychiatric disorders.
Serotonin turnover rises during acute stress in regions such as the amygdala, hypothalamus, and prefrontal cortex, with receptor subtypes exerting distinct effects [16,40]. For example, 5-HT1A receptors have anxiolytic properties, while 5-HT2A receptors appear anxiogenic, and 5-HT1B/2C receptors contribute to adaptive responses to stress [16,41,42].
An extensively examined gene is SLC6A4, which encodes the serotonin transporter (5-HTT). The 5-HTTLPR polymorphism, in particular its short (s) allele genotype, has been linked to heightened emotional reactivity and increased susceptibility to develop bipolar disorder and depressive symptoms in youths exposed to trauma, including sexual and physical abuse, peer victimization, and familial conflict [27,28].
Further genetic variants related to serotonergic function, such as HTR2A (serotonin receptor 2A), have shown associations with stress sensitivity and depression, with evidence for both genetic and epigenetic contributions [13,19]. In particular, Vrshek-Schallhorn et al. conducted a longitudinal study harmonizing data from the Youth Emotion Project (387 young adults followed for 5 years) and a younger adolescent sample [13]. The study investigated how additive genetic risk within serotonin-system polymorphisms (HTR1A, HTR2A, HTR2C, TPH2) interacts with interpersonal stress to predict depressive diseases, supporting a polygenic additive model of psychopathology in the context of major interpersonal stress [13]. Interestingly, in the absence of such stress, carrying a higher genetic risk appeared to be a protective factor [13]. These findings suggest that serotonin-related variants may confer distinct environmental sensitivity, amplifying depression risk under stress but not necessarily in its absence. Such results were replicated in the adolescent sample, strengthening their relevance across developmental stages [13].
3.1.3. Noradrenergic and Dopaminergic System
The noradrenergic system, centered in the locus coeruleus, orchestrates stress responses through projections to the amygdala, hippocampus, hypothalamus, and prefrontal cortex [36,43]. Hyperactivation of this system has been linked to the consolidation of negative emotional memories and to chronic anxiety, while pharmacological blockade of norepinephrine activity may attenuate such effects [44,45]. Imaging studies further suggest that disinhibited norepinephrine signaling enhances amygdala responsiveness to fear, contributing to PTSD pathology [46].
Polymorphisms in COMT (catechol-O-methyltransferase), a key enzyme involved in the degradation of dopamine in the prefrontal cortex, have been shown to influence cognitive control and emotional processing under stress [8]. The common Val158Met polymorphism of COMT is of particular interest, with the Met allele being associated with reduced enzymatic activity, increased dopaminergic tone, and greater stress sensitivity and risk for internalizing disorders in adolescence [8].
Monoamine Oxidase A (MAOA) encodes an enzyme involved in the catabolism of norepinephrine, serotonin, and dopamine. Early-life adversity, such as maternal separation or maltreatment, can induce long-lasting alterations in these neurotransmitter systems, potentially predisposing individuals to aggressive behavior and affective dysregulation [47].
Polymorphisms in the MAOA gene promoter region result in high (MAOA-H) or low (MAOA-L) enzymatic activity. Individuals with the MAOA-L variant may be at risk for blunted or dysregulated cortisol responses under stress, potentially reflecting impaired neuroendocrine adaptation [48].
Interestingly, sex differences have emerged in the behavioral and emotional effects of MAOA polymorphisms in adults. For instance, male carriers of MAOA-H exhibit increased dopamine release and heightened aggressive responses after exposure to violent stimuli, whereas female carriers of MAOA-H show higher vulnerability to burnout and depression following stressful life events [49,50].
3.1.4. BDNF
Hippocampal Brain-Derived Neurotrophic Factor (BDNF) expression supports adaptive responses to chronic stress [16,51]. BDNF, expressed in regions such as the amygdala, hippocampus, prefrontal cortex, and basal forebrain, plays a crucial role in neuronal development, survival, and synaptic plasticity [52]. As shown by Sonoyama et al., loss-of-function mutation in the BDNF gene and its receptor TrkB impairs excitatory synaptogenesis in the hippocampus and is mechanistically linked to a broad spectrum of neurobehavioral abnormalities, including cognitive impairments, memory deficits, and phenotypes associated with neuropsychiatric disorders [53]. Moreover, the BDNF gene, essential for neuronal plasticity and development, has been extensively linked to psychiatric disorders in the adult population, especially PTSD and depression [54,55].
Some studies suggest an involvement of the BDNF, especially for the Val66Met polymorphism, in depression and PTSD among children and adolescents exposed to stressful life events; however, the findings remain inconsistent [14,30,31,32].
3.1.5. Estrogen Receptor
Growing evidence points to estrogen receptor alpha 1 (ESR1), a gene involved in the nuclear regulation of estrogen signaling, as a sensitivity modulator to early life stress and stress-related consequences in youth. Variants in ESR1 were found to attenuate the impact of stress in adolescents with depressive symptoms [13]. Mikhailova revealed a statistically significant difference in allele frequency for rs6557168 in ESR1 by comparing different populations of adolescents born before, during, or after the 1991–1998 Russian socioeconomic crisis [26]. In her assessment, no such difference was identified in other commonly stress-related genes, including MAOA, FKBP5, SLC6A3, and OXTR, although it is important to note that the study did not correlate genetic variants to clinical phenotypes [26].
3.1.6. Excitatory Amino Acid Transporters (EAAT)
The Solute Carrier Family 1 Member 3 (SLC1A3) encodes a glutamate transporter known as EAAT1, predominantly expressed in the central nervous system, playing a central role in the prevention of excitotoxicity through glutamate clearance and maintenance of synaptic neurotransmitter balance [56]. In the literature, SLC1A3 mutations have been linked with neurodevelopmental disorders, especially Attention-Deficit/Hyperactivity Disorder (ADHD) [57].
A case–control association study was performed on more than 100 Indian adolescents (aged 16–19), presenting variable levels of clinically diagnosed stress and depressive symptoms, matched with more than 200 controls [14]. In this population, the T-allele of SLC1A3 C3590T was found to be associated with increased risk of both stress and depression [14], whereas other variants in SLC1A3 and BDNF were not associated.
In parallel, genome-wide association studies (GWAS) have begun to uncover novel loci associated with PTSD and related conditions in trauma-exposed pediatric populations. However, the field faces challenges due to the heterogeneity of clinical phenotypes, limited sample sizes, and varying trauma assessments, resulting in partially inconsistent findings [58]. Nonetheless, these studies collectively support a model of complex, polygenic liability interacting with environmental stressors to shape mental health trajectories during development.
3.2. Epigenetics of SRDs
Epigenetic refers to heritable changes in gene expression that do not modify the underlying DNA sequence. They can be influenced by different environmental factors, including stress and early adverse experiences. Key mechanisms include DNA methylation, non-coding RNAs, and histone modification, which can exert long-term and even transgenerational effects influencing stress response and psychiatric vulnerability [59,60]. Epigenetic processes recently emerged as key mechanisms in the etiology of neurodevelopmental disorders, and various genetic conditions affecting neurodevelopment have been associated with specific methylation patterns [61,62].
Specifically, methylation implies the addition of methyl groups to DNA, usually at CpG sites, leading to a reduction in gene expression; histone modifications include changes to histone proteins influencing gene accessibility; non-coding RNAs such as microRNAs (miRNA) act as gene expression regulators after transcription [63].
Growing evidence links stress exposure (including trauma, physical, emotional, sexual abuse, neglect, bullying, violence), particularly in early life, with epigenetic alterations that can alter neurodevelopment. Epigenetic changes during these critical periods act as a bridge between genetic predisposition and environmental factors, contributing to the emergence of SRD, as well as other psychiatric conditions. In addition, the literature suggests a long-term negative impact on global health, influencing the risk for chronic organic disorders and cancer [11,64]. Childhood maltreatment has been frequently associated with epigenetic alterations in genes regulating the HPA axis. For example, methylation changes of the glucocorticoid receptor gene (NR3C1) observed in adulthood have been consistently associated with exposure to early-life adversity and are increasingly recognized as a potential epigenetic mechanism mediating the development of psychiatric SRD and suicidal behavior [65,66,67].
Longitudinal and population studies reinforce these findings. The TRAILS study, investigating the effects of stressors on 468 adolescents, found higher NR3C1 methylation levels in those who had been exposed to life stress and adverse events during childhood and adolescence, notably without a significant correlation with perinatal stress [21]. They also discovered higher methylation of SLC6A4 after stressful life events in adolescence, with a more pronounced association than during childhood [29].
Similarly, Romens et al. observed that children exposed to physical maltreatment displayed higher methylation levels in the NR3C1 promoter compared to controls [22]. Notably, hypermethylation affected a precise part of the gene within the NGFI-A binding region, a critical site for both brain development and stress regulation [22].
Differential NR3C1 methylation patterns were directly linked to psychopathology in a longitudinal study involving 487 children (mean age 12) by Bosmans et al. [23]. They demonstrated that higher NR3C1 methylation increased vulnerability to developing anxious attachment when exposed to high stress and low maternal support, highlighting an epigenetic sensitivity to early caregiving environments [23].
In addition, a study by Efstathopoulos et al. investigated the relationship between epigenetic changes in NR3C1 and internalizing symptoms in a population of Swedish adolescents aged 13–14 years [24]. In females, NR3C1 hypermethylation was cross-sectionally associated with elevated scores for internalizing symptoms evaluated through the Center for Epidemiologic Studies Depression Scale for Children [24]. Furthermore, those who reported experiences of being bullied or lacking friends showed higher NR3C1 methylation levels [24].
Epigenetic modifications have also been documented in other key stress-regulatory genes.
Hecker et al. [35] identified distinct methylation patterns of the proopiomelanocortin gene (POMC), encoding a precursor protein involved in the regulation of the HPA axis, in Tanzanian children exposed to different degrees of abuse.
FK506 binding protein 5 (FKBP5) encodes a protein involved in the regulation of the HPA axis by modulating the glucocorticoid receptor sensitivity. Growing evidence implicates it in the development of SRD through genetic and epigenetic mechanisms. A study by Parade et al. investigated the longitudinal methylation of FKBP5 among preschoolers exposed to early adversities and stress [68]. They found, consistently over time, low methylation levels in maltreated children [68]. A similar result was uncovered by Non et al., who studied children exposed to early institutionalization, who were shown to have lower methylation levels at FKBP5 and SLC6A4, with levels being related to the duration of institutionalization [20].
Gender differences in epigenetic profiles have been reported in maltreated populations.
For example, differential methylation was found to be associated with gender in the study by Cicchetti et al. [25], which analyzed epigenetic changes in over 500 low-income children, half of whom had documented histories of maltreatment. A significant interaction between maltreatment and gender was observed in the ALDH2 gene: maltreated boys showed increased methylation compared to non-maltreated boys, whereas maltreated girls showed decreased methylation compared to non-maltreated girls [25]. In the ANKK1 gene, an overall gender effect was also identified: girls had higher methylation levels than boys, regardless of maltreatment status [25].
Additional research has identified epigenetic modifications in serotonergic system genes. Parade et al. found that early stress and psychopathology (PTSD and depressive symptoms) were associated with site-specific methylation of the HTR2A gene (serotonin receptor 2A) in preschoolers [19]. Methylation varied by both genotype and stress exposure, with genotype moderating the stress–methylation relationship, suggesting early adversity may influence serotonergic function through epigenetic mechanisms.
Beyond candidate gene approaches, epigenetic changes have also been observed in genome-wide studies. Differential patterns of whole-genome DNA methylation were investigated in a study comparing a small number of institutionalized children aged 7–10 years with a group of children raised by their biological parents in Russia [69]. Institutionalized children showed increased genome methylation, particularly in genes involved in immune response, brain development, neural signaling, learning, and memory [69]. Weder et al. identified methylation in three genes (ID3, GRIN1, TPPP) as predictors of depression in a cohort of maltreated children [34].
Papale et al. identified over 500 differentially methylated loci across 122 genes, many previously linked to stress-related pathways in cohorts of prepubescent girls exposed to varying levels of childhood adversity [70]. Using RNA sequencing, the authors found more than 1400 differentially expressed genes, including FHL3 and NPC2, further supporting the hypothesis that early-life stress leads to widespread and functionally relevant epigenetic changes [70].
Furthermore, Sheerin et al. conducted an epigenome-wide association study in treatment-seeking adolescents to examine the relationship between PTSD symptom severity and DNA methylation [9]. The study identified two CpG sites showing hypomethylation associated with higher PTSD symptoms, as well as one differentially methylated region overlapping the MOBP gene, involved in myelin-related neural processes [9]. One CpG also mapped to MAML3, a transcriptional regulator [9].
Esposito et al. analyzed DNA methylation in a cohort of adolescents who had gone through international adoption from conditions of poverty and disadvantage compared to adolescents raised by their biological parents in adequately resourced environments [71]. They found significant differences in DNA methylation associated with adversity occurring in early childhood, while adversity experienced later in childhood did not show similar epigenetic alterations, highlighting early childhood as a time-sensitive epoch for the epigenetic effects of stress [71].
Together, these findings support a model in which early stress severity triggers complex epigenetic remodeling contributing to the risk of expression of SRDs. Specifically, PTSD symptom severity in adolescents has been linked to epigenetic variations in genes involved in neural signaling and plasticity.
Neuroimaging studies have linked trauma-associated DNA methylation changes to structural brain differences in regions that govern stress responsivity, particularly within the hippocampus, amygdala, and medial prefrontal cortex [72].
To investigate this relationship, Ensink et al. employed a combination of methylome-wide association studies and structural neuroimaging measures in two independent cohorts of children and adolescents (aged 8–18 years) diagnosed with PTSD, compared with a control group [33]. Their work revealed significant methylation differences in children with PTSD regarding multiple genes, including those related to glucocorticoid functioning and Tenascin-XB (TNXB) [33]. TNXB encodes a glycoprotein associated with the extracellular matrix with roles in cell migration and tissue remodeling, interacting with proteins also implicated in hippocampal synaptic plasticity. Furthermore, the study found that methylation of the OLFM3 gene, encoding a neuronal protein involved in the development of microglia, was related to the volume of the anterior hippocampus in both cohorts, being reduced in youth with PTSD [33].
In contrast to these findings, the large-scale Environmental Risk Longitudinal Study, which assessed 2232 twins born in England and Wales at different ages (5, 7, 10, 12, and 18 years), did not provide strong support for the hypothesis that victimization in youth leads to robust changes in DNA methylation [15]. Although the study investigated several forms of abuse (physical, sexual, and emotional) as well as neglect, exposure to violence, bullying, and crime, only sparse and inconsistent associations were observed, suggesting that the relationship between early trauma and DNA methylation may be more subtle or context-dependent than previously hypothesized [15].
4. Genetic Factors and Epigenetics of Resilience During Developmental Age
Resilience refers to the ability to adapt effectively to stress and adversity, maintaining psychological and physical stability [16]. Factors that shape baseline mental health can be distinct from those influencing recovery, stress adaptation, or post-traumatic growth [17]. Recent research has begun to uncover biological, psychological, and developmental factors, including genetics and neurochemistry, that contribute to resilience [16].
The main results of the selected studies have been summarized in Table 2.

Table 2.
Genetic and Epigenetic Factors of Resilience in Children and Adolescents.
4.1. Genetic and Epigenetic Factors Related to Resilience
4.1.1. HPA Axis
As previously reported, the HPA axis is a central neuroendocrine system involved in coordinating the body’s response to stress.
It has been observed that children exposed to repeated stressful events display a blunted cortisol increase, suggesting a form of resilience [16,75]. Meanwhile, low DHEA or DHEA-sulfate (DHEA-S) levels have been connected to depression, and higher DHEA(S) levels have been noted in PTSD cases. Moreover, the DHEA(S)/cortisol ratio has emerged as a meaningful marker for stress vulnerability and resilience [16,80,81].
CRH and its receptors (CRHR-1 and CRHR-2) also play significant roles in stress regulation [44]. In contrast to CHRH-1, CRHR-2, found in regions such as the septum and dorsal raphe, may either amplify or reduce stress effects depending on the context [16,74,82].
Finally, as previously mentioned, ACE (angiotensin I-converting enzyme) also influences HPA activity. Conversely, the I allele, associated with lower ACE activity, has been proposed as a protective factor in some studies, potentially contributing to greater resilience, decreasing neuroendocrine and inflammatory stress responses. In adult population, the ACE I/D polymorphism may interact with environmental stressors in G × E models: individuals with the D/D genotype exposed to chronic stress may be particularly susceptible to HPA dysregulation and emotional dysregulation, while carriers of the I allele may maintain better HPA axis homeostasis under similar conditions, but the results are not univocal in a population of adolescents [26].
4.1.2. Serotoninergic System
As previously discussed, multiple variations in serotonin transporters and receptors are involved not only in shaping vulnerability to psychiatric disorders but are increasingly recognized as contributing to mechanisms of resilience as well [16,41,42].
4.1.3. Noradrenergic and Dopaminergic System
Both the norepinephrine transporter and adrenergic receptors (α and β) have been proposed as biological mediators of susceptibility and resilience to SRDs [16,83,84]. A key role is played by Catechol-O-Methyltransferase (COMT).
In accordance with the findings presented in Section 3.1.3 concerning the Val158Met polymorphism of the COMT gene, individuals homozygous for the Val allele (Val/Val) exhibit attenuated stress responses, suggesting a protective effect [8].
Another key gene in the stress response is monoamine oxidase A (MAOA). In contrast to what has been previously analyzed, female carriers of the 3-repeat (3R) low-activity allele have been associated with greater emotional stability and lower anxiety and depressive symptoms, suggesting sex-specific resilience profiles, even if results are not univocal [26].
4.1.4. BDNF
Exogenous BDNF exerts antidepressant-like effects, enhances neurogenesis, and antidepressant treatments have been shown to upregulate BDNF and TrkB expression in the hippocampus and prefrontal cortex [16,85,86,87]. Still, some antidepressant effects appear independent of BDNF or neurogenesis [16,88,89]. The role of p75 signaling in resilience remains less clear, likely due to its low binding affinity [90]. The contribution of the BDNF Val66Met polymorphism to stress response and resilience remains uncertain.
La Greca et al. investigated how genetic vulnerability interacts with disaster-related stress exposure to predict PTSD and depression symptoms in children following a major natural disaster [76]. In this context, BDNF may represent one of several genes contributing to a broader neurobiological sensitivity to context, which either promotes resilience or enhances vulnerability depending on the quality and intensity of environmental input [76].
4.1.5. NPY
Neuropeptide Y (NPY) plays a key role in stress regulation, exerting anxiety-reducing effects and supporting adaptive responses to adversity [40]. In animal models, reduced NPY expression was found in stress-related brain regions in PTSD-like conditions, while NPY supplementation reversed these effects [40,91]. NPY is widely expressed across key brain regions involved in emotional regulation, including the hypothalamus, amygdala, hippocampus, and locus coeruleus [16,40,92,93]. Genetic differences in the NPY gene have been linked to varying levels of stress resilience. For instance, certain SNPs have been associated with a higher risk of anxiety following early-life stress, likely due to changes in NPY expression and reduced HPA axis regulation [77]. Research has shown that genetic variants—particularly in the promoter region of the NPY gene—can influence NPY levels, with lower expression linked to reduced resilience [94,95]. It also counters the anxiety-inducing effects of CRH [93].
4.1.6. Glutamate, GABA
Glutamate, GABA, and endocannabinoids have been strongly implicated in stress regulation, resilience, and the pathophysiology of mood and anxiety disorders [96,97,98]. Dysregulation within these systems can impair the ability to adapt effectively to both acute and chronic stress. Recent pharmacological studies targeting glutamatergic, GABAergic, and endocannabinoid signaling have yielded encouraging results, pointing to their potential as therapeutic avenues in psychiatric disorders [16,99,100,101]. As an example, CNR1 (cannabinoid receptor type 1) codes for G protein–coupled receptor CB1R, ubiquitously expressed in the central nervous system and in peripheral neurons. Endocannabinoid signaling participates in neural plasticity, learning, and regulation of HPA response to acute or repeated stress. It is believed that the mechanism of development of stressor tolerance is based on the initiation of CB1R signaling caused by repeated stress, thus allowing the risk of negative consequences to be reduced, although results are controversial [26,102,103].
4.1.7. OXTR
Several other genes are involved in influencing individual sensitivity to environmental contexts.
One of the most widely studied genes in this domain is the oxytocin receptor gene (OXTR), which plays a central role in social cognition and emotional regulation. A commonly examined polymorphism within OXTR is rs53576, characterized by an adenine (A) to guanine (G) substitution in the third intron [78]. This SNP has been associated with differential susceptibility to environmental influences, particularly in relation to family dynamics and early caregiving environments.
Individuals carrying the G allele have been found to exhibit higher levels of resilience and positive affect in environments characterized by high warmth and stability, but conversely, lower levels of resilience and well-being in contexts of low support or instability [16,73]. This pattern supports a differential susceptibility model, in which G allele carriers are more sensitive to the quality of their social environment. This heightened social sensitivity may also make G allele carriers more responsive to family-based preventive interventions, but also more vulnerable to the adverse effects of unsupportive parenting.
In line with this, Smearman et al. identified a significant three-way interaction between OXTR genotype, parenting quality, and intervention exposure in predicting telomere length, a biological marker of cellular aging and cumulative stress [78]. Specifically, GG individuals exposed to non-supportive parenting and randomized to the control group (i.e., no intervention) exhibited the shortest telomere lengths, suggesting increased biological stress [78]. In contrast, GG individuals who received the family-based intervention displayed telomere lengths comparable to low-risk groups, indicating a buffering effect of the intervention [78]. Notably, individuals with the A allele did not show significant differences in telomere length across conditions, suggesting reduced sensitivity to environmental variation.
The observed telomere shortening among GG individuals in adverse environments was partially mediated by chronic anger: those exposed to non-supportive parenting and not receiving the intervention showed greater increases in chronic anger over time, which in turn was associated with accelerated telomere erosion [78]. These findings highlight a biological embedding of social experience, with OXTR genotype moderating the extent to which environmental factors shape long-term physiological outcomes.
Also, Mikhailova et al. examined allele distributions of several resilience-related genes, including OXTR rs53576, among Russian adolescents born before, during, and after the socioeconomic crisis of the 1990s, but their findings were not statistically significant [26].
Epigenetic regulation of OXTR, as DNA methylation at the promoter region, has emerged as a mechanism by which early life experiences become biologically embedded, influencing individual trajectories of stress responsivity and resilience. In fact, it has been found that higher levels of OXTR promoter methylation were significantly associated with greater exposure to early adversity, particularly maternal depression and harsh parenting. Importantly, increased methylation was also linked to elevated depressive symptoms, suggesting that OXTR epigenetic silencing may impair the stress-buffering capacity of oxytocin signaling [78]. These findings support a biological pathway by which early social environments influence long-term emotional functioning through epigenetic downregulation of oxytocin signaling. Reduced OXTR expression may weaken the capacity for social affiliation and emotional regulation, both of which are essential components of psychological resilience. Moreover, the interaction between OXTR methylation and early stress may serve as a biomarker for susceptibility to affective disorders in adolescence and beyond. In contrast, lower methylation levels (potentially reflecting greater OXTR expression) have been associated with more adaptive emotional responses, increased social support seeking, and better outcomes following stress exposure, indicating a potential resilience phenotype. This aligns with broader evidence implicating the oxytocinergic system in the modulation of social buffering, especially in contexts of early life adversity [79].
Together, these findings highlight how epigenetic mechanisms mediate the impact of stress on the brain, influencing resilience and susceptibility to psychiatric disorders.
Nonetheless, it is worth noting that most studies on epigenetics and resilience factors are conducted on animals and adult populations.
5. The Role of Psychology and Environment in Coping with Stress
Several psychological and behavioral characteristics contribute to resilience in the face of adversity. High cognitive functioning, effective self-regulation, optimism, active coping, secure attachment, and social connectedness are consistently protective [104]. Positive emotions can also attenuate the expression of genetic vulnerability, such as BDNF Val66Met polymorphism or family history of depression [17,105]. Tendency to experience positive emotions shows moderate heritability (~0.60), but environmental factors, including parenting and daily experiences, strongly shape their expression [17,106,107,108].
6. Conclusions
In conclusion, studies investigating genetic and epigenetic modifications associated with early life stressors and the onset of psychiatric disorders in adolescents are still limited. The impact of genetic and epigenetic factors on the development of SRDs is of considerable relevance, particularly during adolescence, which represents a critical window in which heightened neural plasticity and ongoing epigenetic programming may amplify the effects of early adversity.
Evidence indicates that polymorphisms and epigenetic alterations in genes involved in the HPA axis (such as FKBP5, NR3C1, ADCYAP1R1, ACE), serotoninergic system (SLC6A4, HTR2A), noradrenergic and dopaminergic pathways (COMT, MAOA), as well as BDNF, estrogen receptors and excitatory amino acid transporters, are associated with an increased risk of psychopathology following early trauma, but are also implicated in mechanisms of resilience. It should also be noted that most of these variants are common polymorphisms, generally considered benign, and their individual contribution to psychiatric SRDs appears modest, making the concept of a strong gene × environment interaction still debated. Understanding the psychological substrates and neurobiology underlying factors implicated in coping with stress may help develop strategies aimed at preventing psychopathology after exposure to severe adversity. Furthermore, the mechanisms through which these epigenetic modifications contribute to psychiatric vulnerability—or conversely to resilience—during adolescence are not yet fully understood. Current evidence suggests both overlaps and differences in genetic and epigenetic profiles between adolescents and adults, with substantial interindividual variability. Nonetheless, genetic factors appear to shape both vulnerability and resilience to SRDs. A deeper understanding of gene–environment interactions, and of the neurobiological and psychological substrates involved in stress coping, holds promise for improving prevention strategies and therapeutic interventions. However, further research is needed to translate these findings into precision and personalized medicine, which remains largely underdeveloped in the field of stress-induced disorders.
Author Contributions
Conceptualization, A.R. (Alessia Raffagnato) and I.T.; methodology, A.R. (Alessia Raffagnato), I.T. and C.A.; investigation, A.R. (Alessia Raffagnato), C.A., G.C., L.P., M.F.P. and A.R. (Arianna Raicich); data curation, A.R. (Alessia Raffagnato), C.A., I.B., G.C., L.P. and A.R. (Arianna Raicich); writing—original draft preparation, A.R. (Alessia Raffagnato), C.A., I.B., G.C., L.P. and A.R. (Arianna Raicich); writing—review and editing, I.T., A.R. (Alessia Raffagnato), C.A. and A.G.; supervision, I.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was not necessary since this study does not involve humans. According to Article 1(1) of the Italian Ministerial Decree of 30 January 2023, the competence of ethics committees applies exclusively to clinical trials involving medicinal products, medical devices, or pharmacological observational studies. This study does not involve the collection of original data on human or animal subjects, does not propose any pharmacological or diagnostic interventions, and does not fall under the categories explicitly outlined in the decree. Therefore, ethical committee approval is not required.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halbreich, U. Stress-related physical and mental disorders: A new paradigm. BJPsych Adv. 2021, 27, 145–152. [Google Scholar] [CrossRef]
- Shenk, C.E.; O’Donnell, K.J.; Pokhvisneva, I.; Kobor, M.S.; Meaney, M.J.; Bensman, H.E.; Allen, K.E.; Olson, A.E. Epigenetic Age Acceleration and Risk for Posttraumatic Stress Disorder following Exposure to Substantiated Child Maltreatment. J. Clin. Child Adolesc. Psychol. 2022, 51, 651–661. [Google Scholar] [CrossRef]
- Lee, R.S.; Oswald, L.M.; Wand, G.S. Early Life Stress as a Predictor of Co-Occurring Alcohol Use Disorder and Post-Traumatic Stress Disorder. Alcohol Res. 2018, 39, 147–159. [Google Scholar] [CrossRef]
- Danzi, B.A.A.; La Greca, A.M. Does age matter in genetics? The role of ADCYAP1R1 in sex-specific risk for posttraumatic stress disorder in trauma-exposed preadolescent children. J. Psychiatr. Res. 2023, 164, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Cattane, N.; Rossi, R.; Lanfredi, M.; Cattaneo, A. Borderline personality disorder and childhood trauma: Exploring the affected biological systems and mechanisms. BMC Psychiatry 2017, 17, 221. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S.; Dwivedi, Y. Dissecting early life stress-induced adolescent depression through epigenomic approach. Mol. Psychiatry 2023, 28, 141–153. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Paradise Lost: The Neurobiological and Clinical Consequences of Child Abuse and Neglect. Neuron 2016, 89, 892–909. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.J.; Clukay, C.J.; Matarazzo, A.; Hadfield, K.; Nevell, L.; Dajani, R.; Panter-Brick, C. Novel GxE effects and resilience: A case: Control longitudinal study of psychosocial stress with war-affected youth. PLoS ONE 2022, 17, e0266509. [Google Scholar] [CrossRef]
- Sheerin, C.M.; Lancaster, E.E.; York, T.P.; Walker, J.; Danielson, C.K.; Amstadter, A.B. Epigenome-Wide Study of Posttraumatic Stress Disorder Symptom Severity in a Treatment-Seeking Adolescent Sample. J. Trauma. Stress 2021, 34, 607–615. [Google Scholar] [CrossRef]
- Pagliaccio, D.; Luby, J.L.; Bogdan, R.; Agrawal, A.; Gaffrey, M.S.; Belden, A.C.; Botteron, K.N.; Harms, M.P.; Barch, D.M. Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J. Abnorm. Psychol. 2015, 124, 817–833. [Google Scholar] [CrossRef]
- Dee, G.; Ryznar, R.; Dee, C. Epigenetic Changes Associated with Different Types of Stressors and Suicide. Cells 2023, 12, 1258. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Enoch, M.A.; Acheson, A.; Cohoon, A.J.; Sorocco, K.H.; Hodgkinson, C.A.; Vincent, A.S.; Goldman, D. Early-Life Adversity Interacts with FKBP5 Genotypes: Altered Working Memory and Cardiac Stress Reactivity in the Oklahoma Family Health Patterns Project. Neuropsychopharmacology 2016, 41, 1724–1732. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vrshek-Schallhorn, S.; Stroud, C.B.; Mineka, S.; Zinbarg, R.E.; Adam, E.K.; Redei, E.E.; Hammen, C.; Craske, M.G. Additive genetic risk from five serotonin system polymorphisms interacts with interpersonal stress to predict depression. J. Abnorm. Psychol. 2015, 124, 776–790. [Google Scholar] [CrossRef]
- Ghosh, M.; Ali, A.; Joshi, S.; Srivastava, A.S.; Tapadia, M.G. SLC1A3 C3590T but not BDNF G196A is a predisposition factor for stress as well as depression, in an adolescent eastern Indian population. BMC Med. Genet. 2020, 21, 53. [Google Scholar] [CrossRef] [PubMed]
- Marzi, S.J.; Sugden, K.; Arseneault, L.; Belsky, D.W.; Burrage, J.; Corcoran, D.L.; Danese, A.; Fisher, H.L.; Hannon, E.; Moffitt, T.E.; et al. Analysis of DNA methylation in young people: Limited evidence for an association between victimization stress and epigenetic variation in blood. Am. J. Psychiatry 2018, 175, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feder, A.; Cohen, H.; Kim, J.J.; Calderon, S.; Charney, D.S.; Mathé, A.A. Understanding resilience. Front. Behav. Neurosci. 2013, 7, 10. [Google Scholar] [CrossRef]
- Rutten, B.P.F.; Hammels, C.; Geschwind, N.; Menne-Lothmann, C.; Pishva, E.; Schruers, K.; van den Hove, D.; Kenis, G.; van Os, J.; Wichers, M. Resilience in mental health: Linking psychological and neurobiological perspectives. Acta Psychiatr. Scand. 2013, 128, 3–20. [Google Scholar] [CrossRef]
- Pereira, D.M.B.P.; Grasso, D.J.; Hodgkinson, C.A.; McCarthy, K.J.; Wakschlag, L.S.; Briggs-Gowan, M.J. Maternal posttraumatic stress and FKBP5 Genotype interact to predict trauma-related symptoms in preschool-age offspring. J. Affect. Disord. 2021, 292, 212–216. [Google Scholar] [CrossRef]
- Parade, S.H.; Novick, A.M.; Parent, J.; Seifer, R.; Klaver, S.J.; Marset, C.J.; Gobin, A.P.; Yang, B.Z.; Tyrka, A.R. Stress exposure and psychopathology alter methylation of the serotonin receptor 2A (HTR2A) gene in preschoolers. Dev. Psychopathol. 2017, 29, 1619–1626. [Google Scholar] [CrossRef]
- Non, A.L.; Hollister, B.M.; Humphreys, K.L.; Childebayeva, A.; Esteves, K.; Zeanah, C.H.; Fox, N.A.; Nelson, C.A.; Drury, S.S. DNA methylation at stress-related genes is associated with exposure to early life institutionalization. Am. J. Phys. Anthropol. 2016, 161, 84–93. [Google Scholar] [CrossRef]
- Van Der Knaap, L.J.; Riese, H.; Hudziak, J.J.; Verbiest, M.M.; Verhulst, F.C.; Oldehinkel, A.J.; van Oort, F.V. Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. the TRAILS study. Transl. Psychiatry 2014, 4, e381. [Google Scholar] [CrossRef]
- Romens, S.E.; Mcdonald, J.; Svaren, J.; Pollak, S.D. Associations Between Early Life Stress and Gene Methylation in Children. Child Dev. 2015, 86, 303–309. [Google Scholar] [CrossRef]
- Bosmans, G.; Young, J.F.; Hankin, B.L. NR3C1 methylation as a moderator of the effects of maternal support and stress on insecure attachment development. Dev. Psychol. 2018, 54, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Efstathopoulos, P.; Andersson, F.; Melas, P.A.; Yang, L.L.; Villaescusa, J.C.; Rȕegg, J.; Ekström, T.J.; Forsell, Y.; Galanti, M.R.; Lavebratt, C. NR3C1 hypermethylation in depressed and bullied adolescents. Transl. Psychiatry 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Cicchetti, D.; Handley, E.D. Methylation of the glucocorticoid receptor gene, nuclear receptor subfamily 3, group C, member 1 (NR3C1), in maltreated and nonmaltreated children: Associations with behavioral undercontrol, emotional lability/negativity, and externalizing and internalizing symptoms. Dev. Psychopathol. Dev. Psychopathol. 2017, 29, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, S.V.; Ivanoshchuk, D.E.; Orlov, P.S.; Bairqdar, A.; Anisimenko, M.S.; Denisova, D.V. Assessment of the Genetic Characteristics of a Generation Born during a Long-Term Socioeconomic Crisis. Genes 2023, 14, 2064. [Google Scholar] [CrossRef] [PubMed]
- Etain, B.; Lajnef, M.; Henrion, A.; Dargél, A.A.; Stertz, L.; Kapczinski, F.; Mathieu, F.; Henry, C.; Gard, S.; Kahn, J.P.; et al. Interaction between SLC6A4 promoter variants and childhood trauma on the age at onset of bipolar disorders. Sci. Rep. 2015, 5, 16301. [Google Scholar] [CrossRef]
- Lin, X.; Cao, Y.; Ji, L.; Zhang, W. Inhibitory control mediates the interaction between serotonin transporter gene (5-HTTLPR) and peer victimization on adolescent depressive symptoms. Sci. Rep. 2021, 11, 14640. [Google Scholar] [CrossRef]
- Van Der Knaap, L.J.; Riese, H.; Hudziak, J.J.; Verbiest, M.M.; Verhulst, F.C.; Oldehinkel, A.J.; van Oort, F.V. Adverse life events and allele-specific methylation of the serotonin transporter gene (SLC6A4) in adolescents. Psychosom. Med. 2015, 77, 246–255. [Google Scholar] [CrossRef]
- Simsek, S.; Uysal, C.; Kaplan, I.; Yuksel, T.; Aktas, H. BDNF and cortisol levels in children with or without post-traumatic stress disorder after sustaining sexual abuse. Psychoneuroendocrinology 2015, 56, 45–51. [Google Scholar] [CrossRef]
- Şimşek, Ş.; Yüksel, T.; Kaplan, I.; Uysal, C.; Alaca, R. Examining the levels of BDNF and cortisol in children and adolescent victims of sexual abuse—A preliminary study. Compr. Psychiatry 2015, 61, 23–27. [Google Scholar] [CrossRef]
- Aksu, S.; Unlu, G.; Kardesler, A.C.; Cakaloz, B.; Aybek, H. Altered levels of brain-derived neurotrophic factor, proBDNF and tissue plasminogen activator in children with posttraumatic stress disorder. Psychiatry Res. 2018, 268, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Ensink, J.B.M.; Keding, T.J.; Henneman, P.; Venema, A.; Papale, L.A.; Alisch, R.S.; Westerman, Y.; van Wingen, G.; Zantvoord, J.; Middeldorp, C.M.; et al. Differential DNA Methylation Is Associated With Hippocampal Abnormalities in Pediatric Posttraumatic Stress Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Weder, N.; Weder, N.; Zhang, H.; Jensen, K.; Yang, B.Z.; Simen, A.; Jackowski, A.; Lipschitz, D.; Douglas-Palumberi, H.; Ge, M.; et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 417–424.e5. [Google Scholar] [CrossRef]
- Hecker, T.; Radtke, K.M.; Hermenau, K.; Papassotiropoulos, A.; Elbert, T. Associations among child abuse, mental health, and epigenetic modifications in the proopiomelanocortin gene (POMC): A study with children in Tanzania. Dev. Psychopathol. 2016, 28, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus--norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef]
- Russo, S.J.; Murrough, J.W.; Han, M.H.; Charney, D.S.; Nestler, E.J. Neurobiology of resilience. Nat. Neurosci. 2012, 15, 1475–1484. [Google Scholar] [CrossRef]
- Gerritsen, L.; Milaneschi, Y.; Vinkers, C.; van Hemert, A.M.; van Velzen, L.; Schmaal, L.; Penninx, B.W.J.H. HPA axis genes, and their interaction with childhood maltreatment, are related to cortisol levels and stress-related phenotypes. Neuropsychopharmacology 2017, 42, 2446–2455. [Google Scholar] [CrossRef]
- Korgan, A.C.; Prendergast, K.; Rosenhauer, A.M.; Morrison, K.E.; Jovanovic, T.; Bale, T.L. Trauma and Sensory Systems: Biological Mechanisms Involving the Skin and the 17q21 Gene Cluster. Biol. Psychiatry 2024, 97, 854–861. [Google Scholar] [CrossRef]
- Wu, G.; Feder, A.; Wegener, G.; Bailey, C.; Saxena, S.; Charney, D.; Mathé, A.A. Central functions of neuropeptide Y in mood and anxiety disorders. Expert. Opin. Ther. Targets 2011, 15, 1317–1331. [Google Scholar] [CrossRef]
- Akimova, E.; Lanzenberger, R.; Kasper, S. The Serotonin--1A Receptor in Anxiety Disorders. Biol. Psychiatry 2009, 66, 627–635. [Google Scholar] [CrossRef]
- Benekareddy, M.; Vadodaria, K.C.; Nair, A.R.; Vaidya, V.A. Postnatal serotonin type 2 receptor blockade prevents the emergence of anxiety behavior, dysregulated stress-induced immediate early gene responses, and specific transcriptional changes that arise following early life stress. Biol. Psychiatry 2011, 70, 1024–1032. [Google Scholar] [CrossRef]
- Strawn, J.R.; Geracioti, T.D. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety 2008, 25, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Charney, D.S. Psychobiological Mechanisms of Resilience and Vulnerability: Implications for Successful Adaptation to Extreme Stress. Am. J. Psychiatry 2004, 161, 195–216. [Google Scholar] [CrossRef]
- Feder, A.; Nestler, E.J.; Charney, D.S. Psychobiology and molecular genetics of resilience. Nat. Rev. Neurosci. 2009, 10, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Onur, O.A.; Walter, H.; Schlaepfer, T.E.; Rehme, A.K.; Schmidt, C.; Keysers, C.; Maier, W.; Hurlemann, R. Noradrenergic enhancement of amygdala responses to fear. Soc. Cogn. Affect. Neurosci. 2009, 4, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; McClay, J.; Moffitt, T.E.; Mill, J.; Martin, J.; Craig, I.W.; Taylor, A.; Poulton, R. Role of genotype in the cycle of violence in maltreated children. Science 2002, 297, 851–854. [Google Scholar] [CrossRef]
- Brummett, B.H.; Boyle, S.H.; Siegler, I.C.; Kuhn, C.M.; Surwit, R.S.; Garrett, M.E.; Collins, A.; Ashely-Koch, A.; William, R.B. HPA axis function in male caregivers: Effect of the monoamine oxidase-A gene promoter (MAOA-uVNTR). Biol. Psychol. 2008, 79, 250–255. [Google Scholar] [CrossRef]
- Schlüter, T.; Winz, O.; Henkel, K.; Eggermann, T.; Mohammadkhani-Shali, S.; Dietrich, C.; Heinzel, A.; Decker, M.; Cumming, P.; Zerres, K.; et al. MAOA-VNTR polymorphism modulates context-dependent dopamine release and aggressive behavior in males. Neuroimage 2016, 125, 378–385. [Google Scholar] [CrossRef]
- Plieger, T.; Melchers, M.; Felten, A.; Lieser, T.; Meermann, R.; Reuter, M. Moderator Effects of Life Stress on the Association between MAOA-uVNTR, Depression, and Burnout. Neuropsychobiology 2019, 78, 86–94. [Google Scholar] [CrossRef]
- Taliaz, D.; Loya, A.; Gersner, R.; Haramati, S.; Chen, A.; Zangen, A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci. 2011, 31, 4475–4483. [Google Scholar] [CrossRef]
- Yamada, K.; Nabeshima, T. Brain--Derived Neurotrophic Factor/TrkB Signaling in Memory Processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef]
- Sonoyama, T.; Stadler, L.K.J.; Zhu, M.; Keogh, J.M.; Henning, E.; Hisama, F.; Kirwan, P.; Jura, M.; Blaszczyk, B.K.; DeWitt, D.C.; et al. Human BDNF/TrkB variants impair hippocampal synaptogenesis and associate with neurobehavioural abnormalities. Sci. Rep. 2020, 10, 9028. [Google Scholar] [CrossRef]
- Mahan, A.L.; Ressler, K.J. Fear conditioning, synaptic plasticity and the amygdala: Implications for posttraumatic stress disorder. Trends Neurosci. 2012, 35, 24–35. [Google Scholar] [CrossRef]
- Zwolińska, W.; Dmitrzak-Węglarz, M.; Słopień, A. Biomarkers in Child and Adolescent Depression. Child. Psychiatry Hum. Dev. 2023, 54, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.; Chen, X.; Gu, X.; Wang, M.; Wu, J. A functional variant in SLC1A3 influences ADHD risk by disrupting a hsa-miR-3171 binding site: A two-stage association study. Genes Brain Behav. 2019, 18, e12574. [Google Scholar] [CrossRef]
- Lu, D.; Sapkota, Y.; Valdimarsdóttir, U.A.; Koenen, K.C.; Li, N.; Leisenring, W.M.; Gibson, T.; Wilson, C.L.; Robison, L.L.; Hudson, M.M.; et al. Genome-wide association study of posttraumatic stress disorder among childhood cancer survivors: Results from the Childhood Cancer Survivor Study and the St. Jude Lifetime Cohort. Transl. Psychiatry 2022, 12, 342. [Google Scholar] [CrossRef]
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Bierer, L.M. The relevance of epigenetics to PTSD: Implications for the DSM-V. J. Trauma. Stress 2009, 22, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dall’Aglio, L.; Muka, T.; Cecil, C.A.M.; Bramer, W.M.; Verbiest, M.M.P.J.; Nano, J.; Hidalgo, A.C.; Franco, O.H.; Tiemeier, H. The role of epigenetic modifications in neurodevelopmental disorders: A systematic review. Neurosci. Biobehav. Rev. 2018, 94, 17–30. [Google Scholar] [CrossRef]
- Aref-Eshghi, E.; Kerkhof, J.; Pedro, V.P.; Groupe DI France; Barat-Houari, M.; Ruiz-Pallares, N.; Andrau, J.C.; Lacombe, D.; Van-Gils, J.; Fergelot, P.; et al. Evaluation of DNA Methylation Episignatures for Diagnosis and Phenotype Correlations in 42 Mendelian Neurodevelopmental Disorders. Am. J. Hum. Genet. 2020, 106, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Thumfart, K.M.; Jawaid, A.; Bright, K.; Flachsmann, M.; Mansuy, I.M. Epigenetics of childhood trauma: Long term sequelae and potential for treatment. Neurosci. Biobehav. Rev. 2022, 132, 1049–1066. [Google Scholar] [CrossRef]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Poulter, M.O.; Du, L.; Weaver, I.C.G.; Palkovits, M.; Faludi, G.; Merali, Z.; Szyf, M.; Anisman, H. GABAA Receptor Promoter Hypermethylation in Suicide Brain: Implications for the Involvement of Epigenetic Processes. Biol. Psychiatry 2008, 64, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Du, L.; Hrdina, P.; Palkovits, M.; Faludi, G.; Poulter, M.O.; Anisman, H. Dysregulation in the Suicide Brain: mRNA Expression of Corticotropin-Releasing Hormone Receptors and GABAA Receptor Subunits in Frontal Cortical Brain Region. J. Neurosci. 2004, 24, 1478–1485. [Google Scholar] [CrossRef]
- Parade, S.H.; Parent, J.; Rabemananjara, K.; Seifer, R.; Marsit, C.J.; Yang, B.Z.; Zhang, H.; Tyrka, A.R. Change in FK506 binding protein 5 (FKBP5) methylation over time among preschoolers with adversity. Dev. Psychopathol. 2017, 29, 1627–1634. [Google Scholar] [CrossRef]
- Naumova, O.Y.; Lee, M.; Koposov, R.; Szyf, M.; Dozier, M.; Grigorenko, E.L. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Dev. Psychopathol. 2012, 24, 143–155. [Google Scholar] [CrossRef]
- Papale, L.A.; Seltzer, L.J.; Madrid, A.; Pollak, S.D.; Alisch, R.S. Differentially Methylated Genes in Saliva are linked to Childhood Stress. Sci. Rep. 2018, 8, 10785. [Google Scholar] [CrossRef]
- Esposito, E.A.; Jones, M.J.; Doom, J.R.; MacIsaac, J.L.; Gunnar, M.R.; Kobor, M.S. Differential DNA methylation in peripheral blood mononuclear cells in adolescents exposed to significant early but not later childhood adversity. Dev. Psychopathol. 2016, 28, 1385–1399. [Google Scholar] [CrossRef]
- Walton, E.; Baltramonaityte, V.; Calhoun, V.; Heijmans, B.T.; Thompson, P.M.; Cecil, C.A.M. A systematic review of neuroimaging epigenetic research: Calling for an increased focus on development. Mol. Psychiatry 2023, 28, 2839–2847. [Google Scholar] [CrossRef]
- Bradley, B.; Westen, D.; Mercer, K.B.; Binder, E.B.; Jovanovic, T.; Crain, D.; Wingo, A.; Heim, C. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: Moderation by oxytocin receptor gene. Dev. Psychopathol. 2011, 23, 439–452. [Google Scholar] [CrossRef]
- Binder, E.B.; Nemeroff, C.B. The CRF system, stress, depression and anxietyinsights from human genetic studies. Mol. Psychiatry 2010, 15, 574–588. [Google Scholar] [CrossRef]
- Armbruster, D.; Mueller, A.; Strobel, A.; Lesch, K.P.; Brocke, B.; Kirschbaum, C. Children under stress-COMT genotype and stressful life events predict cortisol increase in an acute social stress paradigm. Int. J. Neuropsychopharmacol. 2012, 15, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- La Greca, A.M.; Lai, B.S.; Joormann, J.; Auslander, B.B.; Short, M.A. Children’s risk and resilience following a natural disaster: Genetic vulnerability, posttraumatic stress, and depression. J. Affect. Disord. 2013, 151, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Donner, J.; Sipilä, T.; Ripatti, S.; Kananen, L.; Chen, X.; Kendler, K.S.; Lönnqvist, J.; Pirkola, S.; Hettema, J.M.; Hovatta, I. Support for involvement of glutamate decarboxylase 1 and neuropeptide y in anxiety susceptibility. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 159B, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Smearman, E.L.; Yu, T.; Brody, G.H. Variation in the oxytocin receptor gene moderates the protective effects of a family-based prevention program on telomere length. Brain Behav. 2016, 6, e00423. [Google Scholar] [CrossRef] [PubMed]
- Hostinar, C.E.; Cicchetti, D.; Rogosch, F.A. Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Dev. Psychopathol. 2014, 26, 465–477. [Google Scholar] [CrossRef][Green Version]
- Morgan, C.A.; Southwick, S.; Hazlett, G.; Rasmusson, A.; Hoyt, G.; Zimolo, Z.; Charney, D.S. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch. Gen. Psychiatry 2004, 61, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, K.; Papadopoulos, A.; Juruena, M.F.; Poon, L.; Pariante, C.M.; Cleare, A.J. The ratio of cortisol/DHEA in treatment resistant depression. Psychoneuroendocrinology 2009, 34, 19–26. [Google Scholar] [CrossRef]
- Hauger, R.L.; Risbrough, V.; Oakley, R.H.; Olivares-Reyes, J.A.; Dautzenberg, F.M. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann. N. Y. Acad. Sci. 2009, 1179, 120–143. [Google Scholar] [CrossRef]
- Krystal, J.H.; Neumeister, A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009, 1293, 13–23. [Google Scholar] [CrossRef]
- Jhaveri, D.J.; Mackay, E.W.; Hamlin, A.S.; Marathe, S.V.; Nandam, L.S.; Vaidya, V.A.; Bartlett, P.F. Norepinephrine directly activates adult hippocampal precursors via β3-adrenergic receptors. J. Neurosci. 2010, 30, 2795–2806. [Google Scholar] [CrossRef]
- Masi, G.; Brovedani, P. The Hippocampus, Neurotrophic Factors and Depression: Possible Implications for the Pharmacotherapy of Depression. CNS Drugs 2011, 25, 913–931. [Google Scholar] [CrossRef]
- Li, Y.; Luikart, B.W.; Birnbaum, S.; Chen, J.; Kwon, C.H.; Kernie, S.G.; Bassel-Duby, R.; Parada, L.F. TrkB Regulates Hippocampal Neurogenesis and Governs Sensitivity to Antidepressive Treatment. Neuron 2008, 59, 399–412. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Petersén, Å.; Wörtwein, G.; Gruber, S.H.M.; El-Khoury, A.; Mathé, A.A. Nortriptyline mediates behavioral effects without affecting hippocampal cytogenesis in a genetic rat depression model. Neurosci. Lett. 2009, 451, 148–151. [Google Scholar] [CrossRef]
- Hansson, A.C.; Rimondini, R.; Heilig, M.; Mathé, A.A.; Sommer, W.H. Dissociation of antidepressant-like activity of escitalopram and nortriptyline on behaviour and hippocampal BDNF expression in female rats. J. Psychopharmacol. 2011, 25, 1378–1387. [Google Scholar] [CrossRef]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar]
- Cohen, H.; Liu, T.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A. The neuropeptide y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 2012, 37, 350–363. [Google Scholar] [CrossRef]
- Sah, R.; Geracioti, T.D. Neuropeptide Y and posttraumatic stress disorder. Mol. Psychiatry 2013, 18, 428–439. [Google Scholar] [CrossRef]
- Sajdyk, T.J.; Shekhar, A.; Gehlert, D.R. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides 2004, 38, 225–234. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, G.; Hariri, A.R.; Enoch, M.A.; Scott, D.; Sinha, R.; Virkkunen, M.; Mash, D.C.; Lipsky, R.H.; Hu, X.Z.; et al. Genetic variation in human NPY expression affects stress response and emotion. Nature 2008, 452, 997–1001. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C. Evaluating the challenges and reproducibility of studies investigating DNA methylation signatures of psychological stress. Epigenomics 2022, 14, 405–421. [Google Scholar] [CrossRef]
- Harvey, B.H.; Shahid, M. Metabotropic and ionotropic glutamate receptors as neurobiological targets in anxiety and stress-related disorders: Focus on pharmacology and preclinical translational models. Pharmacol. Biochem. Behav. 2012, 100, 775–800. [Google Scholar] [CrossRef]
- Hill, M.N. Introduction to the special issue on stress, emotional behavior, and the endocannabinoid system: A decade of research. Neuroscience 2012, 204, 1–3. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef]
- Kirilly, E.; Gonda, X.; Bagdy, G. CB1 receptor antagonists: New discoveries leading to new perspectives. Acta Physiol. 2012, 205, 41–60. [Google Scholar] [CrossRef]
- Mathew, S.J.; Shah, A.; Lapidus, K.; Clark, C.; Jarun, N.; Ostermeyer, B.; Murrough, J.W. Ketamine for treatment-resistant unipolar depression: Current evidence. CNS Drugs 2012, 26, 189–204. [Google Scholar] [CrossRef]
- Mathews, D.C.; Henter, I.D.; Zarate, C.A. Targeting the glutamatergic system to treat major depressive disorder: Rationale and progress to date. Drugs 2012, 72, 1313–1333. [Google Scholar] [CrossRef]
- Hillard, C.J.; Beatka, M.; Sarvaideo, J. Endocannabinoid signaling and the hypothalamic-pituitary-adrenal axis. Compr. Physiol. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Lisboa, S.F.; Niraula, A.; Resstel, L.B.; Guimaraes, F.S.; Godbout, J.P.; Sheridan, J.F. Repeated social defeat-induced neuroinflammation, anxiety-like behavior and resistance to fear extinction were attenuated by the cannabinoid receptor agonist WIN55,212-2. Neuropsychopharmacology 2018, 43, 1924–1933. [Google Scholar] [CrossRef]
- Richardson, G.E. The metatheory of resilience and resiliency. J. Clin. Psychol. 2002, 58, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Wichers, M.C.; Myin-Germeys, I.; Jacobs, N.; Peeters, F.; Kenis, G.; Derom, C.; Vlietinck, R.; Delespaul, P.; van Os, J. Evidence that moment-to-moment variation in positive emotions buffer genetic risk for depression: A momentary assessment twin study. Acta Psychiatr. Scand. 2007, 115, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Boardman, J.D.; Blalock, C.L.; Button, T.M.M. Sex differences in the heritability of resilience. Twin Res. Hum. Genet. 2008, 11, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.M.; Aspinwall, L.G. Emotion regulation across the life span: An integrative perspective emphasizing self-regulation, positive affect, and dyadic processes. Motiv. Emot. 2003, 27, 125–156. [Google Scholar] [CrossRef]
- Hankin, B.L.; Nederhof, E.; Oppenheimer, C.W.; Jenness, J.; Young, J.F.; Abela, J.R.; Smolen, A.; Ormel, J.; Oldehinkel, A.J. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect for better and worse. Transl. Psychiatry 2011, 1, e44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).