Identification of a Novel CLPX Variant in a Mixed-Breed Dog with Anemia and Spinocerebellar Ataxia
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Examination and Ethical Statement
2.2. Pathological Examination
2.3. DNA Extraction
2.4. Whole-Genome Sequencing, Alignment, and Variant Calling
2.5. Variant Filtering
2.6. In Silico Predictions
2.7. Primer Design and Targeted Genotyping
2.8. Breed Determination and Principal Component Analysis
3. Results
3.1. Clinical Examination
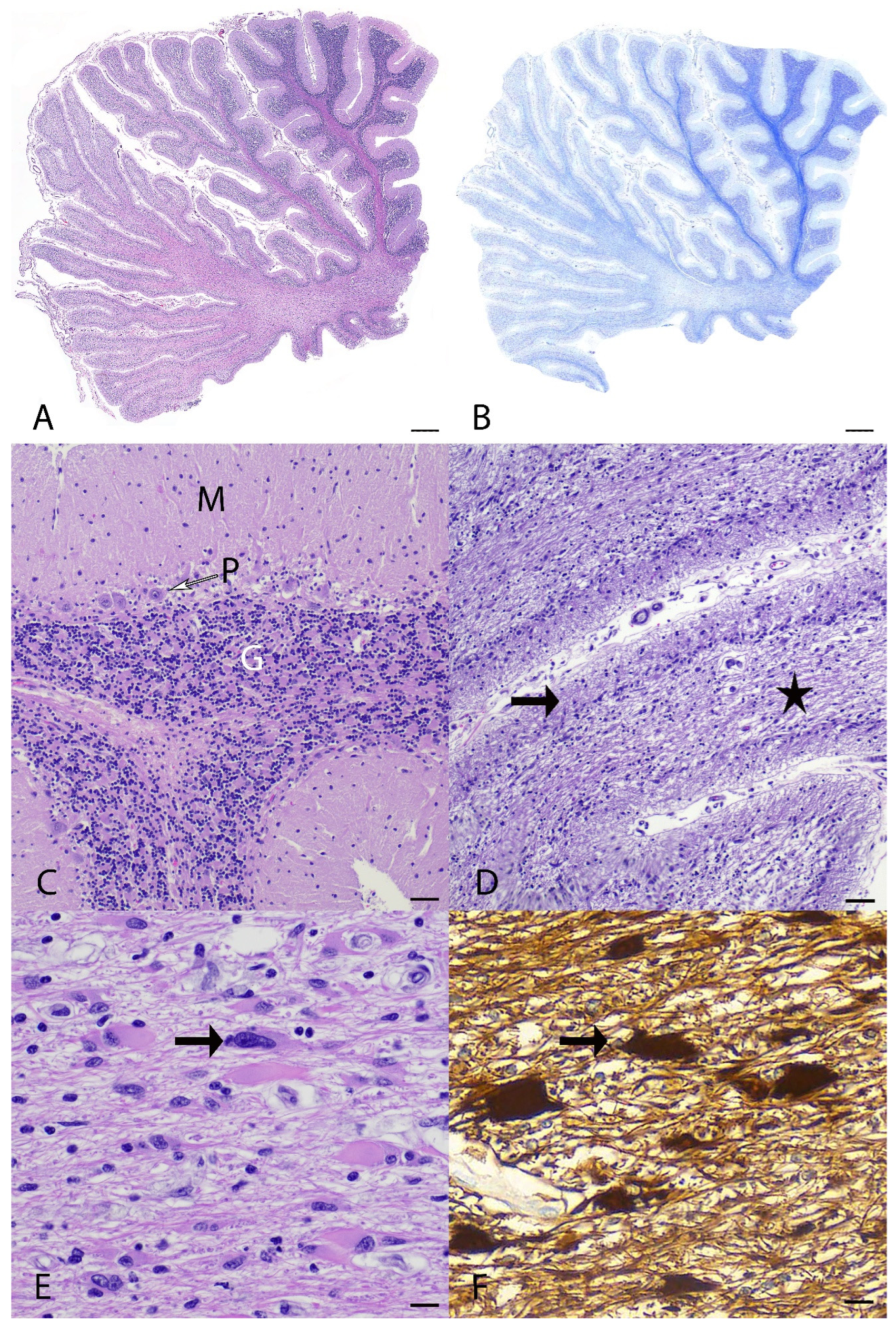
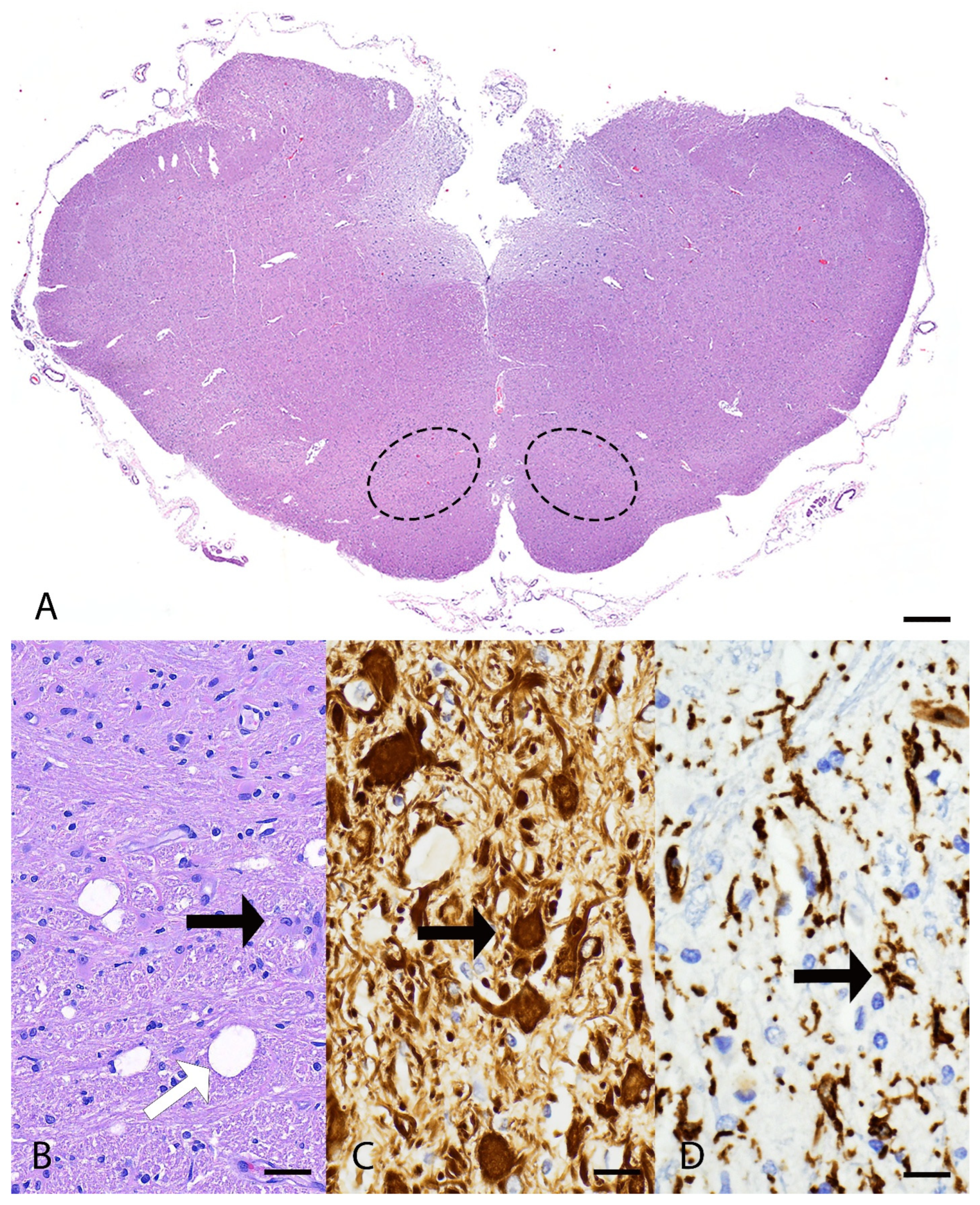
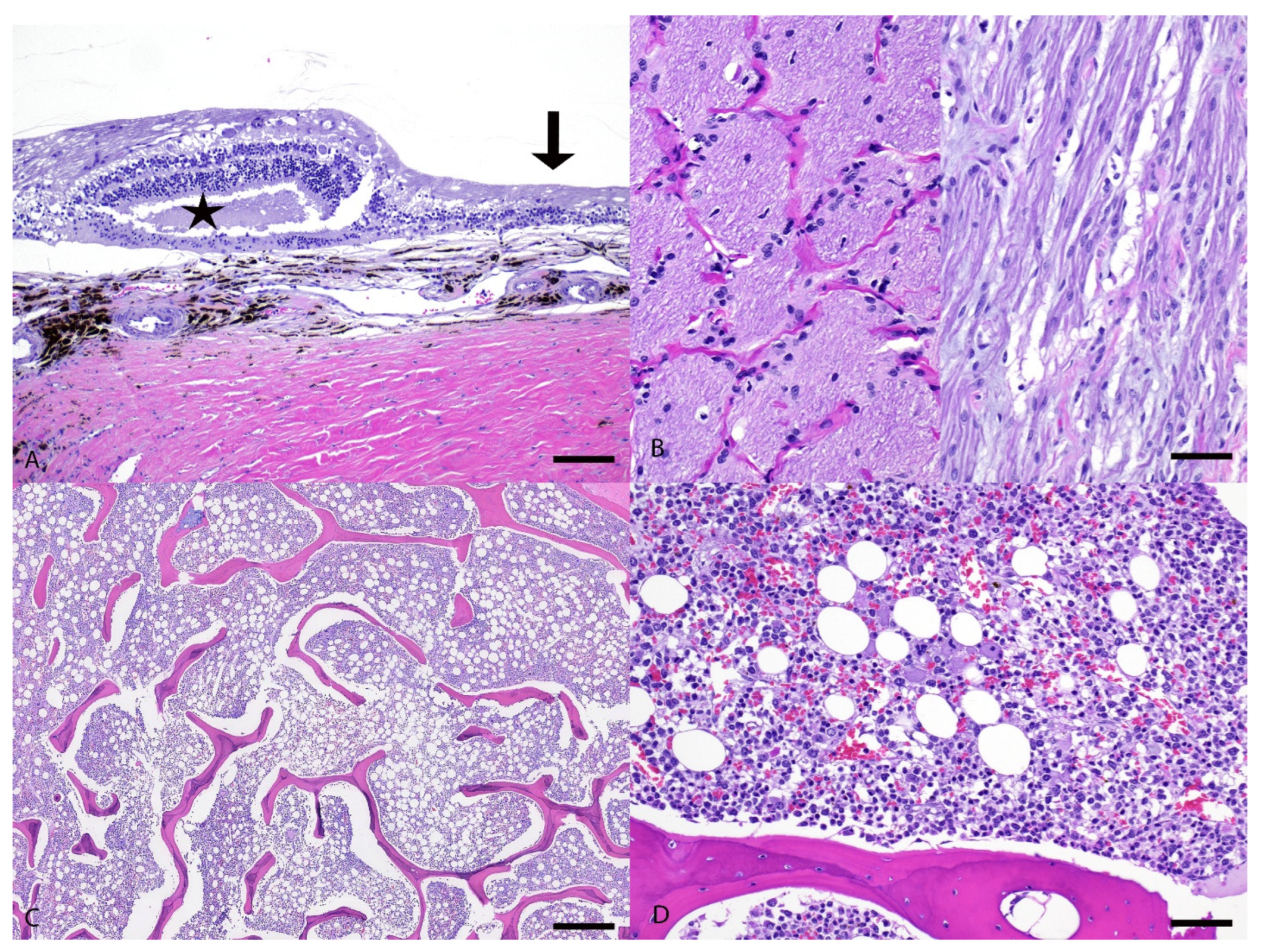
3.2. Pathological Examination
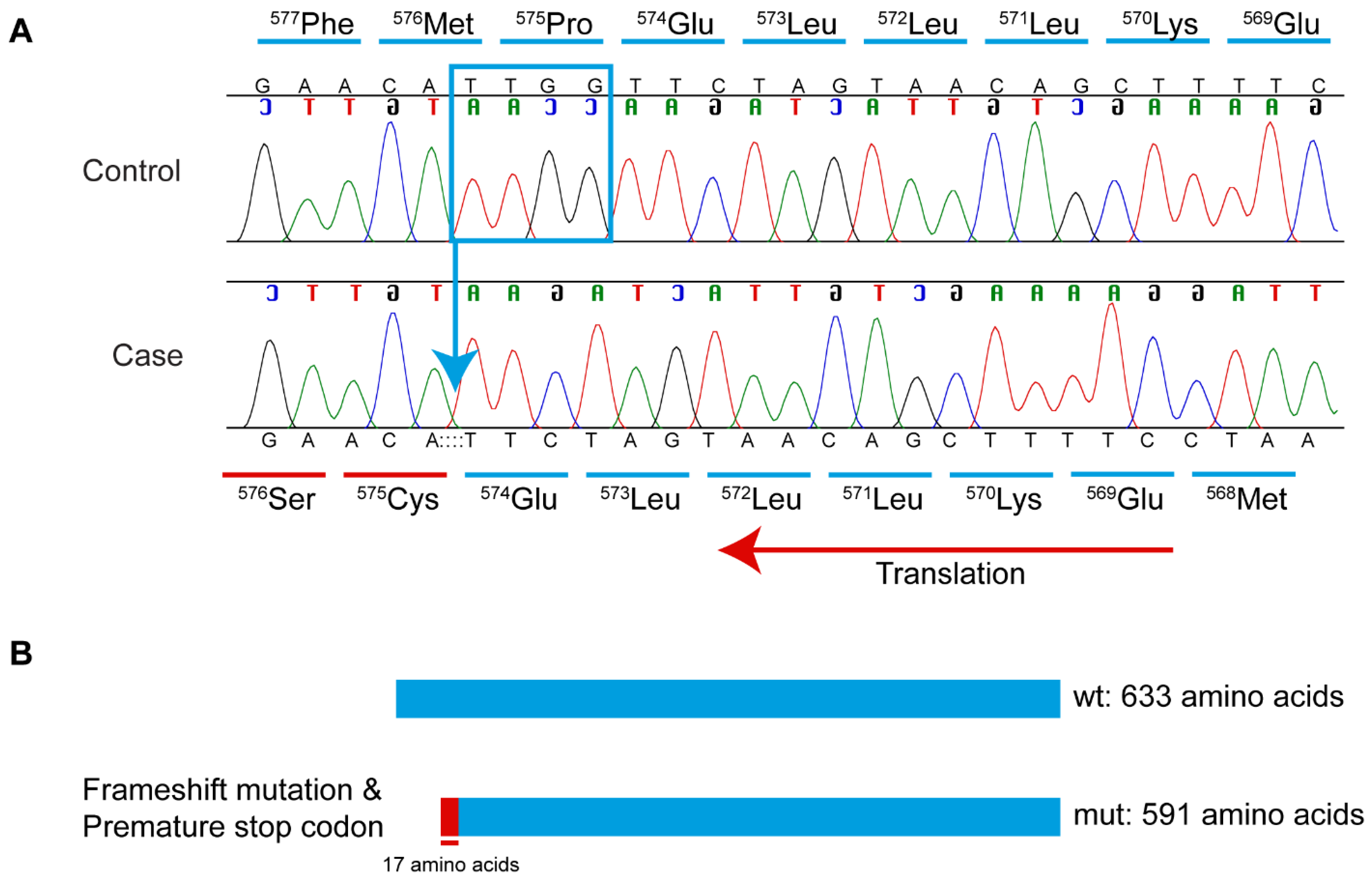
3.3. Whole-Genome Sequence, Variant Filtering, and Variant Confirmation
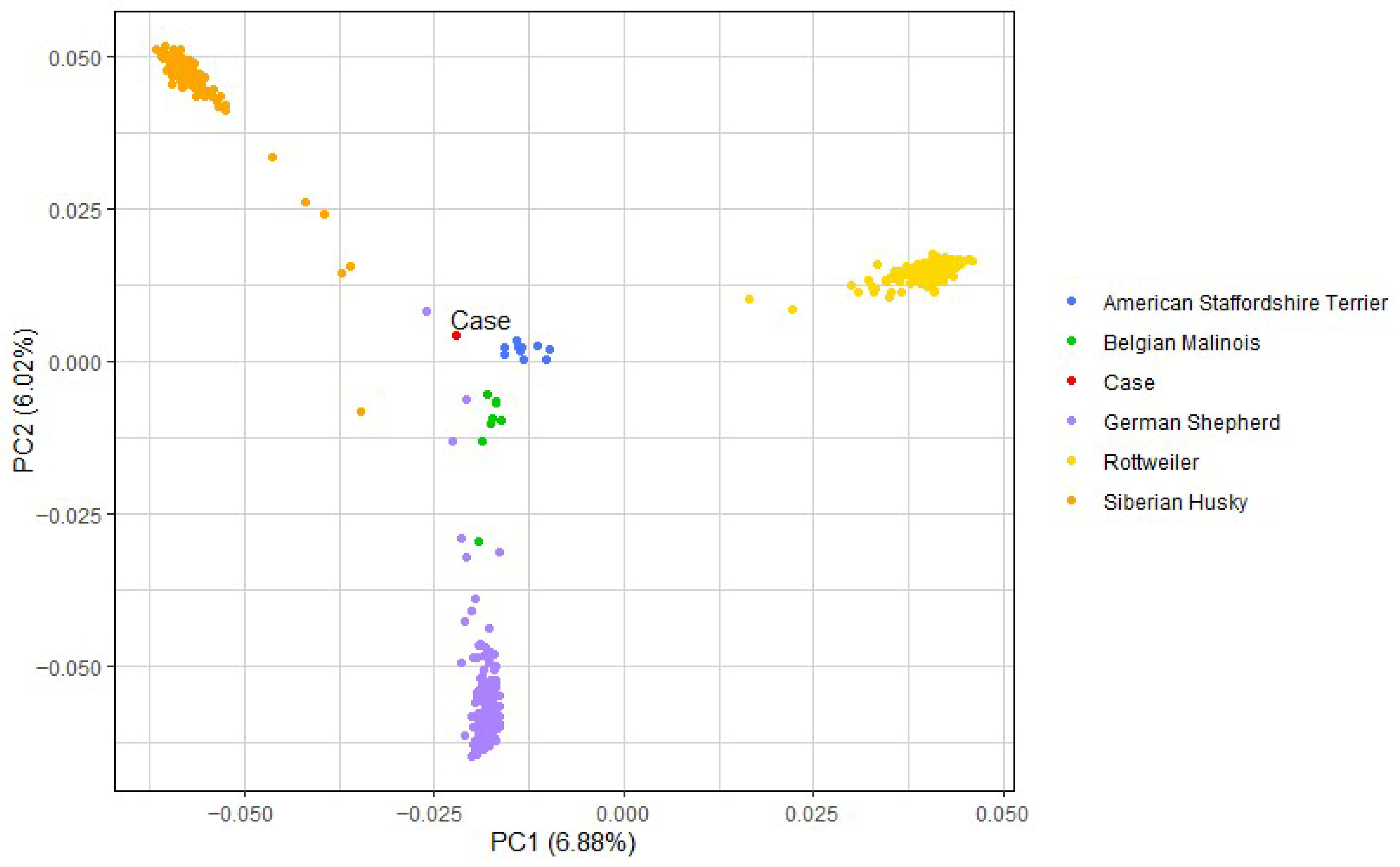
3.4. Breed Determination and Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBC | Complete blood count |
| CNS | Central nervous system |
| DAB | 3,3′-diaminobenzidine tetrahydrochloride |
| GATK | Genome Analysis Toolkit |
| GCTA | Genome-wide Complex Trait Analysis |
| GFAP | Glial fibrillary acidic protein |
| H&E | Hematoxylin and eosin |
| HA | Hereditary ataxia |
| HIER | Heat-induced epitope retrieval |
| FFPE | Formalin-fixed paraffin-embedded |
| IBA-1 | Ionized calcium-binding adaptor molecule 1 |
| LADDL | Louisiana Animal Disease Diagnostic Laboratory |
| LSU VTH | Louisiana State University Veterinary Teaching Hospital |
| M:E | Myeloid to erythroid ratio |
| OMIA | Online Mendelian Inheritance in Animals |
| PCA | Principal component analysis |
| PIMA | Precursor-targeted immune-mediated anemia |
| QC | Quality control |
| RBC | Red blood cell |
| RPE | Retinal pigmented epithelium |
| ROS | Reactive oxygen species |
| SCA | Spinocerebellar ataxia |
| VEP | Variant effect predictor |
| WAGS | Whole Animal Genome Sequencing |
| WGS | Whole-genome sequencing |
Appendix A
References
- Stee, K.; Van Poucke, M.; Lowrie, M.; Van Ham, L.; Peelman, L.; Olby, N.; Bhatti, S.F.M. Phenotypic and genetic aspects of hereditary ataxia in dogs. J. Vet. Intern. Med. 2023, 37, 1306–1322. [Google Scholar] [CrossRef]
- Urkasemsin, G.; Olby, N.J. Canine Hereditary Ataxia. Vet. Clin. Small Anim. Pract. 2014, 44, 1075–1089. [Google Scholar] [CrossRef]
- Harding, A.E. Classification of the Hereditary Ataxias and Paraplegias. Lancet 1983, 321, 1151–1155. [Google Scholar] [CrossRef]
- Soong, B.W.; Morrison, P.J. Spinocerebellar ataxias. Handb. Clin. Neurol. 2018, 155, 143–174. [Google Scholar] [CrossRef]
- Sullivan, R.; Yau, W.Y.; O’Connor, E.; Houlden, H. Spinocerebellar ataxia: An update. J. Neurol. 2019, 266, 533–544. [Google Scholar] [CrossRef]
- Jayadev, S.; Bird, T.D. Hereditary ataxias: Overview. Genet. Med. 2013, 15, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Letko, A.; Dietschi, E.; Nieburg, M.; Jagannathan, V.; Gurtner, C.; Oevermann, A.; Drögemüller, C. A Missense Variant in SCN8A in Alpine Dachsbracke Dogs Affected by Spinocerebellar Ataxia. Genes 2019, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Forman, O.P.; De Risio, L.; Matiasek, K.; Platt, S.; Mellersh, C. Spinocerebellar ataxia in the Italian Spinone dog is associated with an intronic GAA repeat expansion in ITPR1. Mamm. Genome 2015, 26, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, D.; O’Brien, D.P.; Coates, J.R.; Johnson, G.S.; Johnson, G.C.; Mhlanga-Mutangadura, T.; Hansen, L.; Taylor, J.F.; Schnabel, R.D. A homozygous KCNJ10 mutation in Jack Russell Terriers and related breeds with spinocerebellar ataxia with myokymia, seizures, or both. J. Vet. Intern. Med. 2014, 28, 871–877. [Google Scholar] [CrossRef]
- Rohdin, C.; Gilliam, D.; O’Leary, C.A.; O’Brien, D.P.; Coates, J.R.; Johnson, G.S.; Jäderlund, K.H. A KCNJ10 mutation previously identified in the Russell group of terriers also occurs in Smooth-Haired Fox Terriers with hereditary ataxia and in related breeds. Acta Vet. Scand. 2015, 57, 26. [Google Scholar] [CrossRef]
- Stee, K.; Van Poucke, M.; Pumarola, M.; Geerinckx, L.; Van Soens, I.; Bhatti, S.F.M.; Peelman, L.; Cornelis, I. Spinocerebellar ataxia in the Bouvier des Ardennes breed is caused by a KCNJ10 missense variant. J. Vet. Intern. Med. 2023, 37, 216–222. [Google Scholar] [CrossRef]
- Mauri, N.; Kleiter, M.; Leschnik, M.; Högler, S.; Dietschi, E.; Wiedmer, M.; Dietrich, J.; Henke, D.; Steffen, F.; Schuller, S.; et al. A Missense Variant in KCNJ10 in Belgian Shepherd Dogs Affected by Spongy Degeneration with Cerebellar Ataxia (SDCA1). G3 Genes Genomes Genet. 2017, 7, 663–669. [Google Scholar] [CrossRef]
- Van Poucke, M.; Stee, K.; Bhatti, S.F.; Vanhaesebrouck, A.; Bosseler, L.; Peelman, L.J.; Van Ham, L. The novel homozygous KCNJ10 c.986T>C (p.(Leu329Pro)) variant is pathogenic for the SeSAME/EAST homologue in Malinois dogs. Eur. J. Hum. Genet. 2017, 25, 222–226. [Google Scholar] [CrossRef]
- Van Poucke, M.; Stee, K.; Sonck, L.; Stock, E.; Bosseler, L.; Van Dorpe, J.; Van Nieuwerburgh, F.; Deforce, D.; Peelman, L.J.; Van Ham, L.; et al. Truncating SLC12A6 variants cause different clinical phenotypes in humans and dogs. Eur. J. Hum. Genet. 2019, 27, 1561–1568. [Google Scholar] [CrossRef]
- Awano, T.; Johnson, G.S.; Wade, C.M.; Katz, M.L.; Johnson, G.C.; Taylor, J.F.; Perloski, M.; Biagi, T.; Baranowska, I.; Long, S.; et al. Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2009, 106, 2794–2799. [Google Scholar] [CrossRef]
- Cullen, J.N.; Friedenberg, S.G. Whole Animal Genome Sequencing: User-friendly, rapid, containerized pipelines for processing, variant discovery, and annotation of short-read whole genome sequencing data. G3 Genes Genomes Genet. 2023, 13, jkad117. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows—Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- van der Auwera, G.; O’Connor, B.D. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2020. [Google Scholar]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, F.W.; Tammen, I.; Sydney Informatics Hub. Online Mendelian Inheritance in Animals (OMIA) [Dataset]. 2025. Available online: https://www.omia.org/home/ (accessed on 1 July 2024).
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Larson, G.; Kidd, J.M.; von Holdt, B.M.; Ostrander, E.A.; Zhang, Y.P. Dog10K: The International Consortium of Canine Genome Sequencing. Natl. Sci. Rev. 2019, 6, 611–613. [Google Scholar] [CrossRef]
- Stelzer, G.; Plaschkes, I.; Oz-Levi, D.; Alkelai, A.; Olender, T.; Zimmerman, S.; Twik, M.; Belinky, F.; Fishilevich, S.; Nudel, R.; et al. VarElect: The phenotype-based variation prioritizer of the GeneCards Suite. BMC Genom. 2016, 17, 444. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.E.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Talenti, A.; Dreger, D.L.; Frattini, S.; Polli, M.; Marelli, S.; Harris, A.C.; Liotta, L.; Cocco, R.; Hogan, A.N.; Bigi, D.; et al. Studies of modern Italian dog populations reveal multiple patterns for domestic breed evolution. Ecol. Evol. 2018, 8, 2911–2925. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2012, 14, 178–192. [Google Scholar] [CrossRef]
- Bendl, J.; Stourac, J.; Salanda, O.; Pavelka, A.; Wieben, E.D.; Zendulka, J.; Brezovsky, J.; Damborsky, J. PredictSNP: Robust and accurate consensus classifier for prediction of disease-related mutations. PLoS Comput. Biol. 2014, 10, e1003440. [Google Scholar] [CrossRef] [PubMed]
- Pejaver, V.; Urresti, J.; Lugo-Martinez, J.; Pagel, K.A.; Lin, G.N.; Nam, H.J.; Mort, M.; Cooper, D.N.; Sebat, J.; Iakoucheva, L.M.; et al. Inferring the molecular and phenotypic impact of amino acid variants with MutPred2. Nat. Commun. 2020, 11, 5918. [Google Scholar] [CrossRef]
- Johnsen, J. Von Willebrand Disease. In GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 2009. [Google Scholar]
- Meadows, J.R.S.; Kidd, J.M.; Wang, G.D.; Parker, H.G.; Schall, P.Z.; Bianchi, M.; Christmas, M.J.; Bougiouri, K.; Buckley, R.M.; Hitte, C.; et al. Genome sequencing of 2000 canids by the Dog10K consortium advances the understanding of demography, genome function and architecture. Genome Biol. 2023, 24, 187. [Google Scholar] [CrossRef]
- Baker, M.J.; Crameri, J.J.; Thorburn, D.R.; Frazier, A.E.; Stojanovski, D. Mitochondrial biology and dysfunction in secondary mitochondrial disease. Open Biol. 2022, 12, 220274. [Google Scholar] [CrossRef]
- Key, J.; Gispert, S.; Auburger, G. Knockout Mouse Studies Show That Mitochondrial CLPP Peptidase and CLPX Unfoldase Act in Matrix Condensates near IMM, as Fast Stress Response in Protein Assemblies for Transcript Processing, Translation, and Heme Production. Genes 2024, 15, 694. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Sauer, R.T. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim. Biophys. Acta 2012, 1823, 15–28. [Google Scholar] [CrossRef]
- Guo, C.; Xiao, Y.; Gu, J.; Zhao, P.; Hu, Z.; Zheng, J.; Hua, R.; Hai, Z.; Su, J.; Zhang, J.V.; et al. ClpP/ClpX deficiency impairs mitochondrial functions and mTORC1 signaling during spermatogenesis. Commun. Biol. 2023, 6, 1012. [Google Scholar] [CrossRef]
- Kardon, J.R.; Yien, Y.Y.; Huston, N.C.; Branco, D.S.; Hildick-Smith, G.J.; Rhee, K.Y.; Paw, B.H.; Baker, T.A. Mitochondrial ClpX Activates a Key Enzyme for Heme Biosynthesis and Erythropoiesis. Cell 2015, 161, 858–867. [Google Scholar] [CrossRef]
- Rondelli, C.M.; Perfetto, M.; Danoff, A.; Bergonia, H.; Gillis, S.; O’Neill, L.; Jackson, L.; Nicolas, G.; Puy, H.; West, R.; et al. The ubiquitous mitochondrial protein unfoldase CLPX regulates erythroid heme synthesis by control of iron utilization and heme synthesis enzyme activation and turnover. J. Biol. Chem. 2021, 297, 100972. [Google Scholar] [CrossRef]
- Whitman, J.C.; Paw, B.H.; Chung, J. The role of ClpX in erythropoietic protoporphyria. Hematol. Transfus. Cell Ther. 2018, 40, 182–188. [Google Scholar] [CrossRef]
- Lee, S.; Sowa, M.E.; Watanabe, Y.H.; Sigler, P.B.; Chiu, W.; Yoshida, M.; Tsai, F.T. The structure of ClpB: A molecular chaperone that rescues proteins from an aggregated state. Cell 2003, 115, 229–240. [Google Scholar] [CrossRef]
- Jenkinson, E.M.; Rehman, A.U.; Walsh, T.; Clayton-Smith, J.; Lee, K.; Morell, R.J.; Drummond, M.C.; Khan, S.N.; Naeem, M.A.; Rauf, B.; et al. Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am. J. Hum. Genet. 2013, 92, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, D.; Lazzaro, F.; Brusco, A.; Plumari, M.; Battaglia, G.; Pastore, A.; Finardi, A.; Cagnoli, C.; Tempia, F.; Frontali, M.; et al. Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28. Nat. Genet. 2010, 42, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, M.; Jagannathan, V.; Laurent, N.; Noblet, E.; Dutil, G.F.; Troupel, T.; de Dufaure de Citres, C.; Gache, V.; Blot, S.; Escriou, C.; et al. A PNPLA8 frameshift variant in Australian shepherd dogs with hereditary ataxia. Anim. Genet. 2022, 53, 709–712. [Google Scholar] [CrossRef]
- Christen, M.; Rupp, S.; Van Soens, I.; Bhatti, S.F.M.; Matiasek, K.; von Klopmann, T.; Jagannathan, V.; Madden, I.; Batcher, K.; Bannasch, D.; et al. SLC25A12 Missense Variant in Nova Scotia Duck Tolling Retrievers Affected by Cerebellar Degeneration-Myositis Complex (CDMC). Genes 2022, 13, 1223. [Google Scholar] [CrossRef]
- Zeng, R.; Guo, J.; Bullock, G.; Johnson, G.S.; Katz, M.L. Canine Multiple System Degeneration Associated with Sequence Variants in SERAC1. Genes 2024, 15, 1378. [Google Scholar] [CrossRef]
- Lopriore, P.; Ricciarini, V.; Siciliano, G.; Mancuso, M.; Montano, V. Mitochondrial Ataxias: Molecular Classification and Clinical Heterogeneity. Neurol. Int. 2022, 14, 337–356. [Google Scholar] [CrossRef]
- Michalik, A.; Martin, J.J.; Van Broeckhoven, C. Spinocerebellar ataxia type 7 associated with pigmentary retinal dystrophy. Eur. J. Hum. Genet. 2004, 12, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Joo, K.; Woo, S.J. Ophthalmic Manifestations and Genetics of the Polyglutamine Autosomal Dominant Spinocerebellar Ataxias: A Review. Front. Neurosci. 2020, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Beaudin, M.; Matilla-Dueñas, A.; Soong, B.W.; Pedroso, J.L.; Barsottini, O.G.; Mitoma, H.; Tsuji, S.; Schmahmann, J.D.; Manto, M.; Rouleau, G.A.; et al. The Classification of Autosomal Recessive Cerebellar Ataxias: A Consensus Statement from the Society for Research on the Cerebellum and Ataxias Task Force. Cerebellum 2019, 18, 1098–1125. [Google Scholar] [CrossRef]
- Chen, B.S.; Harvey, J.P.; Gilhooley, M.J.; Jurkute, N.; Yu-Wai-Man, P. Mitochondria and the eye-manifestations of mitochondrial diseases and their management. Eye 2023, 37, 2416–2425. [Google Scholar] [CrossRef]
- Evans, J.; Katz, M.L.; Levesque, D.; Shelton, G.D.; de Lahunta, A.; O’Brien, D. A variant form of neuronal ceroid lipofuscinosis in American bulldogs. J. Vet. Intern. Med. 2005, 19, 44–51. [Google Scholar] [CrossRef]
- Saunders, G.K.; Wood, P.A.; Myers, R.K.; Shell, L.G.; Carithers, R. GM1 gangliosidosis in Portuguese water dogs: Pathologic and biochemical findings. Vet. Pathol. 1988, 25, 265–269. [Google Scholar] [CrossRef]
- Assenmacher, T.D.; Jutkowitz, L.A.; Koenigshof, A.M.; de Lucidi, C.A.; Scott, M.A. Clinical features of precursor-targeted immune-mediated anemia in dogs: 66 cases (2004–2013). J. Am. Vet. Med. Assoc. 2019, 255, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Camaschella, C. Hereditary sideroblastic anemias: Pathophysiology, diagnosis, and treatment. Semin. Hematol. 2009, 46, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shi, D.; Yang, S.; Lian, Y.; Li, H.; Cao, M.; He, Y.; Zhang, L.; Qiu, C.; Liu, T.; et al. Mitochondrial tRNA pseudouridylation governs erythropoiesis. Blood 2024, 144, 657–671. [Google Scholar] [CrossRef]
- Yoshimi, A.; Ishikawa, K.; Niemeyer, C.; Grünert, S.C. Pearson syndrome: A multisystem mitochondrial disease with bone marrow failure. Orphanet J. Rare Dis. 2022, 17, 379. [Google Scholar] [CrossRef]
- Yien, Y.Y.; Ducamp, S.; van der Vorm, L.N.; Kardon, J.R.; Manceau, H.; Kannengiesser, C.; Bergonia, H.A.; Kafina, M.D.; Karim, Z.; Gouya, L.; et al. Mutation in human CLPX elevates levels of δ-aminolevulinate synthase and protoporphyrin IX to promote erythropoietic protoporphyria. Proc. Natl. Acad. Sci. USA 2017, 114, E8045–E8052. [Google Scholar] [CrossRef]
- Ahsan, H.; Ali, A.; Ali, R. Oxygen free radicals and systemic autoimmunity. Clin. Exp. Immunol. 2003, 131, 398–404. [Google Scholar] [CrossRef]
- Di Dalmazi, G.; Hirshberg, J.; Lyle, D.; Freij, J.B.; Caturegli, P. Reactive oxygen species in organ-specific autoimmunity. Autoimmun. Highlights 2016, 7, 11. [Google Scholar] [CrossRef]
- Fujii, J.; Kurahashi, T.; Konno, T.; Homma, T.; Iuchi, Y. Oxidative stress as a potential causal factor for autoimmune hemolytic anemia and systemic lupus erythematosus. World J. Nephrol. 2015, 4, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Cheong, A.; Archambault, D.; Degani, R.; Iverson, E.; Tremblay, K.D.; Mager, J. Nuclear-encoded mitochondrial ribosomal proteins are required to initiate gastrulation. Development 2020, 147, dev188714. [Google Scholar] [CrossRef]
- Voith, V.L.; Ingram, E.; Mitsouras, K.; Irizarry, K. Comparison of adoption agency breed identification and DNA breed identification of dogs. J. Appl. Anim. Welf. Sci. 2009, 12, 253–262. [Google Scholar] [CrossRef]
- Voith, V.L.; Trevejo, R.; Dowling-Guyer, S.; Chadik, C.; Marder, A.; Johnson, V.; Irizarry, K. Comparison of visual and DNA breed identification of dogs and inter-observer reliability. Am. J. Sociol. Res. 2013, 3, 1729. [Google Scholar]
- Key, J.; Torres-Odio, S.; Bach, N.C.; Gispert, S.; Koepf, G.; Reichlmeir, M.; West, A.P.; Prokisch, H.; Freisinger, P.; Newman, W.G.; et al. Inactivity of Peptidase ClpP Causes Primary Accumulation of Mitochondrial Disaggregase ClpX with Its Interacting Nucleoid Proteins, and of mtDNA. Cells 2021, 10, 3354. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Kitagawa, Y.; Aihara, M.; Ohki, Y.; Furuyama, K.; Hirokawa, T. Heme-dependent recognition of 5-aminolevulinate synthase by the human mitochondrial molecular chaperone ClpX. FEBS Lett. 2021, 595, 3019–3029. [Google Scholar] [CrossRef] [PubMed]


| Filtering Step | Homozygous Variants | Heterozygous Variants |
|---|---|---|
| All variants compared to reference | 2,586,116 | 3,213,087 |
| Private variants compared to 748 control canine genomes | 3864 | 26,911 |
| Predicted protein-changing private variants | 17 | 175 |
| Located in functional candidate genes for similar phenotypes in other species, with VarElect score ≥ 10 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Cecco, B.S.; Blake, J.M.; Kim, N.J.; Coffey, M.C.; Johnston, A.N.; Miller, A.D.; Ekenstedt, K.J.; Lee, J. Identification of a Novel CLPX Variant in a Mixed-Breed Dog with Anemia and Spinocerebellar Ataxia. Genes 2025, 16, 1359. https://doi.org/10.3390/genes16111359
de Cecco BS, Blake JM, Kim NJ, Coffey MC, Johnston AN, Miller AD, Ekenstedt KJ, Lee J. Identification of a Novel CLPX Variant in a Mixed-Breed Dog with Anemia and Spinocerebellar Ataxia. Genes. 2025; 16(11):1359. https://doi.org/10.3390/genes16111359
Chicago/Turabian Stylede Cecco, Bianca S., Jeanna M. Blake, Namju J. Kim, Madeline C. Coffey, Andrea N. Johnston, Andrew D. Miller, Kari J. Ekenstedt, and Jeongha Lee. 2025. "Identification of a Novel CLPX Variant in a Mixed-Breed Dog with Anemia and Spinocerebellar Ataxia" Genes 16, no. 11: 1359. https://doi.org/10.3390/genes16111359
APA Stylede Cecco, B. S., Blake, J. M., Kim, N. J., Coffey, M. C., Johnston, A. N., Miller, A. D., Ekenstedt, K. J., & Lee, J. (2025). Identification of a Novel CLPX Variant in a Mixed-Breed Dog with Anemia and Spinocerebellar Ataxia. Genes, 16(11), 1359. https://doi.org/10.3390/genes16111359







