Recent Advances in Pepper Fruit Glossiness
Abstract
1. Introduction
2. Pepper Fruit Surface Glossiness
2.1. Definition of Glossiness
2.2. Methods for Measuring Glossiness
2.3. Factors Affecting Glossiness
2.3.1. Surface Appendages
2.3.2. Wax and Cuticle
2.3.3. Fruit Pigment Accumulation
3. Molecular Regulatory Mechanism of Pepper Fruit Glossiness
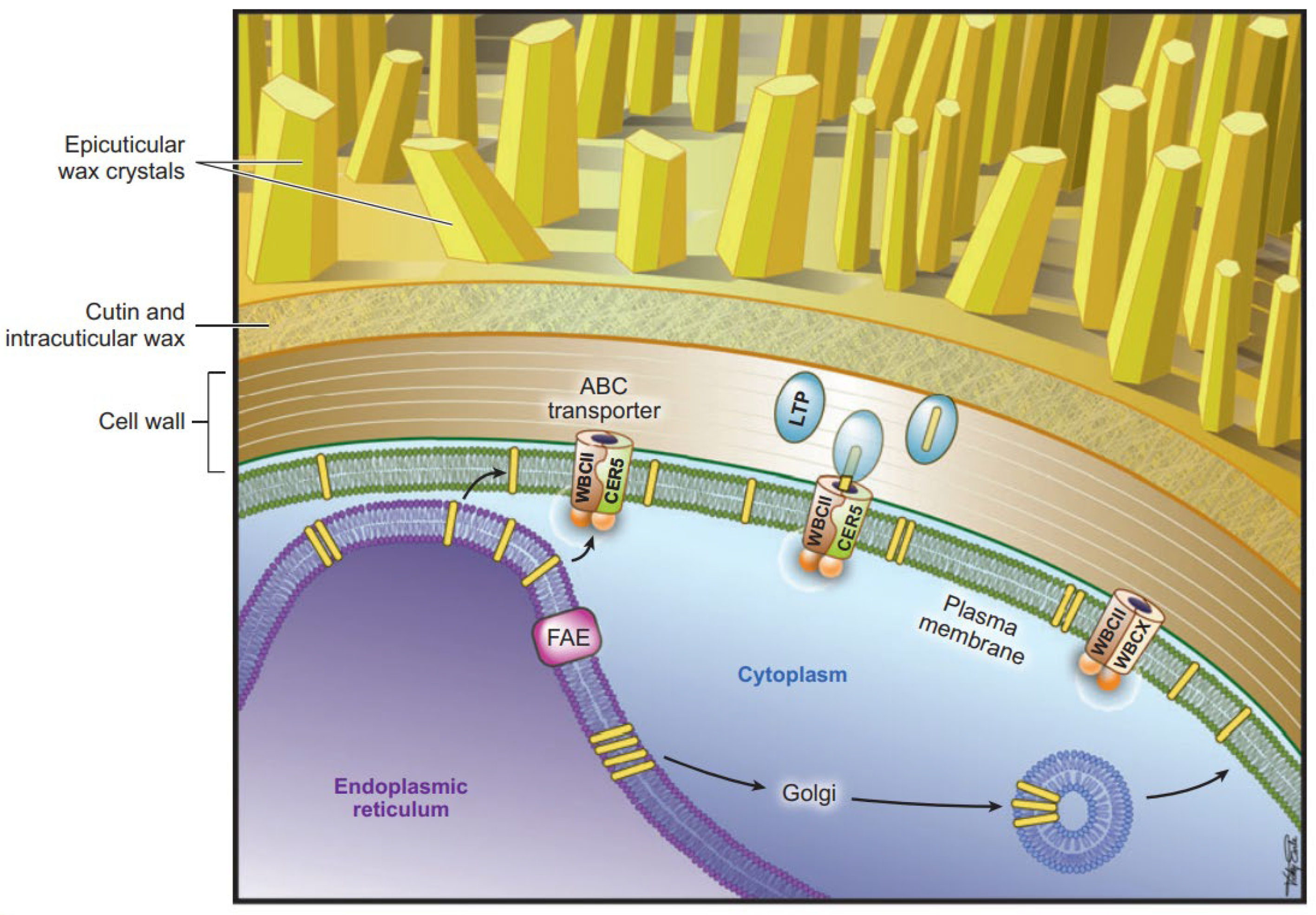
3.1. Biosynthesis and Transport of Wax and Cutin
3.1.1. Biosynthesis and Transport of Cutin
- (1)
- Under the catalysis of fatty acid synthase (FAS), acetyl-CoA and malonyl-acyl carrier protein (malonyl-ACP) undergo repeated condensation and elongation to form C16 or C18 fatty acyl-ACPs. These are subsequently hydrolyzed by fatty acyl-ACP thioesterase (FAT) to yield free C16 or C18 fatty acids [62].
- (2)
- Fatty acids are initially activated by long-chain acyl-CoA synthetases (LACSs) to form fatty acyl-CoAs. These activated intermediates can then be oxidized by cytochrome P450 monooxygenases, specifically CYP86A and CYP77A. CYP86A catalyzes hydroxylation at the terminal carbon, whereas CYP77A mediates mid-chain hydroxylation [63].
- (3)
- Glycerol-3-phosphate acyltransferases (GPATs) catalyze the transfer of an acyl group from acyl-CoA to glycerol-3-phosphate, yielding monoacylglycerol. The typical end product of cutin biosynthesis is 2-monoacylglycerol; however, in the presence of 10,16-dihydroxyhexadecanoic acid, the end product is 2-hydroxy-hexadecanoic monoacylglycerol (2-MHG) [64]. Among cutin monomers in pepper fruit, 10,16-dihydroxyhexadecanoic acid is the most abundant and is likely a fundamental unit for the assembly of cutin polyesters [40].
3.1.2. Biosynthesis and Transport of Wax
3.2. Regulatory Genes of Wax and Cutin
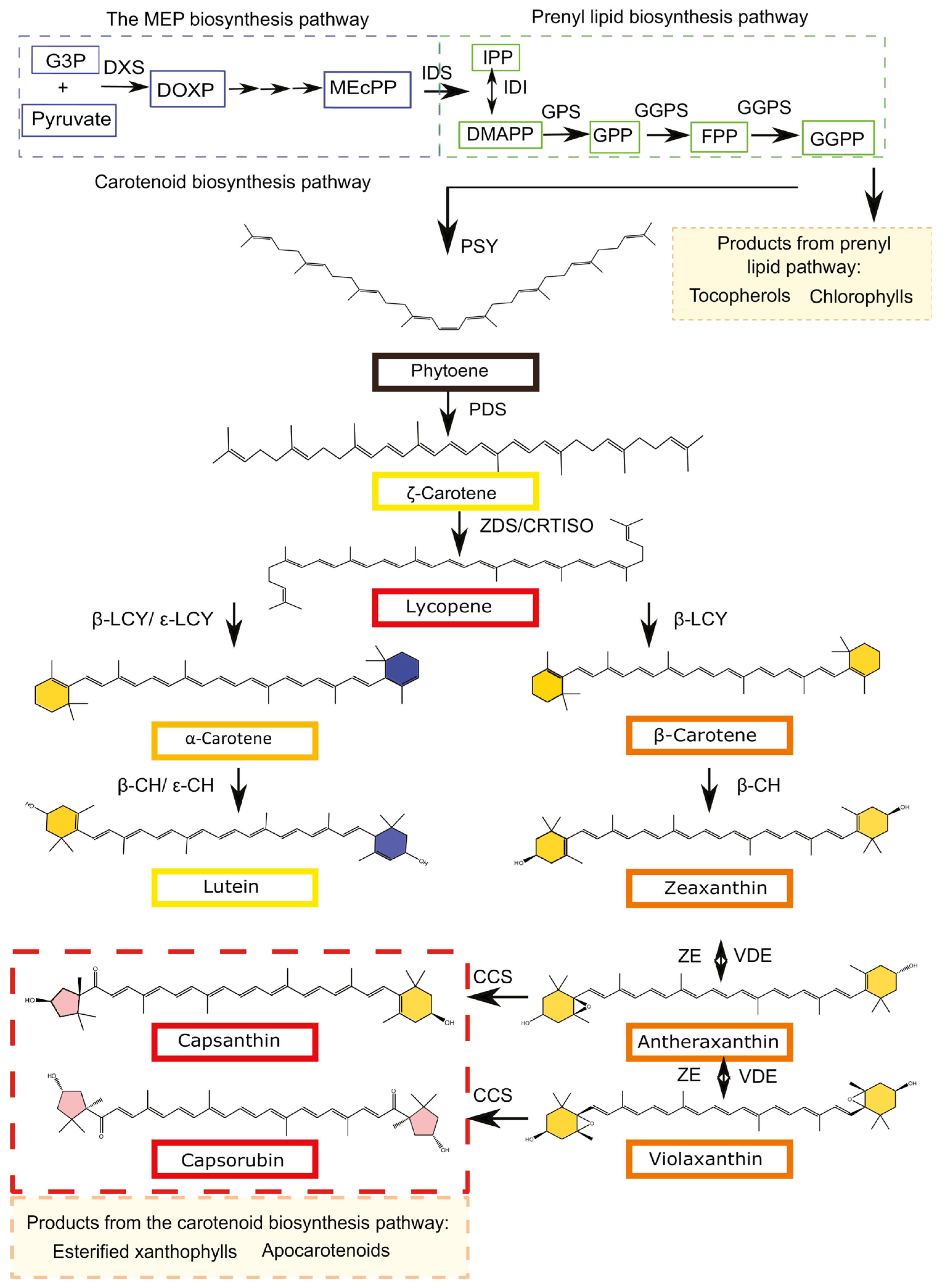
3.3. Carotenoids Biosynthesis and Transport
3.4. Regulatory Genes of Carotenoids
4. Pepper Fruit Glossiness After Harvest
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VLCFAs | very-long-chain fatty acids |
| ROS | reactive oxygen species |
| LOXs | lipoxygenases |
| DXS | 1-deoxy-d-xylulose-5-phosphate synthase |
| MEP | methylerythritol phosphate |
| IPP | isopentenyl diphosphate |
| GPP | geranyl pyrophosphate |
| GGPS | geranylgeranyl pyrophosphate synthase |
| GGPP | geranylgeranyl pyrophosphate |
| PSY | phytoene synthase |
| PDS | Phytoene desaturase |
| ZDS | ζ-carotene desaturase |
References
- Zou, X.X.; Ma, Y.Q.; Dai, X.Z.; Li, X.F.; Yang, S. Spread and Industry Development of Pepper in China. Acta Hortic. Sin. 2020, 47, 1715–1726. [Google Scholar]
- Barboza, G.E.; García, C.C.; de Bem Bianchetti, L.; Romero, M.V.; Scaldaferro, M. Monograph of wild and cultivated chili peppers (Capsicum L., Solanaceae). PhytoKeys. 2022, 200, 1–143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.; Lai, Z.P.; Dong, J.C.; Tan, C.; Wu, Z.M.; Liao, Y.; Chen, C.M.; Song, Z.; Chen, M.X.; Cui, J.J.; et al. Research Progress in Genomics of Pepper (Capsicum spp.). Guangdong Agric. Sci. 2024, 51, 1–12. [Google Scholar]
- Blanke, M. Loss of Gloss: A Fresh Look at Freshness. In Recent Advances in Postharvest Technologies; Springer Nature: Cham, Switzerland, 2024; pp. 1–11. [Google Scholar]
- Mangal, M.; Srivastava, A.; Tomar, B.S. Genetic and molecular regulation of colour and pungency in Hot pepper (Capsicum spp.): A review. Indian J. Agric. Sci. 2018, 88, 343–353. [Google Scholar] [CrossRef]
- Liang, B.; Sun, Y.; Wang, J.; Zheng, Y.; Zhang, W.; Xu, Y.; Li, Q.; Leng, P. Tomato protein phosphatase 2C influences the onset of fruit ripening and fruit glossiness. J. Exp. Bot. 2021, 72, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, P.; Lu, S.; Ou-Yang, Q.; Zhu-Ge, Y.; Tian, R.; Jia, H.; Fang, J. Comparative analysis of cuticular wax in various grape cultivars during berry development and after storage. Front. Nutr. 2021, 8, 817796. [Google Scholar] [CrossRef]
- Gao, L.; Cao, J.; Gong, S.; Hao, N.; Du, Y.; Wang, C.; Wu, T. The COPII subunit CsSEC23 mediates fruit glossiness in cucumber. Plant J. 2023, 116, 524–540. [Google Scholar] [CrossRef]
- Liu, X.; Ge, X.; An, J.; Liu, X.; Ren, H. CsCER6 and CsCER7 influence fruit glossiness by regulating fruit cuticular wax accumulation in cucumber. Int. J. Mol. Sci. 2023, 24, 1135. [Google Scholar] [CrossRef]
- Yang, H.; Mei, W.; Wan, H.; Xu, R.; Cheng, Y. Comprehensive analysis of KCS gene family in Citrinae reveals the involvement of CsKCS2 and CsKCS11 in fruit cuticular wax synthesis at ripening. Plant Sci. 2021, 310, 110972. [Google Scholar] [CrossRef]
- Wang, H.; Nie, Z.; Wang, T.; Yang, S.; Zheng, J. Comparative transcriptome analysis of eggplant (Solanum melongena L.) peels with different glossiness. Agronomy 2024, 14, 3063. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Li, Y.; He, H.; Bie, B.; Ren, G.; Zhao, J.; Wang, Y.; Nie, J.; Pan, J.; et al. High-resolution mapping of the dull fruit skin gene D in cucumber (Cucumis sativus L.). Mol. Breed. 2014, 33, 15–22. [Google Scholar] [CrossRef]
- Zhai, X.; Wu, H.; Wang, Y.; Zhang, Z.; Shan, L.; Zhao, X.; Wang, R.; Liu, C.; Weng, Y.; Wang, Y.; et al. The fruit glossiness locus, dull fruit (D), encodes a C2H2-type zinc finger transcription factor, CsDULL, in cucumber (Cucumis sativus L.). Hortic. Res. 2022, 9, uhac146. [Google Scholar] [CrossRef]
- Hao, Y.; Luo, H.; Wang, Z.; Lu, C.; Ye, X.; Wang, H.; Miao, L. Research progress on the mechanisms of fruit glossiness in cucumber. Gene 2024, 927, 148626. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, J.; Zhang, J.; Song, L.; Wang, Y.; Zhao, L.; Chen, J.; Brotman, Y.; Zhao, T. Exploring the sheen: A review of research advances on fruit glossiness. Front. Plant Sci. 2025, 16, 1629039. [Google Scholar] [CrossRef]
- Honson, V.; Huynh-Thu, Q.; Arnison, M.; Monaghan, D.; Isherwood, Z.J.; Kim, J. Effects of shape, roughness and gloss on the perceived reflectance of colored surfaces. Front. Psychol. 2020, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Determination of the Specular Glossiness of Cowhorn/Sheephorn Pepper—Glossmeter Test Method. Guangxi Nanning, China. 2025. Available online: https://www.ttbz.org.cn/Home/Standard?searchType=3&key=%E5%85%89%E6%B3%BD%E5%BA%A6 (accessed on 8 July 2025).
- Zhou, R.; Zhao, T.M.; Zhao, L.P.; Wang, Y.L.; Song, L.X.; Yu, W.G. High-glossiness tomato breeding and fruit surface glossiness. Jiangsu J. Agric. Sci. 2018, 34, 1437–1440. [Google Scholar]
- Mendoza, F.; Dejmek, P.; Aguilera, J.M. Gloss measurements of raw agricultural products using image analysis. Food Res. Int. 2010, 43, 18–25. [Google Scholar] [CrossRef]
- Althaus, B.; Blanke, M. Non-destructive, opto-electronic determination of the freshness and shrivel of Bell pepper fruits. J. Imaging 2020, 6, 122. [Google Scholar] [CrossRef]
- Kasampalis, D.S.; Tsouvaltzis, P.; Ntouros, K.; Gertsis, A.; Gitas, I.; Siomos, A.S. The use of digital imaging, chlorophyll fluorescence and Vis/NIR spectroscopy in assessing the ripening stage and freshness status of bell pepper fruit. Comput. Electron. Agric. 2021, 187, 106265. [Google Scholar] [CrossRef]
- Wang, L.Q.; Dai, X.Z. Application of colorimeter for testing its color change during the development of hot pepper (Capsicum annuum L.) fruit. J. China Capsicum 2009, 3, 23–26. [Google Scholar]
- Li, L.; Ding, W.K. Chili recognition based on convolution neural network. J. Tianjin Univ. Technol. 2017, 33, 12–15. [Google Scholar]
- Trivedi, P.; Nguyen, N.; Klavins, L.; Kviesis, J.; Heinonen, E.; Remes, J.; Jokipii-Lukkari, S.; Klavins, M.; Karppinen, K.; Jaakola, L.; et al. Analysis of composition, morphology, and biosynthesis of cuticular wax in wild type bilberry (Vaccinium myrtillus L.) and its glossy mutant. Food Chem. 2021, 354, 129517. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. Development of Hairy Gene Markers and Screening of Tomato Elite Inbred Lines. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar]
- Hao, N.; Yao, H.; Suzuki, M.; Li, B.; Wang, C.; Cao, J.; Fujiwara, T.; Wu, T.; Kamiya, T. Novel lignin-based extracellular barrier in glandular trichome. Nat. Plants 2024, 10, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gebretsadik, K.; Qiu, X.; Dong, S.; Miao, H.; Bo, K. Molecular research progress and improvement approach of fruit quality traits in cucumber. Theor. Appl. Genet. 2021, 134, 3535–3552. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Inoue, S.; Igarashi, Y.; Sato, H.; Mizokami, Y. Analysis of Gloss Unevenness and Bidirectional Reflectance Distribution Function in Specular Reflection. J. Imaging 2024, 10, 146. [Google Scholar] [CrossRef]
- Shen, Y.; Mao, L.; Zhou, Y.; Sun, Y.; Lv, J.; Deng, M.; Liu, Z.; Yang, B. Transcriptome analysis reveals key genes involved in trichome formation in pepper (Capsicum annuum L.). Plants 2024, 13, 1090. [Google Scholar] [CrossRef]
- Liu, X.D.; Yan, J.R.; Wang, P.Y.; Zhang, J.Y.; Shen, H.Y. Genetic Inheritance and Gene Mapping of Pepper Trichomes Traits. China Veg. 2015, 12, 19–24. [Google Scholar]
- Ma, D.H.; Pang, J.A.; Wen, X.G.; Li, S.J.; Huo, Z.R.; Lin, S.Q. Study on Characteristic of Glabrous Cucumber (Cucumis sativus L.). Acta Hortic. Sin. 2002, 3, 282–284. [Google Scholar]
- Kim, H.J.; Han, J.H.; Kwon, J.K.; Park, M.; Kim, B.D.; Choi, D. Fine mapping of pepper trichome locus 1 controlling trichome formation in Capsicum annuum L. CM334. Theor. Appl. Genet. 2010, 120, 1099–1106. [Google Scholar] [CrossRef]
- Jeffree, C.E. The fine structure of the plant cuticle. Biol. Plant Cuticle 2006, 23, 11–125. [Google Scholar]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Bourgault, R.; Matschi, S.; Vasquez, M.; Qiao, P.; Sonntag, A.; Charlebois, C.; Mohammadi, M.; Scanlon, M.J.; Smith, L.G.; Molina, I. Constructing functional cuticles: Analysis of relationships between cuticle lipid composition, ultrastructure and water barrier function in developing adult maize leaves. Ann. Bot. 2020, 125, 79–91. [Google Scholar] [CrossRef]
- Petit, J.; Bres, C.; Mauxion, J.P.; Bakan, B.; Rothan, C. Breeding for cuticle-associated traits in crop species: Traits, targets, and strategies. J. Exp. Bot. 2017, 68, 5369–5387. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Biopolyester membranes of plants: Cutin and suberin. Science 1980, 208, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Koch, K.; Ensikat, H.J. The hydrophobic coatings of plant surfaces: Epicuticular wax crystals and their morphologies, crystallinity and molecular self-assembly. Micron 2008, 39, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Qin, K.; Ding, S.; Yang, J.; Jiang, L.; Qin, Y.; Wang, R. Gas chromatography–mass spectrometry metabolite analysis combined with transcriptomic and proteomic provide new insights into revealing cuticle formation during pepper development. J. Agric. Food Chem. 2022, 70, 12383–12397. [Google Scholar] [CrossRef]
- De Rijke, E.; Fellner, C.; Westerveld, J.; Lopatka, M.; Cerli, C.; Kalbitz, K.; De Koster, C.G. Determination of n-alkanes in C. annuum (bell pepper) fruit and seed using GC-MS: Comparison of extraction methods and application to samples of different geographical origin. Anal. Bioanal. Chem. 2015, 407, 5729–5738. [Google Scholar] [CrossRef]
- Ikedou, K.; Yamamoto, H.; Nagashima, H.; Nemoto, N.; Tashiro, K. Crystal structures of n-alkanes with branches of different size in the middle. J. Phys. Chem. B 2005, 109, 10668–10675. [Google Scholar] [CrossRef]
- Cai, Y.; Kiyokawa, H.; Nagai, T.; Haghzare, L.; Arnison, M.; Kim, J. Effects of specular roughness on the perception of color and opacity. J. Opt. Soc. Am. A 2023, 40, A220–A229. [Google Scholar] [CrossRef]
- Natarajan, P.; Akinmoju, T.A.; Nimmakayala, P.; Lopez-Ortiz, C.; Garcia-Lozano, M.; Thompson, B.J.; Stommel, J.; Reddy, U.K. Integrated metabolomic and transcriptomic analysis to characterize cutin biosynthesis between low-and high-cutin genotypes of Capsicum chinense Jacq. Int. J. Mol. Sci. 2020, 21, 1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lv, J.; Liu, Z.; Wang, J.; Yang, B.; Chen, W.; Qu, L.; Dai, X.; Zhang, Z.; Zou, X. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in pepper fruit (Capsicum annuum L.). Food Chem. 2020, 306, 125629. [Google Scholar] [CrossRef]
- D’Souza, M.C.; Singha, S.; Ingle, M. Lycopene concentration of tomato fruit can be estimated from chromaticity values. HortScience 1992, 27, 465–466. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Cao, Y.C.; Yu, H.L.; Wang, L.H.; Zhang, B.X. Genetic control and metabolite composition of fruit quality in Capsicum. Acta Hortic. Sin. 2019, 46, 1825–1841. [Google Scholar]
- Li, L.Y.; Wu, L.L.; Huang, H.R.; Bozhi, Y.A.N.G.; Shudong, Z.H.O.U. Comparative Color and Quality Analyses of Color Mutants and Wild-Type of Pepper at Different Developmental Stages. Food Sci. 2024, 45, 16–21. [Google Scholar]
- Guo, Y.M.; Duan, X.D.; Bai, J.J.; Wang, J.E. Extraction of anthocyanin from Ormamental Pepper fruits and correlation analysis of fruit color. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2021, 41, 48–58. [Google Scholar]
- Berry, H.M.; Rickett, D.V.; Baxter, C.J.; Enfissi, E.M.; Fraser, P.D. Carotenoid biosynthesis and sequestration in red chilli pepper fruit and its impact on colour intensity traits. J. Exp. Bot. 2019, 70, 2637–2650. [Google Scholar] [CrossRef]
- He, J.Y.; An, Y.; Li, J.X.; Xian, J.Q.; Zhou, D.F.; Wang, J.E. Fruit Color Phenotype and Pigment Accumulation Characteristics of Chili Peppers (Capsicum annuum L.). Acta Agric. Boreali-Occident. Sin. 2025, 34, 250–259. [Google Scholar]
- Tao, X.L.; Zhu, H.X.; Zhang, Y.X.; Liu, M.X. Quality Characteristics and Correlation Analysis of Processed Peppers Based on Principal Component Analysis and Cluster Analysis. Food Res. Dev. 2024, 45, 148–156. [Google Scholar]
- Gong, X.F.; Chen, X.; Li, H.; Xu, Y.; Song, Z.F. Non-destructive quality detection method and canonical correlation analysis of processed pepper. Jiangsu Agric. Sci. 2022, 50, 170–176. [Google Scholar]
- Dong, S.C.; Zhang, J.W.; Ling, J.Y.; Hong, J.; Xie, Z.X.; Zhang, S.J.; Song, L.X.; Wang, Y.L.; Zhao, T.M.; Zhao, L.P. Evaluation of fruit glossiness in tomato population and genome-wide association analysis. Jiangsu Agric. Sci. 2024, 52, 36–41. [Google Scholar]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of plant cuticular waxes. In Annual Plant Reviews Volume 23: Biology of the Plant Cuticle; Wiley-Blackwell: Hoboken, NJ, USA, 2006; Volume 23, pp. 145–181. [Google Scholar] [CrossRef]
- Samuels, L.; DeBono, A.; Lam, P.; Wen, M.; Jetter, R.; Kunst, L. Use of Arabidopsis eceriferum mutants to explore plant cuticle biosynthesis. J. Vis. Exp. JoVE 2008, 16, 709. [Google Scholar]
- Beisson, F.; Koo, A.J.; Ruuska, S.; Schwender, J.; Pollard, M.; Thelen, J.J.; Paddock, T.; Salas, J.J.; Savage, L.; Milcamps, A.; et al. Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol. 2003, 132, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.; et al. Acyl-lipid metabolism. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0161. [Google Scholar] [CrossRef]
- Samuels, L.; Jetter, R.; Kunst, L. First steps in understanding the export of lipids to the plant cuticle. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2005, 139, 65–68. [Google Scholar] [CrossRef]
- Kurdyukov, S.; Faust, A.; Nawrath, C.; Bar, S.; Voisin, D.; Efremova, N.; Franke, R.; Schreiber, L.; Saedler, H.; Meétraux, J.; et al. The epidermis-specific extracellular BODYGUARD controls cuticle development and morphogenesis in Arabidopsis. Plant Cell 2006, 18, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Martin, L.B.B.; Viart, H.M.F.; Isaacson, T.; He, Y.; Zhao, L.; Matas, A.J.; Buda, G.J.; Domozych, D.S.; Clausen, M.H.; et al. The identification of cutin synthase: Formation of the plant polyester cutin. Nat. Chem. Biol. 2012, 8, 609–611. [Google Scholar] [CrossRef]
- García-Coronado, H.; Tafolla-Arellano, J.C.; Hernández-Oñate, M.Á.; Burgara-Estrella, A.J.; Robles-Parra, J.M.; Tiznado-Hernández, M.E. Molecular biology, composition and physiological functions of cuticle lipids in fleshy fruits. Plants 2022, 11, 1133. [Google Scholar] [CrossRef]
- Fich, E.A.; Segerson, N.A.; Rose, J. The plant polyester cutin: Biosynthesis, structure, and biological roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef]
- Duan, R.J.; Wang, A.D.; Chen, G.X. Advances in Study of Plant Cuticle Genes. Chin. Bull. Bot. 2017, 52, 637–651. [Google Scholar]
- Borisjuk, N.; Hrmova, M.; Lopato, S. Transcriptional regulation of cuticle biosynthesis. Biotechnol. Adv. 2014, 32, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Suh, M.C. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; You, C.X.; Li, Y.Y.; Hao, Y.J. Advances in biosynthesis, regulation, and function of apple cuticular wax. Front. Plant Sci. 2020, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A.; Clemens, S.; Zachgo, S.; Giblin, E.M.; Taylor, D.C.; Kunst, L. CUT1, an Arabidopsis gene required for cuticular wax biosynthesis and pollen fertility, encodes a very-long-chain fatty acid condensing enzyme. Plant Cell 1999, 11, 825–838. [Google Scholar] [CrossRef]
- Alexander, L.E.; Gilbertson, J.S.; Xie, B.; Song, Z.; Nikolau, B.J. High spatial resolution imaging of the dynamics of cuticular lipid deposition during Arabidopsis flower development. Plant Direct 2021, 5, e00322. [Google Scholar] [CrossRef]
- Ni, F.; Yang, M.; Chen, J.; Guo, Y.; Wan, S.; Zhao, Z.; Yang, S.; Kong, L.; Chu, P.; Guan, R. BnUC1 is a key regulator of epidermal wax biosynthesis and lipid transport in Brassica napus. Int. J. Mol. Sci. 2024, 25, 9533. [Google Scholar] [CrossRef]
- Chen, X.; Goodwin, S.M.; Boroff, V.L.; Liu, X.; Jenks, M.A. Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 2003, 15, 1170–1185. [Google Scholar] [CrossRef]
- Yephremov, A.; Wisman, E.; Huijser, P.; Huijser, C.; Wellesen, K.; Saedler, H. Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 1999, 11, 2187–2201. [Google Scholar] [CrossRef]
- Schnurr, J.; Shockey, J.; Browse, J. The acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 2004, 16, 629–642. [Google Scholar] [CrossRef]
- Yi, T. Cuticle Composition Analysis and Transcriptome Study of Pepper Keratin Deficiency Mutant “Pcd1”. Master’s Thesis, Hunan University, Changsha, China, 2020. [Google Scholar]
- Wang, J.; Shan, Q.; Yi, T.; Ma, Y.; Zhou, X.; Pan, L.; Miao, W.; Zou, X.; Xiong, C.; Liu, F. Fine mapping and candidate gene analysis of CaFCD1 affecting cuticle biosynthesis in Capsicum annuum L. Theor. Appl. Genet. 2023, 136, 46. [Google Scholar] [CrossRef]
- Guzman, I.; Hamby, S.; Romero, J.; Bosland, P.W.; O’Connell, M.A. Variability of carotenoid biosynthesis in orange colored Capsicum spp. Plant Sci. 2010, 179, 49–59. [Google Scholar] [CrossRef]
- del Rocío Gómez-García, M.; Ochoa-Alejo, N. Biochemistry and molecular biology of carotenoid biosynthesis in chili peppers (Capsicum spp.). Int. J. Mol. Sci. 2013, 14, 19025–19053. [Google Scholar] [CrossRef] [PubMed]
- Filyushin, M.A.; Dyachenko, E.A.; Efremov, G.I.; Kochieva, E.Z.; Shchennikova, A.V. Variability and expression pattern of phytoene synthase (PSY) paralogs in pepper species. Russ. J. Genet. 2021, 57, 282–296. [Google Scholar] [CrossRef]
- Berry, H. Elucidation of the Molecular and Biochemical Mechanisms Associated with Colour Intensity and Colour Retention in Fresh and Dry Chilli Peppers. Ph.D. Thesis, University of London, Egham, UK, 2015. [Google Scholar]
- Xue, Q.; Zhang, Q.; Zhang, A.; Li, D.; Liu, Y.; Xu, H.; Yang, Q.; Liu, F.; Han, T.; Tang, X.; et al. Integrated metabolome and transcriptome analysis provides clues to fruit color formation of yellow, orange, and red bell pepper. Sci. Rep. 2024, 14, 29737. [Google Scholar] [CrossRef]
- Tian, S.L.; Li, Z.; Li, L.; Shah, S.N.M.; Gong, Z.H. Analysis of tandem repeat units of the promoter of capsanthin/capsorubin synthase (Ccs) gene in pepper fruit. Physiol. Mol. Biol. Plants 2017, 23, 685–691. [Google Scholar] [CrossRef]
- Tian, S.L.; Li, L.; Shah, S.N.M.; Gong, Z.H. The relationship between red fruit colour formation and key genes of capsanthin biosynthesis pathway in Capsicum annuum. Biol. Plant. 2015, 59, 507–513. [Google Scholar] [CrossRef]
- Venkatesh, J.; Lee, S.Y.; Back, S.; Kim, T.G.; Kim, G.W.; Kim, J.M.; Kwon, J.K.; Kang, B.C. Update on the genetic and molecular regulation of the biosynthetic pathways underlying pepper fruit color and pungency. Curr. Plant Biol. 2023, 35, 100303. [Google Scholar] [CrossRef]
- Ma, X.; Yu, Y.N.; Jia, J.H.; Li, Q.H.; Gong, Z.H. The pepper MYB transcription factor CaMYB306 accelerates fruit coloration and negatively regulates cold resistance. Sci. Hortic. 2022, 295, 110892. [Google Scholar] [CrossRef]
- Song, J.; Sun, B.; Chen, C.; Ning, Z.; Zhang, S.; Cai, Y.; Zheng, X.; Cao, B.; Chen, G.; Jin, D.; et al. An R-R-type MYB transcription factor promotes non-climacteric pepper fruit carotenoid pigment biosynthesis. Plant J. 2023, 115, 724–741. [Google Scholar] [CrossRef]
- Ma, J.; Dai, J.; Liu, X.; Lin, D. The transcription factor CaBBX20 regulates capsanthin accumulation in pepper (Capsicum annuum L.). Sci. Hortic. 2023, 314, 111907. [Google Scholar] [CrossRef]
- Hurtado-Hernandez, H.; Smith, P.G. Inheritance of mature fruit color in Capsicum annuum L. J. Hered. 1985, 76, 211–213. [Google Scholar] [CrossRef]
- Wang, H.; Jia, L.; Li, D.; Manzoor, M.A.; Yan, C.; Ding, Q.; Wang, W.; Hong, X.; Song, T.; Jiang, H. Transcriptomic and Metabolomic Analysis Reveals the Mechanism of H18 Pepper Color Change. Agriculture 2025, 15, 655. [Google Scholar] [CrossRef]
- Jeong, H.B.; Kang, M.Y.; Jung, A.; Han, K.; Lee, J.H.; Jo, J.; Lee, H.Y.; An, J.W.; Kim, S.; Kang, B.C. Single-molecule real-time sequencing reveals diverse allelic variations in carotenoid biosynthetic genes in pepper (Capsicum spp.). Plant Biotechnol. J. 2019, 17, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bai, J.; Duan, X.; Wang, J. Accumulation characteristics of carotenoids and adaptive fruit color variation in ornamental pepper. Sci. Hortic. 2021, 275, 109699. [Google Scholar] [CrossRef]
- Kang, S.H.; Yu, M.S.; Kim, J.M.; Park, S.K.; Lee, C.H.; Lee, H.G.; Kim, S.K. Biochemical, microbiological, and sensory characteristics of stirred yogurt containing red or green pepper (Capsicum annuum cv. Chungyang) juice. Korean J. Food Sci. Anim. Resour. 2018, 38, 451. [Google Scholar] [PubMed]
- Gao, L.J.; Zeng, X.F.; He, X.H.; Yin, X.M. Zeng, Z.H. Research progress on post harvest storage physiology and preservation technology of pepper. South China Agric. 2019, 13, 96–100. [Google Scholar]
- Bin, W.; Wei, L.; Xiao, Y. Research Progress in Mechanism and Preventive Technologies for the Browning of Fresh-Cut Fruits and Vegetables. Shipin Kexue 2025, 46, 367–384. [Google Scholar]
- Maalekuu, K.; Elkind, Y.; Leikin-Frenkel, A.; Lurie, S.; Fallik, E. The relationship between water loss, lipid content, membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biol. Technol. 2006, 42, 248–255. [Google Scholar] [CrossRef]
- Jarén-Galán, M.; Mínguez-Mosquera, M.I. Effect of pepper lipoxygenase activity and its linked reactions on pigments of the pepper fruit. J. Agric. Food Chem. 1999, 47, 4532–4536. [Google Scholar] [CrossRef]
- Lan, Z.; Lin, Y.; Huang, J.; Akutse, K.S. The color matters: Color regulation mechanism of green pepper fruit after harvest. Fruits 2023, 78, 1–7. [Google Scholar] [CrossRef]
- Holden, A.C.; Cohen, H.; Berry, H.M.; Rickett, D.V.; Aharoni, A.; Fraser, P.D. Carotenoid retention during post-harvest storage of Capsicum annuum: The role of the fruit surface structure. J. Exp. Bot. 2024, 75, 1997–2012. [Google Scholar] [CrossRef] [PubMed]
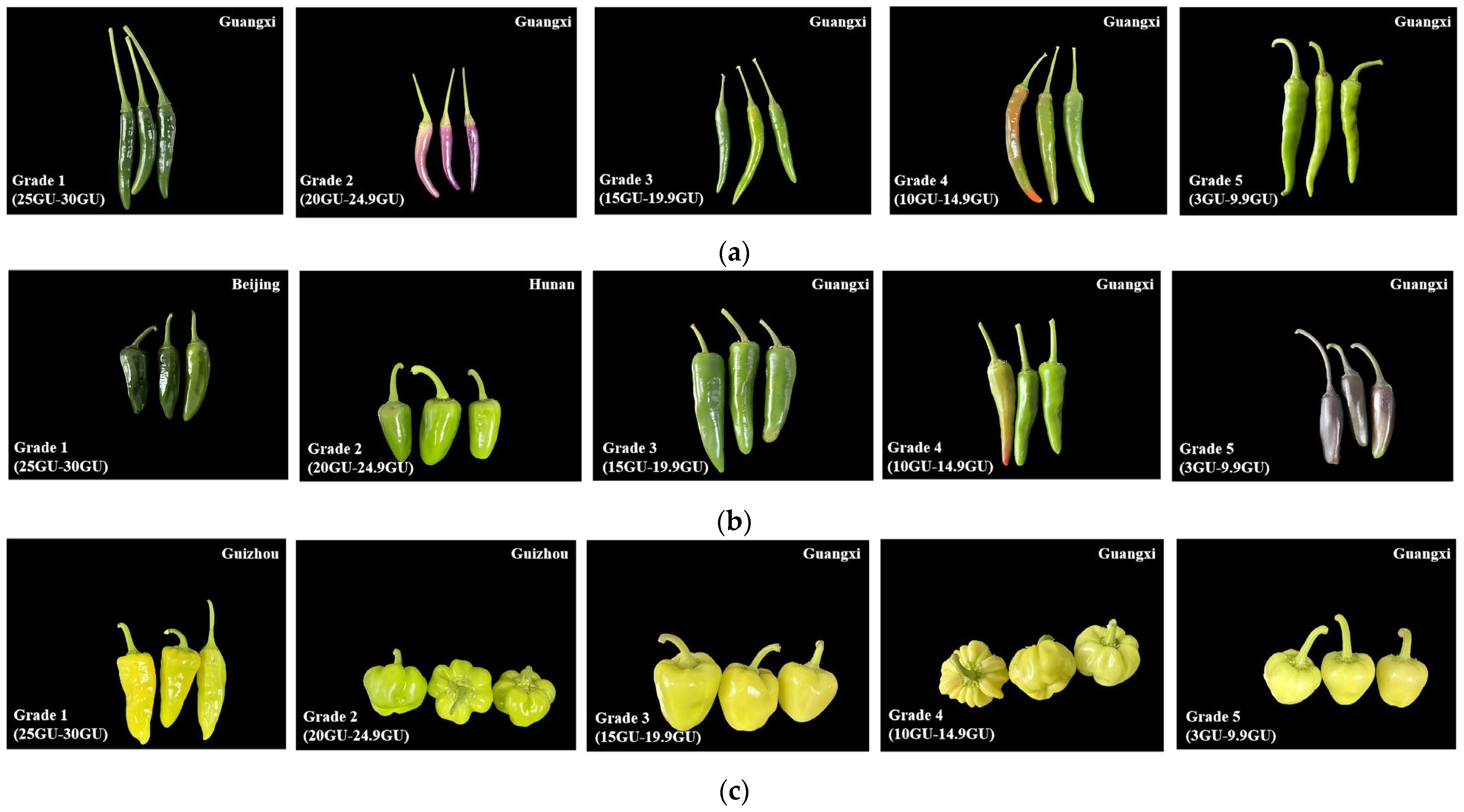
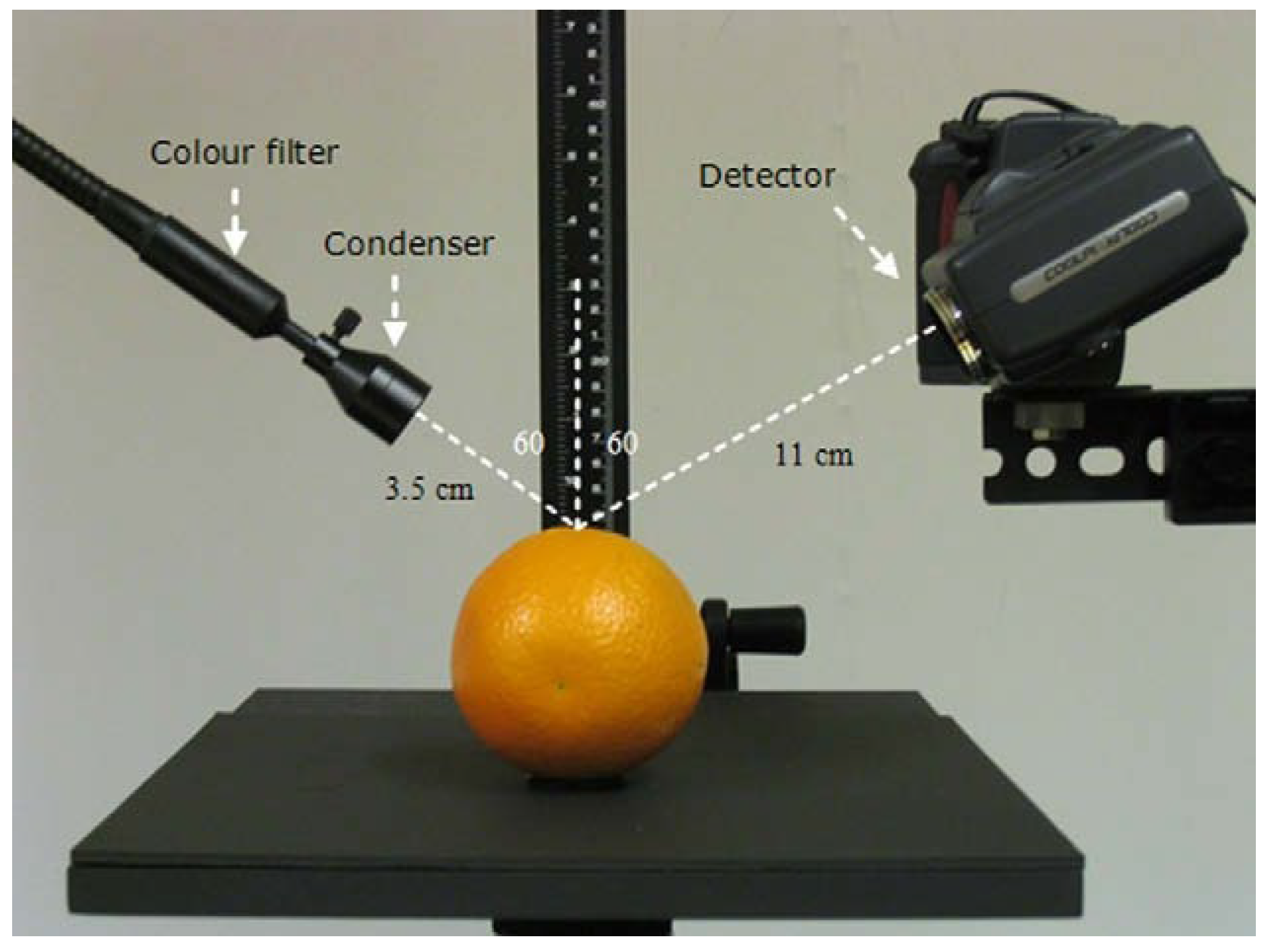
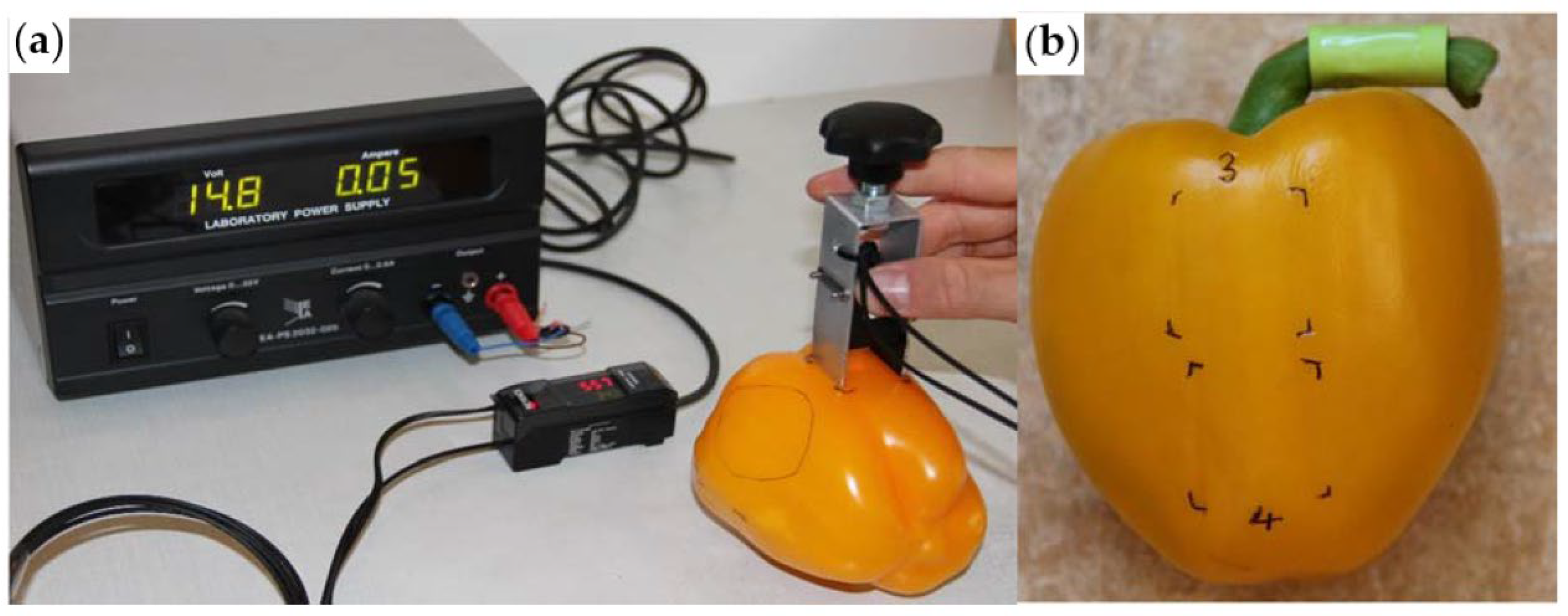



| Methods | Advantages | Disadvantages | Applicable Scenarios |
|---|---|---|---|
| Gloss Meter | High standardization level, easy operation, non-destructive testing | Only reflects macroscopic gloss, fruit shape limitation | Breeding screening, market grading |
| Optical Imaging and Machine Learning | High-throughput detection, strong real-time performance | Dependence on data annotation, environmental sensitivity | Industrial automation |
| Optical Coherence Tomography | non-invasive detection, 3D imaging | resolution limitation, high equipment cost | Storage and preservation research, variety improvement, |
| Colorimeter | Compact size for field operation, cost-effective | Low spectral resolution, relies on manual positioning of measurement areas | screening of color diversity in pepper germplasm resources; rapid on-site detection during fresh pepper procurement |
| Computer Vision System | Non-contact high-throughput analysis, multi-parameter fusion | High equipment cost, requires professional algorithm development and large amounts of training data | Pepper phenomics research, real-time detection in intelligent sorting production lines |
| Locus | Chr | Protein | Trait/Function |
|---|---|---|---|
| PSY1/c2 | 4 | Phytoene synthase 1 | Orange fruit color |
| CCS/y | 6 | Capsanthin capsorubin synthase | Yellow fruit color |
| PRR2/c1 | 1 | Pseudo-response regulator 2 | Lighter fruit color (red, yellow, orange) |
| PSY2 | 2 | Phytoene synthase 2 | Yellow fruit color |
| LCYB | 5 | Phytoene synthase 2 | Fruit color variation (pink to orange) |
| CrtZ-2/ BCH/ CHY2 | 3 | β-Carotene hydroxylase 2 | Orange fruit color |
| ZEP/Or | 2 | β-Carotene hydroxylase 2 | Orange/yellow mature fruit color |
| BBX20 | 6 | B-box (BBX) C-zinc-finger transcription factor (TF) | Regulates a carotenoid synthesis pathway gene (CCS); BBX20 silencing results in orange fruits |
| PP2C35 | 10 | Type 2 C protein phosphatases | Green stripes on fruit surface |
| LOL1 (pc1) | 1 | LOL1 (LSD ONE LIKE1) zinc-finger TF | Null mutation in LOL1 determines light-green fruit color, chloroplast size, and chlorophyll content |
| SGR1 (c) | 1 | Stay-green (SGR) gene, encodes a Magnesium dechelatase | Responsible for the stay-green phenotype |
| GLK2 (pc10) | 10 | GOLDEN2-LIKE TF | Determines light- and dark-green fruit color, chloroplast size, and chlorophyll content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhao, H.; Jing, Z.; Zhao, Z.; Wang, M.; Gong, M.; Wu, X.; He, Z.; Liao, J.; Liu, M.; et al. Recent Advances in Pepper Fruit Glossiness. Genes 2025, 16, 1319. https://doi.org/10.3390/genes16111319
Li Z, Zhao H, Jing Z, Zhao Z, Wang M, Gong M, Wu X, He Z, Liao J, Liu M, et al. Recent Advances in Pepper Fruit Glossiness. Genes. 2025; 16(11):1319. https://doi.org/10.3390/genes16111319
Chicago/Turabian StyleLi, Zongjun, Hu Zhao, Zihuan Jing, Zengjing Zhao, Meng Wang, Mingxia Gong, Xing Wu, Zhi He, Jianjie Liao, Mengjiao Liu, and et al. 2025. "Recent Advances in Pepper Fruit Glossiness" Genes 16, no. 11: 1319. https://doi.org/10.3390/genes16111319
APA StyleLi, Z., Zhao, H., Jing, Z., Zhao, Z., Wang, M., Gong, M., Wu, X., He, Z., Liao, J., Liu, M., Ling, Z., & Wang, R. (2025). Recent Advances in Pepper Fruit Glossiness. Genes, 16(11), 1319. https://doi.org/10.3390/genes16111319





