Sex-Specific Molecular and Genomic Responses to Endocrine Disruptors in Aquatic Species: The Central Role of Vitellogenin
Abstract
1. Introduction
Methodology
2. Endocrine Disruptors in Aquatic Environments
2.1. Main Sources of EDCs
2.2. Exposure Pathways in Aquatic Organisms
2.3. Physiological and Reproductive Effects of EDCs
3. Sex-Specific Molecular Responses to EDCs
3.1. Nuclear Receptor Isoforms (ERα, ERβ, and AR Variants)
3.2. Diversification of vtg Gene Repertoires in Teleosts
3.3. Epigenetic Regulation of Sex-Specific Responses
4. Vitellogenin as a Biomarker
4.1. Structure, Function, and Gene Regulation of VTG
4.2. Mechanisms of Induction by EDCs and Receptor Crosstalk
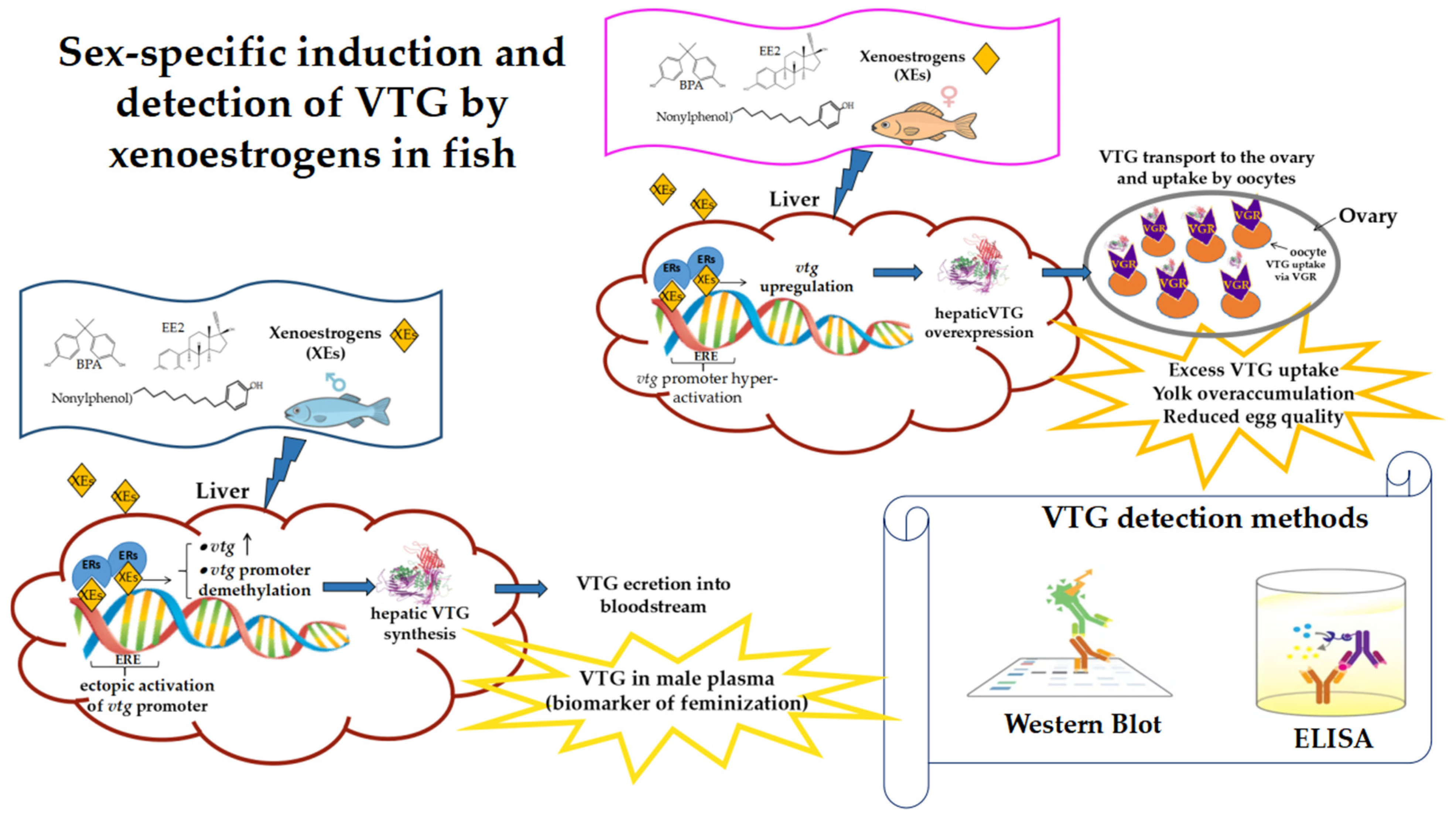
4.3. Sex-Specific Differences in VTG Response
4.4. Applications in Ecotoxicology
4.5. Limitations and Challenges
4.6. Evolutionary and Biotechnological Perspectives of VTG
5. Integration with Other Biomarkers and Molecular Endpoints
5.1. Co-Expression with Hormone Metabolism and Stress Genes
5.2. Epigenetic and Transcriptomic Markers
5.3. Multi-Biomarker and AOP Approaches
6. Ecotoxicological and Ecogenomic Implications for Risk Assessment
6.1. Species-Specific Sensitivity in Marine Fish
6.2. Reproductive Fitness and Population-Level Effects
6.3. Implications for Biodiversity and Ecosystem Health
6.4. Integrating Molecular Data into Risk Assessment
7. Future Perspectives
7.1. Development of Sex-Specific Molecular Biomarkers
7.2. Applications of Multi-Omics and Big Data
7.3. Ecogenomics and Adaptive Evolution
7.4. Regulatory and Sustainability Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-Binding Cassette (transporters) |
| AOP | Adverse Outcome Pathway |
| AOPs | Adverse Outcome Pathways |
| AR | Androgen Receptor |
| ARα | Androgen Receptor alpha |
| ARβ | Androgen Receptor beta |
| ATP | Adenosine Triphosphate |
| AhR | Aryl Hydrocarbon Receptor |
| BDE | Brominated Diphenyl Ether |
| BPA | Bisphenol A |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CYP1A | Cytochrome P450 Family 1 Subfamily A |
| Chg | Choriogenins |
| CpG | Cytosine-phosphate-Guanine dinucleotide |
| DDT | Dichlorodiphenyltrichloroethane |
| DNA | Deoxyribonucleic Acid |
| E2 | 17β-Estradiol |
| EDC | Endocrine-Disrupting Chemical |
| EDCs | Endocrine-Disrupting Chemicals |
| EDSP | Endocrine Disruptor Screening Program (US EPA) |
| EE2 | 17α-Ethinylestradiol |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ER | Estrogen Receptor |
| ERE | Estrogen Response Element |
| ERα | Estrogen Receptor alpha |
| ERβ | Estrogen Receptor beta |
| esr2a | Estrogen Receptor beta a (teleost isoform) |
| esr2b | Estrogen Receptor beta b (teleost isoform) |
| EU | European Union |
| GPER | G Protein-Coupled Estrogen Receptor |
| GWAS | Genome-Wide Association Study |
| HNF4α | Hepatocyte Nuclear Factor 4 alpha |
| HSP70 | Heat Shock Protein 70 |
| HSP90 | Heat Shock Protein 90 |
| HSPs | Heat Shock Proteins |
| HTTr | High-Throughput Transcriptomics |
| MIE | Molecular Initiating Event (AOP framework) |
| OECD | Organisation for Economic Co-operation and Development |
| PCB | Polychlorinated Biphenyl |
| PFAS | Per- and Polyfluoroalkyl Substances |
| PFNA | Perfluorononanoic Acid |
| PXR | Pregnane X Receptor |
| RNA | Ribonucleic Acid |
| RNA-Seq | RNA Sequencing |
| RXR | Retinoid X Receptor |
| SDG | Sustainable Development Goal |
| SLC | Solute Carrier Transporter |
| StAR | Steroidogenic Acute Regulatory Protein |
| tPOD | Transcriptomic Point of Departure |
| VTG | Vitellogenin |
| WoE | Weight of Evidence |
| ZP2 | Zona Pellucida Glycoprotein 2 |
| ZP3 | Zona Pellucida Glycoprotein 3 |
| cyp19a1a | Cytochrome P450 Family 19 Subfamily A Member 1a (ovarian aromatase) |
| cyp19a1b | Cytochrome P450 Family 19 Subfamily A Member 1b (brain aromatase) |
| dnmt | DNA Methyltransferase |
| mPRα | Membrane Progestin Receptor alpha |
| miR-200 | MicroRNA-200 family |
| ncRNA | Non-Coding RNA |
| qPCR | Quantitative Polymerase Chain Reaction |
References
- Wang, Y.; Zhou, J. Endocrine disrupting chemicals in aquatic environments: A potential reason for organism extinction? Aquat. Ecosyst. Health Manag. 2013, 16, 88–93. [Google Scholar] [CrossRef]
- Sumpter, J.P.; Johnson, A.C. Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environ. Sci. Technol. 2005, 39, 4321–4332. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Cren-Olivé, C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ. Pollut. 2011, 159, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Martínez, R.; Navarro-Martín, L.; Kamstra, J.H.; Schwendt, A.; Reynaud, S.; Chalifour, L. Emerging concepts and opportunities for endocrine disruptor screening of the non-EATS modalities. Environ. Res. 2022, 204 Pt A, 111904. [Google Scholar] [CrossRef]
- Windsor, F.M.; Ormerod, S.J.; Tyler, C.R. Endocrine disruption in aquatic systems: Up-scaling research to address ecological consequences. Biol. Rev. 2018, 93, 626–641. [Google Scholar] [CrossRef]
- Crane, M.; Hallmark, N.; Lagadic, L.; Ott, K.; Pickford, D.; Preuss, T.; Thompson, H.; Thorbek, P.; Weltje, L.; Wheeler, J.R. Establishing the relevance of endocrine-disrupting effects for nontarget vertebrate populations. Integr. Environ. Assess. Manag. 2019, 15, 299–300. [Google Scholar] [CrossRef]
- Major, K.M.; DeCourten, B.M.; Li, J.; Britton, M.; Settles, M.L.; Mehinto, A.C.; Connon, R.E.; Brander, S.M. Early-life exposure to environmentally relevant levels of endocrine disruptors drive multigenerational and transgenerational epigenetic changes in a fish model. Front. Mar. Sci. 2020, 7, 471. [Google Scholar] [CrossRef]
- Ayobahan, S.U.; Eilebrecht, E.; Kotthoff, M.; Baumann, L.; Eilebrecht, S.; Teigeler, M.; Hollert, H.; Kalkhof, S.; Schäfers, C. A combined FSTRA-shotgun proteomics approach to identify molecular changes in zebrafish upon chemical exposure. Sci. Rep. 2019, 9, 6599. [Google Scholar] [CrossRef] [PubMed]
- Celino-Brady, F.T.; Lerner, D.T.; Seale, A.P. Experimental approaches for characterizing the endocrine-disrupting effects of environmental chemicals in fish. Front. Endocrinol. 2021, 11, 619361. [Google Scholar] [CrossRef]
- Barra, R.O.; Chiang, G.; Saavedra, M.F.; Orrego, R.; Servos, M.R.; Hewitt, L.M.; McMaster, M.E.; Bahamonde, P.; Tucca, F.; Munkittrick, K.R.; et al. Endocrine disruptor impacts on fish from Chile: The influence of wastewaters. Front. Endocrinol. 2021, 12, 611281. [Google Scholar] [CrossRef]
- Tseng, P.W.; Wu, G.C.; Kuo, W.L.; Tseng, Y.C.; Chang, C.F. The ovarian transcriptome at the early stage of testis removal-induced male-to-female sex change in the protandrous black porgy Acanthopagrus schlegelii. Front. Genet. 2022, 13, 816955. [Google Scholar] [CrossRef]
- Matozzo, V.; Gagné, F.; Marin, M.G.; Ricciardi, F.; Blaise, C. Vitellogenin as a biomarker of exposure to estrogenic compounds in aquatic invertebrates: A review. Environ. Int. 2008, 34, 531–545. [Google Scholar] [CrossRef]
- Hutchinson, T.H.; Ankley, G.T.; Segner, H.; Tyler, C.R. Screening and testing for endocrine disruption in fish—Biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006, 114 (Suppl. S1), 106–114. [Google Scholar] [CrossRef]
- Bowman, C.J.; Kroll, K.J.; Hemmer, M.J.; Folmar, L.C.; Denslow, N.D. Estrogen-Induced Vitellogenin mRNA and Protein in Sheepshead Minnow (Cyprinodon variegatus). Gen. Comp. Endocrinol. 2000, 120, 300–313. [Google Scholar] [CrossRef]
- Brown, R.J.; Panter, G.H.; Burden, N.; Salinas, E.R.; Weltje, L.; Wheeler, J.R.; Wolf, Y.; Lagadic, L. Are changes in vitellogenin concentrations in fish reliable indicators of chemical-induced endocrine activity? Ecotoxicol. Environ. Saf. 2023, 266, 115563. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.K.A.; Yu, R.M.K.; Islam, R.; Nguyen, T.H.T.; Bui, T.L.H.; Kong, R.Y.C.; O’Connor, W.A.; Leusch, F.D.L.; Andrew-Priestley, M.; MacFarlane, G.R. The utility of vitellogenin as a biomarker of estrogenic endocrine disrupting chemicals in molluscs. Environ. Pollut. 2019, 248, 1067–1078. [Google Scholar] [CrossRef]
- Ketata, I.; Denier, X.; Hamza-Chaffai, A.; Minier, C. Endocrine-related reproductive effects in molluscs. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 147, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Medesani, D.A.; Fingerman, M. Endocrine disruption in crustaceans due to pollutants: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.D.; Huth, L.K.; Pieto, D.L.; Selcer, K.W. Vitellogenin as a biomarker for xenobiotic estrogens in an amphibian model system. Environ. Toxicol. Chem. 1998, 17, 30–36. [Google Scholar] [CrossRef]
- Tompsett, A.R.; Wiseman, S.; Higley, E.; Pryce, S.; Chang, H.; Giesy, J.P.; Hecker, M. Effects of 17α-ethynylestradiol on sexual differentiation and development of the African clawed frog (Xenopus laevis). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2012, 156, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.M.; Ball, J.S.; Williams, T.D.; Wu, H.; Ortega, F.; Van Aerle, R.; Katsiadaki, I.; Falciani, F.; Viant, M.R.; Chipman, J.K.; et al. Identifying health impacts of exposure to copper using transcriptomics and metabolomics in a fish model. Environ. Sci. Technol. 2010, 44, 820–826. [Google Scholar] [CrossRef]
- Viant, M.R.; Rosenblum, E.S.; Tjeerdema, R.S. NMR-based metabolomics: A powerful approach for characterizing the effects of environmental stressors on organism health. Environ. Sci. Technol. 2003, 37, 4982–4989. [Google Scholar] [CrossRef]
- Marjan, P.; Martyniuk, C.J.; Arlos, M.J.; Servos, M.R.; Ruecker, N.J.; Munkittrick, K.R. Identifying transcriptomic indicators of tertiary treated municipal effluent in longnose dace (Rhinichthys cataractae) caged under semi-controlled conditions in experimental raceways. Sci. Total Environ. 2024, 923, 171257. [Google Scholar] [CrossRef]
- United Nations. Sustainable Development Goal 14: Life Below Water. Available online: https://sdgs.un.org/goals/goal14 (accessed on 24 August 2025).
- Kloas, W.; Urbatzka, R.; Opitz, R.; Würtz, S.; Behrends, T.; Hermelink, B.; Hofmann, F.; Jagnytsch, O.; Kroupova, H.; Lorenz, C.; et al. Endocrine disruption in aquatic vertebrates. Ann. N.Y. Acad. Sci. 2009, 1163, 187–200. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bencic, D.C.; Breen, M.S.; Collette, T.W.; Conolly, R.B.; Denslow, N.D.; Edwards, S.W.; Ekman, D.R.; Garcia-Reyero, N.; LaLone, C.A.; et al. Endocrine disrupting chemicals in fish: Developing exposure indicators and predictive models of effects based on mechanism of action. Aquat. Toxicol. 2009, 92, 168–178. [Google Scholar] [CrossRef]
- Porte, C.; Janer, G.; Lorusso, L.C.; Ortiz-Zarragoitia, M.; Cajaraville, M.P.; Fossi, M.C.; Canesi, L. Endocrine disruptors in marine organisms: Approaches and perspectives. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 143, 303–315. [Google Scholar] [CrossRef]
- Tasselli, S.; Marziali, L.; Roscioli, C.; Guzzella, L. Legacy Dichlorodiphenyltrichloroethane (DDT) Pollution in a River Ecosystem: Sediment Contamination and Bioaccumulation in Benthic Invertebrates. Sustainability 2023, 15, 6493. [Google Scholar] [CrossRef]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; de Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S.; et al. Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 2011, 127, 64–73. [Google Scholar] [CrossRef] [PubMed]
- de Lima Oliveira, W.; Mendonça Mota, T.F.; da Silva, A.P.; de Lima Oliveira, R.D.; Comelli, C.L.; Orlandini, N.D.; Zimmer, D.F.; de Oliveira, E.C.; de Castilhos Ghisi, N. Does the atrazine increase animal mortality: Unraveling through a meta-analytic study. Sci. Total Environ. 2024, 951, 175553. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Chen, S.; Zeng, C.; Fan, Y.; Ge, W.; Chen, W. Estrogenic and non-estrogenic effects of bisphenol A and its action mechanism in the zebrafish model: An overview of the past two decades of work. Environ. Int. 2023, 176, 107976. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.R.; Lange, A.; Paull, G.C.; Katsu, Y.; Iguchi, T. The roach (Rutilus rutilus) as a sentinel for assessing endocrine disruption. Environ. Sci. 2007, 14, 235–253. [Google Scholar] [CrossRef]
- Balbi, T.; Franzellitti, S.; Fabbri, R.; Montagna, M.; Fabbri, E.; Canesi, L. Impact of bisphenol A (BPA) on early embryo development in the marine mussel Mytilus galloprovincialis: Effects on gene transcription. Environ. Pollut. 2016, 218, 996–1004. [Google Scholar] [CrossRef]
- Fernández-González, L.E.; Sánchez-Marín, P.; Gestal, C.; Beiras, R.; Diz, A.P. Vitellogenin gene expression in marine mussels exposed to ethinylestradiol: No induction at the transcriptional level. Mar. Environ. Res. 2021, 168, 105315. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad. Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef]
- Blanchfield, P.J.; Kidd, K.A.; Docker, M.F.; Palace, V.P.; Park, B.J.; Postma, L.D. Recovery of a wild fish population from whole-lake additions of a synthetic estrogen. Environ. Sci. Technol. 2015, 49, 3136–3144. [Google Scholar] [CrossRef]
- Marlatt, V.L.; Bayen, S.; Castaneda-Cortès, D.; Delbès, G.; Grigorova, P.; Langlois, V.S.; Martyniuk, C.J.; Metcalfe, C.D.; Parent, L.; Rwigemera, A.; et al. Impacts of endocrine disrupting chemicals on reproduction in wildlife and humans. Environ. Res. 2022, 208, 112584. [Google Scholar] [CrossRef]
- Hahn, M.E. Aryl hydrocarbon receptors: Diversity and evolution. Chem. Biol. Interact. 2002, 141, 131–160. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, G.; Yu, X.; Du, Y.; Man, Q.; Wang, W.C. Perfluorooctanoic acid disrupts thyroid hormone biosynthesis by altering glycosylation of Na+/I− symporter in larval zebrafish. Ecotoxicol. Environ. Saf. 2025, 297, 118249. [Google Scholar] [CrossRef]
- Yu, Q.; Cai, L.; Liu, H.; Hu, Z.; Wu, H.; Chen, X.; Ren, C.; Zhang, J. Bioaccumulation and bioelimination of per- and polyfluoroalkyl substances in aquatic organisms: A review. J. Water Process Eng. 2025, 75, 108065. [Google Scholar] [CrossRef]
- Fabrello, J.; Targhetta, F.; Ciscato, M.; Asnicar, D.; Bernardini, I.; Milan, M.; Patarnello, T.; Marin, M.G.; Matozzo, V. First Evidence of In Vitro Effects of C6O4—A Substitute of PFOA—On Haemocytes of the Clam Ruditapes philippinarum. Toxics 2021, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Aquilina-Beck, A.A.; Reiner, J.L.; Chung, K.W.; DeLise, M.J.; Key, P.B.; DeLorenzo, M.E. Uptake and Biological Effects of Perfluorooctane Sulfonate Exposure in the Adult Eastern Oyster Crassostrea virginica. Arch. Environ. Contam. Toxicol. 2020, 79, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Romersi, R.F.; Nicklisch, S.C.T. Interactions of Environmental Chemicals and Natural Products with ABC and SLC Transporters in the Digestive System of Aquatic Organisms. Front. Physiol. 2022, 12, 767766. [Google Scholar] [CrossRef]
- Bieczynski, F.; Painefilú, J.C.; Venturino, A.; Luquet, C.M. Expression and Function of ABC Proteins in Fish Intestine. Front. Physiol. 2021, 12, 791834. [Google Scholar] [CrossRef]
- Ervin, S.M.; Li, H.; Lim, L.; Roberts, L.R.; Liang, X.; Mani, S.; Redinbo, M.R. Gut Microbial β-Glucuronidases Reactivate Estrogens as Components of the Estrobolome. J. Biol. Chem. 2019, 294, 18586–18599. [Google Scholar] [CrossRef]
- Ma, L.; Yates, S.R. Degradation and Metabolite Formation of 17β-Estradiol-3-Glucuronide and 17β-Estradiol-3-Sulphate in River Water and Sediment. Water Res. 2018, 139, 1–9. [Google Scholar] [CrossRef]
- Monteverdi, G.H.; Di Giulio, R.T. In Vitro and In Vivo Association of 2,3,7,8-Tetrachlorodibenzo-p-dioxin and Benzo[a]pyrene with the Yolk-Precursor Protein Vitellogenin. Environ. Toxicol. Chem. 2000, 19, 2502–2511. [Google Scholar] [CrossRef]
- Heiden, T.K.; Hutz, R.J.; Carvan, M.J. Accumulation, Tissue Distribution, and Maternal Transfer of Dietary 2,3,7,8-Tetrachlorodibenzo-p-dioxin: Impacts on Reproductive Success of Zebrafish. Toxicol. Sci. 2005, 87, 497–507. [Google Scholar] [CrossRef]
- Honda, M.; Muta, A.; Akasaka, T.; Inoue, Y.; Shimasaki, Y.; Kannan, K.; Okino, N.; Oshima, Y. Identification of Perfluorooctane Sulfonate Binding Protein in the Plasma of Tiger Pufferfish Takifugu rubripes. Ecotoxicol. Environ. Saf. 2014, 104, 409–413. [Google Scholar] [CrossRef]
- Point, A.D.; Crimmins, B.S.; Holsen, T.M.; Fernando, S.; Hopke, P.K.; Darie, C.C. Can Blood Proteome Diversity among Fish Species Help Explain Perfluoroalkyl Acid Trophodynamics in Aquatic Food Webs? Sci. Total Environ. 2023, 875, 162337. [Google Scholar] [CrossRef]
- Molnár, S.; Kulcsár, G.; Perjési, P. Determination of Steroid Hormones in Water Samples by Liquid Chromatography Electrospray Ionization Mass Spectrometry Using Parallel Reaction Monitoring. Microchem. J. 2022, 175, 107105. [Google Scholar] [CrossRef]
- Shao, Z.; Guagliardo, P.; Jiang, H.; Wang, W.-X. Intra- and Intercellular Silver Nanoparticle Translocation and Transformation in Oyster Gill Filaments: Coupling Nanoscale SIMS and Dual Stable Isotope Tracing. Environ. Sci. Technol. 2021, 55, 433–446. [Google Scholar] [CrossRef]
- Ikuta, T.; Tame, A.; Takahashi, T.; Nomaki, H.; Nakajima, R. Microplastic Particles Are Phagocytosed in Gill Cells of Deep-Sea and Coastal Mussels. Front. Mar. Sci. 2022, 9, 1034950. [Google Scholar] [CrossRef]
- Dong, L.; Sun, Y.; Chu, M.; Xie, Y.; Wang, P.; Li, B.; Li, Z.; Xu, X.; Feng, Y.; Sun, G.; et al. Exploration of Response Mechanisms in the Gills of Pacific Oyster (Crassostrea gigas) to Cadmium Exposure through Integrative Metabolomic and Transcriptomic Analyses. Animals 2024, 14, 2318. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Escauriaza, R.; Lozano, V.; Pérez-Parallé, M.L.; Blanco, J.; Sánchez, J.L.; Pazos, A.J. Expression Analyses of Genes Related to Multixenobiotic Resistance in Mytilus galloprovincialis after Exposure to Dinophysis acuminata. Toxins 2021, 13, 614. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Wu, L.; Fu, S.; Que, H.; Shi, B. Transcriptomic Analysis Reveals the Mechanisms of Cadmium Transport and Detoxification in Portuguese Oysters (Crassostrea angulata). Animals 2025, 15, 1041. [Google Scholar] [CrossRef]
- Banaee, M.; Zeidi, A.; Mikušková, N.; Faggio, C. Assessing Metal Toxicity on Crustaceans in Aquatic Ecosystems: A Comprehensive Review. Biol. Trace Elem. Res. 2024, 202, 5743–5761. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Du, J.; Hu, J.; Bao, Z.; Qu, Z. Effects of Methyl Farnesoate on the Growth and Antioxidant Capacity of Neocaridina denticulata. Antioxidants 2025, 14, 635. [Google Scholar] [CrossRef]
- Rodríguez, E.M. Endocrine Disruption in Crustaceans: New Findings and Perspectives. Mol. Cell. Endocrinol. 2024, 585, 112189. [Google Scholar] [CrossRef]
- Jones, S.E.; Gutkowski, N.; Demick, S.; Curello, M.; Pavia, A.; Robuck, A.R.; Li, M.-L. Assessing Bivalves as Biomonitors of Per- and Polyfluoroalkyl Substances in Coastal Environments. Environ. Sci. Technol. 2025, 59, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Oikari, A. Use of early juvenile zebrafish (Danio rerio) for in vivo assessment of endocrine modulation by xenoestrogens. J. Environ. Anal. Toxicol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Piferrer, F.; Anastasiadi, D.; Valdivieso, A.; Sánchez-Baizán, N.; Moraleda-Prados, J.; Ribas, L. The model of the conserved epigenetic regulation of sex. Front. Genet. 2019, 10, 857. [Google Scholar] [CrossRef]
- Dang, Z.C. Fish biomarkers for regulatory identification of endocrine disrupting chemicals. Environ. Pollut. 2014, 185, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Sun, L.X.; Zhu, J.J.; Zhao, Y.; Wang, H.; Liu, H.J.; Ji, X.S. Epigenetic control of cyp19a1a expression is critical for high temperature induced Nile tilapia masculinization. J. Therm. Biol. 2017, 69, 76–84. [Google Scholar] [CrossRef]
- Thomas, P. Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics. Cells 2022, 11, 1785. [Google Scholar] [CrossRef]
- Nagata, J.; Mushirobira, Y.; Nishimiya, O.; Yamaguchi, Y.; Fujita, T.; Hiramatsu, N.; Hara, A.; Todo, T. Hepatic estrogen-responsive genes relating to oogenesis in cutthroat trout (Oncorhynchus clarki): Transcriptional induction in primary cultured hepatocytes and in vitro promoter transactivation in response to estradiol-17β. Gen. Comp. Endocrinol. 2021, 310, 113812. [Google Scholar] [CrossRef]
- Maradonna, F.; Carnevali, O. Vitellogenin, zona radiata protein, cathepsin D and heat shock protein 70 as biomarkers of exposure to xenobiotics. Biomarkers 2007, 12, 240–255. [Google Scholar] [CrossRef]
- Canesi, L.; Betti, M.; Lorusso, L.C.; Ciacci, C.; Gallo, G. In vivo effects of bisphenol A in Mytilus hemocytes: Modulation of kinase-mediated signalling pathways. Aquat. Toxicol. 2005, 71, 73–84. [Google Scholar] [CrossRef]
- Qu, M.; Xu, J.; Yang, Y.; Li, R.; Li, T.; Chen, S.; Di, Y. Assessment of sulfamethoxazole toxicity to marine mussels (Mytilus galloprovincialis): Combining p38-MAPK signaling pathway modulation with histopathological alterations. Ecotoxicol. Environ. Saf. 2023, 249, 114365. [Google Scholar] [CrossRef]
- Kundu, S.; Ray, A.; Das Gupta, S.; Biswas, A.; Roy, S.; Tiwari, N.K.; Kumar, V.S.; Das, B.K. Environmental bisphenol A disrupts methylation of steroidogenic genes in the ovary of paradise threadfin (Polynemus paradiseus) via abnormal DNA methylation: Implications for human exposure and health risk assessment. Chemosphere 2024, 351, 141236. [Google Scholar] [CrossRef] [PubMed]
- Breves, J.P.; Duffy, T.A.; Einarsdottir, I.E.; Björnsson, B.T.; McCormick, S.D. In vivo effects of 17α-ethinylestradiol, 17β-estradiol and 4-nonylphenol on insulin-like growth factor-binding proteins (IGFBPs) in Atlantic salmon. Aquat. Toxicol. 2018, 203, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; van Aerle, R.; Santos, E.; Brighty, G. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2006, 114 (Suppl. S1), 32–39. [Google Scholar] [CrossRef] [PubMed]
- Aluru, N.; Hallanger, I.G.; McMonagle, H.; Harju, M. Hepatic gene expression profiling of Atlantic cod (Gadus morhua) liver after exposure to organophosphate flame retardants revealed altered cholesterol biosynthesis and lipid metabolism. Environ. Toxicol. Chem. 2021, 40, 1639–1648. [Google Scholar] [CrossRef]
- Falciani, F.; Diab, A.M.; Sabine, V.; Williams, T.; Ortega, F.; George, S.G.; Chipman, J. Hepatic transcriptomic profiles of European flounder (Platichthys flesus) from field sites and computational approaches to predict site from stress gene responses following exposure to model toxicants. Aquat. Toxicol. 2008, 90, 92–101. [Google Scholar] [CrossRef]
- Blalock, B.; Robinson, W.E.; Loguinov, A.; Vulpe, C.D.; Krick, K.S.; Poynton, H.C. Transcriptomic and network analyses reveal mechanistic-based biomarkers of endocrine disruption in the marine mussel, Mytilus edulis. Environ. Sci. Technol. 2018, 52, 9419–9430. [Google Scholar] [CrossRef]
- Jubeaux, G.; Audouard-Combe, F.; Simon, R.; Tutundjian, R.; Salvador, A.; Geffard, O.; Chaumot, A. Vitellogenin-like proteins among invertebrate species diversity: Potential of proteomic mass spectrometry for biomarker development. Environ. Sci. Technol. 2012, 46, 6315–6323. [Google Scholar] [CrossRef]
- Breitholtz, M. Crustaceans. In Endocrine Disrupters; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 116–142. [Google Scholar] [CrossRef]
- Vogt, É.L.; Model, J.F.A.; Vinagre, A.S. Effects of organotins on crustaceans: Update and perspectives. Front. Endocrinol. 2018, 9, 65. [Google Scholar] [CrossRef]
- Mazurová, E.; Hilscherová, K.; Triebskorn, R.; Köhler, H.R. Endocrine regulation of reproduction in crustaceans: Identification of potential targets for toxicants and environmental contaminants. Biologia 2008, 63, 139–150. [Google Scholar] [CrossRef]
- LeBlanc, G.A. Crustacean endocrine toxicology: A review. Ecotoxicology 2007, 16, 61–81. [Google Scholar] [CrossRef]
- Toyota, K.; Yamane, F.; Ohira, T. Impacts of Methyl Farnesoate and 20-Hydroxyecdysone on Larval Mortality and Metamorphosis in the Kuruma Prawn Marsupenaeus japonicus. Front. Endocrinol. 2020, 11, 475. [Google Scholar] [CrossRef]
- Knigge, T.; LeBlanc, G.A.; Ford, A.T. A crab is not a fish: Unique aspects of the crustacean endocrine system and considerations for endocrine toxicology. Front. Endocrinol. 2021, 12, 587608. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 229: Fish Short Term Reproduction Assay; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2012. [Google Scholar] [CrossRef]
- OECD. Test No. 234: Fish Sexual Development Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2011. [Google Scholar] [CrossRef]
- Filby, A.L.; Tyler, C.R. Molecular characterization of estrogen receptors 1, 2a, and 2b and their tissue and ontogenic expression profiles in fathead minnow (Pimephales promelas). Biol. Reprod. 2005, 73, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Bardet, P.; Horard, B.; Robinson-Rechavi, M.; Laudet, V.; Vanacker, J. Characterization of oestrogen receptors in zebrafish (Danio rerio). J. Mol. Endocrinol. 2002, 28, 153–163. [Google Scholar] [CrossRef]
- Griffin, L.B.; January, K.E.; Ho, K.W.; Cotter, K.A.; Callard, G.V. Morpholino-mediated knockdown of ERα, ERβa, and ERβb mRNAs in zebrafish (Danio rerio) embryos reveals differential regulation of estrogen-inducible genes. Endocrinology 2013, 154, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.I.; Estêvão, M.D.; Power, D.M. Effects of estrogens and estrogenic disrupting compounds on fish mineralized tissues. Mar. Drugs 2014, 12, 4474–4494. [Google Scholar] [CrossRef]
- Ryu, T.; Okamoto, K.; Ansai, S.; Nakao, M.; Kumar, A.; Iguchi, T.; Ogino, Y. Gene duplication of androgen receptor as an evolutionary driving force underlying the diversity of sexual characteristics in teleost fishes. Zool. Sci. 2024, 41, 68–76. [Google Scholar] [CrossRef]
- Jolly, C.; Katsiadaki, I.; Morris, S.; Le Belle, N.; Dufour, S.; Mayer, I.; Pottinger, T.G.; Scott, A.P. Detection of the anti-androgenic effect of endocrine disrupting environmental contaminants using in vivo and in vitro assays in the three-spined stickleback. Aquat. Toxicol. 2009, 92, 228–239. [Google Scholar] [CrossRef][Green Version]
- Pang, Y.; Thomas, P. Role of G protein-coupled estrogen receptor 1 (GPER) in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev. Biol. 2010, 342, 194–206. [Google Scholar] [CrossRef]
- Aizen, J.; Pang, Y.; Harris, C.; Converse, A.; Zhu, Y.; Aguirre, M.A.; Thomas, P. Roles of progesterone receptor membrane component 1 and membrane progestin receptor alpha in regulation of zebrafish oocyte maturation. Gen. Comp. Endocrinol. 2018, 263, 51–61. [Google Scholar] [CrossRef]
- He, L.; Shi, X.; Zeng, X.; Zhou, F.; Lan, T.; Chen, M.; Han, K. Characterization of the glucocorticoid receptor of large yellow croaker (Larimichthys crocea) and its expression in response to salinity and immune stressors. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2022, 265, 111124. [Google Scholar] [CrossRef]
- Bemanian, V.; Male, R.; Goksøyr, A. The aryl hydrocarbon receptor-mediated disruption of vitellogenin synthesis in the fish liver: Cross-talk between AhR- and ERα-signalling pathways. Comp. Hepatol. 2004, 3, 2. [Google Scholar] [CrossRef]
- Mortensen, A.S.; Arukwe, A. Interactions between estrogen- and Ah-receptor signalling pathways in primary culture of salmon hepatocytes exposed to nonylphenol and 3,3′,4,4′-tetrachlorobiphenyl (congener 77). Comp. Hepatol. 2007, 6, 2. [Google Scholar] [CrossRef]
- Mahoney, H.; Xie, Y.; Brinkmann, M.; Giesy, J.P. Next generation per- and poly-fluoroalkyl substances: Status and trends, aquatic toxicity, and risk assessment. Eco Environ. Health 2022, 1, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, J.T.; Emelyanov, A.; Korzh, V.; Gong, Z. Hepatic and extrahepatic expression of vitellogenin genes in the zebrafish, Danio rerio. Gene 2005, 356, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Kristoffersen, B.A. Vertebrate vitellogenin gene duplication in relation to the “3R hypothesis”: Correlation to the pelagic egg and the oceanic radiation of teleosts. PLoS ONE 2007, 2, e169. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Patinote, A.; Nguyen, T.; Com, E.; Pineau, C.; Bobe, J. Genome editing reveals reproductive and developmental dependencies on specific types of vitellogenin in zebrafish (Danio rerio). Mol. Reprod. Dev. 2019, 86, 1168–1188. [Google Scholar] [CrossRef]
- Trichet, V.; Buisine, N.; Mouchel, N.; Le Pennec, J.P.; Wolff, J. Genomic analysis of the vitellogenin locus in rainbow trout (Oncorhynchus mykiss) reveals a complex history of gene amplification and retroposon activity. Mol. Gen. Genet. 2000, 263, 828–837. [Google Scholar] [CrossRef]
- Carducci, F.; Biscotti, M.A.; Canapa, A. Vitellogenin gene family in vertebrates: Evolution and functions. Eur. Zool. J. 2019, 86, 233–240. [Google Scholar] [CrossRef]
- Finn, R.N.; Kolarevic, J.; Kongshaug, H.; Nilsen, F. Evolution and differential expression of a vertebrate vitellogenin gene cluster. BMC Evol. Biol. 2009, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, N.; Matsubara, T.; Fujita, T.; Sullivan, C.V.; Hara, A. Multiple piscine vitellogenins: Biomarkers of fish exposure to estrogenic endocrine disruptors in aquatic environments. Mar. Biol. 2006, 149, 35–47. [Google Scholar] [CrossRef]
- Xiong, Z.; Yang, J.; Zhang, S.; Wang, Y.; Xu, S.; Guo, C.; Wang, D. Expression and localization of vitellogenin genes (VTG) and receptor (VGR) in the gonad development of silver pomfret Pampus argenteus. J. Oceanol. Limnol. 2023, 41, 1575–1592. [Google Scholar] [CrossRef]
- Yushko, L.V.; Shevlyakov, A.D.; Romazeva, M.A.; Medvedeva, A.V.; Sokolova, O.A. The Role of DNA Methylation in Zebrafish Models of CNS Diseases. J. Evol. Biochem. Physiol. 2024, 60, 973–987. [Google Scholar] [CrossRef]
- Kocerha, J.; Denslow, N.D. Steroidogenic acute regulatory protein in fish. J. Endocrinol. 2025, 265, e240232. [Google Scholar] [CrossRef]
- Wang, X.; Hill, D.; Tillitt, D.E.; Bhandari, R.K. Bisphenol A and 17α-ethinylestradiol-induced transgenerational differences in expression of osmoregulatory genes in the gill of medaka (Oryzias latipes). Aquat. Toxicol. 2019, 211, 227–234. [Google Scholar] [CrossRef]
- Xiong, S.; Ma, W.; Jing, J.; Zhang, J.; Dan, C.; Gui, J.F.; Mei, J. An miR-200 Cluster on Chromosome 23 Regulates Sperm Motility in Zebrafish. Endocrinology 2018, 159, 1982–1991. [Google Scholar] [CrossRef]
- Lee, J.; Kho, Y.; Kim, P.-G.; Ji, K. Exposure to bisphenol S alters the expression of microRNA in male zebrafish. Toxicol. Appl. Pharmacol. 2018, 338, 191–196. [Google Scholar] [CrossRef]
- Dourdin, T.S.; Rivière, G.; Cormier, A.; Di Poi, C.; Guyomard, K.; Rabiller, M.; Akcha, F.; Sadialiou, T.B.; Le Monier, P.; Sussarellu, R. Molecular and phenotypic effects of early exposure to an environmentally relevant pesticide mixture in the Pacific oyster, Crassostrea gigas. Environ. Pollut. 2023, 326, 121472. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Feswick, A.; Munkittrick, K.R.; Dreier, D.A.; Denslow, N.D. Twenty years of transcriptomics, 17α-ethinylestradiol, and fish. Gen. Comp. Endocrinol. 2020, 286, 113325. [Google Scholar] [CrossRef]
- Hemmer, M.J.; Bowman, C.J.; Hemmer, B.L.; Friedman, S.D.; Marcovich, D.; Kroll, K.J.; Denslow, N.D. Vitellogenin mRNA regulation and plasma clearance in male sheepshead minnows (Cyprinodon variegatus) after cessation of exposure to 17β-estradiol and p-nonylphenol. Aquat. Toxicol. 2002, 58, 99–112. [Google Scholar] [CrossRef]
- Geraudie, P.; Gerbron, M.; Hill, E.; Minier, C. Roach (Rutilus rutilus) reproductive cycle: A study of biochemical and histological parameters in a low contaminated site. Fish Physiol. Biochem. 2010, 36, 767–777. [Google Scholar] [CrossRef]
- Yadetie, F.; Karlsen, O.A.; Eide, M.; Hogstrand, C.; Goksøyr, A. Liver Transcriptome Analysis of Atlantic Cod (Gadus morhua) Exposed to PCB 153 Indicates Effects on Cell Cycle Regulation and Lipid Metabolism. BMC Genom. 2014, 15, 481. [Google Scholar] [CrossRef]
- Abd Elkader, H.-T.A.E.; Al-Shami, A.S. Unveiling the impact of bisphenol A on date mussels: Insights into oxidative stress, hormonal imbalance, gonadal atresia, and immune resilience. Mar. Environ. Res. 2025, 208, 107143. [Google Scholar] [CrossRef]
- Ji, C.; Wei, L.; Zhao, J.; Wu, H. Metabolomic analysis revealed that female mussel Mytilus galloprovincialis was sensitive to bisphenol A exposures. Environ. Toxicol. Pharmacol. 2014, 37, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wu, H.; Wei, L.; Zhao, J.; Yu, J. Proteomic and metabolomic analysis reveal gender-specific responses of mussel Mytilus galloprovincialis to 2,2′,4,4′-tetrabromodiphenyl ether (BDE 47). Aquat. Toxicol. 2013, 140–141, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Villeneuve, D.L. The fathead minnow in aquatic toxicology: Past, present and future. Aquat. Toxicol. 2006, 78, 91–102. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Patinote, A.; Com, E.; Pineau, C.; Bobe, J. Knock out of specific maternal vitellogenins in zebrafish (Danio rerio) evokes vital changes in egg proteomic profiles that resemble the phenotype of poor quality eggs. BMC Genom. 2021, 22, 308. [Google Scholar] [CrossRef]
- Zha, W.; Hu, W.; Ge, C.; Chen, J.; Cao, Z. Zebrafish as a model system for studying reproductive diseases. Front. Cell Dev. Biol. 2024, 12, 1481634. [Google Scholar] [CrossRef]
- Meng, X.; Bartholomew, C.; Craft, J.A. Differential expression of vitellogenin and oestrogen receptor genes in the liver of zebrafish, Danio rerio. Anal. Bioanal. Chem. 2010, 396, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, S.; Chen, J.; Zhang, Q.; Liu, Y.; You, C.; Monroig, Ó.; Tocher, D.R.; Li, Y. Hepatocyte Nuclear Factor 4α (HNF4α) Is a Transcription Factor of Vertebrate Fatty Acyl Desaturase Gene as Identified in Marine Teleost Siganus canaliculatus. PLoS ONE 2016, 11, e0160361. [Google Scholar] [CrossRef] [PubMed]
- Maglich, J.M.; Caravella, J.A.; Lambert, M.H.; Willson, T.M.; Moore, J.T.; Ramamurthy, L. The First Completed Genome Sequence from a Teleost Fish (Fugu rubripes) Adds Significant Diversity to the Nuclear Receptor Superfamily. Nucleic Acids Res. 2003, 31, 4051–4058. [Google Scholar] [CrossRef] [PubMed]
- Bainy, A.C.D.; Kubota, A.; Goldstone, J.V.; Lille-Langøy, R.; Karchner, S.I.; Celander, M.C.; Hahn, M.E.; Goksøyr, A.; Stegeman, J.J. Functional Characterization of a Full-Length Pregnane X Receptor, Expression in vivo, and Identification of PXR Alleles in Zebrafish (Danio rerio). Aquat. Toxicol. 2013, 142–143, 447–457. [Google Scholar] [CrossRef]
- Brion, F.; Nilsen, B.M.; Eidem, J.K.; Goksøyr, A.; Porcher, J.M. Development and validation of an enzyme-linked immunosorbent assay to measure vitellogenin in the zebrafish (Danio rerio). Environ. Toxicol. Chem. 2002, 21, 1699–1708. [Google Scholar] [CrossRef]
- Jobling, S.; Nolan, M.; Tyler, C.R.; Brighty, G.; Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998, 32, 2498–2506. [Google Scholar] [CrossRef]
- Markell, L.K.; Mingoia, R.T.; Peterson, H.M.; Yao, J.; Waters, S.M.; Finn, J.P.; Nabb, D.L.; Han, X. Endocrine disruption screening by protein and gene expression of vitellogenin in freshly isolated and cryopreserved rainbow trout hepatocytes. Chem. Res. Toxicol. 2014, 27, 1450–1457. [Google Scholar] [CrossRef]
- Gräns, J.; Wassmur, B.; Celander, M.C. One-way inhibiting cross-talk between arylhydrocarbon receptor (AhR) and estrogen receptor (ER) signaling in primary cultures of rainbow trout hepatocytes. Aquat. Toxicol. 2010, 100, 263–270. [Google Scholar] [CrossRef]
- Ricciardi, F.; Matozzo, V.; Marin, M.G. Effects of 4-nonylphenol exposure in mussels (Mytilus galloprovincialis) and crabs (Carcinus aestuarii) with particular emphasis on vitellogenin induction. Mar. Pollut. Bull. 2008, 57, 365–372. [Google Scholar] [CrossRef]
- Nolte, T.M.; De Cooman, W.; Vink, J.P.M.; Elst, R.; Ryken, E.; Ragas, A.M.J.; Hendriks, A.J. Bioconcentration of Organotin Cations during Molting Inhibits Heterocypris incongruens Growth. Environ. Sci. Technol. 2020, 54, 14288–14301. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Tian, X.; Zeng, B.; Wang, B.; Yu, F. The Retinoid X Receptor from Mud Crab: New Insights into Its Role in Vitellogenesis. Sci. Rep. 2016, 6, 23654. [Google Scholar] [CrossRef] [PubMed]
- Fenske, M.; van Aerle, R.; Brack, S.; Tyler, C.R.; Segner, H. Development and validation of a homologous zebrafish vitellogenin ELISA and its application for studies on estrogenic chemicals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 129, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Barucca, M.; Canapa, A.; Olmo, E.; Regoli, F. Analysis of vitellogenin gene induction as a valuable biomarker of estrogenic exposure in various Mediterranean fish species. Environ. Res. 2006, 101, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, U.; Maitra, S. Impact of metabolic disrupting chemicals on redox homeostasis, energy sensors, receptor modulation, and hormone metabolism: A comparative account in teleost and mammalian model organisms. Aquac. Fish. 2024, 9, 455–485. [Google Scholar] [CrossRef]
- Kinnberg, K.; Toft, G. Effects of estrogenic and antiandrogenic compounds on the testis structure of adult male guppies (Poecilia reticulata). Ecotoxicol. Environ. Saf. 2003, 54, 16–24. [Google Scholar] [CrossRef]
- Levi, L.; Pekarski, I.; Gutman, E.; Fortina, P.; Hyslop, T.; Biran, J.; Levavi-Sivan, B.; Lubzens, E. Revealing genes associated with vitellogenesis in the liver of the zebrafish (Danio rerio) by transcriptome profiling. BMC Genom. 2009, 10, 141. [Google Scholar] [CrossRef]
- Dorts, J.; Richter, C.A.; Wright-Osment, M.K.; Ellersieck, M.R.; Carter, B.J.; Tillitt, D.E. The genomic transcriptional response of female fathead minnows (Pimephales promelas) to an acute exposure to the androgen, 17β-trenbolone. Aquat. Toxicol. 2009, 91, 44–53. [Google Scholar] [CrossRef][Green Version]
- Rios-Sicairos, J.; Betancourt-Lozano, M.; Leal-Tarin, B.; Hernandez-Cornejo, R.; Aguilar-Zarate, G.; Garcia-De-La-Parra, L.M.; Gutierrez, J.N.; Marquez-Rocha, F.; Garcia-Gasca, A. Heat-shock protein (Hsp70) and cytochrome P-450 (CYP1A) in the white mullet Mugil curema (Pisces: Mugilidae) as biomarkers to assess environmental quality in coastal lagoons. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 68–74. [Google Scholar] [CrossRef]
- Kolli, R.T.; Glenn, T.C.; Bringolf, R.B.; Henderson, M.; Cummings, B.S.; Kenneke, J.F. Changes in CpG Methylation of the vtg1 Promoter in Adult Male Zebrafish after Exposure to 17α-Ethinylestradiol. Environ. Toxicol. Chem. 2024, 43, 1547–1556. [Google Scholar] [CrossRef]
- Borcier, E.; Artigaud, S.; Gaillard, J.C.; Armengaud, J.; Charrier, G.; Couteau, J.; Receveur, J.; Ouddane, B.; Diop, M.; Amara, R.; et al. Coupling caging and proteomics on the European flounder (Platichthys flesus) to assess the estuarine water quality at micro scale. Sci. Total Environ. 2019, 695, 133760. [Google Scholar] [CrossRef]
- See, M.J.; Bencic, D.C.; Flick, R.W.; Lazorchak, J.; Biales, A.D. Characterization of vitellogenin concentration in male fathead minnow mucus compared to plasma and liver mRNA. Ecotoxicol. Environ. Saf. 2022, 236, 113428. [Google Scholar] [CrossRef] [PubMed]
- Hennies, M.; Wiesmann, M.; Allner, B.; Sauerwein, H. Vitellogenin in carp (Cyprinus carpio) and perch (Perca fluviatilis): Purification, characterization and development of an ELISA for the detection of estrogenic effects. Sci. Total Environ. 2003, 309, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.A.; Singh, R.; Pandey, A.; Chauhan, R.S. Role of Sex-Biased miRNAs in Teleosts—A Review. Aquac. Res. 2021, 52, 5071–5086. [Google Scholar] [CrossRef]
- Hamilton, P.B.; Baynes, A.; Nicol, E.; Harris, G.; Uren Webster, T.M.; Beresford, N.; Straszkiewicz, M.; Jobling, S.; Tyler, C.R. Feminizing effects of ethinylestradiol in roach (Rutilus rutilus) populations with different estrogenic pollution exposure histories. Aquat. Toxicol. 2022, 249, 106229. [Google Scholar] [CrossRef]
- Baynes, A.; Lange, A.; Beresford, N.; Bryden, E.; Whitlock, K.; Tyler, C.R.; Jobling, S. Endocrine Disruption Is Reduced but Still Widespread in Wild Roach (Rutilus rutilus) Living in English Rivers. Environ. Sci. Technol. 2023, 57, 12632–12641. [Google Scholar] [CrossRef]
- Kirby, M.F.; Allen, Y.T.; Dyer, R.A.; Feist, S.W.; Katsiadaki, I.; Matthiessen, P.; Scott, A.P.; Smith, A.; Stentiford, G.D.; Thain, J.E.; et al. Surveys of Plasma Vitellogenin and Intersex in Male Flounder (Platichthys flesus) as Measures of Endocrine Disruption in UK Estuaries: Temporal Trends, 1996–2001. Environ. Toxicol. Chem. 2004, 23, 748–758. [Google Scholar] [CrossRef]
- George, S.; Gubbins, M.; MacIntosh, A.; Reynolds, W.; Sabine, V.; Scott, A.; Thain, J. A comparison of pollutant biomarker responses with transcriptional responses in European flounders (Platichthys flesus) subjected to estuarine pollution. Mar. Environ. Res. 2004, 58, 571–575. [Google Scholar] [CrossRef]
- Ríos-Sicairos, J.; García-Rodríguez, F.; Páez-Osuna, F.; Sánchez-Como, J.; Acosta-Vargas, B.; Frías-Espericueta, M.G. HSP70 and CYP1A in White Mullet (Mugil curema) as Biomarkers to Assess Environmental Quality in Coastal Lagoons. Mar. Environ. Res. 2010, 70, 11–16. [Google Scholar] [CrossRef]
- Cocci, P.; Mosconi, G.; Palermo, F.A. An In Silico and In Vitro Study for Investigating Estrogenic Endocrine Effects of Emerging Persistent Pollutants Using Primary Hepatocytes from Grey Mullet (Mugil cephalus). Environments 2021, 8, 58. [Google Scholar] [CrossRef]
- Ramos-Júdez, S.; Estévez, A.; González-López, W.Á.; Duncan, N. Lipid and fatty acid composition of muscle, liver, ovary, and peritoneal fat in wild flathead grey mullet (Mugil cephalus) according to ovarian development. Theriogenology 2023, 198, 317–326. [Google Scholar] [CrossRef]
- Ortiz-Zarragoitia, M.; Bizarro, C.; Rojo-Bartolomé, I.; Diaz de Cerio, O.; Cajaraville, M.P.; Cancio, I. Mugilid Fish Are Sentinels of Exposure to Endocrine Disrupting Compounds in Coastal and Estuarine Environments. Mar. Drugs 2014, 12, 4756–4782. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, D.; Vandeputte, M.; Sánchez-Baizán, N.; Allal, F.; Piferrer, F. Dynamic epimarks in sex-related genes predict gonad phenotype in the European sea bass, a fish with mixed genetic and environmental sex determination. Epigenetics 2018, 13, 988–1011. [Google Scholar] [CrossRef] [PubMed]
- Yadetie, F.; Zhang, X.; Hanna, E.M.; Aranguren-Abadía, L.; Eide, M.; Blaser, N.; Brun, M.; Jonassen, I.; Goksøyr, A.; Karlsen, O.A. RNA-Seq Analysis of Atlantic Cod Liver Slices Exposed to Benzo[a]pyrene and 17α-Ethinylestradiol. Aquat. Toxicol. 2018, 201, 174–186. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Du, J.; Liu, C.; Zhou, G.; Li, M.; Yan, Z. Application of Multi-Omics Techniques in Aquatic Ecotoxicology: A Review. Toxics 2025, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Blackwell, B.R.; Cavallin, J.E.; Doering, J.A.; Feifarek, D.J.; Jensen, K.M.; Kahl, M.D.; LaLone, C.A.; Poole, S.T.; Randolph, E.C.; et al. Adverse outcome pathway network-based assessment of the interactive effects of an androgen receptor agonist and an aromatase inhibitor on fish endocrine function. Environ. Toxicol. Chem. 2020, 39, 913–922. [Google Scholar] [CrossRef]
- Valdivieso, A.; Anastasiadi, D.; Ribas, L.; Piferrer, F. Development of Epigenetic Biomarkers for the Identification of Sex and Thermal Stress in Fish Using DNA Methylation Analysis and Machine Learning Procedures. Mol. Ecol. Resour. 2023, 23, 453–470. [Google Scholar] [CrossRef]
- Cannea, F.B.; Follesa, M.C.; Porcu, C.; Rossino, R.; Olianas, A.; Rescigno, A.; Padiglia, A. Antibodies targeting the European lobster (Palinurus elephas) vitellogenin developed by mRNA isolation and in-silico-designed antigenic peptides. Biol. Open 2022, 11, bio059019. [Google Scholar] [CrossRef]
- De Wit, M.; Keil, D.; van der Ven, K.; Vandamme, S.; Witters, E.; De Coen, W. An integrated transcriptomic and proteomic approach characterizing estrogenic and metabolic effects of 17α-ethinylestradiol in zebrafish (Danio rerio). Gen. Comp. Endocrinol. 2010, 167, 190–201. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Q.; Wang, H.; Wei, L.; Wang, S.; Li, S.; Yuan, D.; Wang, Z. Integrated transcriptomic and metabolomic analyses identify key factors in the vitellogenesis of juvenile Sichuan bream (Sinibrama taeniatus). Front. Mar. Sci. 2023, 10, 1243767. [Google Scholar] [CrossRef]
- Pinto, P.I.; Anjos, L.; Estêvão, M.D.; Santos, S.; Santa, C.; Manadas, B.; Monsinjon, T.; Canário, A.V.M.; Power, D.M. Proteomics of sea bass skin-scales exposed to the emerging pollutant fluoxetine compared to estradiol. Sci. Total Environ. 2022, 814, 152671. [Google Scholar] [CrossRef]
- Frelih, M.; Ayobahan, S.U.; Marghany, F.; Essfeld, F.; Eilebrecht, S. Toxicogenomic signatures of estrogen-related modes of action in the zebrafish embryo. Environ. Toxicol. Chem. 2025, 44, 2568–2579. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, D.L.; Bush, K.; Hazemi, M.; Hoang, J.X.; Le, M.; Blackwell, B.R.; Stacy, E.; Flynn, K.M. Derivation of Transcriptomics-Based Points of Departure for 20 Per- or Polyfluoroalkyl Substances Using a Larval Fathead Minnow (Pimephales promelas) Reduced Transcriptome Assay. Environ. Toxicol. Chem. 2024, 44, 2455–2469. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.M.; Le, M.; Hazemi, M.; Biales, A.; Bencic, D.C.; Blackwell, B.R.; Bush, K.; Flick, R.; Hoang, J.X.; Martinson, J.; et al. Comparing Transcriptomic Points of Departure to Apical Effect Concentrations for Larval Fathead Minnow Exposed to Chemicals with Four Different Modes of Action. Arch. Environ. Contam. Toxicol. 2024, 86, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, D.L.; Le, M.; Hazemi, M.; Biales, A.; Bencic, D.C.; Bush, K.; Flick, R.; Martinson, J.; Morshead, M.; Rodriguez, K.S.; et al. Pilot Testing and Optimization of a Larval Fathead Minnow High-Throughput Transcriptomics Assay. Curr. Res. Toxicol. 2022, 4, 100099. [Google Scholar] [CrossRef]
- O’Brien, J.; Mitchell, C.; Auerbach, S.; Doonan, L.; Ewald, J.; Everett, L.; Faranda, A.; Johnson, K.; Reardon, A.; Rooney, J.; et al. Bioinformatic Workflows for Deriving Transcriptomic Points of Departure: Current Status, Data Gaps, and Research Priorities. Toxicol. Sci. 2025, 203, 147–159. [Google Scholar] [CrossRef]
- Gasser, L.; Schür, C.; Perez-Cruz, F.; Schirmer, K.; Baity-Jesi, M. Machine Learning-Based Prediction of Fish Acute Mortality: Implementation, Interpretation, and Regulatory Relevance. Environ. Sci. Adv. 2024, 3, 1124–1138. [Google Scholar] [CrossRef]
- Nam, S.-E.; Kim, J.-H.; Kim, H.-J.; Park, S.; Lee, S.; Kim, B.-M.; Park, J.-C. Multi-Omics Integration Reveals Underlying Toxicological Mechanisms of Triclosan in the Freshwater Fish Zacco platypus. Aquat. Toxicol. 2025, 287, 107522. [Google Scholar] [CrossRef]
- Whitehead, A.; Pilcher, W.; Champlin, D.; Nacci, D. Common mechanism underlies repeated evolution of extreme pollution tolerance. Proc. R. Soc. B 2012, 279, 427–433. [Google Scholar] [CrossRef]
- Whitehead, A.; Clark, B.W.; Reid, N.M.; Hahn, M.E.; Nacci, D. When evolution is the solution to pollution: Key principles, and lessons from rapid repeated adaptation of killifish (Fundulus heteroclitus) populations. Evol. Appl. 2017, 10, 762–783. [Google Scholar] [CrossRef]
- Ajisafe, O.M.; Adekunle, Y.A.; Egbon, E.; Ogbonna, C.E.; Olawade, D.B. The role of machine learning in predictive toxicology: A review of current trends and future perspectives. Life Sci. 2025, 378, 123821. [Google Scholar] [CrossRef]
- Conolly, R.B.; Ankley, G.T.; Cheng, W.; Mayo, M.L.; Miller, D.H.; Perkins, E.J.; Villeneuve, D.L.; Watanabe, K.H. Quantitative adverse outcome pathways and their application to predictive toxicology. Environ. Sci. Technol. 2017, 51, 4661–4672. [Google Scholar] [CrossRef]
- Piccardo, M.; Vellani, V.; Anselmi, S.; Grazioli, E.; Renzi, M.; Terlizzi, A.; Pittura, L.; D’Errico, G.; Regoli, F.; Bevilacqua, S. The application of the weight-of-evidence approach for an integrated ecological risk assessment of marine protected sites. Ecol. Indic. 2024, 159, 111676. [Google Scholar] [CrossRef]
- OECD. Endocrine Disrupting Chemicals in Freshwater: Monitoring and Regulating Water Quality; OECD Studies on Water; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Wernersson, A.S.; Carere, M.; Maggi, C.; Tusil, P.; Soldan, P.; James, A.; Sanchez, W.; Dulio, V.; Broeg, K.; Reifferscheid, G.; et al. The European technical report on aquatic effect-based monitoring tools under the Water Framework Directive. Environ. Sci. Eur. 2015, 27, 7. [Google Scholar] [CrossRef]
| Taxonomic Group | EDCs | Main Effects | Sex-Specific Effects | Representative References |
|---|---|---|---|---|
| Fish (P. promelas) | 17α-ethinylestradiol (EE2) | VTG induction, intersex development, population collapse in whole-lake experiment | Males strongly induced; females affected at reproductive stages | [38,39] |
| Fish (R. rutilus) | Nonylphenol, wastewater effluents | VTG induction, intersex gonads, feminization of wild populations | Feminization in males; females within physiological baseline | [34,35] |
| Fish (various freshwater species) | Atrazine | Aromatase induction, altered sex steroid balance, feminization | Males overexpress aromatase; feminization observed | [32,33] |
| Fish (G. morhua) | 17α-ethinylestradiol (EE2) | Hepatic reprogramming of lipid metabolism and immune pathways (multi-omics) | Sex-biased transcriptional shifts, stronger in males | [76] |
| Fish (P. flesus) | Field exposure in polluted estuaries | Co-induction of VTG, choriogenins, HSP70/90; evidence of estrogenic disruption | Male induction of VTG/Chg; females less responsive | [77] |
| Mollusks (Mytilus spp.) | Bisphenol A (BPA), EE2 | Disrupted embryogenesis, altered lipid metabolism and vitellogenesis; inconsistent VTG-like induction | Sex-dependent responses unclear; variable results among studies | [36,37,40,41,71] |
| Mollusks (Crassostrea gigas) | Xenoestrogens (e.g., BPA, EE2) | Altered DNA methylation and non-coding RNAs; disruption of gametogenesis | Epigenetic changes may affect gametes differently between sexes | [45] |
| Crustaceans (general, e.g., crabs, amphipods) | Organotins, pesticides | Disruption of EcR/RXR signaling; impaired molting, vitellogenesis, and reproduction | Sex-dependent reproductive impairment observed | [79,80,81,82,83] |
| Crustaceans (Marsupenaeus japonicus) | Methyl farnesoate (MF), 20-hydroxyecdysone (20E) | Experimental exposure confirmed disruption of molting and reproduction (EcR/RXR axis) | Disruption of hormone balance affects both sexes differently | [84] |
| Gene | Gene Product and Function | Main EDCs | Regulation by EDCs | Molecular Mechanism | Sex-Specific Responses | References |
|---|---|---|---|---|---|---|
| vtg (D. rerio, salmonids, R. rutilus) | VTG (yolk precursor protein) | EE2, BPA, NP | Strongly upregulated | ERα → EREs; promoter hypomethylation enhances induction | Negligible baseline in males → strong induction under EDCs; physiological in females | [100,101,102,103,104,105,106,107] |
| zp2, zp3 (teleosts) | ZP2/ZP3 (zona pellucida proteins, egg envelope) | EE2, BPA | Upregulated | ER-dependent transcription; co-regulated with vtg | Female-biased; weak/no expression in males under control conditions | [88,90] |
| chg (teleosts) | Chg (choriogenins, egg envelope glycoproteins) | EE2, NP | Upregulated | ERα-mediated transcription | Strong biomarker in males (ectopic induction) | [106,107] |
| cyp19a1a (teleosts, amphibians) | CYP19A1A (Aromatase A, estrogen synthesis) | Atrazine, BPA | Induced | Steroidogenesis; conversion androgens → estrogens | Overexpression in males → feminization | [91,110] |
| cyp1a (teleosts, invertebrates) | CYP1A (cytochrome P450 1A, detoxification enzyme) | Dioxins, PCBs | Upregulated | AhR/ARNT → XREs; antagonizes ER signaling | Suppression of vtg in males | [97,98] |
| star (teleosts) | StAR (steroidogenic acute regulatory protein, cholesterol transport) | BPA, metals | Altered | Steroid hormone biosynthesis | Affects sex steroid balance in both sexes | [109] |
| hsd family (teleosts) | HSDs (hydroxysteroid dehydrogenases, steroid metabolism) | BPA, metals | Modulated | Steroidogenesis | Key in androgen/estrogen ratio; dysregulation impacts fertility | [91,110] |
| dnmt (teleosts, mollusks) | DNMTs (DNA methyltransferases, epigenetic regulators) | BPA, EE2 | Altered | DNA methylation changes at promoters (vtg1, cyp19a1a) | Sex-specific promoter methylation (e.g., vtg1 hypo in females, hyper in males) | [108,113] |
| miR-200 family (D. rerio) | miR-200 family (epigenetic regulators of spermatogenesis) | EE2 | Upregulated | p53–miR-200 axis | Reduced sperm motility in males | [111,112] |
| vtg-like (bivalves, e.g., Mytilus spp.) | VTG-like yolk precursor proteins | BPA, EE2 | Inconsistent induction | Estrogenic signaling vs. oxidative stress cross-talk | Variable; induction sometimes absent in males/females | [118,119,120] |
| EcR/RXR (crustaceans) | Ecdysone receptor/Retinoid X receptor (molting, reproduction) | Organotins, pesticides | Disrupted | Interference with 20E–MF axis | Impaired molting and vitellogenesis; sex-dependent reproductive impairment | [92,93,121,122] |
| VTG Genes and Paralogs (Species) | Physiological Induction (Females) | Induction Under EDC Exposure (Males and Juveniles) | Main Regulatory Pathway | Notes | References |
|---|---|---|---|---|---|
| vtgAa, vtgAb (D. rerio) | Strong induction during vitellogenesis under estrogen control | Robust induction by EE2, BPA, Nonylphenol | ERα binding EREs; promoter demethylation | Essential for oocyte maturation and fertility | [100,102,123,125,129,130,137] |
| vtgC (D. rerio) | Weakly responsive in females | Minimal induction under EDCs | Weak ERα responsiveness | Secondary role; subfunctionalized paralog | [101,104,105] |
| multiple vtg paralogs (salmonids, e.g., Salmo salar) | Several paralogs highly induced; others weak | Differential induction depending on paralog | ERα/EREs; subfunctionalization | Large gene arrays due to genome duplication | [103,105,106] |
| VTG-like proteins (Mytilus spp.) | Baseline expression variable | Sometimes induced by BPA, Nonylphenol, EE2 (inconsistent) | ER-related transcripts; stress pathways | Biomarker value debated, not consistent | [118,119,120,133] |
| VTG-like transcripts (crustaceans, e.g., crabs, shrimps) | VTG expression increases during female vitellogenesis under hormonal control | Limited induction after organotin and other EDC exposure; responses vary across taxa | RXR/EcR pathways; interaction with methyl farnesoate and 20-hydroxyecdysone | VTG investigated as a potential biomarker in crustaceans, but responses remain variable across taxa | [21,84,85,135] |
| Biomarker | Species and Taxa | Response in Males | Response in Females | Assay | Ecotoxicological Relevance | References |
|---|---|---|---|---|---|---|
| VTG | Fish (Cyprinis, Salmonids) | Strong induction | Baseline fluctuation | ELISA, qPCR, Western blot | Sensitive biomarker of estrogenic exposure | [15,16,17,18,106,136,137,146] |
| ERα/ERβ isoforms | Teleosts | Differential modulation | Variable (cycle-dependent) | qPCR, RNA-Seq | Key regulators of VTG and reproduction | [88,89,90,125] |
| miR-200 cluster | D. rerio | Reduced sperm motility | Not determined | qPCR, small RNA-seq | Epigenetic marker of fertility disruption | [111,112,147] |
| DNMTs/ncRNAs | Mollusks (Mytilus, Crassostrea) | Altered methylation, disrupted ncRNAs | Gametogenesis impairment | qPCR, methylome analysis | Epigenetic biomarkers of endocrine disruption in invertebrates | [20,36,37,113] |
| EcR/RXR signaling | Crustaceans (crabs, amphipods, shrimps) | Impaired molting and reproduction | Altered vitellogenesis | Transcriptomics, receptor assays | Biomarkers of endocrine disruption via the MF/20E hormonal pathway | [21,60,84,85,135] |
| Omics Approach | Advantages | Limitations | Representative Examples | Relevance to Sex-Specific Responses and VTG | References |
|---|---|---|---|---|---|
| Transcriptomics | Genome-wide sensitivity; reveals estrogenic signatures; identifies co-regulated pathways | High data complexity; requires bioinformatic pipelines | EE2—D. rerio (estrogenic signatures); RNA-Seq—G. morhua (immune/metabolic reprogramming); M. edulis (biomarkers of endocrine disruption) | Sex-biased regulation of vtg, zp2/3, chg; stronger shifts in males due to lower baseline estrogen | [24,117,148,149] |
| Proteomics | Links directly to protein biomarkers; detects post-translational modifications | Requires species-specific antibodies; less standardized across taxa | Male C. variegatus—hepatic remodeling under EE2; P. flesus (field proteomics: VTG, Chg, HSP70/90); M. galloprovincialis (oxidative stress) | Confirms VTG isoform induction; links sex-specific stress to protein remodeling | [11,12,120,140,141] |
| Metabolomics | Captures functional metabolic shifts; sensitive to physiological disruption | Lower mechanistic specificity; metabolites can be transient | R. rutilus—EE2 reduced circulating steroids; G. morhua (multi-omics: systemic reprogramming); mussels (BDE-47/TBBPA: altered energy metabolism) | Sex-dependent metabolic trade-offs; VTG synthesis linked to lipid/energy metabolism | [24,25,119] |
| Epigenomics | Reveals persistent and transgenerational effects; potential biomarkers | Limited cross-species data; methods still emerging in ecotox | D. rerio—vtg1 promoter methylation under EE2; O. latipes—methylation shifts (BPA/EE2); M. galloprovincialis—altered dnmt; C. gigas—ncRNA disruption | Explains sex-specific sensitivity (promoter methylation differences); reveals heritable effects beyond VTG induction | [10,73,111,112,143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannea, F.B.; Porcu, C.; Follesa, M.C.; Padiglia, A. Sex-Specific Molecular and Genomic Responses to Endocrine Disruptors in Aquatic Species: The Central Role of Vitellogenin. Genes 2025, 16, 1317. https://doi.org/10.3390/genes16111317
Cannea FB, Porcu C, Follesa MC, Padiglia A. Sex-Specific Molecular and Genomic Responses to Endocrine Disruptors in Aquatic Species: The Central Role of Vitellogenin. Genes. 2025; 16(11):1317. https://doi.org/10.3390/genes16111317
Chicago/Turabian StyleCannea, Faustina Barbara, Cristina Porcu, Maria Cristina Follesa, and Alessandra Padiglia. 2025. "Sex-Specific Molecular and Genomic Responses to Endocrine Disruptors in Aquatic Species: The Central Role of Vitellogenin" Genes 16, no. 11: 1317. https://doi.org/10.3390/genes16111317
APA StyleCannea, F. B., Porcu, C., Follesa, M. C., & Padiglia, A. (2025). Sex-Specific Molecular and Genomic Responses to Endocrine Disruptors in Aquatic Species: The Central Role of Vitellogenin. Genes, 16(11), 1317. https://doi.org/10.3390/genes16111317








