Effects of Exercise Addiction and the COL1A1 Gene rs1800012 Polymorphism on Injury Susceptibility in Elite Female Volleyball Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
2.3. Experimental Approach
2.4. Genotyping
2.5. Evaluation of Exercise Addiction
2.6. Statistical Analyses
3. Results
3.1. Association Between Exercise Addiction and Injury Susceptibility
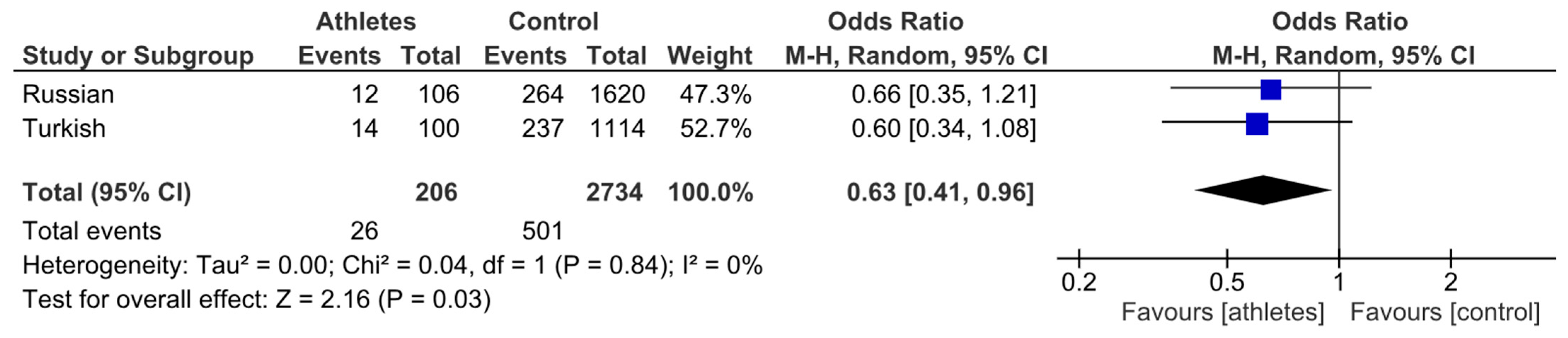
3.2. Association Between the COL1A1 rs1800012 Polymorphism, Volleyball Player Status, and Injury Susceptibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACL | Anterior cruciate ligament |
| COL1A1 | Collagen Type I Alpha 1 Chain |
| DNA | Deoxyribonucleic acid |
| EAS | Exercise Addiction Scale |
| PCR | Polymerase chain reaction |
| SNP | Single-nucleotide polymorphism |
References
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A.; Bahr, R.; Beynnon, B.D.; Demaio, M.; Dick, R.W.; Engebretsen, L.; Garrett, W.E., Jr.; Hannafin, J.A.; et al. Understanding and Preventing Noncontact Anterior Cruciate Ligament Injuries: A Review of the Hunt Valley II Meeting, January 2005. Am. J. Sports Med. 2006, 34, 1512–1532. [Google Scholar] [CrossRef]
- Emery, C.A.; Pasanen, K. Current Trends in Sport Injury Prevention. Best Pract. Res. Clin. Rheumatol. 2019, 33, 3–15. [Google Scholar] [CrossRef]
- Lohmander, L.S.; Ostenberg, A.; Englund, M.; Roos, H. High Prevalence of Knee Osteoarthritis, Pain, and Functional Limitations in Female Soccer Players Twelve Years after Anterior Cruciate Ligament Injury. Arthritis Rheum. 2004, 50, 3145–3152. [Google Scholar] [CrossRef]
- Podlog, L.; Eklund, R.C. Return to Sport after Serious Injury: A Retrospective Examination of Motivation and Psychological Outcomes. J. Sport Rehabil. 2005, 14, 20–34. [Google Scholar] [CrossRef]
- Appaneal, R.N.; Levine, B.R.; Perna, F.M.; Roh, J.L. Measuring Postinjury Depression among Male and Female Competitive Athletes. J. Sport Exerc. Psychol. 2009, 31, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Soligard, T.; Myklebust, G.; Steffen, K.; Holme, I.; Silvers, H.; Bizzini, M.; Junge, A.; Dvorak, J.; Bahr, R.; Andersen, T.E. Comprehensive Warm-Up Programme to Prevent Injuries in Young Female Footballers: Cluster Randomised Controlled Trial. BMJ 2008, 337, a2469. [Google Scholar] [CrossRef] [PubMed]
- Emery, C.A.; Meeuwisse, W.H. The Effectiveness of a Neuromuscular Prevention Strategy to Reduce Injuries in Youth Soccer: A Cluster-Randomised Controlled Trial. Br. J. Sports Med. 2010, 44, 555–562. [Google Scholar] [CrossRef]
- Vriend, I.; Gouttebarge, V.; Finch, C.F.; van Mechelen, W.; Verhagen, E.A.L.M. Intervention Strategies Used in Sport Injury Prevention Studies: A Systematic Review Identifying Studies Applying the Haddon Matrix. Sports Med. 2017, 47, 2027–2043. [Google Scholar] [CrossRef] [PubMed]
- John, G.; AlNadwi, A.; Georges Abi Antoun, T.; Ahmetov, I.I. Injury Prevention Strategies in Female Football Players: Addressing Sex-Specific Risks. Sports 2025, 13, 39. [Google Scholar] [CrossRef]
- Hewett, T.E.; Myer, G.D.; Ford, K.R. Reducing Knee and Anterior Cruciate Ligament Injuries among Female Athletes: A Systematic Review of Neuromuscular Training Interventions. J. Knee Surg. 2005, 18, 82–88. [Google Scholar] [CrossRef]
- Gabbett, T.J. The Training-Injury Prevention Paradox: Should Athletes Be Training Smarter and Harder? Br. J. Sports Med. 2016, 50, 273–280. [Google Scholar] [CrossRef]
- Bertelsen, M.L.; Hulme, A.; Petersen, J.; Brund, R.K.; Sørensen, H.; Finch, C.F.; Parner, E.T.; Nielsen, R.O. A Framework for the Etiology of Running-Related Injuries. Scand. J. Med. Sci. Sports 2017, 27, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, A.; Johnson, U.; Andersen, M.B.; Tranaeus, U.; Stenling, A.; Lindwall, M. Psychosocial Factors and Sport Injuries: Meta-Analyses for Prediction and Prevention. Sports Med. 2017, 47, 353–365. [Google Scholar] [CrossRef]
- Chhabra, B.; Nazlıgül, M.D.; Szabo, A. Exercise Addiction in Team Sports: A Systematic Literature Review. Scand. J. Psychol. 2024, 65, 846–857. [Google Scholar] [CrossRef]
- Juwono, I.D.; Tolnai, N.; Szabo, A. Exercise Addiction in Athletes: A Systematic Review of the Literature. Int. J. Ment. Health Addict. 2022, 20, 3113–3127. [Google Scholar] [CrossRef]
- Berczik, K.; Szabó, A.; Griffiths, M.D.; Kurimay, T.; Kun, B.; Urbán, R.; Demetrovics, Z. Exercise Addiction: Symptoms, Diagnosis, Epidemiology, and Etiology. Subst. Use Misuse 2012, 47, 403–417. [Google Scholar] [CrossRef]
- Lichtenstein, M.B.; Nielsen, R.O.; Gudex, C.; Hinze, C.J.; Jørgensen, U. Exercise Addiction Is Associated with Emotional Distress in Injured and Non-Injured Regular Exercisers. Addict. Behav. Rep. 2018, 8, 33–39. [Google Scholar] [CrossRef]
- Collins, M.; Posthumus, M.; Schwellnus, M.P. The COL1A1 Gene and Acute Soft Tissue Ruptures. Br. J. Sports Med. 2010, 44, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, H.; Chen, K.; Wu, B.; Liu, H. Association of Polymorphisms Rs1800012 in COL1A1 with Sports-Related Tendon and Ligament Injuries: A Meta-Analysis. Oncotarget 2017, 8, 27627–27634. [Google Scholar] [CrossRef]
- Brazier, J.; Antrobus, M.; Stebbings, G.K.; Day, S.H.; Heffernan, S.M.; Cross, M.J.; Williams, A.G. Tendon and Ligament Injuries in Elite Rugby: The Potential Genetic Influence. Sports 2019, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Bulgay, C.; Kasakolu, A.; Bıyıklı, T.; Koncagul, S.; Kazan, H.H.; Ahmetov, I.I.; Ergun, M.A.; Griffiths, M.D.; Szabo, A. Genome-Wide Association Study of Exercise Addiction among Elite Wrestlers. Brain Sci. 2025, 15, 102. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; John, G.; Semenova, E.A.; Hall, E.C.R. Genomic Predictors of Physical Activity and Athletic Performance. Adv. Genet. 2024, 111, 311–408. [Google Scholar] [CrossRef]
- Boulygina, E.A.; Borisov, O.V.; Valeeva, E.V.; Semenova, E.A.; Kostryukova, E.S.; Kulemin, N.A.; Larin, A.K.; Nabiullina, R.M.; Mavliev, F.A.; Akhatov, A.M.; et al. Whole Genome Sequencing of Elite Athletes. Biol. Sport 2020, 37, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fuku, N.; Miyamoto-Mikami, E.; Tanaka, M.; Miyachi, M.; Murakami, H.; Mitchell, B.; Morrison, E.; Ahmetov, I.; Sportgene Research Group; et al. Multi-Phase, Multi-Ethnic GWAS Uncovers Putative Loci in Predisposition to Elite Sprint and Power Performance, Health and Disease. Biol. Sport 2025, 42, 141–159. [Google Scholar] [CrossRef]
- Maciejewska-Skrendo, A.; Sawczuk, M.; Cięszczyk, P.; Ahmetov, I.I. Genes and power athlete status. In Sports, Exercise, and Nutritional Genomics: Current Status and Future Directions; Barh, D., Ahmetov, I., Eds.; Academic Press: London, UK, 2019; pp. 41–72. [Google Scholar] [CrossRef]
- Moreland, E.; Borisov, O.V.; Semenova, E.A.; Larin, A.K.; Andryushchenko, O.N.; Andryushchenko, L.B.; Generozov, E.V.; Williams, A.G.; Ahmetov, I.I. Polygenic profile of elite strength athletes. J. Strength Cond. Res. 2022, 36, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Massidda, M.; Voisin, S.; Culigioni, C.; Piras, F.; Cugia, P.; Yan, X.; Eynon, N.; Calò, C.M. ACTN3 R577X Polymorphism Is Associated with the Incidence and Severity of Injuries in Professional Football Players. Clin. J. Sport Med. 2019, 29, 57–61. [Google Scholar] [CrossRef]
- Feldmann, D.C.; Rahim, M.; Suijkerbuijk, M.A.M.; Laguette, M.N.; Cieszczyk, P.; Ficek, K.; Huminska-Lisowska, K.; Häger, C.K.; Stattin, E.; Nilsson, K.G.; et al. Investigation of Multiple Populations Highlight VEGFA Polymorphisms to Modulate Anterior Cruciate Ligament Injury. J. Orthop. Res. 2022, 40, 1604–1612. [Google Scholar] [CrossRef]
- Murtagh, C.F.; Hall, E.C.R.; Brownlee, T.E.; Drust, B.; Williams, A.G.; Erskine, R.M. The Genetic Association with Athlete Status, Physical Performance, and Injury Risk in Soccer. Int. J. Sports Med. 2023, 44, 941–960. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Roos, T.R.; Roos, A.K.; Kleimeyer, J.P.; Ahmed, M.A.; Goodlin, G.T.; Fredericson, M.; Ioannidis, J.P.; Avins, A.L.; Dragoo, J.L. Genome-Wide Association Screens for Achilles Tendon and ACL Tears and Tendinopathy. PLoS ONE 2017, 12, e0170422. [Google Scholar] [CrossRef]
- Gibbon, A.; Saunders, C.J.; Collins, M.; Gamieldien, J.; September, A.V. Defining the Molecular Signatures of Achilles Tendi-nopathy and Anterior Cruciate Ligament Ruptures: A Whole-Exome Sequencing Approach. PLoS ONE 2018, 13, e0205860. [Google Scholar] [CrossRef]
- Mann, V.; Hobson, E.E.; Li, B.; Stewart, T.L.; Grant, S.F.; Robins, S.P.; Aspden, R.M.; Ralston, S.H. A COL1A1 Sp1 Binding Site Polymorphism Predisposes to Osteoporotic Fracture by Affecting Bone Density and Quality. J. Clin. Investig. 2001, 107, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Stewart, T.L.; Hof, R.V.; Reid, D.M.; Aspden, R.M.; Ralston, S. A Rare Haplotype in the Upstream Regulatory Region of COL1A1 Is Associated with Reduced Bone Quality and Hip Fracture. J. Bone Miner. Res. 2009, 24, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Leźnicka, K.; Żyżniewska-Banaszak, E.; Gębska, M.; Machoy-Mokrzyńska, A.; Krajewska-Pędzik, A.; Maciejewska-Skrendo, A.; Leońska-Duniec, A. Interactions between Gene Variants within the COL1A1 and COL5A1 Genes and Musculoskeletal Injuries in Physically Active Caucasians. Genes 2021, 12, 1056. [Google Scholar] [CrossRef]
- Stępien-Słodkowska, M.; Ficek, K.; Eider, J.; Leońska-Duniec, A.; Maciejewska-Karłowska, A.; Sawczuk, M.; Zarębska, A.; Jastrzębski, Z.; Grenda, A.; Kotarska, K.; et al. The +1245G/T Polymorphisms in the Collagen Type I Alpha 1 (COL1A1) Gene in Polish Skiers with Anterior Cruciate Ligament Injury. Biol. Sport 2013, 30, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Vijayvargiya, P.; Camilleri, M.; Cima, R.R. COL1A1 Mutations Presenting as Descending Perineum Syndrome in a Young Patient with Hypermobility Syndrome. Mayo Clin. Proc. 2018, 93, 386–391. [Google Scholar] [CrossRef]
- Saito, M.; Ginszt, M.; Semenova, E.A.; Massidda, M.; Huminska-Lisowska, K.; Michałowska-Sawczyn, M.; Homma, H.; Cięszczyk, P.; Okamoto, T.; Larin, A.K.; et al. Is COL1A1 Gene Rs1107946 Polymorphism Associated with Sport Climbing Status and Flexibility? Genes 2022, 13, 403. [Google Scholar] [CrossRef]
- Barbitoff, Y.A.; Khmelkova, D.N.; Pomerantseva, E.A.; Slepchenkov, A.V.; Zubashenko, N.A.; Mironova, I.V.; Kaimonov, V.S.; Polev, D.E.; Tsay, V.V.; Glotov, A.S.; et al. Expanding the Russian allele frequency reference via cross-laboratory data integration: Insights from 7452 exome samples. Natl. Sci. Rev. 2024, 11, nwae326. [Google Scholar] [CrossRef]
- RUSeq Database. Available online: http://ruseq.ru (accessed on 24 August 2025).
- Demir, G.T.; Hazar, Z.; Cicioğlu, H.İ. Exercise Addiction Scale (EAS): A Study of Validity and Reliability. Kastamonu Educ. J. 2018, 26, 865–874. [Google Scholar]
- Ordu, F. Egzersiz Bağımlılığı: Bir Güncelleme. Bağımlılık Derg. 2022, 23, 536–546. [Google Scholar] [CrossRef]
- Szabo, A.; Griffiths, M.D.; de la Vega Marcos, R.; Mervó, B.; Demetrovics, Z. Methodological and Conceptual Limitations in Exercise Addiction Research. Yale J. Biol. Med. 2015, 88, 303–308. [Google Scholar]
- Caru, M.; Poulnais, S.; Gorwood, P.; Kern, L. Exercise Addiction, Pain and Injuries in Amateur Athletes. Sport Sci. Health 2022, 18, 1253–1261. [Google Scholar] [CrossRef]
- Weinstein, A.; Szabo, A. Exercise Addiction: A Narrative Overview of Research Issues. Dialogues Clin. Neurosci. 2023, 25, 1–13. [Google Scholar] [CrossRef]
- Egorov, A.Y.; Szabo, A. The Exercise Paradox: An Interactional Model for a Clearer Conceptualization of Exercise Addiction. J. Behav. Addict. 2013, 2, 199–208. [Google Scholar] [CrossRef]
- de la Vega, R.; Parastatidou, I.S.; Ruíz-Barquín, R.; Szabo, A. Exercise Addiction in Athletes and Leisure Exercisers: The Moderating Role of Passion. J. Behav. Addict. 2016, 5, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Wright, C.; Winrow, D. Exercise Dependence and Social Physique Anxiety in Competitive and Non-Competitive Runners. Int. J. Sport Exerc. Psychol. 2010, 8, 61–69. [Google Scholar] [CrossRef]
- Szabo, A.; de la Vega, R.; Ruiz-Barquín, R.; Rivera, O. Exercise Addiction in Spanish Athletes: Investigation of the Roles of Gender, Social Context and Level of Involvement. J. Behav. Addict. 2013, 2, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Schorb, A.; Niebauer, J.; Aichhorn, W.; Schiepek, G.; Scherr, J.; Claussen, M.C. Overtraining from a Sports Psychiatry Perspective. Dtsch. Z. Sportmed. 2021, 72, 271–279. [Google Scholar] [CrossRef]
- Khoschnau, S.; Melhus, H.; Jacobson, A. Type I Collagen Alpha1 Sp1 Polymorphism and the Risk of Cruciate Ligament Ruptures or Shoulder Dislocations. Am. J. Sports Med. 2008, 36, 2432–2436. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Downs, D.S. Exercise Dependence: A Systematic Review. Psychol. Sport Exerc. 2002, 3, 89–123. [Google Scholar] [CrossRef]
- Varillas-Delgado, D.; Del Coso, J.; Gutiérrez-Hellín, J.; Aguilar-Navarro, M.; Muñoz, A.; Maestro, A.; Morencos, E. Genetics and Sports Performance: The Present and Future in the Identification of Talent for Sports Based on DNA Testing. Eur. J. Appl. Physiol. 2022, 122, 1811–1830. [Google Scholar] [CrossRef]
- Lee, J.; Bridge, J.E.; Clark, D.R.; Stewart, C.E.; Erskine, R.M. Collagen Supplementation Augments Changes in Patellar Tendon Properties in Female Soccer Players. Front. Physiol. 2023, 14, 1089971. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tanaka, M.; Eynon, N.; North, K.N.; Williams, A.G.; Collins, M.; Moran, C.N.; Britton, S.L.; Fuku, N.; Ashley, E.A.; et al. The Future of Genomic Research in Athletic Performance and Adaptation to Training. Genet. Sport 2016, 61, 55–67. [Google Scholar] [CrossRef]
- Eynon, N.; Nasibulina, E.S.; Banting, L.K.; Cieszczyk, P.; Maciejewska-Karlowska, A.; Sawczuk, M.; Bondareva, E.A.; Shagimardanova, R.R.; Raz, M.; Sharon, Y.; et al. The FTO A/T polymorphism and elite athletic performance: A study involving three groups of European athletes. PLoS ONE 2013, 8, e60570. [Google Scholar] [CrossRef] [PubMed]
- Varillas-Delgado, D.; Gutierrez-Hellín, J.; Maestro, A. Genetic Profile in Genes Associated with Sports Injuries in Elite Endurance Athletes. Int. J. Sports Med. 2023, 44, 64–71. [Google Scholar] [CrossRef]
- Massidda, M.; Flore, L.; Cugia, P.; Piras, F.; Scorcu, M.; Kikuchi, N.; Cięszczyk, P.; Maciejewska-Skrendo, A.; Tocco, F.; Calò, C.M. Association Between Total Genotype Score and Muscle Injuries in Top-Level Football Players: A Pilot Study. Sports Med. Open 2024, 10, 22. [Google Scholar] [CrossRef]
- Maestro, A.; Del Coso, J.; Aguilar-Navarro, M.; Gutiérrez-Hellín, J.; Morencos, E.; Revuelta, G.; Ruiz Casares, E.; Perucho, T.; Varillas-Delgado, D. Genetic Profile in Genes Associated with Muscle Injuries and Injury Etiology in Professional Soccer Players. Front. Genet. 2022, 13, 1035899. [Google Scholar] [CrossRef]
- Del Coso, J.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F.; Herrero, D.; Puente, C. Polygenic Profile and Exercise-Induced Muscle Damage by a Competitive Half-Ironman. J. Strength Cond. Res. 2020, 34, 1400–1408. [Google Scholar] [CrossRef] [PubMed]

| Variable | Value | |
|---|---|---|
| Age | ||
| Mean ± SD | 26.2 ± 4.65 | |
| Median (min–max) | 25.5 (19–38) | |
| Height (cm) | ||
| Mean ± SD | 178.9 ± 8.54 | |
| Median (min–max) | 180.0 (158–199) | |
| Weight (kg) | ||
| Mean ± SD | 67.9 ± 7.6 | |
| Median (min–max) | 68.5 (50–83) | |
| Position | n | % |
| Libero | 12 | 24.0 |
| Middle player | 15 | 30.0 |
| Setter | 9 | 18.0 |
| Spiker | 14 | 28.0 |
| Duration of play | ||
| 4–7 years | 3 | 6.0 |
| 8–10 years | 6 | 12.0 |
| ≥11 years | 41 | 82.0 |
| Collagen supplementation | ||
| Yes | 37 | 74.0 |
| No | 13 | 26.0 |
| Variable | n | % | |
|---|---|---|---|
| Severe Injury | 1–5 | 42 | 84.0 |
| None | 8 | 16.0 | |
| Surgery | Yes | 13 | 26.0 |
| No | 37 | 74.0 | |
| Return to sport following injury | 1–3 weeks | 15 | 31.25 |
| 1–2 months | 9 | 18.75 | |
| 3–4 months | 3 | 6.25 | |
| ≥5 months | 12 | 25.00 | |
| No injuries | 9 | 18.75 | |
| EAS Parameter | Min | Max | Mean ± SD |
|---|---|---|---|
| Total EAS score | 40.0 | 71.0 | 56.12 ± 7.49 |
| EAS–Extreme focus and emotional transformation | 15.0 | 34.0 | 26.02 ± 4.04 |
| EAS–Delaying of personal social needs and conflict | 11.0 | 28.0 | 18.42 ± 3.65 |
| EAS–Development of tolerance and passion | 6.0 | 18.0 | 11.68 ± 2.53 |
| Variable | Experienced Severe Injury | n | Mean ± SD | Z | p and r |
|---|---|---|---|---|---|
| EAS (Total) | 1–5 | 42 | 56.76 ± 7.63 | −1.524 | 0.128; −0.22 |
| None | 8 | 52.75 ± 5.99 | |||
| EAS–Extreme focus and emotional transformation | 1–5 | 42 | 25.95 ± 4.29 | −0.160 | 0.873; −0.02 |
| None | 8 | 26.38 ± 2.56 | |||
| EAS–Delaying of personal social needs and conflict | 1–5 | 42 | 18.90 ± 3.61 | −2.099 | 0.036 *; −0.30 |
| None | 8 | 15.88 ± 2.80 | |||
| EAS–Development of tolerance and passion | 1–5 | 42 | 11.90 ± 2.53 | −1.378 | 0.168; −0.19 |
| None | 8 | 10.50 ± 2.33 |
| Variable | Collagen Supplementation | n | Mean ± SD | Z | p and r |
|---|---|---|---|---|---|
| EAS (Total) | Yes | 37 | 55.70 ± 7.15 | −0.875 | 0.382; −0.12 |
| No | 13 | 57.31 ± 8.57 | |||
| EAS–Extreme focus and emotional transformation | Yes | 37 | 25.59 ± 3.83 | −1.515 | 0.130; −0.21 |
| No | 13 | 27.23 ± 4.53 | |||
| EAS–Delaying of personal social needs and conflict | Yes | 37 | 18.92 ± 3.74 | −1.61 | 0.107; −0.23 |
| No | 13 | 17.0 ± 3.08 | |||
| EAS–Development of tolerance and passion | Yes | 37 | 11.19 ± 2.26 | −2.561 | 0.01 *; −0.36 |
| No | 13 | 13.08 ± 2.81 |
| Group | n | T Allele | G Allele | T Allele Frequency, % | G Allele Frequency, % |
|---|---|---|---|---|---|
| Turkish athletes | 50 | 14 | 86 | 14.0 | 86.0 |
| Turkish controls | 557 | 237 | 877 | 21.3 | 78.7 |
| Russian athletes | 53 | 12 | 94 | 11.3 | 88.7 |
| Russian controls | 810 | 264 | 1356 | 16.3 | 83.7 |
| Return to Sport Following Injury | COL1A1 rs1800012 Genotypes | p | |
|---|---|---|---|
| GG | GT | ||
| 1–3 weeks | 10 | 5 | 0.009 * |
| 1–2 months | 8 | 1 | |
| 3–4 months | 0 | 3 | |
| ≥5 months | 7 | 5 | |
| No injuries | 9 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piri, M.M.; Cerit, M.; Anılır, M.; Polat, T.; Karaduman, A.A.; Szabo, A.; Antoun, T.G.A.; John, G.; Semenova, E.A.; Larin, A.K.; et al. Effects of Exercise Addiction and the COL1A1 Gene rs1800012 Polymorphism on Injury Susceptibility in Elite Female Volleyball Players. Genes 2025, 16, 1300. https://doi.org/10.3390/genes16111300
Piri MM, Cerit M, Anılır M, Polat T, Karaduman AA, Szabo A, Antoun TGA, John G, Semenova EA, Larin AK, et al. Effects of Exercise Addiction and the COL1A1 Gene rs1800012 Polymorphism on Injury Susceptibility in Elite Female Volleyball Players. Genes. 2025; 16(11):1300. https://doi.org/10.3390/genes16111300
Chicago/Turabian StylePiri, Muhammed Mustafa, Mesut Cerit, Murat Anılır, Tolga Polat, Aynur Ayşe Karaduman, Attila Szabo, Tiffany Georges Abi Antoun, George John, Ekaterina A. Semenova, Andrey K. Larin, and et al. 2025. "Effects of Exercise Addiction and the COL1A1 Gene rs1800012 Polymorphism on Injury Susceptibility in Elite Female Volleyball Players" Genes 16, no. 11: 1300. https://doi.org/10.3390/genes16111300
APA StylePiri, M. M., Cerit, M., Anılır, M., Polat, T., Karaduman, A. A., Szabo, A., Antoun, T. G. A., John, G., Semenova, E. A., Larin, A. K., Kulemin, N. A., Generozov, E. V., & Ahmetov, I. I. (2025). Effects of Exercise Addiction and the COL1A1 Gene rs1800012 Polymorphism on Injury Susceptibility in Elite Female Volleyball Players. Genes, 16(11), 1300. https://doi.org/10.3390/genes16111300









