Identification of a Four-Gene Signature Based on Metal Metabolism for Alzheimer’s Disease Diagnosis
Abstract
1. Introduction
2. Materials and Methods
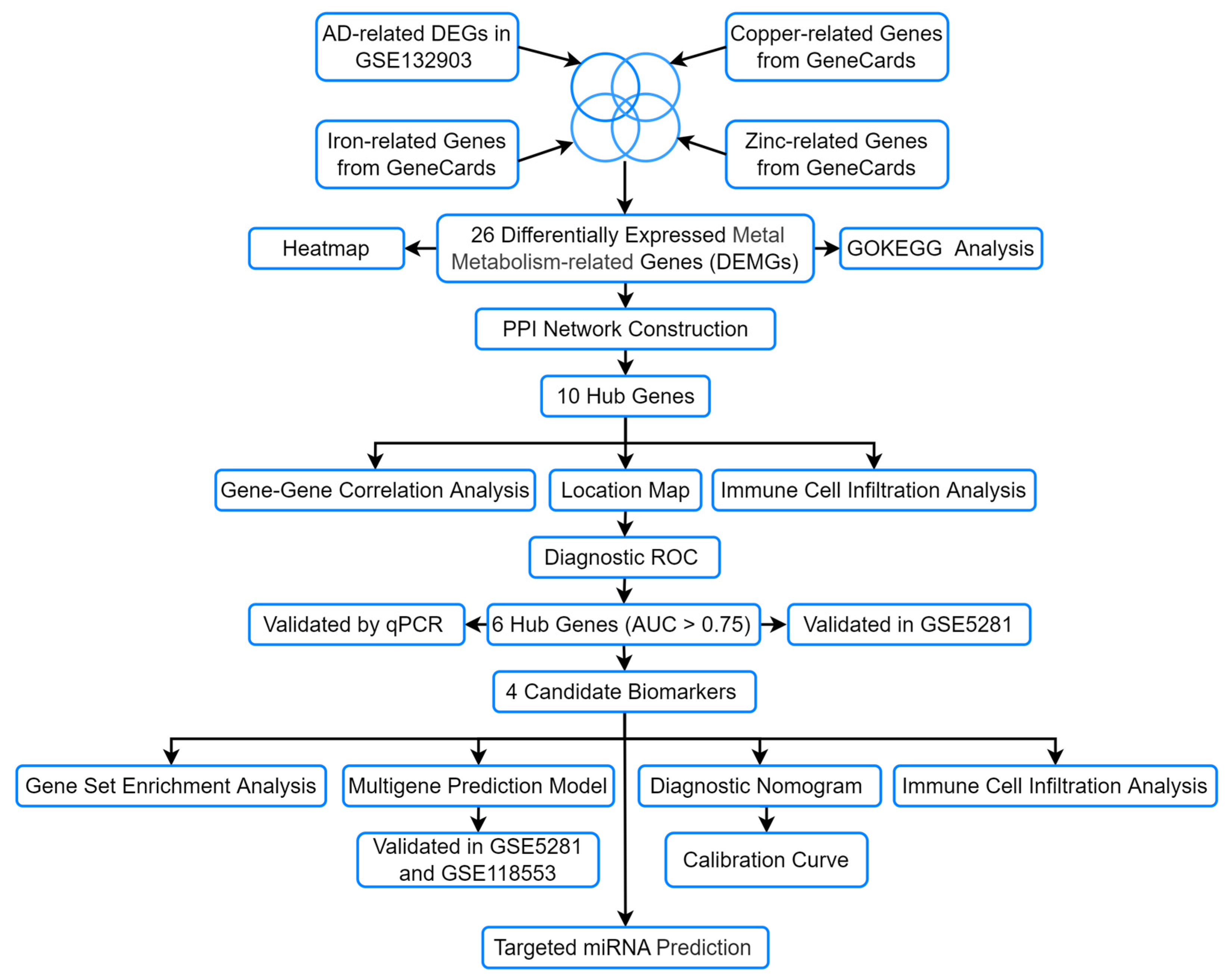
2.1. Data Acquisition and Preprocessing
2.2. Identification of DEGs and DEMGs
2.3. Biological Functional and Enrichment Analysis of DEMGs
2.4. PPI Network Construction and Hub Gene Identification
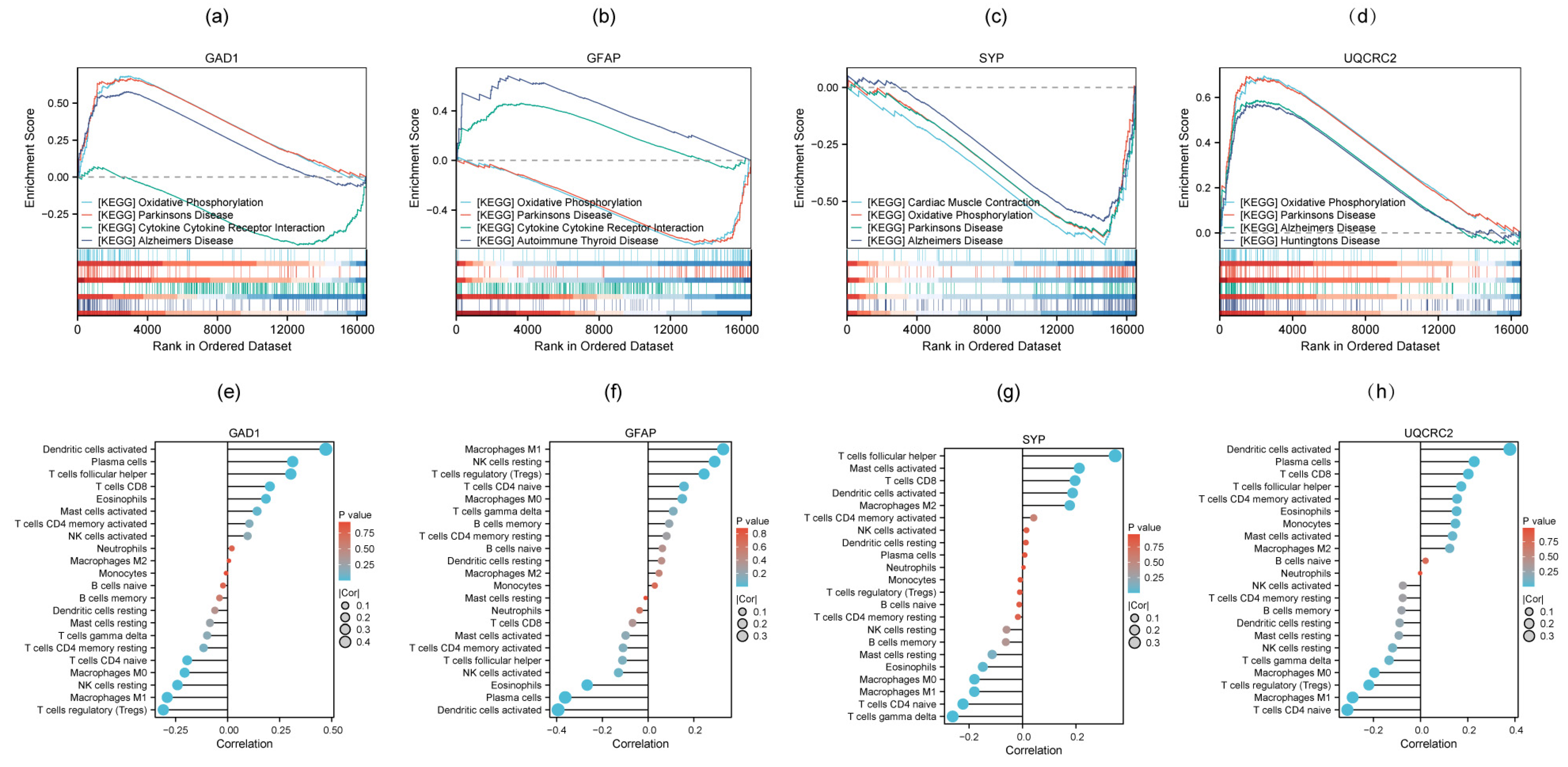
2.5. Immune Cell Infiltration Analysis
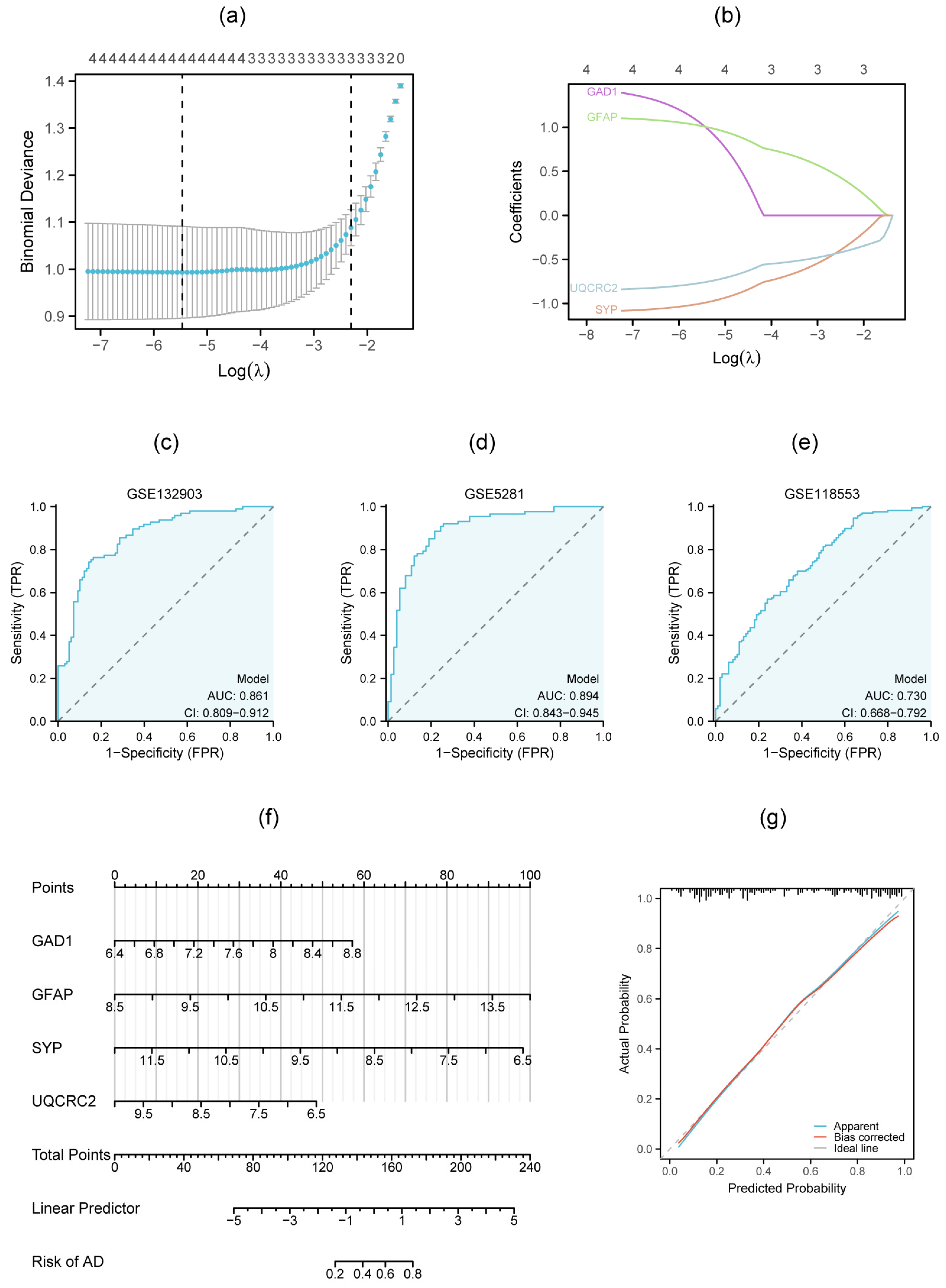
2.6. Construction of ROC Curves and a Diagnostic Model
2.7. Cell Culture and qRT-PCR
2.8. External Dataset Validation
2.9. Gene Set Enrichment Analysis (GSEA)
2.10. Exploration of microRNAs Targeting the Hub Genes
3. Results
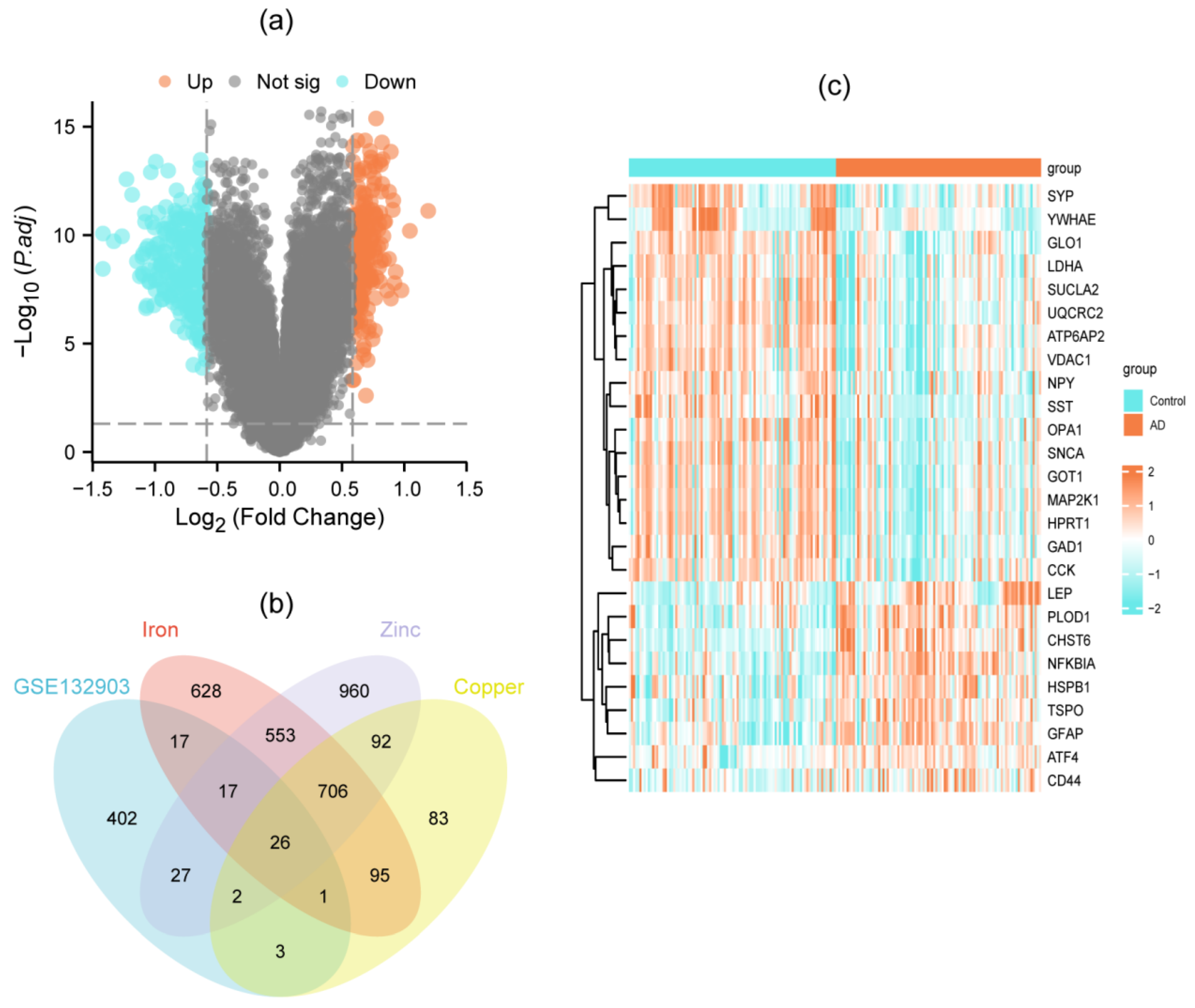
3.1. Identification of DEMGs
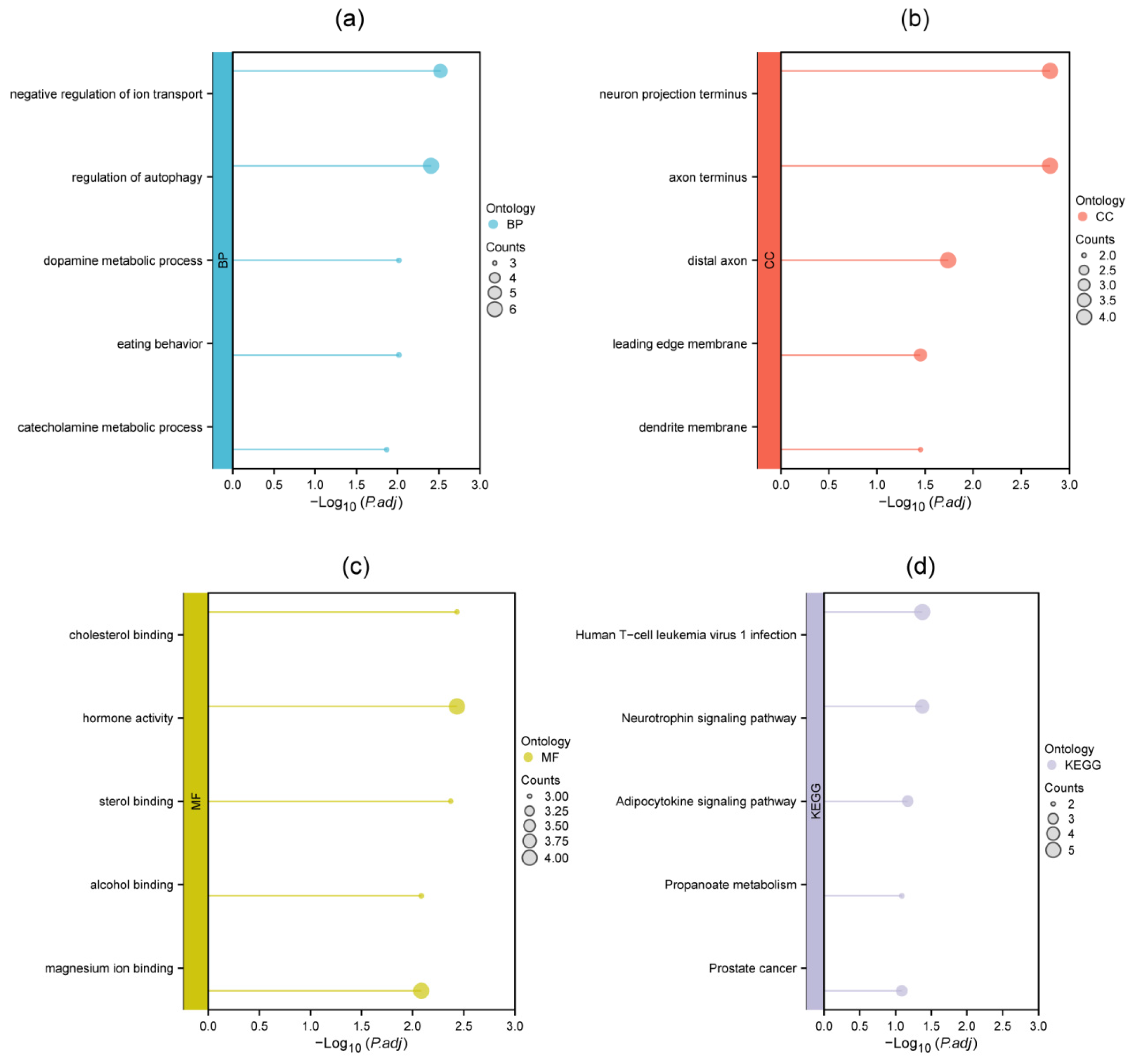
3.2. GO and KEGG Enrichment Analysis of DEMGs
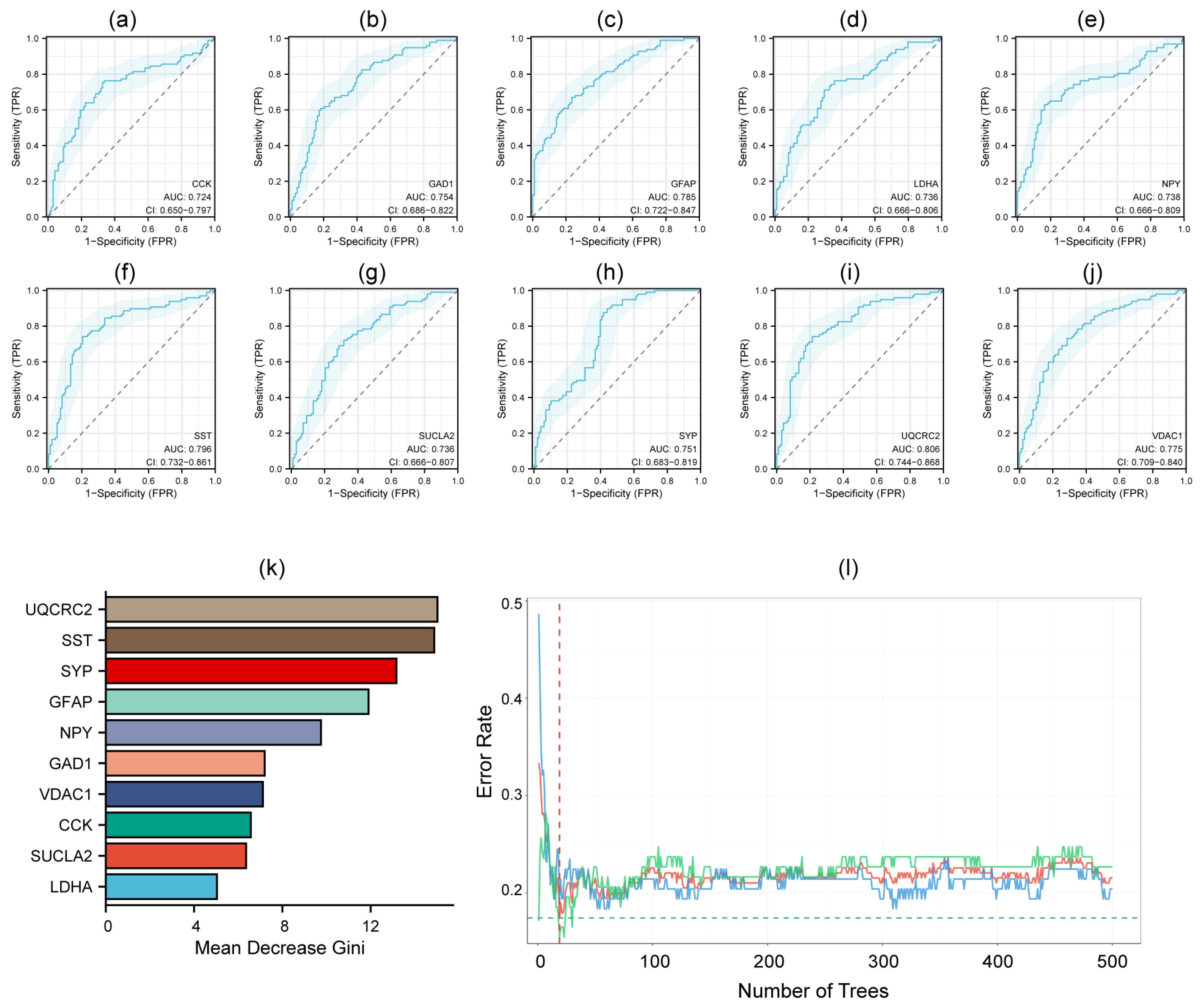
3.3. PPI Network Construction and Hub Genes Identification
3.4. Diagnostic ROC Model for Hub Genes
3.5. Validation of Hub Genes by qRT-PCR and External Dataset
3.6. GSEA and Immune Cell Infiltration Analysis of Four Candidate Biomarkers
3.7. Multigenic Prediction Model and Nomogram Construction
3.8. Identification of miRNAs Targeting Metal Metabolism-Related Biomarkers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayton, S.; Lei, P.; Bush, A.I. Metallostasis in Alzheimer’s disease. Free Radic. Biol. Med. 2013, 62, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, X.S.; Ren, K.D.; Peng, J.; Luo, X.J. Restoration of metal homeostasis: A potential strategy against neurodegenerative diseases. Ageing Res. Rev. 2023, 87, 101931. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Gu, W.; Fu, S.; Zou, G.; Jiang, Z. Zinc homeostasis regulates caspase activity and inflammasome activation. PLoS Pathog. 2024, 20, e1012805. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Liu, J.; Zhang, X.; Huang, Y.; Zhu, Z.; Tang, S.; Wang, W.; Ge, Z.; Yu, H.; He, M. Association of antioxidants use with the risk of dementia among community-dwelling adults in the United Kingdom biobank. Front. Nutr. 2023, 10, 1270179. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Tapia, V.S.; White, C.S.; Daniels, M.J.D.; Drinkall, S.; Kennedy, P.T.; Spence, H.G.; Yu, S.; Green, J.P.; Hoyle, C.; et al. Zinc Status Alters Alzheimer’s Disease Progression through NLRP3-Dependent Inflammation. J. Neurosci. 2021, 41, 3025–3038. [Google Scholar] [CrossRef]
- Kozin, S.A. Role of Interaction between Zinc and Amyloid Beta in Pathogenesis of Alzheimer’s Disease. Biochemistry 2023, 88, S75–S87. [Google Scholar] [CrossRef]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Crosstalk of copper and zinc in the pathogenesis of vascular dementia. J. Clin. Biochem. Nutr. 2022, 71, 7–15. [Google Scholar] [CrossRef]
- Kawahara, M.; Tanaka, K.I.; Kato-Negishi, M. Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia. Int. J. Mol. Sci. 2021, 22, 7242. [Google Scholar] [CrossRef]
- Everett, J.; Lermyte, F.; Brooks, J.; Tjendana-Tjhin, V.; Plascencia-Villa, G.; Hands-Portman, I.; Donnelly, J.M.; Billimoria, K.; Perry, G.; Zhu, X.; et al. Biogenic metallic elements in the human brain? Sci. Adv. 2021, 7, eabf6707. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Shi, W.; Lu, L.; Zhou, Q.; Pu, Y.; Yin, L. Copper exposure induces neurotoxicity through ferroptosis in C. elegans. Chem. Biol. Interact. 2025, 407, 111369. [Google Scholar] [CrossRef]
- Wang, T.; Xu, S.F.; Fan, Y.G.; Li, L.B.; Guo, C. Iron Pathophysiology in Alzheimer’s Diseases. Adv. Exp. Med. Biol. 2019, 1173, 67–104. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, J. Iron Metabolism, Ferroptosis, and the Links With Alzheimer’s Disease. Front. Neurosci. 2019, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chang, X.; Lang, M. Iron Homeostasis Disorder and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2442. [Google Scholar] [CrossRef] [PubMed]
- Everett, J.; Brooks, J.; Tjendana Tjhin, V.; Lermyte, F.; Hands-Portman, I.; Plascencia-Villa, G.; Perry, G.; Sadler, P.J.; O’Connor, P.B.; Collingwood, J.F.; et al. Label-Free In Situ Chemical Characterization of Amyloid Plaques in Human Brain Tissues. ACS Chem. Neurosci. 2024, 15, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Su, X.; Qin, J.; Jin, Y.; Zhang, N.; Huang, S. Identification of Autophagy-Related Biomarkers and Diagnostic Model in Alzheimer’s Disease. Genes 2024, 15, 1027. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Li, Z.; Wang, S.; Yu, G.; Wang, L. Identification ferroptosis-related hub genes and diagnostic model in Alzheimer’s disease. Front. Mol. Neurosci. 2023, 16, 1280639. [Google Scholar] [CrossRef]
- Yan, R.; Wang, W.; Yang, W.; Huang, M.; Xu, W. Mitochondria-Related Candidate Genes and Diagnostic Model to Predict Late-Onset Alzheimer’s Disease and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2024, 99, S299–S315. [Google Scholar] [CrossRef]
- Zhang, Y.; Kiryu, H. Identification of oxidative stress-related genes differentially expressed in Alzheimer’s disease and construction of a hub gene-based diagnostic model. Sci. Rep. 2023, 13, 6817. [Google Scholar] [CrossRef]
- Piras, I.S.; Krate, J.; Delvaux, E.; Nolz, J.; Mastroeni, D.F.; Persico, A.M.; Jepsen, W.M.; Beach, T.G.; Huentelman, M.J.; Coleman, P.D. Transcriptome Changes in the Alzheimer’s Disease Middle Temporal Gyrus: Importance of RNA Metabolism and Mitochondria-Associated Membrane Genes. J. Alzheimer’s Dis. 2019, 70, 691–713. [Google Scholar] [CrossRef]
- Liang, W.S.; Dunckley, T.; Beach, T.G.; Grover, A.; Mastroeni, D.; Walker, D.G.; Caselli, R.J.; Kukull, W.A.; McKeel, D.; Morris, J.C.; et al. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol. Genom. 2007, 28, 311–322. [Google Scholar] [CrossRef]
- Liang, W.S.; Dunckley, T.; Beach, T.G.; Grover, A.; Mastroeni, D.; Ramsey, K.; Caselli, R.J.; Kukull, W.A.; McKeel, D.; Morris, J.C.; et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: A reference data set. Physiol. Genom. 2008, 33, 240–256. [Google Scholar] [CrossRef]
- Liang, W.S.; Reiman, E.M.; Valla, J.; Dunckley, T.; Beach, T.G.; Grover, A.; Niedzielko, T.L.; Schneider, L.E.; Mastroeni, D.; Caselli, R.; et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 4441–4446. [Google Scholar] [CrossRef]
- Patel, H.; Hodges, A.K.; Curtis, C.; Lee, S.H.; Troakes, C.; Dobson, R.J.B.; Newhouse, S.J. Transcriptomic analysis of probable asymptomatic and symptomatic Alzheimer brains. Brain Behav. Immun. 2019, 80, 644–656. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Xu, Q.; Cheng, H.; Tan, X. The Efficacy and Pharmacological Mechanism of Zn7MT3 to Protect against Alzheimer’s Disease. Sci. Rep. 2017, 7, 13763. [Google Scholar] [CrossRef]
- Cui, C.; Zhong, B.; Fan, R.; Cui, Q. HMDD v4.0: A database for experimentally supported human microRNA-disease associations. Nucleic Acids Res. 2024, 52, D1327–D1332. [Google Scholar] [CrossRef]
- Lu, L.; Dai, W.Z.; Zhu, X.C.; Ma, T. Analysis of Serum miRNAs in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2021, 36, 15333175211021712. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Lu, Y.; Zhang, T.; Zhao, W. Serum aberrant expression of miR-24-3p and its diagnostic value in Alzheimer’s disease. Biomark. Med. 2021, 15, 1499–1507. [Google Scholar] [CrossRef]
- Wu, H.Z.Y.; Thalamuthu, A.; Cheng, L.; Fowler, C.; Masters, C.L.; Sachdev, P.; Mather, K.A. Differential blood miRNA expression in brain amyloid imaging-defined Alzheimer’s disease and controls. Alzheimer’s Res. Ther. 2020, 12, 59. [Google Scholar] [CrossRef]
- Zhang, N.; Li, W.W.; Lv, C.M.; Gao, Y.W.; Liu, X.L.; Zhao, L. miR-16-5p and miR-19b-3p prevent amyloid β-induced injury by targeting BACE1 in SH-SY5Y cells. NeuroReport 2020, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, P.; Xi, J.; Cui, J.; Campbell, M.; Pham, W.; Matsubara, J.A. MicroRNAs in tear fluids predict underlying molecular changes associated with Alzheimer’s disease. Life Sci. Alliance 2023, 6, e202201757. [Google Scholar] [CrossRef] [PubMed]
- Dobricic, V.; Schilling, M.; Schulz, J.; Zhu, L.S.; Zhou, C.W.; Fuß, J.; Franzenburg, S.; Zhu, L.Q.; Parkkinen, L.; Lill, C.M.; et al. Differential microRNA expression analyses across two brain regions in Alzheimer’s disease. Transl. Psychiatry 2022, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Serpente, M.; Fenoglio, C.; D’Anca, M.; Arcaro, M.; Sorrentino, F.; Visconte, C.; Arighi, A.; Fumagalli, G.G.; Porretti, L.; Cattaneo, A.; et al. MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer’s Disease. Cells 2020, 9, 1443. [Google Scholar] [CrossRef]
- Ghiam, S.; Eslahchi, C.; Shahpasand, K.; Habibi-Rezaei, M.; Gharaghani, S. Exploring the role of non-coding RNAs as potential candidate biomarkers in the cross-talk between diabetes mellitus and Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 955461. [Google Scholar] [CrossRef]
- Qin, H.; Hu, C.; Zhao, X.; Tian, M.; Zhu, B. Usefulness of candidate mRNAs and miRNAs as biomarkers for mild cognitive impairment and Alzheimer’s disease. Int. J. Neurosci. 2023, 133, 89–102. [Google Scholar] [CrossRef]
- Hajjri, S.N.; Sadigh-Eteghad, S.; Mehrpour, M.; Moradi, F.; Shanehbandi, D.; Mehdizadeh, M. Beta-Amyloid-Dependent miRNAs as Circulating Biomarkers in Alzheimer’s Disease: A Preliminary Report. J. Mol. Neurosci. 2020, 70, 871–877. [Google Scholar] [CrossRef]
- De Felice, B.; Montanino, C.; Oliva, M.; Bonavita, S.; Di Onofrio, V.; Coppola, C. MicroRNA Expression Signature in Mild Cognitive Impairment Due to Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 4408–4416. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Zhang, C.; Qin, L.; Liao, X.; Zhao, L. The close relationship between trace elements (Cu, Fe, Zn, Se, Rb, Si, Cr, and V) and Alzheimer’s disease: Research progress and insights. J. Trace Elem. Med Biol 2025, 90, 127692. [Google Scholar] [CrossRef]
- Garza, N.M.; Swaminathan, A.B.; Maremanda, K.P.; Zulkifli, M.; Gohil, V.M. Mitochondrial copper in human genetic disorders. Trends Endocrinol. Metab. 2023, 34, 21–33. [Google Scholar] [CrossRef]
- Cendrowska-Pinkosz, M.; Ostrowska-Lesko, M.; Ognik, K.; Krauze, M.; Juskiewicz, J.; Dabrowska, A.; Szponar, J.; Mandziuk, S. Dietary Copper Deficiency Leads to Changes in Gene Expression Indicating an Increased Demand for NADH in the Prefrontal Cortex of the Rat’s Brain. Int. J. Mol. Sci. 2022, 23, 6706. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, J.; Liu, T.; Jia, R.; Yang, L.; Sun, P.; Zhao, W. Copper metabolism and hepatocellular carcinoma: Current insights. Front. Oncol. 2023, 13, 1186659. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Shin, S.; Yang, S.J. Iron Homeostasis and Energy Metabolism in Obesity. Clin. Nutr. Res. 2022, 11, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Panghal, A.; Jadhav, K.; Thakur, A.; Verma, R.K.; Singh, C.; Goyal, M.; Kumar, J.; Namdeo, A.G. Recent Advances in Targeting Transition Metals (Copper, Iron, and Zinc) in Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 10916–10940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dischler, A.; Glover, K.; Qin, Y. Neuronal signalling of zinc: From detection and modulation to function. Open Biol. 2022, 12, 220188. [Google Scholar] [CrossRef]
- Tsang, B.L.; Holsted, E.; McDonald, C.M.; Brown, K.H.; Black, R.; Mbuya, M.N.N.; Grant, F.; Rowe, L.A.; Manger, M.S. Effects of Foods Fortified with Zinc, Alone or Cofortified with Multiple Micronutrients, on Health and Functional Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1821–1837. [Google Scholar] [CrossRef]
- Gale, J.; Aizenman, E. The physiological and pathophysiological roles of copper in the nervous system. Eur. J. Neurosci. 2024, 60, 3505–3543. [Google Scholar] [CrossRef]
- Carello-Collar, G.; Bellaver, B.; Ferreira, P.C.L.; Ferrari-Souza, J.P.; Ramos, V.G.; Therriault, J.; Tissot, C.; De Bastiani, M.A.; Soares, C.; Pascoal, T.A.; et al. The GABAergic system in Alzheimer’s disease: A systematic review with meta-analysis. Mol. Psychiatry 2023, 28, 5025–5036. [Google Scholar] [CrossRef]
- Wan, L.; Zhong, P.; Li, P.; Ren, Y.; Wang, W.; Yu, M.; Feng, H.Y.; Yan, Z. CRISPR-based epigenetic editing of Gad1 improves synaptic inhibition and cognitive behavior in a Tauopathy mouse model. Neurobiol. Dis. 2025, 206, 106826. [Google Scholar] [CrossRef]
- Peretti, D.E.; Boccalini, C.; Ribaldi, F.; Scheffler, M.; Marizzoni, M.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Frisoni, G.B.; Garibotto, V. Association of glial fibrillary acid protein, Alzheimer’s disease pathology and cognitive decline. Brain 2024, 147, 4094–4104. [Google Scholar] [CrossRef]
- Leipp, F.; Vialaret, J.; Mohaupt, P.; Coppens, S.; Jaffuel, A.; Niehoff, A.C.; Lehmann, S.; Hirtz, C. Glial fibrillary acidic protein in Alzheimer’s disease: A narrative review. Brain Commun. 2024, 6, fcae396. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ye, S.; Wang, Y.; Chen, H.; Che, P.; Chen, J.; Zhang, N. Plasma biomarkers for diagnosis and differentiation and their cognitive correlations in patients with Alzheimer’s disease. Brain Commun. 2025, 7, fcaf094. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Z.; Liu, Y.; Duan, K.; Guan, J. Delivery of miR-15b-5p via magnetic nanoparticle-enhanced bone marrow mesenchymal stem cell-derived extracellular vesicles mitigates diabetic osteoporosis by targeting GFAP. Cell Biol. Toxicol. 2024, 40, 52. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Kim, J.A.; Park, G.; Cho, U. Aberrant synaptophysin expression in classic Hodgkin lymphoma. Diagn. Pathol. 2022, 17, 90. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, A.; Yang, H.; Wang, Q.; Ren, B.; Guo, T.; Qiang, J.; Cao, H.; Gao, Y.J.; Xu, L.; et al. “Olfactory three-needle” acupuncture enhances synaptic function in Aβ1-42-induced Alzheimer’s disease via activating PI3K/AKT/GSK-3β signaling pathway. J. Integr. Neurosci. 2021, 20, 55–65. [Google Scholar] [CrossRef]
- Chen, F.; Bai, J.; Zhong, S.; Zhang, R.; Zhang, X.; Xu, Y.; Zhao, M.; Zhao, C.; Zhou, Z. Molecular Signatures of Mitochondrial Complexes Involved in Alzheimer’s Disease via Oxidative Phosphorylation and Retrograde Endocannabinoid Signaling Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 9565545. [Google Scholar] [CrossRef]
- Younas, A.; Younas, N.; Iqbal, M.J.; Ferrer, I.; Zerr, I. Comparative interactome mapping of Tau-protein in classical and rapidly progressive Alzheimer’s disease identifies subtype-specific pathways. Neuropathol. Appl. Neurobiol. 2024, 50, e12964. [Google Scholar] [CrossRef]
- Firdous, S.M.; Khan, S.A.; Maity, A. Oxidative stress-mediated neuroinflammation in Alzheimer’s disease. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 8189–8209. [Google Scholar] [CrossRef]
- Budhkar, A.; Tang, Z.; Liu, X.; Zhang, X.; Su, J.; Song, Q. xSiGra: Explainable model for single-cell spatial data elucidation. Brief. Bioinform. 2024, 25, bbae388. [Google Scholar] [CrossRef]



| Dataset | GSE132903 | GSE5281 | GSE118553 | ||||
| Type | Microarray | Microarray | Microarray | ||||
| Platform | GPL10558 | GPL570 | GPL10558 | ||||
| Genetic source | Middle temporal gyrus | Entorhinal cortex, hippocampus, medial temporal gyrus, posterior cingulate, superior frontal gyrus, primary visual cortex | frontal cortex, temporal cortex, entorhinal cortex, cerebellum | ||||
| Groups | Control | AD | Control | AD | Control | AsymAD | AD |
| Number | 98 | 97 | 74 | 87 | 100 | 134 | 167 |
| Age | 84.98 ± 6.90 | 85.02 ± 6.75 | 79.51 ± 8.92 | 79.32 ± 7.25 | 70.44 ± 15.79 | 86.28 ± 8.59 | 82.92 ± 10.20 |
| Gender | |||||||
| Male | 50 | 49 | 53 | 51 | 55 | 40 | 69 |
| Female | 48 | 48 | 21 | 36 | 45 | 94 | 98 |
| Gene Symbol | logFC | p.adj | Description |
|---|---|---|---|
| CCK | −0.61 | 3.64 × 10−7 | Cholecystokinin |
| GAD1 | −0.99 | 2.51 × 10−9 | Glutamate decarboxylase 1 |
| GFAP | 1.04 | 6.36 × 10−11 | Glial fibrillary acidic protein |
| LDHA | −0.61 | 1.73 × 10−8 | Lactate dehydrogenase A |
| NPY | −0.65 | 1.23 × 10−7 | Neuropeptide Y |
| SST | −0.81 | 5.91 × 10−10 | Somatostatin |
| SUCLA2 | −0.67 | 6.31 × 10−9 | Succinate-CoA ligase ADP-forming subunit beta |
| SYP | −0.95 | 2.21 × 10−9 | Synaptophysin |
| UQCRC2 | −0.66 | 7.29 × 10−12 | Ubiquinol-cytochrome c reductase core protein 2 |
| VDAC1 | −0.64 | 1.13 × 10−9 | Voltage dependent anion channel 1 |
| Gene Symbol | CCK | GAD1 | GFAP | LDHA | NPY | SST | SUCLA2 | SYP | UQCRC2 | VDAC1 |
|---|---|---|---|---|---|---|---|---|---|---|
| CCK | 0.83 | −0.64 | 0.58 | 0.67 | 0.65 | 0.63 | 0.61 | 0.57 | 0.67 | |
| GAD1 | 0.83 | −0.66 | 0.68 | 0.65 | 0.77 | 0.72 | 0.57 | 0.70 | 0.74 | |
| GFAP | −0.64 | −0.66 | −0.49 | −0.40 | −0.50 | −0.66 | −0.20 | −0.65 | −0.57 | |
| LDHA | 0.58 | 0.68 | −0.49 | 0.61 | 0.63 | 0.89 | 0.55 | 0.85 | 0.89 | |
| NPY | 0.67 | 0.65 | −0.40 | 0.61 | 0.76 | 0.64 | 0.57 | 0.62 | 0.67 | |
| SST | 0.65 | 0.77 | −0.50 | 0.63 | 0.76 | 0.67 | 0.67 | 0.64 | 0.70 | |
| SUCLA2 | 0.63 | 0.72 | −0.66 | 0.89 | 0.64 | 0.67 | 0.50 | 0.92 | 0.92 | |
| SYP | 0.61 | 0.57 | −0.20 | 0.55 | 0.57 | 0.67 | 0.50 | 0.48 | 0.64 | |
| UQCRC2 | 0.57 | 0.70 | −0.65 | 0.85 | 0.62 | 0.64 | 0.92 | 0.48 | 0.90 | |
| VDAC1 | 0.67 | 0.74 | −0.57 | 0.89 | 0.67 | 0.70 | 0.92 | 0.64 | 0.90 |
| Gene Symbol | miRNA Name | Description |
|---|---|---|
| GAD1 | hsa-miR-24-3p | Down-regulated in AD [29]; has certain value in the diagnosis of AD and may be a potential biomarker [30]. |
| GFAP | hsa-miR-15b-5p | Showed consistent differential expression in AD compared to controls [31]. |
| GFAP | hsa-miR-16-5p | Relieved amyloid beta-induced injury by targeting BACE1 in SH-SY5Y cells; protective agents for treatment of Alzheimer’s disease [32]. |
| GFAP | hsa-miR-15a-5p | Showed significant up-regulations in the tear fluids of transgenic mice with disease progression, as tracked by cortical Abeta load and reactive astrogliosis [33]. |
| GFAP | hsa-miR-195-5p | Upregulation of miR-195-5p might be related to tau pathology [34]. |
| SYP | hsa-miR-190a-3p | Significantly upregulated in small neural-derived extracellular vesicles from AD patients [35]. |
| SYP | hsa-miR-423-5p | Proposed as new candidate biomarkers in the cross-talk between Diabetes Mellitus and Alzheimer’s Disease [36]. |
| SYP | hsa-miR-6734-5p | Remarkably upregulated in the MCI and AD groups; miR-6734-3p and its target mRNA CYTH4 might be used as novel biomarkers for MCI and AD [37]. |
| UQCRC2 | hsa-miR-4422 | Reduced expression of miR4422 that targets GSAP and BACE1 expression can lead to an increase in the formation of Abeta plaque; could be a reliable biomarker for Alzheimer’s diagnosis [38]. |
| UQCRC2 | hsa-miR-567 | Differentially expressed in cerebrospinal fluid samples, blood and serum from MCI-AD patients [39]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Huang, S.; Gao, Y.; Yin, L.; Pan, L.; Xu, W. Identification of a Four-Gene Signature Based on Metal Metabolism for Alzheimer’s Disease Diagnosis. Genes 2025, 16, 1287. https://doi.org/10.3390/genes16111287
Huang D, Huang S, Gao Y, Yin L, Pan L, Xu W. Identification of a Four-Gene Signature Based on Metal Metabolism for Alzheimer’s Disease Diagnosis. Genes. 2025; 16(11):1287. https://doi.org/10.3390/genes16111287
Chicago/Turabian StyleHuang, Dandan, Shasha Huang, Yunhan Gao, Linxi Yin, Lijun Pan, and Wei Xu. 2025. "Identification of a Four-Gene Signature Based on Metal Metabolism for Alzheimer’s Disease Diagnosis" Genes 16, no. 11: 1287. https://doi.org/10.3390/genes16111287
APA StyleHuang, D., Huang, S., Gao, Y., Yin, L., Pan, L., & Xu, W. (2025). Identification of a Four-Gene Signature Based on Metal Metabolism for Alzheimer’s Disease Diagnosis. Genes, 16(11), 1287. https://doi.org/10.3390/genes16111287






