Abstract
Background. PMP22-related neuropathies comprise a spectrum of predominantly demyelinating disorders, most commonly Charcot–Marie–Tooth type 1A (CMT1A; 17p12 duplication) and hereditary neuropathy with liability to pressure palsies (HNPP; 17p12 deletion), with rarer phenotypes due to PMP22 sequence variants (CMT1E, Dejerine–Sottas syndrome [DSS]). Methods. We conducted a PRISMA-compliant systematic review (PROSPERO ID: 1139921) of PubMed and Scopus (January 2015–August 2025). Eligible studies reported genetically confirmed PMP22-related neuropathies with clinical and/or neurophysiological data. Owing to heterogeneous reporting, we synthesized pooled counts and proportions without meta-analysis, explicitly tracking missing denominators. Results. One hundred twenty-seven studies (n = 4493 patients) were included. Sex was available for 995 patients (males 53.8% [535/995]; females 46.2% [460/995]); mean age at onset was 23.7 years in males and 16.4 years in females. Phenotypic classification was reported for 4431/4493 (75.4% CMT1A, 20.9% HNPP, 2.6% CMT1E, 1.2% DSS). Across phenotypes, weakness/foot drop was the leading presenting symptom when considering only cohorts that explicitly reported it (e.g., 65.3% in CMT1A; 76.0% in HNPP); sensory complaints (numbness, paresthesia/dysesthesia) were variably documented. Neurophysiology consistently showed demyelinating patterns, with median and ulnar nerves most frequently abnormal among assessed nerves; in HNPP, deep peroneal and sural involvement were also common in evaluated subsets. Comorbidities clustered by phenotype: orthopedic/neuromuscular features (pes cavus/hammer toes, scoliosis/kyphosis, tremor) in CMT1A and DSS; broader metabolic/autoimmune and neurodevelopmental associations in HNPP; and higher syndromic/ocular/hearing involvement in CMT1E. Genetically, 75.6% (3241/4291) had 17p12 duplication, 19.6% (835/4291) 17p12 deletion, and 4.8% (215/4291) PMP22 sequence variants with marked allelic heterogeneity. Among 2571 cases with available methods, MLPA was most used (41.9%), followed by NGS (20.4%) and Sanger sequencing (17.8%). Main limitations include heterogeneous and incomplete reporting across studies (especially symptoms and nerve-specific data) and the absence of a formal risk-of-bias appraisal, which preclude meta-analysis and may skew phenotype proportions toward more frequently reported entities (e.g., CMT1A). Conclusions. Recent literature confirms that PMP22 copy-number variants account for the vast majority of cases, while sequence-level variants underpin a minority with distinct phenotypes (notably CMT1E/DSS). Routine MLPA, complemented by targeted/NGS, optimizes diagnostic yield. Standardized reporting of nerve-conduction parameters and symptom denominators is urgently needed to enable robust cross-study comparisons in both pediatric and adult populations.
1. Introduction
Peripheral myelin protein 22 (PMP22) is a transmembrane glycoprotein, predominantly expressed in Schwann cells after birth. In particular, it plays a fundamental role in the formation and maintenance of myelin [1,2]. The most common hereditary neuropathy, CMT1A (Charcot-Marie-Tooth 1A), is caused by a trisomy of the human PMP22 gene. Other alterations affecting the gene may include large heterozygous deletions, which cause HNPP (Hereditary Neuropathy with Liability to Pressure Palsy), and point mutations (more frequently causing subtypes such as CMT1E, Dejerine-Sottas Disease/DSS) [3,4,5]. In CMT1A we can see an hereditary neuropathy, both sensory and motor, with a neurophysiological finding characterized by demyelinating polyneuropathy [6,7]. Patients generally present with alterations such as reduced muscle trophism, loss of sensitivity, paresthesia, pain and strength deficits [8,9]. Another group of neuropathies associated with large deletions in the PMP22 gene are HNPP (Hereditary neuropathy with liability to pressure palsy). In this case, the pattern, which is always demyelinating, tends to affect nerves that are prone to entrapment, primarily the ulnar and deep peroneal nerves, followed by the radial and other nerves. Patients generally present with loss of strength confined to the region innervated by the affected nerve(s), sensory deficits, pain and paresthesia [10]. Forms such as CMT1E and DSS/DSS are rarer than others. However, with the advent of new techniques for performing genetic testing (such as Whole Exome Sequencing), mutations are emerging that can determine these clinical pictures [11]. The forms described above may be associated with comorbidities such as scoliosis, kyphosis, hammer toes and hollow feet, eye abnormalities, and sensorineural deafness [12]. In this systematic review of the literature, we will try to emphasize the most important aspects of the aforementioned neuropathies, taking into account 10 years of global scientific literature in order to provide as complete a picture as possible of PMP22-related neuropathies.
2. Materials and Methods
2.1. Protocol and Registration
This systematic review was conducted according to PRISMA 2020 guidelines [13]. The review protocol was registered a priori on PROSPERO (ID: 1139921). The selection process is summarized in the PRISMA flow diagram (Figure 1) (Figure S1: PRISMA 2020 checklist).
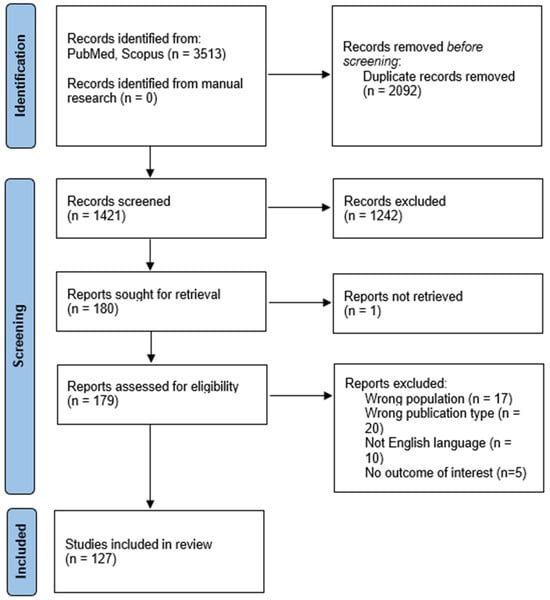
Figure 1.
PRISMA 2020 flow diagram.
2.2. Eligibility Criteria
We included studies that fulfilled the following criteria:
- Population: patients of any age with a confirmed genetic diagnosis of PMP22-related neuropathy.
- Phenotypes: CMT1A (17p12 duplication), HNPP (17p12 deletion), CMT1E (single-nucleotide or indel variants in PMP22 classified as pathogenic or likely pathogenic), and DSS/Dejerine–Sottas attributed to PMP22.
- Data required: at minimum, genetic confirmation plus either (i) clinical description or (ii) electrophysiological features.
- Study design: case reports, case series, cohort or registry studies.
- Language: English.
- Publication years: 2015–August 2025.
We excluded: review articles, conference abstracts without extractable data, animal/in vitro studies, mixed cohorts without separate PMP22-related neuropathies cases, reports without both genetic confirmation and at least one clinical/electrophysiological descriptor, and duplicate publications of the same cohort (in which case the most informative or complete version was retained).
2.3. Information Sources and Search Strategy
A systematic literature search was conducted in PubMed and Scopus databases, covering the period from January 2015 to August 2025. The search strategy combined controlled vocabulary and free-text terms related to PMP22-associated neuropathies, using the following keywords: (“PMP22” OR “Peripheral Myelin Protein 22”) AND (“Charcot-Marie-Tooth Disease” OR “Charcot-Marie-Tooth” OR “CMT1A” OR “CMT1E” OR “Dejerine–Sottas” OR “DSS” OR “HNPP” OR “Hereditary Neuropathy with Liability to Pressure Palsies” OR “neuropath*” OR “peripheral neuropathy” OR “polyneuropath*”). Reference lists of included articles and relevant reviews were also screened to identify additional eligible studies.
2.4. Study Selection
Four reviewers independently screened titles/abstracts and then full texts against eligibility criteria. Disagreements were resolved by consensus. Reasons for exclusion at full-text stage were recorded. Duplicates were removed before screening.
2.5. Data Extraction
For each study, we collected information on:
- Demographics: number of patients, sex distribution, family or sporadic cases.
- Genetics: type of variant (duplication, deletion, point mutation), technique of detection (MLPA, array-CGH/SNP array, NGS, WES, Sanger, or other methods), inheritance pattern, and parental origin when available. Variant nomenclature was standardized according to HGVS recommendations, and ACMG/AMP or ACMG/ClinGen classifications were reported when provided by the authors. Cases originally classified as “CMT1 with point mutation” were reassigned to the CMT1E subgroup or to the DSS group based on data availability (Supplementary Table S4). CMT1E was defined as a demyelinating form of CMT associated with a pathogenic or likely pathogenic PMP22 sequence variant, with typical childhood or adolescent onset and motor nerve conduction velocity (NCV) < 38 m/s. DSS was defined as early-infantile onset (<2 years) with severe demyelinating neuropathy, marked motor delay, and nerve hypertrophy when reported. Cases carrying PMP22 variants were also assigned to the DSS group if the clinical presentation fulfilled criteria for severe infantile disease. Mixed or uncertain cases were jointly reviewed by two investigators and classified by consensus.
- Clinical features: age at onset (harmonized into predefined bands: infancy 0–1 year, childhood 2–11 years, school age 6–12 years, teenage 13–19 years; midpoints calculated for ranges), presenting symptoms, comorbidities.
- Neurophysiology: motor and sensory conduction studies, including nerve-specific data when reported. When values were only provided as group-level conduction patterns, data were recorded as not available (NA).
When multiple affected individuals were described within a family but case-level details were unavailable, only those with sufficient clinical and/or neurophysiological information were included. For clinical and comorbidity data, denominators varied depending on the subset of patients undergoing full neurological examination; this heterogeneity was carefully retained, with unavailable information coded as NA. Features specifically excluded by the authors were coded as 0.
Frequencies of testing methods reflect reporting practices rather than a one-to-one mapping between assay and case count: MLPA is often applied broadly in registry/clinic cohorts (yielding many CMT1A/HNPP diagnoses), whereas NGS/WES is typically reserved for MLPA-negative cases and identifies fewer, sequence-variant–driven phenotypes (e.g., CMT1E/DSS). Sanger cannot detect CNVs and was used for sequence confirmation.
2.6. Data Synthesis
Descriptive statistics were used to summarize the frequency of genetic variants, inheritance patterns, parental origin, clinical manifestations, and investigation methods. Due to the heterogeneity of reporting, especially for clinical and neurophysiological features, no formal meta-analysis was attempted. Instead, results are presented as pooled counts and proportions, with explicit acknowledgment of missing denominators when applicable.
Descriptive statistics were used to summarize the frequency of genetic variants, inheritance patterns, parental origin, clinical manifestations, and investigation methods. Due to the heterogeneity of reporting, especially for clinical and neurophysiological features, no formal meta-analysis was attempted. Instead, results are presented as pooled counts and proportions, with explicit acknowledgment of missing denominators when applicable. All statistical analyses were performed using RStudio (version 4.5.1).
2.7. Ethics and Reporting Standards
All data were extracted from previously published studies; therefore, no new ethics approval or informed consent was required. Reporting of variants adhered to HGVS standards. Clinical, genetic, and neurophysiological data were abstracted exactly as described in the original studies, and coded as NA when insufficiently specified or as 0 when a feature was explicitly excluded.
2.8. Risk of Bias Assessment
A formal risk of bias appraisal was not performed, as the included studies mainly consisted of case reports, case series, and heterogeneous cohort descriptions, which are not amenable to standardized quality assessment tools. Instead, methodological limitations were acknowledged and discussed, including the retrospective design of most studies, incomplete or inconsistent reporting of clinical and neurophysiological features, and variability in genetic testing approaches. These factors should be considered when interpreting the findings of this review.
3. Results
We collected data from 127 studies [3,4,7,8,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134], including a total of 4493 patients with PMP22-related neuropathies. The full list of included studies, with details on study design and patient characteristics, is provided in Supplementary Table S1.
3.1. Demographic and Clinical Data
Sex distribution was available for 995 patients across these studies, of whom 535 were male and 460 female. The mean age at onset was 23.7 years in males and 16.4 years in females (Table 1).

Table 1.
Demographic data.
Phenotypic classification was available for 4431 patients, distributed as follows: CMT1A n = 3340 (75.4%), HNPP n = 924 (20.9%), CMT1E n = 114 (2.6%), DSS n = 53 (1.2%) (Table 2).

Table 2.
Distribution of patients by phenotype. Phenotypic data were not uniformly reported across all studies; percentages are calculated on the 4431 patients with available classification.
Sex distribution was further analyzed in the subset of studies reporting a single phenotype to minimize overlap with mixed cohorts. Among these, a higher proportion of males was found in CMT1A (56.2%, n = 91/162) and HNPP (56.9%, n = 189/332). CMT1E showed a slight predominance of females (60.0%, n = 15/25), whereas DSS, although based on a limited number of cases, was more frequently reported in males (75.0%, n = 3/4) (Table 3).

Table 3.
Distribution of patients by phenotype and sex in the subset of studies reporting a single phenotype.
The overall distribution of phenotypes is shown in Figure 2, while Figure 3 displays the sex distribution by phenotype in the subset of single-phenotype studies.
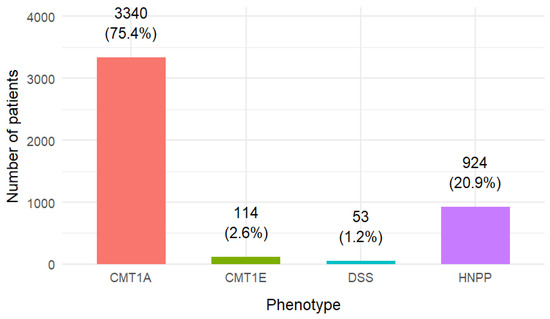
Figure 2.
Distribution of patients by phenotype.
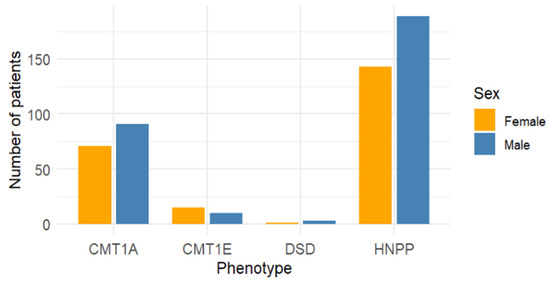
Figure 3.
Sex distribution by phenotype in single-phenotype studies only.
3.2. Onset Symptoms by Phenotype
Data on onset symptoms were analyzed separately for each phenotype, using two complementary approaches (Table 4). First, percentages were calculated using the total number of patients within each phenotype as denominator, since missing values (NA) in the original reports did not necessarily indicate absence of the symptom but rather a lack of specification. Second, the same variables were recalculated on the subset of studies providing explicit numerical data, to control for underreporting bias.

Table 4.
Onset symptoms by phenotype—total vs. available-data denominators. Percentages are shown using two denominators: Total = total number of patients per phenotype (including NA as “not reported”); Available = sum of patients in the subset of studies that explicitly reported a numeric value for the symptom. Zeros indicate explicitly reported absence; NA indicates not reported.
In CMT1A, weakness/foot drop was the most frequent presenting symptom, observed in 2.9% of all patients (98/3340) and in 65.3% of those with data available (98/150). Abnormal sensibility was reported in 1.5% (50/3340; 38.8% of available data), paresthesia/dysesthesia in 0.8% (28/3340; 21.1% of available), and hypotrophy/atrophy in 0.3% (11/3340; 8.0% of available).
In CMT1E, weakness/foot drop was present in 19.0% (23/121; 82.1% of available), abnormal sensibility in 11.6% (14/121; 60.9% of available), paresthesia/dysesthesia in 2.5% (3/121; 13.0% of available), and hypotrophy/atrophy in 10.7% (13/121; 48.1% of available).
In HNPP, weakness/foot drop occurred in 17.1% of all cases (158/924) and 76.0% of those with available data, followed by abnormal sensibility (8.9%/45.3%), paresthesia/dysesthesia (8.7%/46.2%), and hypotrophy/atrophy (0.4%/2.5%).
In DSS, weakness/foot drop was found in 5.7% of patients (3/53; 37.5% of available), abnormal sensibility in 1.9% (1/53; 12.5% of available), while paresthesia/dysesthesia and hypotrophy/atrophy were not reported (0/53 each).
3.3. Nerve Involvement by Phenotype
Using the total number of patients within each phenotype as denominator, the pattern of nerve involvement showed distinct distributions across subtypes (Table 5). In CMT1A (n = 3340), the most frequently affected nerves were the median motor (10.2%, 340/3340) and median sensory (7.9%, 263/3340), followed by ulnar motor (4.5%) and ulnar sensory (4.1%); less frequent findings involved deep peroneal motor (1.0%), sural (1.0%), posterior tibial motor (0.6%), superficial peroneal sensory (0.1%), and radial motor (0.1%). In CMT1E (n = 121), abnormalities were most often observed in the median motor (18.2%), ulnar motor/sensory (14.9% each), and median sensory (14.0%), followed by sural (12.4%), posterior tibial motor (10.7%), and deep peroneal motor (9.9%); superficial peroneal sensory (4.1%) and radial motor (3.3%) were less common, and “other nerves” were not reported. In DSS (n = 53), a relatively uniform distribution was seen (5.7% for ulnar motor, ulnar sensory, median motor, median sensory, and posterior tibial motor; 1.9% for deep peroneal motor, sural, and superficial peroneal sensory; 0% for radial motor). In HNPP (n = 924), involvement was broader and more frequent across multiple nerves (e.g., median motor 26.9%, ulnar motor 23.4%, deep peroneal motor 21.1%, sural 19.5%, median sensory 15.7%, ulnar sensory 14.7%, posterior tibial motor 13.0%).

Table 5.
(A). Nerve involvement by phenotype (total denominator). Percentages were calculated using the total number of patients in each phenotype as denominator. Missing data (NA) were not excluded, as they did not necessarily indicate absence of nerve involvement. Values therefore represent conservative estimates of nerve abnormalities within each subtype. (B). Nerve involvement by phenotype (available-data denominator). Percentages were recalculated using only the subset of patients for whom information on each specific nerve was reported (“available-data denominator”). These values reflect the proportion of abnormalities among evaluated cases and should not be interpreted as prevalence across the entire phenotype. (C). Summary of “other” nerves reported as affected across PMP22-related phenotypes.
Because NA in the original reports did not necessarily indicate absence, we also recalculated proportions using only the subset of patients for whom a given nerve was specifically reported (the “available-data denominator,” Table 5). As expected, percentages were higher within evaluated subsets—for example, CMT1A median sensory 87.4% (263/301) and median motor 83.1% (340/409); CMT1E ulnar sensory 85.7% (18/21) and median motor 81.5% (22/27); in DSS, several nerves reached 75–100% among the few assessed cases; and in HNPP, deep peroneal motor 59.6% (195/327), median motor 73.7% (249/338), sural 59.2% (180/304), and ulnar motor 66.3% (216/326). These values reflect reporting among assessed patients and should not be interpreted as phenotype-wide prevalence.
Beyond the routinely tested nerves, additional “other nerves” were occasionally reported as affected (Table 5). Such findings were primarily documented in CMT1A and HNPP, while none were reported in CMT1E. In CMT1A, isolated abnormalities involved the phrenic, plantar, and radial sensory branches, as well as alterations of the blink reflex (10 total mentions). DSS included rare involvement of the tibialis anterior and first dorsal interosseous nerves. The broadest extension occurred in HNPP, with additional abnormalities affecting axillary, facial, radial sensory, trigeminal, abducens, musculocutaneous, suprascapular, femoral, lateral cutaneous, sciatic, tibial sensory, plantar, and muscolospiral nerves.
3.4. Comorbidities by Phenotype
Comorbidities were extracted from all eligible studies and classified by domain and phenotype (Table 6, Figure 4). Their distribution and content differed markedly across PMP22-related syndromes.

Table 6.
Frequency of comorbidities in single-phenotype cohorts. Summary of comorbidity domains by phenotype. Legend: ✓ present; ✓✓ prominent across series; “–” not reported.
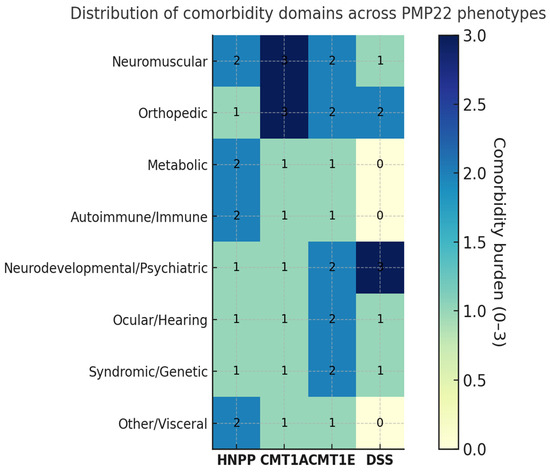
Figure 4.
Heatmap of comorbidities across PMP22-related neuropathies. Distribution and relative frequency of comorbidities among patients with different PMP22-related phenotypes. Each cell represents the proportion of patients with a given comorbidity within each phenotype, color-coded according to percentage values. Missing data were not considered as absence. The heat intensity increases with higher reported frequency.
HNPP showed the broadest systemic spectrum, including metabolic disorders (type 2 diabetes, MODY6), autoimmune or immune dysregulation (e.g., immune dysfunctions, bullous pemphigoid), and overlap with immune-mediated neuropathies (CIDP, Guillain–Barré). Additional findings comprised neurodevelopmental and psychiatric features (autism, psychosis), skeletal anomalies (clubfoot, marfanoid habitus, syndactyly, short stature), and ocular or syndromic associations (glaucoma with nystagmus, Waardenburg syndrome).
In CMT1A, comorbidities clustered within neuromuscular and orthopedic domains—particularly pes cavus/hammer toes and scoliosis or kyphoscoliosis—together with tremor/postural tremor and frequent entrapment neuropathies (carpal/cubital tunnel), consistent with chronic length-dependent involvement. Extra-neurological comorbidities were infrequent and heterogeneously reported across studies.
CMT1E displayed a comparatively higher rate of syndromic and developmental associations (including delayed motor milestones), with ocular abnormalities, hearing loss, dysautonomia, and pes cavus variably reported across series.
Dejerine–Sottas syndrome (DSS), albeit supported by smaller cohorts, showed early and severe disease with developmental delay, skeletal deformities (scoliosis, pes cavus), and occasional hearing or ocular involvement, consistent with profound demyelination from infancy.
Compared with length-dependent CMT phenotypes (CMT1A/1E/DSS), HNPP showed a compression-prone pattern with higher reporting of deep peroneal, radial and ulnar involvement among assessed nerves, and a broader extra-neurological spectrum. Conversely, orthopedic deformities were most frequent in CMT1A/DSS.
Overall, neuromuscular and orthopedic comorbidities represented the most consistent domains across PMP22 phenotypes (most prominent in CMT1A and DSS), whereas metabolic and autoimmune involvement appeared largely confined to HNPP. Detailed quantitative data are provided in Supplementary Table S2.
3.5. Genetic Findings
Variant type was reported in all studies (127/127). The PMP22 duplication represented the most frequent genetic mechanism (n = 3241; 75.6%), followed by the reciprocal deletion (n = 835; 19.6%) and sequence-level variants (n = 215; 4.8%) (Table 7, Figure 5).

Table 7.
Genetic findings by variant type and phenotype.
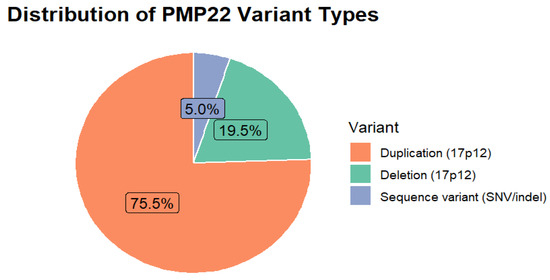
Figure 5.
Distribution of PMP22 Variant Types.
A total of 215 patients carried PMP22 point mutations or small indels, encompassing a highly heterogeneous spectrum of missense, nonsense, frameshift, and splice-site variants. Most mutations were described in single families, with only a few recurring across cohorts (e.g., p.Trp39Cys, p.Ala67Pro, p.Gly107Asp/Val, p.Ser72Leu). A comprehensive list of all unique variants is provided in Supplementary Table S3. Given the substantial variability in reporting—particularly regarding the number of affected relatives and inconsistent nomenclature—these data should be interpreted as a descriptive catalog rather than as a precise estimate of prevalence.
When stratified by phenotype (Table 7), PMP22 mutations displayed distinct patterns:
- CMT1A was exclusively associated with the 17p12 duplication, with no single-nucleotide variants identified.
- HNPP was mainly caused by the reciprocal 17p12 deletion, but 13 patients (1.4%) carried loss-of-function sequence variants such as p.Trp28**, c.178+2T>C, and recurrent frameshifts (p.Leu145Argfs10*), reproducing the classical phenotype without a genomic deletion.
- CMT1E exhibited the broadest allelic heterogeneity, including recurrent missense (p.Trp39Cys, p.Gly107Asp/Val) and truncating (p.Cys85**, p.Gly94Alafs17*) variants, often occurring de novo.
- Dejerine–Sottas syndrome (DSS) was linked to early and severe disease forms, frequently associated with p.Ser72Leu, p.Ala67Pro, p.Gly150Asp/Val, or small in-frame deletions (p.Phe84del, p.Ala115_Thr118del), and occasionally exon-level deletions overlapping 17p12.
Information on the diagnostic technique was available for 2571 patients (out of 4291 with genetic data). MLPA represented the most frequently reported approach (41.9%), followed by NGS (20.4%) and Sanger sequencing (17.8%). WES (0.4%) and CGH/SNP array (1.4%) were rarely used, while other methods accounted for 18.1% (Table 8).

Table 8.
Genetic investigation methods used across included studies. “Other methods” included a variety of less frequently used approaches, such as PCR-based assays (PCR/RFLP, multiplex/qPCR, real-time PCR, TaqMan CN assay, quantitative DNA dosage), fluorescence in situ hybridization (FISH), RNA-based analyses (RT-PCR on fibroblasts, RNA from skin biopsy), microsatellite/STR analysis, and molecular modeling.
The available data did not allow for precise quantification of the sequencing strategies applied across studies. However, several reports indicated that MLPA was typically used to detect 17p12 copy-number variants, whereas sequence-level variants were identified through Sanger sequencing or, in more recent publications, through NGS or WES. Some studies described stepwise diagnostic workflows combining MLPA and sequencing approaches, occasionally complemented by confirmatory assays such as FISH, qPCR, microsatellite analysis, or RNA-based studies. These methodological details were inconsistently reported, limiting comparative assessment across cohorts.
4. Discussion
PMP22-related neuropathies constitute a heterogeneous group of diseases, as evidenced by the data we have collected in this systematic review of the literature.
About the gender of patients, where we noted a higher incidence of PMP22-related neuropathies in males (53.8%). We would like to emphasize that HNPP has a higher incidence in males than that seen in other forms of PMP22-related neuropathies. This data coincides with the fact that males have a fairly clear prevalence for HNPP, which was already analyzed by Manganelli and colleagues in 2013 [135], where it was found that this could be due not only to aging, but also to intrinsic predisposing factors in men compared to women. For example, still within the context of the male-female dichotomy, it is also known that progesterone, by increasing the expression of PMP22, can positively regulate myelin formation [136] and may have a protective function in HNPP, where PMP22 deficiency makes axons more susceptible to injury [137].
For a clinician (neurologist, general practitioner, emergency room physician, pediatrician), it is important to assess the symptoms and physical characteristics that the patient presents upon arrival at the ward or clinic. Among the most commonly reported symptoms, we find weakness, followed by abnormal sensibility. Weakness should be emphasized especially in individuals with HNPP (in 76% of cases where the clinic was available), especially in nerves more prone to entrapment, such as the common peroneal nerve (characteristic foot drop) and neuropathies affecting the ulnar nerve; this does not exclude the possibility that the patient may also have sensory symptoms, such as numbness and paresthesia [138].
Almost all of the neuropathies described in this systematic review of the literature are considered demyelinating. The fact that some nerves are not evoked, both motor and sensory, could lead to the mistaken belief that some PMP22 neuropathies are often axonal. However, as well described by Moss KR and colleagues in 2020 [139], even in CMT1A-HNPP-CMT1E, which are demyelinating forms, there is axonal involvement due to secondary axonal degeneration. The pathophysiological mechanisms underlying secondary axonal degeneration can vary, so we can talk, for example, about a lack of trophic support or greater vulnerability to toxic insults (e.g., chemotherapy) in CMT1A [140,141,142,143,144,145], involvement of the cytoskeletal organization in HNPP and CMT1E [146,147,148,149]. In very rare cases, point mutations on PMP22 can cause more axonal than demyelinating symptoms, compatible with CMT2, as described in the article by Antoniadi et al., published in 2015 [14].
From a neurophysiological point of view, characteristics of sensory-motor demyelinating neuropathies are both motor and sensory involvement, nerve conduction velocity less than 38 m/s, increased distal latencies, reduced amplitudes [150]. The nerves that most frequently show pathological characteristics are the motor median nerve and the motor ulnar nerve. Both of these nerves are the most commonly analyzed, probably because they are easy to locate when performing NCS, and because they are involved in both demyelinating forms of CMT (where we may have sensory-motor polyneuropathy) and localized forms that are more prone to entrapment (as in the case of HNPP) [151].
As regards comorbidities, the most common are pes cavus and hammer toes, followed by scoliosis. Among the pathological comorbidities encountered, one of the most interesting is that described by Shimizu in 2016, with CIDP (Chronic Inflammatory Demyelinating Neuropathy) [101]. This is based on the fact that the patient showed conduction blocks on NCS, which are not typical of CMT1A [152]. Based on this, and given the chronic nature of the condition, laboratory tests were performed which confirmed the clinical suspicion. In fact, conduction block in CMT1A is not often described in the literature, although it appears to be possible, as described by Chen and colleagues in 2022 (who describe partial conduction blocks) [23].
Also interesting is the possibility of overlap with Smith Magenis syndrome, in cases where the PMP22 deletion also includes the RAI1 gene [122]. These cases, like others described in the review in question, make it more difficult to characterize neurological symptoms/signs that are free from the influence of the comorbidity found.
As regards the genetic tests used, MLPA (41.9%) and array techniques such as CGH arrays and SNP arrays should be highlighted for their effectiveness and ability to identify duplications and deletions that underlie the symptoms of most of the individuals described. The fact that MLPA is the most widely used technique shows that, from a clinical and neurophysiological point of view, these diseases are well codified and allow us to adopt a very precise diagnostic technique. However, especially in cases of multiple comorbidities, we must not make the mistake of not conducting genetic tests to detect other mutations (e.g., point mutations), at the risk of missing a double diagnosis.
Other types of genetic investigation techniques used, like NGS (Next Generation Sequencing) gene panels (20.4%) focused on neuropathies, capable of detecting point mutations that might otherwise be missed by other laboratory techniques. Among point mutations, those on PMP22 are not the most frequent cause of neuropathies described in this systematic review, but they still constitute a significant proportion of our sample (4.8%).
Although our study takes a sample of relevant articles, it has some limitations. In particular, as already mentioned, not all of the nerves examined were analyzed for each patient, so the statistics are affected by a procedure that is not standardized worldwide and that is operator-dependent.
The other limitation relates to the family history of the patients. We have reported cases of positive family history, understood as maternal or paternal heredity; however, genetic testing of parents was not performed in all the cases described, sometimes due to refusal by the individuals concerned, other times due to the patient’s advanced age, etc.
As for the symptoms reported by patients, they were not described in many of the studies analyzed. While they were well characterized in case reports/case series, we found difficulties regarding the clinical presentation characteristics (strength deficit, loss of sensation, paraesthesia, hypoatrophy) in articles describing large patient cohorts.
It is possible that some nerves appear to be less frequently affected simply because they are less frequently tested with NCS or not reported; the heterogeneity in the quality and completeness of reporting limits the ability to estimate true prevalence.
5. Conclusions
As in any medical situation, whether genetic or not, the most important thing to do is to take a medical history and perform a physical examination. The presence of pes cavus and distal atrophy of the lower limbs should always prompt the clinician to interview the patient for any signs of neuropathy and, if necessary, to investigate further with a Nerve Conduction Study (NCS) examination. Family history should always be investigated in situations compatible with HNPP/CMT, as some symptoms, sometimes underestimated by patients, can point us towards the diagnosis. The use of increasingly advanced genetic tests, such as NGS and WES, can lead us to rarer diagnoses of conditions attributable to point mutations, taking care to investigate, including within the family, certain comorbidities such as scoliosis, sensorineural hearing loss, and eye disorders. The drafting of a standardized protocol for gathering information about NCS in patients with CMT could greatly assist in standardizing data and making information more statistically reliable, both in children and adults.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes16111279/s1, Figure S1: PRISMA 2020 checklist; Table S1: data analyzed; Table S2 (A–D): comorbidities in HNPP, CMT1A, CMT1E, DSS; Table S3 (A–D): PMP22 point mutations and small indels across phenotypes; Table S4: Differences between CMT1E and DSS [153,154,155].
Author Contributions
Conceptualization: C.A.C. and C.F.; methodology: G.P., M.G., A.P., S.R., C.A.C., D.F. and C.F.; data curation: C.A.C., L.C., G.P., G.S. and M.G.; writing—original draft preparation: C.A.C. and G.P.; writing—review and editing: C.A.C. and C.F.; supervision: C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review was not required for this study as it is a scoping review that analyses previously published data.
Informed Consent Statement
Informed consent was not required for this study as it is a scoping review that analyses previously published data.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roth, A.R.; Li, J.; Dortch, R.D. Candidate imaging biomarkers for PMP22-related inherited neuropathies. Ann. Clin. Transl. Neurol. 2022, 9, 925–935. [Google Scholar] [CrossRef]
- Li, J.; Parker, B.; Martyn, C.; Natarajan, C.; Guo, J. The PMP22 gene and its related diseases NIH public access author manuscript. Mol. Neurobiol. 2013, 47, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Savasta, S.; Serra, F.; Galimberti, L.; Comisi, F.F.; Cossu, M.; Vannelli, A.; Masala, M.; Tanca, S.; Murru, S. Case Report: Hereditary neuropathy with liability to pressure palsy (HNPP): The role of genetic investigation in diagnostic assessment. Front. Genet. 2025, 16, 1613022. [Google Scholar] [CrossRef]
- Ait El Cadi, C.; Dafrallah, L.; Amalou, G.; Charif, M.; Charoute, H.; Araqi-Houssaini, A.; Lakhiari, H.; Lenaers, G.; Barakat, A. A case report of two Moroccan patients with hereditary neurological disorders and molecular modeling study on the S72L de novo PMP22 variant. Rev. Neurol. 2023, 179, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.Y.; Kwon, H.M.; Nam, D.E.; Tamanna, N.; Lee, A.J.; Kim, S.B.; Choi, B.-O.; Chung, K.W. Peripheral myelin protein 22 gene mutations in Charcot-Marie-Tooth disease type 1E patients. Genes 2022, 13, 1219. [Google Scholar] [CrossRef] [PubMed]
- Berciano, J.; García, A.; Gallardo, E.; Peeters, K.; Pelayo-Negro, A.L.; Álvarez-Paradelo, S.; Gazulla, J.; Martínez-Tames, M.; Infante, J.; Jordanova, A. Intermediate Charcot-Marie-Tooth disease: An electrophysiological reappraisal and systematic review. J. Neurol. 2017, 264, 1655–1677. [Google Scholar] [CrossRef]
- Volodarsky, M.; Kerkhof, J.; Stuart, A.; Levy, M.; Brady, L.I.; Tarnopolsky, M.; Lin, H.; Ainsworth, P.; Sadikovic, B. Comprehensive genetic sequence and copy number analysis for Charcot-Marie-Tooth disease in a Canadian cohort of 2517 patients. J. Med. Genet. 2021, 58, 284–288. [Google Scholar] [CrossRef]
- Wu, R.; Lv, H.; Zhang, W.; Wang, Z.; Zuo, Y.; Liu, J.; Yuan, Y. Clinical and Pathological Variation of Charcot-Marie-Tooth 1A in a Large Chinese Cohort. Biomed. Res. Int. 2017, 2017, 6481367. [Google Scholar] [CrossRef]
- Padua, L.; Cavallaro, T.; Pareyson, D.; Quattrone, A.; Vita, G.; Schenone, A.; Italian CMT QoL Study Group. Charcot-Marie-Tooth and pain: Correlations with neurophysiological, clinical, and disability findings. Neurol. Sci. 2008, 29, 193–194. [Google Scholar] [CrossRef]
- Chrestian, N. Hereditary Neuropathy with Liability to Pressure Palsies; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2020. [Google Scholar]
- Kim, Y.G.; Kwon, H.; Park, J.H.; Nam, S.H.; Ha, C.; Shin, S.; Heo, W.Y.; Kim, H.J.; Chung, K.W.; Jang, J.H.; et al. Whole-genome sequencing in clinically diagnosed Charcot-Marie-Tooth disease undiagnosed by whole-exome sequencing. Brain Commun. 2023, 5, fcad139. [Google Scholar] [CrossRef]
- Nagappa, M.; Sharma, S.; Taly, A.B. Charcot-Marie-Tooth Disease; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Antoniadi, T.; Buxton, C.; Dennis, G.; Forrester, N.; Smith, D.; Lunt, P.; Burton-Jones, S. Application of targeted multi-gene panel testing for the diagnosis of inherited peripheral neuropathy provides a high diagnostic yield with unexpected phenotype-genotype variability. BMC Med. Genet. 2015, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti-Stella, A.; Chiaro, G.; Calandra-Buonaura, G.; Contin, M.; Scaglione, C.; Barletta, G.; Cecere, A.; Garagnani, P.; Tieri, P.; Ferrarini, A.; et al. A patient with PMP22-related hereditary neuropathy and DBH-gene-related dysautonomia. J. Neurol. 2015, 262, 2373–2381. [Google Scholar] [CrossRef]
- Baudou, E.; Cances, C.; Magdelaine, C.; Latour, P.; Louvier, U.W.; Juntas-Morales, R.; Cintas, P.; Rivier, F. Unexpected Intermediate Nerve Conduction Velocity Findings in Charcot-Marie-Tooth Syndromes Classified as Demyelinated or Axonal in a Pediatric Population. Neuropediatrics 2022, 53, 182–187. [Google Scholar] [CrossRef]
- Benquey, T.; Fockens, E.; Kouton, L.; Delmont, E.; Martini, N.; Levy, N.; Attarian, S.; Bonello-Palot, N. A New Point Mutation in the PMP22 Gene in a Family Suffering from Atypical HNPP. J. Neuromuscul. Dis. 2020, 7, 505–510. [Google Scholar] [CrossRef]
- Cakar, A.; Candayan, A.; Bagırova, G.; Uyguner, Z.O.; Ceylaner, S.; Durmus, H.; Battaloglu, E.; Parman, Y. Delineating the genetic landscape of Charcot-Marie-tooth disease in Türkiye: Distinct distribution, rare phenotypes, and novel variants. Eur. J. Neurol. 2025, 32, e16572. [Google Scholar] [CrossRef]
- Campagnolo, M.; Taioli, F.; Cacciavillani, M.; Ruiz, M.; Luigetti, M.; Salvalaggio, A.; Castellani, F.; Testi, S.; Ferrarini, M.; Cavallaro, T.; et al. Sporadic hereditary neuropathies misdiagnosed as chronic inflammatory demyelinating polyradiculoneuropathy: Pitfalls and red flags. J. Peripher. Nerv. Syst. 2020, 25, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Castellucci, G.; Figueroa, M.; Sivaswamy, L. Hereditary Neuropathy with Liability to Pressure Palsy Detected During the Use of Recreational Drugs. Neurohospitalist 2023, 13, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Cea, G.; Contreras, J.P.; Aguilar, S.; Vera, J. Wrist drop in an arcade dancing game: Unusual sudden bilateral radial palsy. Neuromuscul. Disord. 2019, 29, 398–400. [Google Scholar] [CrossRef]
- Chen, C.X.; Dong, H.L.; Wei, Q.; Li, L.X.; Yu, H.; Li, J.Q.; Liu, G.L.; Li, H.F.; Bai, G.; Ma, H.; et al. Genetic spectrum and clinical profiles in a southeast Chinese cohort of Charcot-Marie-Tooth disease. Clin. Genet. 2019, 96, 439–448. [Google Scholar] [CrossRef]
- Chen, Z.; Saini, M.; Neo, S.X.M.; Ng, P.S.; Koh, J.S.; Prasad, K.; Verma, K.; Davila, S.; Lim, W.K.; Phua, Z.; et al. Acute to Subacute Atraumatic Entrapment Neuropathies in Patients with CMT1A: A Report of a Distinct Phenotypic Variant of CMT1A. Front. Neurol. 2022, 13, 826634. [Google Scholar] [CrossRef]
- Choi, K.; Ahn, S.H.; Baek, S.H.; Kim, J.S.; Choi, S.J.; Shin, J.Y.; Kim, S.M.; Hong, Y.H.; Sung, J.J. Spinobulbar muscular atrophy combined with atypical hereditary neuropathy with liability to pressure palsy. J. Clin. Neurosci. 2018, 48, 90–92. [Google Scholar] [CrossRef]
- Chompoopong, P.; Niu, Z.; Shouman, K.; Madigan, N.N.; Sandroni, P.; Berini, S.E.; Shin, A.Y.; Brault, J.S.; Boon, A.J.; Laughlin, R.S.; et al. Utility of Carpal Tunnel Release and Ulnar Decompression in CMT1A and HNPP. Muscle Nerve 2022, 66, 479–486. [Google Scholar] [CrossRef]
- Chrestian, N.; McMillan, H.; Poulin, C.; Campbell, C.; Vajsar, J. Hereditary neuropathy with liability to pressure palsies in childhood: Case series and literature update. Neuromuscul. Disord. 2015, 25, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Cornett, K.M.D.; Menezes, M.P.; Shy, R.R.; Moroni, I.; Pagliano, E.; Pareyson, D.; Estilow, T.; Yum, S.W.; Bhandari, T.; Muntoni, F.; et al. Natural history of Charcot-Marie-Tooth disease during childhood. Ann. Neurol. 2017, 82, 353–359. [Google Scholar] [CrossRef]
- D’Arrigo, S.; Tessarollo, V.; Taroni, F.; Baratta, S.; Pantaleoni, C.; Schiaffi, E.; Ciano, C. A Case of Severe Early-Onset Neuropathy Caused by a Compound Heterozygous Deletion of the PMP22 Gene: Clinical and Neurographic Aspects. Neuropediatrics 2020, 51, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Davion, J.B.; Cassim, F.; Péréon, Y.; Nguyen The Tich, S. Young infants with PMP22 duplication can have minor nerve conduction study abnormalities. Neurophysiol. Clin. 2022, 52, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Davion, J.B.; Devaux, J.; Tard, C.; Magot, A.; Cassim, F.; Péréon, Y. Guillain-Barré syndrome in patients with Charcot-Marie-Tooth type 1A disease, probably a non-random association. Neurophysiol. Clin. 2025, 55, 103071. [Google Scholar] [CrossRef]
- De Kock, L.; Van der Cruyssen, F.; Gruijthuijsen, L.; Politis, C. Facial Paresthesia, a Rare Manifestation of Hereditary Neuropathy with Liability to Pressure Palsies: A Case Report. Front. Neurol. 2021, 12, 726437. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.P.; Pereira, R.C.; Onofre, P.T.; Marques, V.D.; de Andrade, G.B.; Barreira, A.A.; Marques Junior, W. Clinical and neurophysiological features of the hereditary neuropathy with liability to pressure palsy due to the 17p11.2 deletion. Arq. Neuropsiquiatr. 2016, 74, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Doğan, Y.; Gül, Ş.; Ceylan, A.C.; Kutsal, Y.G. A special association between Charcot-Marie-Tooth type 1A disease and relapsing remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 35, 83–85. [Google Scholar] [CrossRef]
- Dukefoss, T.T.; Kleggetveit, I.P.; Helås, T.; Jørum, E. Pain and small-fiber affection in hereditary neuropathy with liability to pressure palsies (HNPP). Scand. J. Pain 2019, 20, 61–68. [Google Scholar] [CrossRef]
- Endres, D.; Maier, S.J.; Ziegler, C.; Nickel, K.; Riering, A.N.; Berger, B.; Lambeck, J.; Fritz, M.; Gläser, B.; Stock, F.; et al. Schizophrenia and Hereditary Polyneuropathy: PMP22 Deletion as a Common Pathophysiological Link? Front. Psychiatry 2019, 10, 270. [Google Scholar] [CrossRef]
- Ferese, R.; Campopiano, R.; Scala, S.; D’Alessio, C.; Storto, M.; Buttari, F.; Centonze, D.; Logroscino, G.; Zecca, C.; Zampatti, S.; et al. Cohort Analysis of 67 Charcot-Marie-Tooth Italian Patients: Identification of New Mutations and Broadening of Phenotype Expression Produced by Rare Variants. Front. Genet. 2021, 12, 682050. [Google Scholar] [CrossRef]
- Fernandes, M.; Caetano, A.; Castelhano, L.; Santos, L. Characterization of a Portuguese family with Charcot-Marie-Tooth disease type 1E due to a novel point mutation in the PMP22 gene. Clin. Neurol. Neurosurg. 2021, 208, 106829. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.M.; Peciña, A.; Muñoz-Cabello, B.; Antiñolo, G.; Borrego, S. Co-segregation of a homozygous SMN1 deletion and a heterozygous PMP22 duplication in a patient. Clin. Case Rep. 2016, 4, 879–884. [Google Scholar] [CrossRef]
- Fritz, N.E.; Chen, Y.; Waters, L.; Saba, S.; Hackett, M.; Mada, F.C.; Li, J. Fatigue in patients with hereditary neuropathy with liability to pressure palsies. Ann. Clin. Transl. Neurol. 2020, 7, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Fusco, C.; Spagnoli, C.; Salerno, G.G.; Pavlidis, E.; Frattini, D.; Pisani, F. Hereditary neuropathy with liability to pressure palsy (HNPP): Report of a family with a new point mutation in PMP22 gene. Ital. J. Pediatr. 2017, 43, 97. [Google Scholar] [CrossRef] [PubMed]
- Gazulla, J.; Almárcegui, C.; Berciano, J. Reversible inflammatory neuropathy superimposed on Charcot-Marie-Tooth type 1A disease. Neurol. Sci. 2018, 39, 793–794. [Google Scholar] [CrossRef]
- Gentile, L.; Russo, M.; Fabrizi, G.M.; Taioli, F.; Ferrarini, M.; Testi, S.; Alfonzo, A.; Aguennouz, M.; Toscano, A.; Vita, G.; et al. Charcot-Marie-Tooth disease: Experience from a large Italian tertiary neuromuscular center. Neurol. Sci. 2020, 41, 1239–1243. [Google Scholar] [CrossRef]
- Gogou, M.; Pavlidou, E.; Pavlou, E.; Papageorgiou, T.; Tragiannidis, A.; Giannopoulos, A.; Hatzipantelis, E. Charcot-Marie-Tooth 1A concurrent with anaplastic ependymoma in a toddler: When an acute event unmasks a chronic condition. Turk. J. Pediatr. 2019, 61, 428–430. [Google Scholar] [CrossRef]
- Hachisuka, A.; Matsushima, Y.; Hachisuka, K.; Saeki, S. A Case of Apoplexy Attack-Like Neuropathy due to Hereditary Neuropathy with Liability to Pressure Palsies in a Patient Diagnosed with Chronic Cerebral Infarction. J. Stroke Cerebrovasc. Dis. 2016, 25, e83–e85. [Google Scholar] [CrossRef]
- Han, L.; Huang, Y.; Nie, Y.; Li, J.; Chen, G.; Tu, S.; Shen, P.; Chen, C. A novel PMP22 insertion mutation causing Charcot-Marie-Tooth disease type 3: A case report. Medicine 2021, 100, e25163. [Google Scholar] [CrossRef]
- Hauw, F.; Fargeot, G.; Adams, D.; Attarian, S.; Cauquil, C.; Chanson, J.B.; Créange, A.; Gendre, T.; Deiva, K.; Delmont, E.; et al. Charcot-Marie-Tooth disease misdiagnosed as chronic inflammatory demyelinating polyradiculoneuropathy: An international multicentric retrospective study. Eur. J. Neurol. 2021, 28, 2846–2854. [Google Scholar] [CrossRef]
- He, J.; Guo, L.; Xu, G.; Xu, L.; Lin, S.; Chen, W.; Wang, N. Clinical and genetic investigation in Chinese patients with demyelinating Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 2018, 23, 216–226. [Google Scholar] [CrossRef]
- Hoebeke, C.; Bonello-Palot, N.; Audic, F.; Boulay, C.; Tufod, D.; Attarian, S.; Chabrol, B. Retrospective study of 75 children with peripheral inherited neuropathy: Genotype-phenotype correlations. Arch. Pediatr. 2018, 25, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Lin, K.P.; Guo, Y.C.; Tsai, Y.S.; Liao, Y.C.; Lee, Y.C. Mutation spectrum of Charcot-Marie-Tooth disease among the Han Chinese in Taiwan. Ann. Clin. Transl. Neurol. 2019, 6, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Meng, L.; Jiang, L.; Wang, Z.; Chen, L.; Yuan, Y. Evaluation of the median nerve by shear wave elastography in patients with Charcot-Marie-Tooth disease type 1A. Med. Ultrason. 2023, 25, 161–167. [Google Scholar] [CrossRef]
- Hughes, H.; Tubridy, N.; Connolly, S. A Life-Saving Palsy: Hereditary Neuropathy with Liability to Pressure Palsies (HNPP) Presenting As Hand Weakness during Cardiopulmonary Resuscitation (CPR) Training. Ir. Med. J. 2018, 111, 808. [Google Scholar] [PubMed]
- Isik, K.; Odabaşı, Z. An interesting cause of wrist drop: The crow position in yoga and hereditary neuropathy with liability to pressure palsies. Turk. J. Phys. Med. Rehabil. 2024, 70, 282–284. [Google Scholar] [CrossRef]
- Ismail, H.; Anand, B.; Hughes, T.; Thomas, B. Charcot-Marie-Tooth Disease Presenting in the Postpartum Period: A Case Report. Cureus 2024, 16, e76077. [Google Scholar] [CrossRef]
- Ivanovic, V.; Brankovic, M.; Bjelica, B.; Kacar, A.; Tubic, R.; Jankovic, M.; Marjanovic, A.; Novakovic, I.; Rakocevic-Stojanovic, V.; Peric, S. Yield of the PMP22 deletion analysis in patients with compression neuropathies. J. Neurol. 2020, 267, 3617–3623. [Google Scholar] [CrossRef] [PubMed]
- Jaffry, M.; Bouchachi, S.; Ahmed, M.; Gad, S.N.; Sathe, S.; Souayah, N. Expanding the phenotypic spectrum of Dejerine-Sottas syndrome caused by the trembler mutation. Neurogenetics 2022, 23, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Jerath, N.U.; Kamholz, J.; Grider, T.; Harper, A.; Swenson, A.; Shy, M.E. Coexistence of a T118M PMP22 missense mutation and chromosome 17 (17p11.2-p12) deletion. Muscle Nerve 2015, 52, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Kawarai, T.; Yamazaki, H.; Miyamoto, R.; Takamatsu, N.; Mori, A.; Osaki, Y.; Orlacchio, A.; Nodera, H.; Hashiguchi, A.; Higuchi, Y.; et al. PMP22-related disease: A novel splice site acceptor variant and intrafamilial phenotype variability. Neuromuscul. Disord. 2019, 29, 422–426. [Google Scholar] [CrossRef]
- Khadilkar, S.V.; Bala, M.; Shah, S. Magnetic resonance imaging neurography depicting radiological anticipation in a family with PMP22 duplication. Neurol. India 2018, 66, 1159–1160. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.H.; Park, J.Y.; Koo, H.; Park, K.D.; Hong, Y.B.; Chung, K.W.; Chot, B.O. A longitudinal clinicopathological study of two unrelated patients with Charcot-Marie-Tooth disease type 1E. Neurol. India 2017, 65, 893–895. [Google Scholar] [CrossRef]
- Kim, K.E.; Yeom, J. Proximal arm weakness is the most common presentation in young Korean soldiers diagnosed as having hereditary neuropathy with liability to pressure palsy (HNPP). J. R. Army Med. Corps 2016, 162, 352–354. [Google Scholar] [CrossRef]
- Li, L.X.; Zhao, S.Y.; Liu, Z.J.; Ni, W.; Li, H.F.; Xiao, B.G.; Wu, Z.Y. Improving molecular diagnosis of Chinese patients with Charcot-Marie-Tooth by targeted next-generation sequencing and functional analysis. Oncotarget 2016, 7, 27655–27664. [Google Scholar] [CrossRef][Green Version]
- Li, L.X.; Dong, H.L.; Xiao, B.G.; Wu, Z.Y. A Novel Missense Mutation in Peripheral Myelin Protein-22 Causes Charcot-Marie-Tooth Disease. Chin. Med. J. 2017, 130, 1779–1784. [Google Scholar] [CrossRef]
- Liao, Y.C.; Tsai, P.C.; Lin, T.S.; Hsiao, C.T.; Chao, N.C.; Lin, K.P.; Lee, Y.C. Clinical and Molecular Characterization of PMP22 point mutations in Taiwanese patients with Inherited Neuropathy. Sci. Rep. 2017, 7, 15363. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Duan, X.; Zhang, Y.; Fan, D. Clinical and Genetic Diversity of PMP22 Mutations in a Large Cohort of Chinese Patients with Charcot-Marie-Tooth Disease. Front. Neurol. 2020, 11, 630. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Del Tedesco, F.; Cambise, C.; Coraci, D.; Donati, F.; Santilli, V.; Padua, L. Fibular nerve palsy after hip replacement: Not only surgeon responsibility. Hereditary neuropathy with liability to pressure palsies (HNPP) a rare cause of nerve liability. Orthop. Traumatol. Surg. Res. 2016, 102, 529–531. [Google Scholar] [CrossRef]
- Lorefice, L.; Murru, M.R.; Coghe, G.; Fenu, G.; Corongiu, D.; Frau, J.; Tranquilli, S.; Tacconi, P.; Vannelli, A.; Marrosu, G.; et al. Charcot-Marie-Tooth disease: Genetic subtypes in the Sardinian population. Neurol. Sci. 2017, 38, 1019–1025, Erratum in Neurol. Sci. 2017, 38, 1027. https://doi.org/10.1007/s10072-017-2944-3. [Google Scholar] [CrossRef]
- Lorenzoni, P.J.; Kay, C.S.; Cavalet, C.; Arndt, R.C.; Werneck, L.C.; Scola, R.H. Hereditary Neuropathy with Liability to Pressure Palsies: A Single-Center Experience in Southern Brazil. Neurol. Int. 2016, 8, 6677. [Google Scholar] [CrossRef]
- Lousa, M.; Vázquez-Huarte-Mendicoa, C.; Gutiérrez, A.J.; Saavedra, P.; Navarro, B.; Tugores, A. Genetic epidemiology, demographic, and clinical characteristics of Charcot-Marie-tooth disease in the island of Gran Canaria (Spain). J. Peripher. Nerv. Syst. 2019, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Duan, X.; Liu, X.; Fan, D. Clinical and mutational spectrum of paediatric Charcot-Marie-Tooth disease in a large cohort of Chinese patients. Front. Genet. 2023, 14, 1188361. [Google Scholar] [CrossRef]
- Machado, R.I.L.; Souza, P.V.S.; Farias, I.B.; Badia, B.M.L.; Filho, J.M.V.A.; Lima, R.J.V.; Pinto, W.B.V.R.; Oliveira, A.S.B. Clinical and Genetic Aspects of Childhood-Onset Demyelinating Charcot-Marie-Tooth’s Disease in Brazil. J. Pediatr. Genet. 2022, 12, 301–307. [Google Scholar] [CrossRef]
- Maghsoodi, N.; Crook, M.A. A case of charcot-marie-Tooth (CMT) disease with hypercholesterolaemia and statin side-effects: A case report and literature review. J. Clin. Neurosci. 2017, 38, 57–59. [Google Scholar] [CrossRef]
- Mahungu, A.C.; Steyn, E.; Floudiotis, N.; Wilson, L.A.; Vandrovcova, J.; Reilly, M.M.; Record, C.J.; Benatar, M.; Wu, G.; Raga, S.; et al. The mutational profile in a South African cohort with inherited neuropathies and spastic paraplegia. Front. Neurol. 2023, 14, 1239725. [Google Scholar] [CrossRef]
- Maltese, G.; Tan, S.V.; Bruno, E.; Brackenridge, A.; Thomas, S. Peripheral neuropathy in diabetes: It’s not always what it looks like. Diabet. Med. 2018, 35, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
- Martinez Thompson, J.M.; Klein, C.J. Thirty Years Later, Case Closed: A Case of PMP22 Triplication from Anticipation. Mayo Clin. Proc. 2016, 91, 687–688. [Google Scholar] [CrossRef] [PubMed]
- Marttila, M.; Kytövuori, L.; Helisalmi, S.; Kallio, M.; Laitinen, M.; Hiltunen, M.; Kärppä, M.; Majamaa, K. Molecular Epidemiology of Charcot-Marie-Tooth Disease in Northern Ostrobothnia, Finland: A Population-Based Study. Neuroepidemiology 2017, 49, 34–39. [Google Scholar] [CrossRef]
- Matsuda, N.; Ootsuki, K.; Kobayashi, S.; Nemoto, A.; Kubo, H.; Usami, S.I.; Kanani, K. A novel case of concurrent occurrence of demyelinating-polyneuropathy-causing PMP22 duplication and SOX10 gene mutation producing severe hypertrophic neuropathy. BMC Neurol. 2021, 21, 243. [Google Scholar] [CrossRef]
- Milley, G.M.; Varga, E.T.; Grosz, Z.; Nemes, C.; Arányi, Z.; Boczán, J.; Diószeghy, P.; Molnár, M.J.; Gál, A. Genotypic and phenotypic spectrum of the most common causative genes of Charcot-Marie-Tooth disease in Hungarian patients. Neuromuscul. Disord. 2018, 28, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Mota, P.T.D.; Maio, M.; Sapage, R.; Branco, C.; Pintado, C. Hereditary Neuropathy with Liability to Pressure Palsies: A Rare Condition That Presents with Common Symptoms: A Case Report. JBJS Case Connect. 2018, 8, e95. [Google Scholar] [CrossRef]
- Nagappa, M.; Sharma, S.; Govindaraj, P.; Chickabasaviah, Y.T.; Siram, R.; Shroti, A.; Debnath, M.; Sinha, S.; Bindu, P.S.; Taly, A.B. PMP22 Gene-Associated Neuropathies: Phenotypic Spectrum in a Cohort from India. J. Mol. Neurosci. 2020, 70, 778–789. [Google Scholar] [CrossRef]
- Nan, H.; Wu, Y.; Cui, S.; Sun, H.; Wang, J.; Li, Y.; Meng, L.; Nagasaka, T.; Wu, L. Coexistence of Charcot-Marie-Tooth 1A and nondystrophic myotonia due to PMP22 duplication and SCN4A pathogenic variants: A case report. BMC Neurol. 2022, 22, 17. [Google Scholar] [CrossRef]
- Narvaez-Caicedo, C.; Jacob, S.M.; Wu, L.; Patel, C. Co-Existent Central and Peripheral Demyelination: Related or Coincidental? Neurol. Int. 2024, 16, 1666–1673. [Google Scholar] [CrossRef]
- Nghia, H.T.T.; Umapathi, T.; Duc, N.M.; Hieu, N.L.T.; Thao, M.P. Genetic landscape of Charcot-Marie-Tooth disease in Vietnam: A prospective multicenter study. J. Neuromuscul. Dis. 2025, 12, 22143602251313722. [Google Scholar] [CrossRef]
- Nguyen-Le, T.H.; Do, M.D.; Le, L.H.G.; Nhat, Q.N.N.; Hoang, N.T.T.; Van Le, T.; Mai, T.P. Genotype-phenotype characteristics of Vietnamese patients diagnosed with Charcot-Marie-Tooth disease. Brain Behav. 2022, 12, e2744. [Google Scholar] [CrossRef] [PubMed]
- Nozue, K.; Sugeno, N.; Ishiyama, S.; Yoshida, M.; Aoki, M. Recurrent Ipsilateral C5 Nerve Palsy Associated with Hereditary Neuropathy with Liability to Pressure Palsy. Cureus 2024, 16, e55948. [Google Scholar] [CrossRef]
- Padilha, J.P.D.; Brasil, C.S.; Hoefel, A.M.L.; Winckler, P.B.; Donis, K.C.; Brusius-Facchin, A.C.; Saute, J.A.M. Diagnostic yield of targeted sequential and massive panel approaches for inherited neuropathies. Clin. Genet. 2020, 98, 185–190. [Google Scholar] [CrossRef]
- Palu, E.; Järvilehto, J.; Pennonen, J.; Huber, N.; Herukka, S.K.; Haapasalo, A.; Isohanni, P.; Tyynismaa, H.; Auranen, M.; Ylikallio, E. Rare PMP22 variants in mild to severe neuropathy uncorrelated to plasma GDF15 or neurofilament light. Neurogenetics 2023, 24, 291–301. [Google Scholar] [CrossRef]
- Palumbo, F.; Yamamoto, M.; Hirata, H. Multiple tendon transfer for a case of radial nerve palsy in hereditary neuropathy with liability to pressure palsy. Nagoya J. Med. Sci. 2023, 85, 204–210. [Google Scholar] [CrossRef]
- Park, D.; Ryu, J.S.; Kim, K.J. Compression of Root Level in a Patient with Hereditary Neuropathy with Liability to Pressure Palsy Diagnosed by Magnetic Resonance Imaging. Am. J. Phys. Med. Rehabil. 2016, 95, e140–e144. [Google Scholar] [CrossRef]
- Pisciotta, C.; Bertini, A.; Tramacere, I.; Manganelli, F.; Fabrizi, G.M.; Schenone, A.; Tozza, S.; Cavallaro, T.; Taioli, F.; Ferrarini, M.; et al. Clinical spectrum and frequency of Charcot-Marie-Tooth disease in Italy: Data from the National CMT Registry. Eur. J. Neurol. 2023, 30, 2461–2470. [Google Scholar] [CrossRef]
- Pridmore, M.; Castoro, R.; McCollum, M.S.; Kang, H.; Li, J.; Dortch, R. Length-dependent MRI of hereditary neuropathy with liability to pressure palsies. Ann. Clin. Transl. Neurol. 2020, 7, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Record, C.J.; Pipis, M.; Skorupinska, M.; Blake, J.; Poh, R.; Polke, J.M.; Eggleton, K.; Nanji, T.; Zuchner, S.; Cortese, A.; et al. Whole genome sequencing increases the diagnostic rate in Charcot-Marie-Tooth disease. Brain 2024, 147, 3144–3156. [Google Scholar] [CrossRef]
- Robert-Varvat, F.; Jousserand, G.; Bouhour, F.; Vial, C.; Cintas, P.; Echaniz-Laguna, A.; Delmont, E.; Clavelou, P.; Chauplannaz, G.; Jomir, L.; et al. Hereditary neuropathy with liability to pressure palsy in patients under 30 years old: Neurophysiological data and proposed electrodiagnostic criteria. Muscle Nerve 2018, 57, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Rudnik-Schöneborn, S.; Tölle, D.; Senderek, J.; Eggermann, K.; Elbracht, M.; Kornak, U.; von der Hagen, M.; Kirschner, J.; Leube, B.; Müller-Felber, W.; et al. Diagnostic algorithms in Charcot-Marie-Tooth neuropathies: Experiences from a German genetic laboratory on the basis of 1206 index patients. Clin. Genet. 2016, 89, 34–43. [Google Scholar] [CrossRef]
- Saffra, N.A.; Emborgo, T.S.; Laureta, E.C.; Kirsch, D.S.; Guarini, L. Asymptomatic Retinal Vein Occlusion in a 13-Year-Old with Heterozygous Deletion of the PMP22 Gene and a Diagnosis of Hereditary Neuropathy with Liability to Pressure Palsies. J. Neuroophthalmol. 2022, 42, e367–e370. [Google Scholar] [CrossRef]
- Sagnelli, A.; Scaioli, V.; Piscosquito, G.; Salsano, E.; Dalla Bella, E.; Gellera, C.; Pareyson, D. Spinal and bulbar muscular atrophy and Charcot-Marie-Tooth type 1A: Co-existence of two rare neuromuscular genetic diseases in the same patient. Neuromuscul. Disord. 2015, 25, 800–801. [Google Scholar] [CrossRef]
- Sato, T.; Maekawa, R.; Mitsutake, A.; Katsumata, J.; Seki, T.; Hideyama, T.; Shiio, Y. Elderly-onset case of hereditary neuropathy with liability to pressure palsies: A case report. Neurol. Clin. Neurosci. 2018, 6, 123–155. [Google Scholar] [CrossRef]
- Sherlaw-Sturrock, C.A.; Cassidy, G.; Glover, K.; Naik, S. A case report of trisomy 17 mosaicism: PMP22 gene duplication as a result of trisomy 17 associated with Charcot-Marie-Tooth disease. Clin. Dysmorphol. 2020, 29, 65–68. [Google Scholar] [CrossRef]
- Shibuya, K.; Yoshida, T.; Misawa, S.; Sekiguchi, Y.; Beppu, M.; Amino, H.; Suzuki, Y.I.; Suichi, T.; Tsuneyama, A.; Nakamura, K.; et al. Hidden Charcot-Marie-Tooth 1A as Revealed by Peripheral Nerve Imaging. Intern. Med. 2019, 58, 3157–3161. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.; Iyer, V.G.; Zhang, Y.P.; Shields, C.B. Heterogeneous Presentation of Hereditary Neuropathy with Liability to Pressure Palsies: Clinical and Electrodiagnostic Findings in Three Patients. Cureus 2022, 14, e32296. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.B.E.; Iyer, V.G.; Zhang, Y.P.; Shields, C.B. Nerve Ultrasound Findings before and after Surgery in a Patient with Charcot-Marie-Tooth Disease Type 1A and Comorbid Carpal Tunnel Syndrome. Case Rep. Neurol. 2022, 14, 111–116. [Google Scholar] [CrossRef]
- Shimizu, K.; Hanajima, R.; Shimizu, T.; Usui, R.; Yanagida, A.; Akutsu, T.; Iizuka, T.; Nishiyama, K. Coexistence of Charcot–Marie–Tooth disease type 1A and chronic inflammatory demyelinating polyradiculoneuropathy with conduction blocks. Neurol. Clin. Neurosci. 2016, 4, 192–194. [Google Scholar] [CrossRef]
- Sidhu, A.; Hankerd, M.; Kennelly, K.; Kristofice, M.; Ebrahim, S. Co-occurrence of Xp21 microduplication encompassing the DMD locus in conjunction with 17p12/PMP22 microduplication in a female with Charcot–Marie–Tooth disease type 1A. Egypt. J. Med. Hum. Genet. 2015, 16, 199–204, ISSN 1110-8630. [Google Scholar] [CrossRef][Green Version]
- Sun, A.P.; Tang, L.; Liao, Q.; Zhang, H.; Zhang, Y.S.; Zhang, J. Coexistent Charcot-Marie-Tooth type 1A and type 2 diabetes mellitus neuropathies in a Chinese family. Neural Regen. Res. 2015, 10, 1696–1699. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Chen, Z.; Ling, L.; Yang, F.; Huang, X. Clinical and genetic spectra of Charcot-Marie-Tooth disease in Chinese Han patients. J. Peripher. Nerv. Syst. 2017, 22, 13–18. [Google Scholar] [CrossRef]
- Takahashi, S.; Chum, M.; Kimpinski, K. Electrodiagnostic Characterization of Hereditary Neuropathy with Liability to Pressure Palsies. J. Clin. Neuromuscul. Dis. 2017, 18, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Takegami, N.; Hamada, M.; Yamaguchi-Takegami, N.; Sakuishi, K.; Toda, T. An Elderly Woman with Complaints of Pain and Hearing Loss, Diagnosed with CMT1A with PMP22 Duplication. Intern. Med. 2024, 63, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Thongsing, A.; Pho-Iam, T.; Limwongse, C.; Likasitwattanakul, S.; Sanmaneechai, O. Case series: Childhood Charcot-Marie-Tooth: Predominance of axonal subtype. eNeurologicalSci 2019, 16, 100200. [Google Scholar] [CrossRef]
- Tohge, R.; Shinoto, Y.; Takahashi, M. Case of hereditary neuropathy with liability to pressure palsies presenting progressive muscular atrophy with lower motor neuron degeneration in the spinal cord and the brainstem. Neurol. Clin. Neurosci. 2015, 4, 19–21. [Google Scholar] [CrossRef]
- Tomaselli, P.J.; Blake, J.; Polke, J.M.; do Nascimento, O.J.M.; Reilly, M.M.; Marques Júnior, W.; Laurá, M. Intermediate conduction velocity in two cases of Charcot-Marie-Tooth disease type 1A. Eur. J. Neurol. 2024, 31, e16199. [Google Scholar] [CrossRef]
- Uchôa Cavalcanti, E.B.; Santos, S.C.L.; Martins, C.E.S.; de Carvalho, D.R.; Rizzo, I.M.P.O.; Freitas, M.C.D.N.B.; da Silva Freitas, D.; de Souza, F.S.; Junior, A.M.; do Nascimento, O.J.M. Charcot-Marie-Tooth disease: Genetic profile of patients from a large Brazilian neuromuscular reference center. J. Peripher. Nerv. Syst. 2021, 26, 290–297. [Google Scholar] [CrossRef]
- Vill, K.; Kuhn, M.; Gläser, D.; Müller-Felber, W. Overlap phenotype between CMT1A and hereditary neuropathy with liability to pressure palsies caused by the novel small in-frame deletion c.407_418del12 in PMP22 gene. Neuropediatrics 2015, 46, 44–48. [Google Scholar] [CrossRef]
- Vogt, B.; Chahin, N.; Wiszniewski, W.; Ragole, T.; Karam, C. Screening for genetic mutations in patients with neuropathy without definite etiology is useful. J. Neurol. 2020, 267, 2648–2654. [Google Scholar] [CrossRef]
- Wang, D.S.; Wu, X.; Bai, Y.; Zaidman, C.; Grider, T.; Kamholz, J.; Lupski, J.R.; Connolly, A.M.; Shy, M.E. PMP22 exon 4 deletion causes ER retention of PMP22 and a gain-of-function allele in CMT1E. Ann. Clin. Transl. Neurol. 2017, 4, 236–245. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Chen, Y.; Qi, S.; Wang, M. A case report of a MODY6 patient coexistence with Charcot-Marie-Toothe 1A syndrome. Front. Endocrinol. 2025, 16, 1502783. [Google Scholar] [CrossRef]
- Wang, R.; He, J.; Li, J.J.; Ni, W.; Wu, Z.Y.; Chen, W.J.; Wang, Y. Clinical and genetic spectra in a series of Chinese patients with Charcot-Marie-Tooth disease. Clin. Chim. Acta 2015, 451 Pt B, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.S.; Ptak, C.P.; Pashkova, N.; Grider, T.; Peterson, T.A.; Pareyson, D.; Pisciotta, C.; Saveri, P.; Moroni, I.; Laura, M.; et al. Charcot-Marie-Tooth disease type 1E: Clinical natural history and molecular impact of PMP22 variants. Brain 2025, awaf219. [Google Scholar] [CrossRef]
- Wong, E.; DeOrchis, V.S.; Stein, B.; Herskovitz, S. Davidenkow syndrome: A phenotypic variant of hereditary neuropathy with liability to pressure palsies. Muscle Nerve 2018, 57, E108–E110. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Fu, J.; Meng, L.; Lv, H.; Wang, Z.; Zhirong, J.; Yuan, Y. Homozygous splice-site mutation c.78 + 5G>A in PMP22 causes congenital hypomyelinating neuropathy. Neuropathology 2019, 39, 441–446. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, Z.; Liu, L.; Li, X.; Huang, S.; Zhao, H.; Wang, B.; Zeng, S.; Cao, W.; Li, L.; et al. Genotype and phenotype distribution of 435 patients with Charcot-Marie-Tooth disease from central south China. Eur. J. Neurol. 2021, 28, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, F.G.; Nurlu, G.; Özdamar, S.E.; Temuçin, Ç.M. The Neurophysiologic Frequency of Hereditary Neuropathy with Liability to Pressure Palsy in Entrapment Neuropathies. Turk. J. Neurol. 2016, 22, 177–180. [Google Scholar] [CrossRef]
- Yoshimura, A.; Yuan, J.H.; Hashiguchi, A.; Ando, M.; Higuchi, Y.; Nakamura, T.; Okamoto, Y.; Nakagawa, M.; Takashima, H. Genetic profile and onset features of 1005 patients with Charcot-Marie-Tooth disease in Japan. J. Neurol. Neurosurg. Psychiatry 2019, 90, 195–202. [Google Scholar] [CrossRef]
- Yuan, B.; Neira, J.; Gu, S.; Harel, T.; Liu, P.; Briceño, I.; Elsea, S.H.; Gómez, A.; Potocki, L.; Lupski, J.R. Nonrecurrent PMP22-RAI1 contiguous gene deletions arise from replication-based mechanisms and result in Smith-Magenis syndrome with evident peripheral neuropathy. Hum. Genet. 2016, 135, 1161–1174. [Google Scholar] [CrossRef]
- Yuan, B.; Harel, T.; Gu, S.; Liu, P.; Burglen, L.; Chantot-Bastaraud, S.; Gelowani, V.; Beck, C.R.; Carvalho, C.M.; Cheung, S.W.; et al. Nonrecurrent 17p11.2p12 Rearrangement Events that Result in Two Concomitant Genomic Disorders: The PMP22-RAI1 Contiguous Gene Duplication Syndrome. Am. J. Hum. Genet. 2015, 97, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Zambon, A.A.; Pitt, M.; Laurà, M.; Polke, J.M.; Reilly, M.M.; Muntoni, F. A novel homozygous variant extending the peripheral myelin protein 22 by 9 amino acids causes early-onset Charcot-Marie-Tooth disease with predominant severe sensory ataxia. J. Peripher. Nerv. Syst. 2020, 25, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zi, X.; Hu, Z.; Peng, Y.; Wu, L.; Li, X.; Jiang, M.; Liu, L.; Xie, Y.; Xia, K.; et al. PMP22-Related neuropathies and other clinical manifestations in Chinese han patients with charcot-marie-tooth disease type 1. Muscle Nerve 2015, 52, 69–75. [Google Scholar] [CrossRef]
- Zhang, B.; Lyu, S. Ultrasonographic manifestations of Charcot-Marie-Tooth disease due to a mutation in the PMP22 gene: A case image. J. Clin. Ultrasound 2025, 53, 179–181. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Zhao, F.; Chang, X.; Wang, J.; Zhang, J.; Pang, X.; Shi, J.; Guo, J.; Zhang, W. Clinical, neurophysiological and genetic characteristics of Charcot-Marie-Tooth from a research center in northern China. Neurol. Asia 2024, 29, 133–144. [Google Scholar] [CrossRef]
- Zhao, M.; Du, Q.; Ding, Y.; Wang, W. Hereditary neuropathy with liability to pressure palsies with recurrent facial paralysis as main clinical manifestation in a Chinese patient: A case report. Medicine 2025, 104, e43192. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, S. Narcolepsy with cataplexy in a child with Charcot-Marie-Tooth disease. Case Report. Neuro Endocrinol. Lett. 2016, 37, 265–268. [Google Scholar]
- Zhu, J.; Tong, X.; Li, Y.; Li, G.; Pi, Z. Hereditary neuropathy with liability to pressure palsies misdiagnosed as Guillain-Barré Syndrome: A case report. Medicine 2022, 101, e30768. [Google Scholar] [CrossRef]
- Zhu, G.; Nie, X.; Qi, W.; Ma, Y.; Hao, L.; Guo, X. Tibial neuropathy, a rare manifestation of hereditary neuropathy with liability to pressure palsy: A case report. Heliyon 2023, 9, e18340. [Google Scholar] [CrossRef]
- Zhu, J.; Dai, S.; Li, Y.; Ma, M.; Chu, M.; Lin, Z.; Sun, L.; Zhou, J.C. Two Cases of Charcot-Marie-Tooth Disease Diagnosed in a 53-Year-Old Mother and a 24-Year-Old Daughter. Am. J. Case Rep. 2025, 26, e947400. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.W.D.; Jerath, N.U. T118M Variant of PMP22 Gene Presents with Painful Peripheral Neuropathy and Varying Charcot-Marie-Tooth Features: A Case Series and Review of the Literature. Case Rep. Genet. 2018, 2018, 2618071. [Google Scholar] [CrossRef]
- Li, J.; Niu, B.; Wang, X.; Hu, H.; Cao, B. A case report of hereditary neuropathy with liability to pressure palsies accompanied by type 2 diabetes mellitus and psoriasis. Medicine 2017, 96, e6922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manganelli, F.; Pisciotta, C.; Dubbioso, R.; Maruotti, V.; Iodice, R.; Notturno, F.; Ruggiero, L.; Vitale, C.; Nolano, M.; Uncini, A.; et al. Electrophysiological comparison between males and females in HNPP. Neurol. Sci. 2013, 34, 1429–1432. [Google Scholar] [CrossRef]
- Sereda, M.W.; Meyer zu Hörste, G.; Suter, U.; Uzma, N.; Nave, K.A. Therapeutic administration of progesterone antagonist in a model of Charcot-Marie-Tooth disease (CMT-1A). Nat. Med. 2003, 9, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, X.; Katona, I.; Saporta, M.A.; Shy, M.E.; O’Malley, H.A.; Isom, L.L.; Suter, U.; Li, J. Conduction block in PMP22 deficiency. J. Neurosci. 2010, 30, 600–608. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grossman, M.J.; Feinberg, J.; DiCarlo, E.F.; Birchansky, S.B.; Wolfe, S.W. Hereditary neuropathy with liability to pressure palsies: Case report and discussion. HSS J. 2007, 3, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Moss, K.R.; Bopp, T.S.; Johnson, A.E.; Höke, A. New evidence for secondary axonal degeneration in demyelinating neuropathies. Neurosci. Lett. 2021, 744, 135595. [Google Scholar] [CrossRef]
- Sahenk, Z.; Nagaraja, H.N.; McCracken, B.S.; King, W.M.; Freimer, M.L.; Cedarbaum, J.M.; Mendell, J.R. NT-3 promotes nerve regeneration and sensory improvement in CMT1A mouse models and in patients. Neurology 2005, 65, 681–689. [Google Scholar] [CrossRef]
- Vigo, T.; Nobbio, L.; Hummelen, P.V.; Abbruzzese, M.; Mancardi, G.; Verpoorten, N.; Verhoeven, K.; Sereda, M.W.; Nave, K.A.; Timmerman, V.; et al. Experimental Charcot-Marie-Tooth type 1A: A cDNA microarrays analysis. Mol. Cell Neurosci. 2005, 28, 703–714. [Google Scholar] [CrossRef]
- Aghajan, Y.; Yoon, J.M.; Crawford, J.R. Severe vincristine-induced polyneuropathy in a teenager with anaplastic medulloblastoma and undiagnosed Charcot-Marie-Tooth disease. BMJ Case Rep. 2017, 2017, bcr-2016. [Google Scholar] [CrossRef]
- Cil, T.; Altintas, A.; Tamam, Y.; Battaloglu, E.; Isikdogan, A. Low dose vincristine-induced severe polyneuropathy in a Hodgkin lymphoma patient: A case report (vincristine-induced severe polyneuropathy). J. Pediatr. Hematol. Oncol. 2009, 31, 787–789. [Google Scholar] [CrossRef]
- Graf, W.D.; Chance, P.F.; Lensch, M.W.; Eng, L.J.; Lipe, H.P.; Bird, T.D. Severe vincristine neuropathy in Charcot-Marie-Tooth disease type 1A. Cancer 1996, 77, 1356–1362. [Google Scholar] [CrossRef]
- Jariwal, R.; Shoua, B.; Sabetian, K.; Natarajan, P.; Cobos, E. Unmasking a Case of Asymptomatic Charcot-Marie-Tooth Disease (CMT1A) with Vincristine. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618758349. [Google Scholar] [CrossRef]
- Debruyne, J.; Dehaene, I.; Martin, J.J. Hereditary pressure-sensitive neuropathy. J. Neurol. Sci. 1980, 47, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Kalfakis, N.; Panas, M.; Karadima, G.; Floroskufi, P.; Kokolakis, N. Vassilopoulos D, Hereditary neuropathy with liability to pressure palsies emerging during vincristine treatment. Neurology 2002, 59, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Sahenk, Z.; Chen, L.; Freimer, M. A novel PMP22 point mutation causing HNPP phenotype: Studies on nerve xenografts. Neurology 1998, 51, 702–707. [Google Scholar] [CrossRef]
- Abe, K.T.; Lino, A.M.; Hirata, M.T.; Pavanello, R.C.; Brotto, M.W.; Marchiori, P.E.; Zatz, M. A novel stop codon mutation in the PMP22 gene associated with a variable phenotype. Neuromuscul. Disord. 2004, 14, 313–320. [Google Scholar] [CrossRef]
- Manganelli, F.; Pisciotta, C.; Reilly, M.M.; Tozza, S.; Schenone, A.; Fabrizi, G.M.; Cavallaro, T.; Vita, G.; Padua, L.; Gemignani, F.; et al. Nerve conduction velocity in CMT1A: What else can we tell? Eur. J. Neurol. 2016, 23, 1566–1571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, L.; Zhang, H.; Li, C.; Yang, N.; Wang, J.; Liang, J. Literature review of clinical analysis of hereditary neuropathy with liability to pressure palsies. J. Neurol. 2024, 272, 41. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, H.J.; Lee, E.R. Electrophysiological evaluation of chronic inflammatory demyelinating polyneuropathy and charcot-marie-tooth type 1: Dispersion and correlation analysis. J. Phys. Ther. Sci. 2013, 25, 1265–1268. [Google Scholar] [CrossRef][Green Version]
- Benstead, T.J.; Kuntz, N.L.; Miller, R.G.; Daube, J.R. The electrophysiologic profile of Dejerine-Sottas disease (HMSN III). Muscle Nerve 1990, 13, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Gabreëls-Festen, A. Dejerine-Sottas syndrome grown to maturity: Overview of genetic and morphological heterogeneity and follow-up of 25 patients. J. Anat. 2002, 200, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Roa, B.B.; Dyck, P.J.; Marks, H.G.; Chance, P.F.; Lupski, J.R. Dejerine-Sottas syndrome associated with point mutation in the peripheral myelin protein 22 (PMP22) gene. Nat. Genet. 1993, 5, 269–273. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).