RNAi-Based Bioinsecticides for Controlling Vector-Borne Diseases
Abstract
1. Introduction
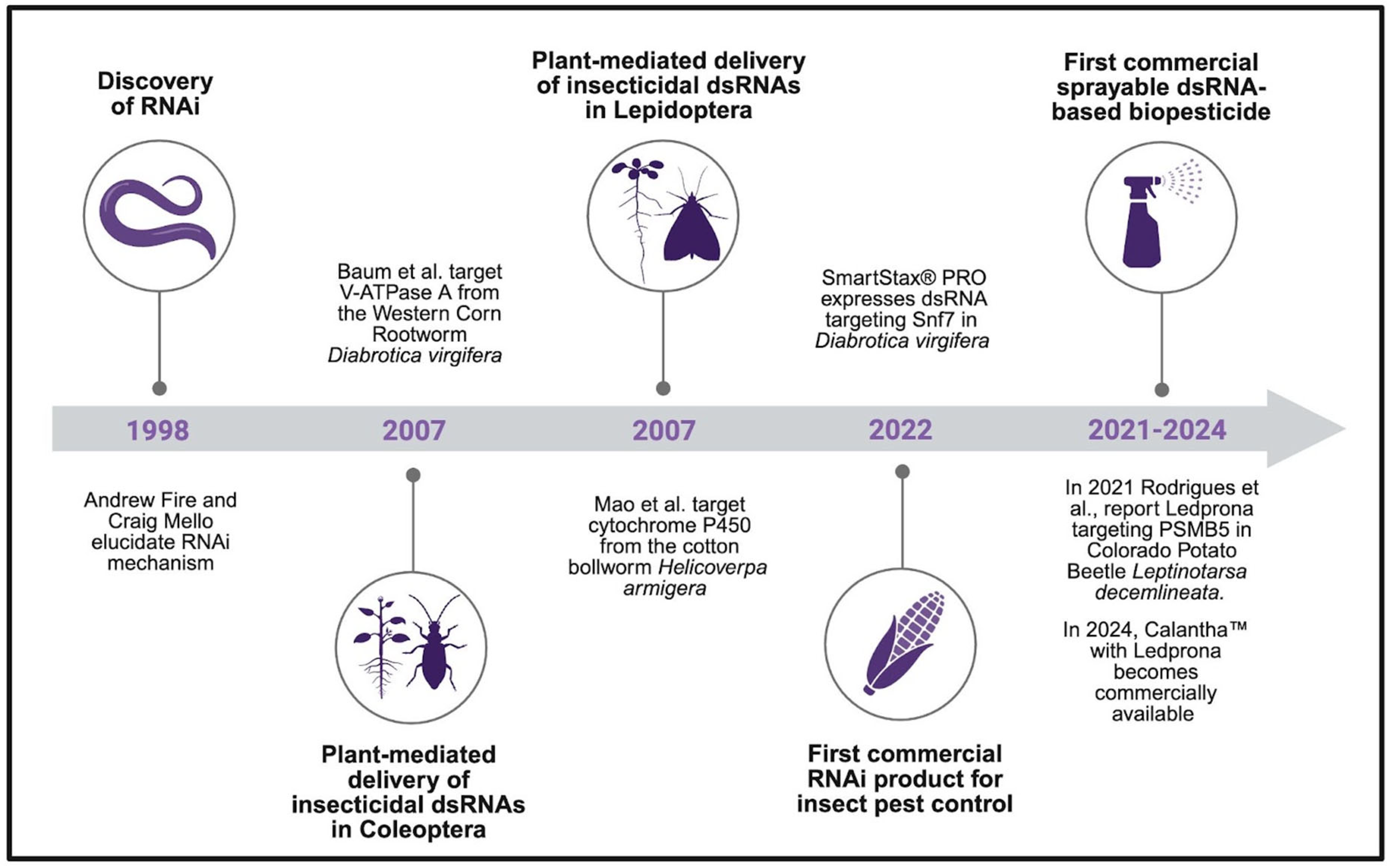
2. RNAi-Based Approaches in Agriculture
Is Gene Selection the Key to Effective RNAi-Based Approaches?
3. Potential Genes for Suppression of Insect Vector Populations Using RNAi-Based Insecticides
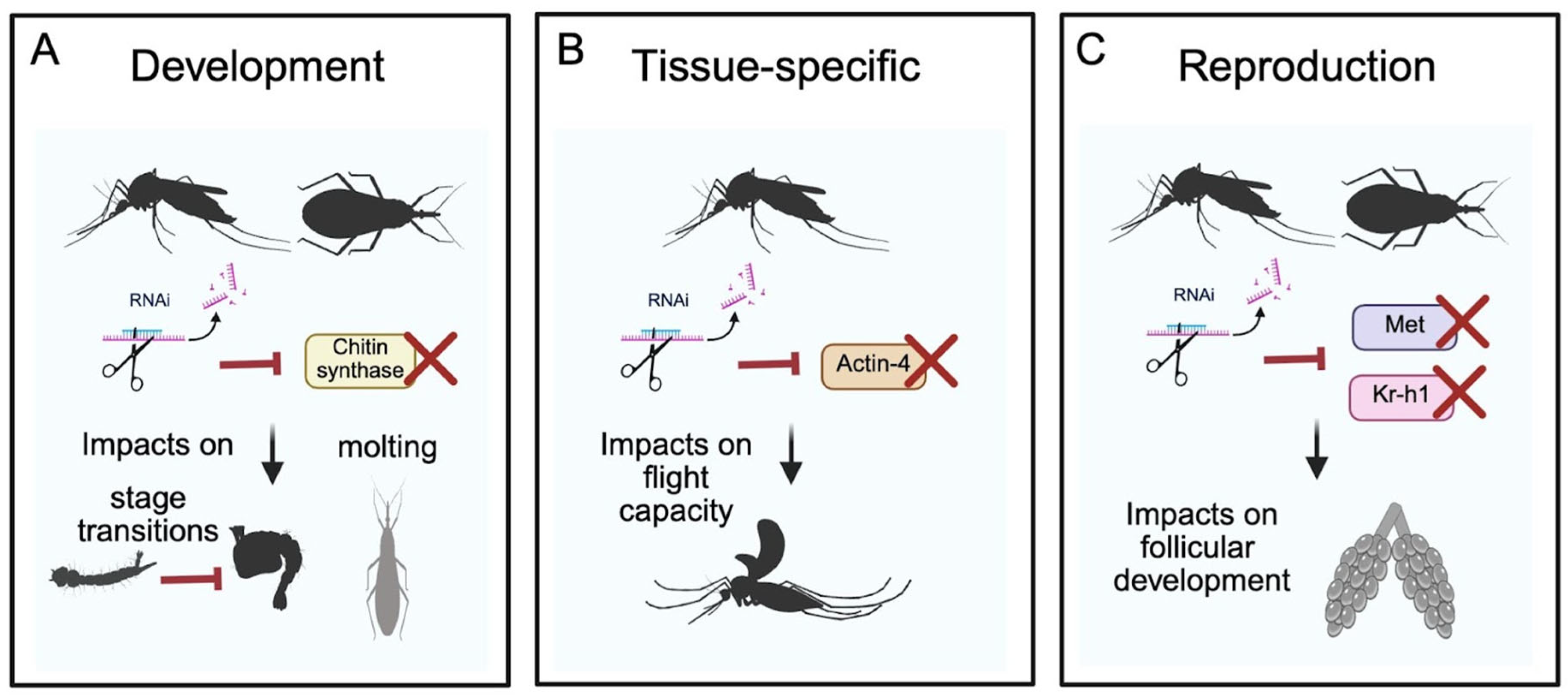
3.1. Targeting Development-Related Genes
3.1.1. Chitin Metabolism
3.1.2. Actin Silencing to Block Flight Capacity
3.2. Targeting Genes Related to Reproduction
4. Advances and Challenges in dsRNA Delivery in Vectors
4.1. Delivery Approaches
4.1.1. Soaking and Oral Feeding (Naked dsRNA)
4.1.2. Nanoparticles
4.1.3. Liposomes
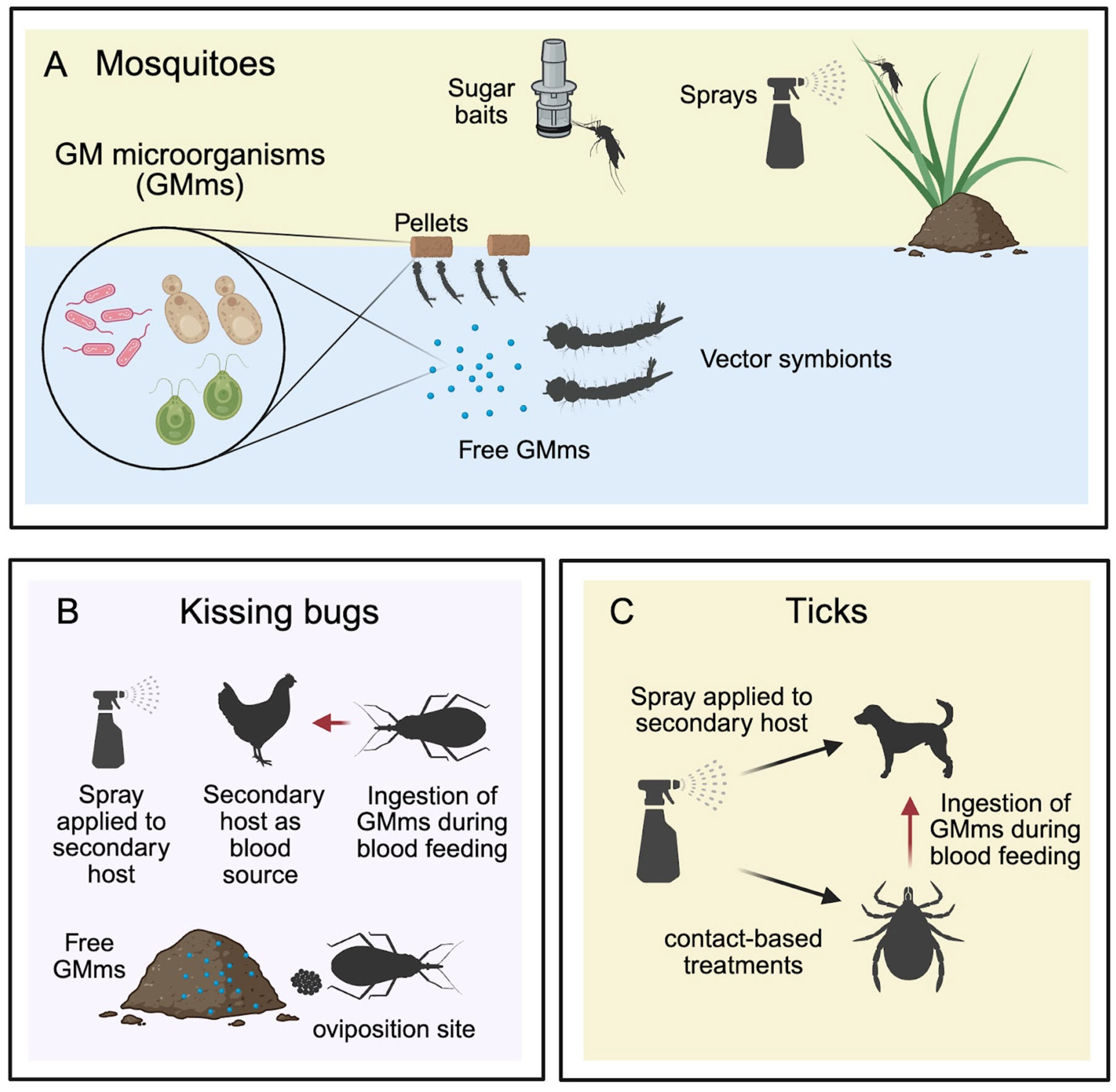
4.1.4. Microorganisms Delivery Systems
Genetically Modified Microorganisms (Bacteria, Yeast and Alga)
Heat-Killed Genetically Modified Microorganisms (Bacteria and Yeast)
Paratransgenesis
4.2. Advances and Limitations of dsRNA Delivery for Targeting Insect Vectors in the Field
Potential Strategies for Field Delivery of dsRNA Targeting Vectors
5. dsRNA-Based Insecticides: Advantages in Specificity over Conventional Pesticides
6. Perspectives of RNAi-Based Insecticides to Control Insect Vectors
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151297-8.
- Van Den Berg, H.; Da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent Trends in Global Insecticide Use for Disease Vector Control and Potential Implications for Resistance Management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- World Health Organization. Global Insecticide Use for Vector-Borne Disease Control: A 10-Year Assessment (2010–2019), 6th ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003203-3.
- Zhou, W.; Li, M.; Achal, V. A Comprehensive Review on Environmental and Human Health Impacts of Chemical Pesticide Usage. Emerg. Contam. 2025, 11, 100410. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a Promising Alternative to Synthetic Pesticides: A Case for Microbial Pesticides, Phytopesticides, and Nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Tikhe, C.V.; Dimopoulos, G. Mosquito Antiviral Immune Pathways. Dev. Comp. Immunol. 2021, 116, 103964. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA In Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A Perspective on RNAi-Based Biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of Coleopteran Insect Pests through RNA Interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a Cotton Bollworm P450 Monooxygenase Gene by Plant-Mediated RNAi Impairs Larval Tolerance of Gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO Maize against Western Corn Rootworm and Northern Corn Rootworm: Efficacy and Resistance Management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.; et al. First Sprayable Double-Stranded RNA-Based Biopesticide Product Targets Proteasome Subunit Beta Type-5 in Colorado Potato Beetle (Leptinotarsa Decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef] [PubMed]
- GreenLight Biosciences, Memorandum: Posting EPA-HQ-OPP-2021-0271 to Regulations.Gov for Public Access 2023. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2021-0271-0003 (accessed on 1 October 2025).
- Ni, M.; Ma, W.; Wang, X.; Gao, M.; Dai, Y.; Wei, X.; Zhang, L.; Peng, Y.; Chen, S.; Ding, L.; et al. Next-generation Transgenic Cotton: Pyramiding RNAi and Bt Counters Insect Resistance. Plant Biotechnol. J. 2017, 15, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Full Crop Protection from an Insect Pest by Expression of Long Double-Stranded RNAs in Plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef]
- Zha, W.; Peng, X.; Chen, R.; Du, B.; Zhu, L.; He, G. Knockdown of Midgut Genes by dsRNA-Transgenic Plant-Mediated RNA Interference in the Hemipteran Insect Nilaparvata Lugens. PLoS ONE 2011, 6, e20504. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.; Beeman, R.W. The Tribolium Chitin Synthase Genes TcCHS1 and TcCHS2 Are Specialized for Synthesis of Epidermal Cuticle and Midgut Peritrophic Matrix. Insect Mol. Biol. 2005, 14, 453–463. [Google Scholar] [CrossRef]
- Chen, W.; Cao, P.; Liu, Y.; Yu, A.; Wang, D.; Chen, L.; Sundarraj, R.; Yuchi, Z.; Gong, Y.; Merzendorfer, H.; et al. Structural Basis for Directional Chitin Biosynthesis. Nature 2022, 610, 402–408. [Google Scholar] [CrossRef]
- Cortés, J.C.G.; Curto, M.-Á.; Carvalho, V.S.D.; Pérez, P.; Ribas, J.C. The Fungal Cell Wall as a Target for the Development of New Antifungal Therapies. Biotechnol. Adv. 2019, 37, 107352. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Avino, M.; Zhou, Q.; Fugelstad, J.; Clergeot, P.-H.; Bulone, V. Chitin Synthases from Saprolegnia Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs. PLoS Pathog. 2010, 6, e1001070. [Google Scholar] [CrossRef] [PubMed]
- Hogenkamp, D.G.; Arakane, Y.; Zimoch, L.; Merzendorfer, H.; Kramer, K.J.; Beeman, R.W.; Kanost, M.R.; Specht, C.A.; Muthukrishnan, S. Chitin Synthase Genes in Manduca Sexta: Characterization of a Gut-Specific Transcript and Differential Tissue Expression of Alternately Spliced mRNAs during Development. Insect Biochem. Mol. Biol. 2005, 35, 529–540. [Google Scholar] [CrossRef]
- Kato, N.; Mueller, C.R.; Fuchs, J.F.; Wessely, V.; Lan, Q.; Christensen, B.M. Regulatory Mechanisms of Chitin Biosynthesis and Roles of Chitin in Peritrophic Matrix Formation in the Midgut of Adult Aedes Aegypti. Insect Biochem. Mol. Biol. 2006, 36, 1–9. [Google Scholar] [CrossRef]
- Kelkenberg, M.; Odman-Naresh, J.; Muthukrishnan, S.; Merzendorfer, H. Chitin Is a Necessary Component to Maintain the Barrier Function of the Peritrophic Matrix in the Insect Midgut. Insect Biochem. Mol. Biol. 2015, 56, 21–28. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Li, S.; Zhu, K.Y.; Ma, E.; Zhang, J. Characterization of a Midgut-Specific Chitin Synthase Gene (LmCHS2) Responsible for Biosynthesis of Chitin of Peritrophic Matrix in Locusta Migratoria. Insect Biochem. Mol. Biol. 2012, 42, 902–910. [Google Scholar] [CrossRef]
- Lu, Z.-J.; Huang, Y.-L.; Yu, H.-Z.; Li, N.-Y.; Xie, Y.-X.; Zhang, Q.; Zeng, X.-D.; Hu, H.; Huang, A.-J.; Yi, L.; et al. Silencing of the Chitin Synthase Gene Is Lethal to the Asian Citrus Psyllid, Diaphorina Citri. Int. J. Mol. Sci. 2019, 20, 3734. [Google Scholar] [CrossRef]
- Shi, J.-F.; Mu, L.-L.; Chen, X.; Guo, W.-C.; Li, G.-Q. RNA Interference of Chitin Synthase Genes Inhibits Chitin Biosynthesis and Affects Larval Performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef]
- Wu, J.-J.; Chen, Z.-C.; Wang, Y.-W.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. Silencing Chitin Deacetylase 2 Impairs Larval–Pupal and Pupal–Adult Molts in Leptinotarsa decemlineata. Insect Mol. Biol. 2019, 28, 52–64. [Google Scholar] [CrossRef]
- Shao, L.; Devenport, M.; Jacobs-Lorena, M. The Peritrophic Matrix of Hematophagous Insects. Arch. Insect Biochem. Physiol. 2001, 47, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Dana, A.N.; Hong, Y.S.; Kern, M.K.; Hillenmeyer, M.E.; Harker, B.W.; Lobo, N.F.; Hogan, J.R.; Romans, P.; Collins, F.H. Gene Expression Patterns Associated with Blood-Feeding in the Malaria Mosquito Anopheles Gambiae. BMC Genom. 2005, 6, 5. [Google Scholar] [CrossRef]
- Ibrahim, G.H.; Smartt, C.T.; Kiley, L.M.; Christensen, B.M. Cloning and Characterization of a Chitin Synthase cDNA from the Mosquito Aedes Aegypti. Insect Biochem. Mol. Biol. 2000, 30, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Park, Y.; Zhu, K.Y. Identification and Characterization of Two Chitin Synthase Genes in African Malaria Mosquito, Anopheles Gambiae. Insect Biochem. Mol. Biol. 2012, 42, 674–682. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Liu, J.; Xu, J.; Zhang, R.; Zheng, J.; Shen, B.; Sun, Y.; Zhou, D. Adverse Effects of Knocking down Chitin Synthase A on Female Reproduction in Culex pipiens pallens (Diptera: Culicidae). Pest Manag. Sci. 2023, 79, 4463–4473. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Yin, Q.; Zhang, H.; Yin, H.; Sun, Y.; Ma, L.; Zhou, D.; Shen, B. Physiological Characterization of Chitin Synthase A Responsible for the Biosynthesis of Cuticle Chitin in Culex Pipiens Pallens (Diptera: Culicidae). Parasit. Vectors 2021, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.F.; Alvarenga, E.S.L.; Figueira-Mansur, J.; Franco, T.A.; Ramos, I.B.; Masuda, H.; Melo, A.C.A.; Moreira, M.F. Effects of Chitin Synthase Double-Stranded RNA on Molting and Oogenesis in the Chagas Disease Vector Rhodnius Prolixus. Insect Biochem. Mol. Biol. 2014, 51, 110–121. [Google Scholar] [CrossRef]
- Chaplain, R.A. On the Role of Actin in the Contractile Complex of Insect Flight Muscle. Arch. Biochem. Biophys. 1968, 130, 70–79. [Google Scholar] [CrossRef]
- Celestino-Montes, A.; Hernández-Martínez, S.; Rodríguez, M.H.; Cázares-Raga, F.E.; Vázquez-Calzada, C.; Lagunes-Guillén, A.; Chávez-Munguía, B.; Rubio-Miranda, J.Á.; Hernández-Cázares, F.D.J.; Cortés-Martínez, L.; et al. Development of the Indirect Flight Muscles of Aedes Aegypti, a Main Arbovirus Vector. BMC Dev. Biol. 2021, 21, 11. [Google Scholar] [CrossRef]
- An, H.-S.; Mogami, K.; Yoo, M.-A.; Lee, W.-H. Characterization of Drosophila Melanogaster Act88F Actin Allele with Point Mutation in Myosin Binding Site. Mol. Cells 1993, 3, 433–443. [Google Scholar] [CrossRef]
- Muñoz, D.; Jimenez, A.; Marinotti, O.; James, A.A. The AeAct-4 Gene Is Expressed in the Developing Flight Muscles of Female Aedes Aegypti. Insect Mol. Biol. 2004, 13, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.L.; Christensen, B.M. Flight Muscle-Specific Expression of act88F: GFP in Transgenic Culex Quinquefasciatus Say (Diptera: Culicidae). Parasitol. Int. 2004, 53, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Payá, D.; Flis, I.; Anderson, M.A.E.; Hawes, P.; Li, M.; Akbari, O.S.; Basu, S.; Alphey, L. Targeting Female Flight for Genetic Control of Mosquitoes. PLoS Negl. Trop. Dis. 2020, 14, e0008876. [Google Scholar] [CrossRef]
- Paiz-Reyes, C.; Taracena, M.L.; Flores-Ayuso, P.; Hunt, C.; Benedict, A.C.; Illescas, K.; Morales, M.; Padilla, N.; Dotson, E.M.; Pennington, P.M. Oral Delivery of dsRNA Targeting a Female-Biased Flight Muscle Actin Impairs Flight in the Malaria Vector, Anopheles Albimanus. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Basnet, S.; Kamble, S.T. RNA Interference of the Muscle Actin Gene in Bed Bugs: Exploring Injection Versus Topical Application for dsRNA Delivery. J. Insect Sci. 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xu, W.; Fu, K.; Guo, W.; Kim, D.S.; Zhang, J. Positional Effects of Double-Stranded RNAs Targeting β-Actin Gene Affect RNA Interference Efficiency in Colorado Potato Beetle. Pestic. Biochem. Physiol. 2022, 184, 105121. [Google Scholar] [CrossRef]
- Sattar, M.N.; Naqqash, M.N.; Rezk, A.A.; Mehmood, K.; Bakhsh, A.; Elshafie, H.; Al-Khayri, J.M. Sprayable RNAi for Silencing of Important Genes to Manage Red Palm Weevil, Rhynchophorus Ferrugineus (Coleoptera: Curculionidae). PLoS ONE 2024, 19, e0308613. [Google Scholar] [CrossRef]
- Santos, C.G.; Humann, F.C.; Hartfelder, K. Juvenile Hormone Signaling in Insect Oogenesis. Curr. Opin. Insect Sci. 2019, 31, 43–48. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Cardoso-Jaime, V.; Nouzova, M.; Michalkova, V.; Ramirez, C.E.; Fernandez-Lima, F.; Noriega, F.G. Juvenile Hormone Controls Ovarian Development in Female Anopheles Albimanus Mosquitoes. Sci. Rep. 2019, 9, 2127. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Mayoral, J.G.; Li, Y.; Noriega, F.G. Role of Juvenile Hormone and Allatotropin on Nutrient Allocation, Ovarian Development and Survivorship in Mosquitoes. J. Insect Physiol. 2007, 53, 230–234. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Rivera-Perez, C.; Nouzova, M.; Noriega, F.G. Coordinated Changes in JH Biosynthesis and JH Hemolymph Titers in Aedes Aegypti Mosquitoes. J. Insect Physiol. 2015, 72, 22–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saha, T.T.; Roy, S.; Pei, G.; Dou, W.; Zou, Z.; Raikhel, A.S. Synergistic Action of the Transcription Factors Krüppel Homolog 1 and Hairy in Juvenile Hormone/Methoprene-Tolerant-Mediated Gene-Repression in the Mosquito Aedes Aegypti. PLOS Genet. 2019, 15, e1008443. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Sambucaro, M.J.; Riccillo, F.L.; Calderón-Fernández, G.M.; Sterkel, M.; Diambra, L.A.; Ronderos, J.R. Genomic and Functional Characterization of a Methoprene-Tolerant Gene in the Kissing-Bug Rhodnius Prolixus. Gen. Comp. Endocrinol. 2015, 216, 1–8. [Google Scholar] [CrossRef]
- Leyria, J.; Orchard, I.; Lange, A.B. Impact of JH Signaling on Reproductive Physiology of the Classical Insect Model, Rhodnius Prolixus. Int. J. Mol. Sci. 2022, 23, 13832. [Google Scholar] [CrossRef]
- Ramos, F.O.; Nouzova, M.; Fruttero, L.L.; Leyria, J.; Ligabue-Braun, R.; Noriega, F.G.; Canavoso, L.E. Role of Methoprene-Tolerant in the Regulation of Oogenesis in Dipetalogaster Maxima. Sci. Rep. 2022, 12, 14195. [Google Scholar] [CrossRef]
- Abrisqueta, M.; Süren-Castillo, S.; Maestro, J.L. Insulin Receptor-Mediated Nutritional Signalling Regulates Juvenile Hormone Biosynthesis and Vitellogenin Production in the German Cockroach. Insect Biochem. Mol. Biol. 2014, 49, 14–23. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Palli, S.R. Molecular Analysis of Nutritional and Hormonal Regulation of Female Reproduction in the Red Flour Beetle, Tribolium Castaneum. Insect Biochem. Mol. Biol. 2011, 41, 294–305. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, Z.; Zhao, H.; Wu, X.; Cai, N.; Ma, T.; Tang, B.; Chen, G.; Wang, S. RNAi-Mediated FoxO Silencing Inhibits Reproduction in Locusta Migratoria. Insects 2024, 15, 891. [Google Scholar] [CrossRef]
- Hansen, I.A.; Sieglaff, D.H.; Munro, J.B.; Shiao, S.-H.; Cruz, J.; Lee, I.W.; Heraty, J.M.; Raikhel, A.S. Forkhead Transcription Factors Regulate Mosquito Reproduction. Insect Biochem. Mol. Biol. 2007, 37, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Denlinger, D.L. Insulin Signaling and FOXO Regulate the Overwintering Diapause of the Mosquito Culex Pipiens. Proc. Natl. Acad. Sci. USA 2008, 105, 6777–6781. [Google Scholar] [CrossRef]
- Su, C.; Liu, S.; Sun, M.; Yu, Q.; Li, C.; Graham, R.I.; Wang, X.; Wang, X.; Xu, P.; Ren, G. Delivery of Methoprene-Tolerant dsRNA to Improve RNAi Efficiency by Modified Liposomes for Pest Control. ACS Appl. Mater. Interfaces 2023, 15, 13576–13588. [Google Scholar] [CrossRef]
- Clifton, M.E.; Lopez, K. Assessing Insect Growth Regulator Resistance Using Bioassays: A Systematic Review and Meta-Analysis of Methoprene and Pyriproxyfen Inhibition of Emergence in Three Vector Mosquito Species. Trop. Med. Infect. Dis. 2025, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.P. Environmental Safety Review of Methoprene and Bacterially-Derived Pesticides Commonly Used for Sustained Mosquito Control. Ecotoxicol. Environ. Saf. 2017, 139, 335–343. [Google Scholar] [CrossRef]
- Tielemans, E.; Prullage, J.; Knaus, M.; Visser, M.; Manavella, C.; Chester, S.T.; Young, D.; Everett, W.R.; Rosentel, J. Efficacy of a Novel Topical Combination of Fipronil, (S)-Methoprene, Eprinomectin, and Praziquantel, against the Ticks, Ixodes Ricinus and Ixodes Scapularis, on Cats. Vet. Parasitol. 2014, 202, 59–63. [Google Scholar] [CrossRef]
- Cruz, B.C.; Teixeira, W.F.P.; Gomes, L.V.C.; Maciel, W.G.; Felippelli, G.; Buzzulini, C.; Ferreira, L.L.; Santos, T.R.D.; Soares, V.E.; Sakamoto, C.A.M.; et al. Does Bathing Affect Tick and Flea Burdens and Ectoparasiticide Effectiveness of a Spot-on Formulation (Fipronil + (S)-Methoprene) for Dogs? Vet. Parasitol. 2020, 283, 109192. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Santos, M.A.; Dantas-Torres, F.; Pugliese, N.; Miglianti, M.; Rhimi, W.; Cafarchia, C.; Otranto, D. Susceptibility of Rhipicephalus Rutilus Ticks from Dogs to Different Acaricides. Ticks Tick-Borne Dis. 2025, 16, 102493. [Google Scholar] [CrossRef]
- Bailly, T.P.; Kohlmeier, P. Chemosensory and Behavioral Effects of Methoprene, a Commonly Used Juvenile Hormone Analog and Insect Pesticide. Curr. Opin. Insect Sci. 2025, 71, 101392. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, J.; Yee, D.A. Non-Target Effects of Methoprene and Larvicidal Surface Films on Invertebrate Predators of Mosquito Larvae. J. Vector Ecol. 2023, 48, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Tikhe, C.V.; Cardoso-Jaime, V.; Dong, S.; Rutkowski, N.; Dimopoulos, G. Trypsin-like Inhibitor Domain (TIL)-Harboring Protein Is Essential for Aedes Aegypti Reproduction. Int. J. Mol. Sci. 2022, 23, 7736. [Google Scholar] [CrossRef]
- Moreau, J.; Desouhant, E.; Louâpre, P.; Goubault, M.; Rajon, E.; Jarrige, A.; Menu, F.; Thiéry, D. How Host Plant and Fluctuating Environments Affect Insect Reproductive Strategies? In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 81, pp. 259–287. ISBN 978-0-12-803318-0. [Google Scholar]
- Dusfour, I.; Chaney, S.C. Success, Failure and Expectations in the Context of Arbovirus Expansion and Emergence. In Mosquitopia: The Place of Pests in a Healthy World; Routledge: New York, NY, USA, 2021; p. 21. ISBN 978-1-003-05603-4. [Google Scholar]
- Takken, W.; Verhulst, N.O. Host Preferences of Blood-Feeding Mosquitoes. Annu. Rev. Entomol. 2013, 58, 433–453. [Google Scholar] [CrossRef]
- Wong, M.L.; Zulzahrin, Z.; Vythilingam, I.; Lau, Y.L.; Sam, I.-C.; Fong, M.Y.; Lee, W.-C. Perspectives of Vector Management in the Control and Elimination of Vector-Borne Zoonoses. Front. Microbiol. 2023, 14, 1135977. [Google Scholar] [CrossRef]
- Aljamali, M.N.; Sauer, J.R.; Essenberg, R.C. RNA Interference: Applicability in Tick Research. Exp. Appl. Acarol. 2002, 28, 89–96. [Google Scholar] [CrossRef]
- Blandin, S.; Moita, L.F.; Köcher, T.; Wilm, M.; Kafatos, F.C.; Levashina, E.A. Reverse Genetics in the Mosquito Anopheles gambiae: Targeted Disruption of the Defensin Gene. EMBO Rep. 2002, 3, 852–856. [Google Scholar] [CrossRef]
- Araujo, R.N.; Santos, A.; Pinto, F.S.; Gontijo, N.F.; Lehane, M.J.; Pereira, M.H. RNA Interference of the Salivary Gland Nitrophorin 2 in the Triatomine Bug Rhodnius Prolixus (Hemiptera: Reduviidae) by dsRNA Ingestion or Injection. Insect Biochem. Mol. Biol. 2006, 36, 683–693. [Google Scholar] [CrossRef]
- Lopez, S.B.G.; Guimarães-Ribeiro, V.; Rodriguez, J.V.G.; Dorand, F.A.P.S.; Salles, T.S.; Sá-Guimarães, T.E.; Alvarenga, E.S.L.; Melo, A.C.A.; Almeida, R.V.; Moreira, M.F. RNAi-Based Bioinsecticide for Aedes Mosquito Control. Sci. Rep. 2019, 9, 4038. [Google Scholar] [CrossRef]
- Lopez-Martinez, G.; Meuti, M.; Denlinger, D.L. Rehydration Driven RNAi: A Novel Approach for Effectively Delivering dsRNA to Mosquito Larvae. J. Med. Entomol. 2012, 49, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Sharma, A.; Lu, C.; Gulia-Nuss, M. RNAi by Soaking Aedes Aegypti Pupae in dsRNA. Insects 2021, 12, 634. [Google Scholar] [CrossRef]
- Whyard, S.; Erdelyan, C.; Partridge, A.L.; Singh, A.D.; Beebe, N.W.; Capina, R. Silencing the Buzz: A New Approach to Population Suppression of Mosquitoes by Feeding Larvae Double-Stranded RNAs. Parasit. Vectors 2015, 8, 96. [Google Scholar] [CrossRef]
- Soares, C.A.G.; Lima, C.M.R.; Dolan, M.C.; Piesman, J.; Beard, C.B.; Zeidner, N.S. Capillary Feeding of Specific dsRNA Induces Silencing of the Isac Gene in Nymphal Ixodes scapularis Ticks. Insect Mol. Biol. 2005, 14, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Giesbrecht, D.; Heschuk, D.; Wiens, I.; Boguski, D.; LaChance, P.; Whyard, S. RNA Interference Is Enhanced by Knockdown of Double-Stranded RNases in the Yellow Fever Mosquito Aedes Aegypti. Insects 2020, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, N.; Thiruvengadam, V.; Sushil, S. Nanoparticle-Mediated dsRNA Delivery for Precision Insect Pest Control: A Comprehensive Review. Mol. Biol. Rep. 2024, 51, 355. [Google Scholar] [CrossRef]
- Mysore, K.; Flannery, E.M.; Tomchaney, M.; Severson, D.W.; Duman-Scheel, M. Disruption of Aedes Aegypti Olfactory System Development through Chitosan/siRNA Nanoparticle Targeting of Semaphorin-1a. PLoS Negl. Trop. Dis. 2013, 7, e2215. [Google Scholar] [CrossRef]
- Mysore, K.; Andrews, E.; Li, P.; Duman-Scheel, M. Chitosan/siRNA Nanoparticle Targeting Demonstrates a Requirement for Single-Minded during Larval and Pupal Olfactory System Development of the Vector Mosquito Aedes Aegypti. BMC Dev. Biol. 2014, 14, 9. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/Double-stranded RNA Nanoparticle-mediated RNA Interference to Silence Chitin Synthase Genes through Larval Feeding in the African Malaria Mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef]
- Zhang, X.; Mysore, K.; Flannery, E.; Michel, K.; Severson, D.W.; Zhu, K.Y.; Duman-Scheel, M. Chitosan/Interfering RNA Nanoparticle Mediated Gene Silencing in Disease Vector Mosquito Larvae. J. Vis. Exp. 2015, 97, e52523. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Debnath, N.; Cui, Y.; Unrine, J.; Palli, S.R. Chitosan, Carbon Quantum Dot, and Silica Nanoparticle Mediated dsRNA Delivery for Gene Silencing in Aedes aegypti: A Comparative Analysis. ACS Appl. Mater. Interfaces 2015, 7, 19530–19535. [Google Scholar] [CrossRef] [PubMed]
- Ramesh Kumar, D.; Saravana Kumar, P.; Gandhi, M.R.; Al-Dhabi, N.A.; Paulraj, M.G.; Ignacimuthu, S. Delivery of Chitosan/dsRNA Nanoparticles for Silencing of Wing Development Vestigial (vg) Gene in Aedes Aegypti Mosquitoes. Int. J. Biol. Macromol. 2016, 86, 89–95. [Google Scholar] [CrossRef]
- Attia, M.M.; Khalf, M.A.; Abou-Okada, M.; Shamseldean, M.S.; Salem, M.A.; Al-Sabi, M.N.S. Chitosan–Silver Nanocomposites as a Promising Tool for Controlling the Bed Bug: Cimex lectularius (Heteroptera: Cimicidae). J. Bioact. Compat. Polym. 2023, 38, 178–187. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Lu, Y.-H.; Liu, Y.; Lin, Y.-C.; Su, Y.-J.; Henry, D.; Lacarriere, V.; Haller, S.; Lin, Y.-H.; Chiang, P.-C.; Hsu, C.-I.; et al. Lipoplex-Based RNA Delivery System Enhances RNAi Efficiency for Targeted Pest Control in Spodoptera Litura. Int. J. Biol. Macromol. 2025, 319, 145091. [Google Scholar] [CrossRef]
- Rodríguez-Almazán, C.; Reyes, E.Z.; Zúñiga-Navarrete, F.; Muñoz-Garay, C.; Gómez, I.; Evans, A.M.; Likitvivatanavong, S.; Bravo, A.; Gill, S.S.; Soberón, M. Cadherin Binding Is Not a Limiting Step for Bacillus thuringiensis Subsp. Israelensis Cry4Ba Toxicity to Aedes aegypti Larvae. Biochem. J. 2012, 443, 711–717. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, J.; Zhou, Y.; Cao, J.; Gong, H.; Zhang, H.; Zhou, J. Liposome Mediated Double-Stranded RNA Delivery to Silence Ribosomal Protein P0 in the Tick Rhipicephalus Haemaphysaloides. Ticks Tick-Borne Dis. 2018, 9, 638–644. [Google Scholar] [CrossRef]
- Papić, L.; Rivas, J.; Toledo, S.; Romero, J. Double-Stranded RNA Production and the Kinetics of Recombinant Escherichia Coli HT115 in Fed-Batch Culture. Biotechnol. Rep. 2018, 20, e00292. [Google Scholar] [CrossRef]
- Ongvarrasopone, C.; Roshorm, Y.; Panyim, S. A Simple and Cost Effective Method to Generate dsRNA for RNAi Studies in Invertebrates. ScienceAsia 2007, 33, 035–039. [Google Scholar] [CrossRef]
- Taracena, M.L.; Oliveira, P.L.; Almendares, O.; Umaña, C.; Lowenberger, C.; Dotson, E.M.; Paiva-Silva, G.O.; Pennington, P.M. Genetically Modifying the Insect Gut Microbiota to Control Chagas Disease Vectors through Systemic RNAi. PLoS Negl. Trop. Dis. 2015, 9, e0003358. [Google Scholar] [CrossRef]
- Hapairai, L.K.; Mysore, K.; Chen, Y.; Harper, E.I.; Scheel, M.P.; Lesnik, A.M.; Sun, L.; Severson, D.W.; Wei, N.; Duman-Scheel, M. Lure-and-Kill Yeast Interfering RNA Larvicides Targeting Neural Genes in the Human Disease Vector Mosquito Aedes Aegypti. Sci. Rep. 2017, 7, 13223. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Sun, L.; Hapairai, L.K.; Wang, C.-W.; Igiede, J.; Roethele, J.B.; Scheel, N.D.; Scheel, M.P.; Li, P.; Wei, N.; et al. A Yeast RNA-Interference Pesticide Targeting the Irx Gene Functions as a Broad-Based Mosquito Larvicide and Adulticide. Insects 2021, 12, 986. [Google Scholar] [CrossRef]
- Souza, R.S.; Virginio, F.; Riback, T.I.S.; Suesdek, L.; Barufi, J.B.; Genta, F.A. Microorganism-Based Larval Diets Affect Mosquito Development, Size and Nutritional Reserves in the Yellow Fever Mosquito Aedes Aegypti (Diptera: Culicidae). Front. Physiol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Promprao, J.; Sanevas, N.; Kraichak, E.; Saraphol, S.; Klankeo, P.; Kaewmee, S.; Mano, C.; Soe, B.K.; Rujeerapaiboon, A.; Preativatanyou, K.; et al. Microalgae Diversity in Aedes Aegypti Larvae Guts and Breeding Sites in Nakhon Si Thammarat, Thailand Revealed by Light Microscopy and Metabarcoding. Metabarcoding Metagenom. 2025, 9, e152948. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, S.; Ou, R.; Samrakandi, M.; Beerntsen, B.T.; Sayre, R.T. Development of an RNAi Based Microalgal Larvicide to Control Mosquitoes. Malar. World J. 2013, 4, 6. [Google Scholar] [CrossRef]
- Fei, X.; Zhang, Y.; Ding, L.; Xiao, S.; Xie, X.; Li, Y.; Deng, X. Development of an RNAi-Based Microalgal Larvicide for the Control of Aedes aegypti. Parasites Vectors 2021, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Xiao, S.; Huang, X.; Li, Z.; Li, X.; He, C.; Li, Y.; Zhang, X.; Deng, X. Control of Aedes Mosquito Populations Using Recombinant Microalgae Expressing Short Hairpin RNAs and Their Effect on Plankton. PLoS Negl. Trop. Dis. 2023, 17, e0011109. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Huang, X.; Li, Z.; Li, X.; He, C.; Xiao, S.; Li, Y.; Zhang, X.; Deng, X. Effect of Marker-Free Transgenic Chlamydomonas on the Control of Aedes Mosquito Population and on Plankton. Parasit. Vectors 2023, 16, 18. [Google Scholar] [CrossRef]
- Yang, W.; Wang, B.; Lei, G.; Chen, G.; Liu, D. Advances in Nanocarriers to Improve the Stability of dsRNA in the Environment. Front. Bioeng. Biotechnol. 2022, 10, 974646. [Google Scholar] [CrossRef]
- Mysore, K.; Hapairai, L.K.; Sun, L.; Harper, E.I.; Chen, Y.; Eggleson, K.K.; Realey, J.S.; Scheel, N.D.; Severson, D.W.; Wei, N.; et al. Yeast Interfering RNA Larvicides Targeting Neural Genes Induce High Rates of Anopheles Larval Mortality. Malar. J. 2017, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Taracena, M.; Hunt, C.; Pennington, P.; Andrew, D.; Jacobs-Lorena, M.; Dotson, E.; Wells, M. Effective Oral RNA Interference (RNAi) Administration to Adult Anopheles Gambiae Mosquitoes. J. Vis. Exp. 2022, 181, e63266. [Google Scholar] [CrossRef]
- Stewart, A.T.M.; Mysore, K.; Njoroge, T.M.; Winter, N.; Feng, R.S.; Singh, S.; James, L.D.; Singkhaimuk, P.; Sun, L.; Mohammed, A.; et al. Demonstration of RNAi Yeast Insecticide Activity in Semi-Field Larvicide and Attractive Targeted Sugar Bait Trials Conducted on Aedes and Culex Mosquitoes. Insects 2023, 14, 950. [Google Scholar] [CrossRef]
- Brizzee, C.; Mysore, K.; Njoroge, T.M.; McConnell, S.; Hamid-Adiamoh, M.; Stewart, A.T.M.; Kinder, J.T.; Crawford, J.; Duman-Scheel, M. Targeting Mosquitoes through Generation of an Insecticidal RNAi Yeast Strain Using Cas-CLOVER and Super PiggyBac Engineering in Saccharomyces Cerevisiae. J. Fungi 2023, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.A.; Furtado Pacheco, J.P.; Dyson, P.; Castro, H.C.; Gonzalez, M.S.; Azambuja, P.; Mello, C.B. Overview of Paratransgenesis as a Strategy to Control Pathogen Transmission by Insect Vectors. Parasit. Vectors 2022, 15, 112. [Google Scholar] [CrossRef]
- Koosha, M.; Vatandoost, H.; Karimian, F.; Choubdar, N.; Oshaghi, M.A. Delivery of a Genetically Marked Serratia AS1 to Medically Important Arthropods for Use in RNAi and Paratransgenic Control Strategies. Microb. Ecol. 2019, 78, 185–194. [Google Scholar] [CrossRef]
- Whitten, M.M.A.; Facey, P.D.; Del Sol, R.; Fernández-Martínez, L.T.; Evans, M.C.; Mitchell, J.J.; Bodger, O.G.; Dyson, P.J. Symbiont-Mediated RNA Interference in Insects. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160042. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cui, C.; Wang, G.; Wei, G.; Bai, L.; Li, Y.; Sun, P.; Dong, L.; Liu, Z.; Yun, J.; et al. Engineered Gut Symbiotic Bacterium-Mediated RNAi for Effective Control of Anopheles Mosquito Larvae. Microbiol. Spectr. 2023, 11, e0166623. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA Uptake in Insects and Potential of RNAi for Pest Control: A Review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Barrozo, R.B. Food Recognition in Hematophagous Insects. Curr. Opin. Insect Sci. 2019, 34, 55–60. [Google Scholar] [CrossRef] [PubMed]
- CDC Tick Lifecycles; CDC: Atlanta, GA, USA, 2024.
- Stevens, L.; Dorn, P.L.; Schmidt, J.O.; Klotz, J.H.; Lucero, D.; Klotz, S.A. Kissing Bugs. The Vectors of Chagas. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 75, pp. 169–192. ISBN 978-0-12-385863-4. [Google Scholar]
- Ghassemi, M.; Akhavan, A.A.; Zahraei-Ramazani, A.; Yakhchali, B.; Arandian, M.H.; Jafari, R.; Akhlaghi, M.; Shirani-Bidabadi, L.; Azam, K.; Koosha, M.; et al. Rodents as Vehicle for Delivery of Transgenic Bacteria to Make Paratransgenic Sand Fly Vectors of Cutaneous Leishmaniasis in Field Condition. Sci. Rep. 2023, 13, 14912. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Zhou, H.; Chao, Z.; Yan, S.; Shen, J. Nanocarrier-delivered dsRNA Suppresses Wing Development of Green Peach Aphids. Insect Sci. 2022, 29, 669–682. [Google Scholar] [CrossRef]
- Zhan, C.; Jiao, B.; Xu, L.; Peng, Y.; Zhao, Y. Topical RNA Interference Induces Mortality in the Cotton–Melon Aphid Aphis Gossypii with No Adverse Effect on the Predator Propylea Japonica. Insects 2025, 16, 276. [Google Scholar] [CrossRef]
- Araújo, M.F.; Castanheira, E.M.S.; Sousa, S.F. The Buzz on Insecticides: A Review of Uses, Molecular Structures, Targets, Adverse Effects, and Alternatives. Molecules 2023, 28, 3641. [Google Scholar] [CrossRef]
- Meier, C.J.; Rouhier, M.F.; Hillyer, J.F. Chemical Control of Mosquitoes and the Pesticide Treadmill: A Case for Photosensitive Insecticides as Larvicides. Insects 2022, 13, 1093. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Zheng, X.; Bai, M.; Hu, D. Review on Structures of Pesticide Targets. Int. J. Mol. Sci. 2020, 21, 7144. [Google Scholar] [CrossRef]
- Roy, S.; Saha, T.T.; Zou, Z.; Raikhel, A.S. Regulatory Pathways Controlling Female Insect Reproduction. Annu. Rev. Entomol. 2018, 63, 489–511. [Google Scholar] [CrossRef]
- Smykal, V.; Dolezel, D. Evolution of Proteins Involved in the Final Steps of Juvenile Hormone Synthesis. J. Insect Physiol. 2023, 145, 104487. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Lu, W.; Yin, X.; An, S. Application Progress of Plant-Mediated RNAi in Pest Control. Front. Bioeng. Biotechnol. 2022, 10, 963026. [Google Scholar] [CrossRef] [PubMed]
- Dahmana, H.; Mediannikov, O. Mosquito-Borne Diseases Emergence/Resurgence and How to Effectively Control It Biologically. Pathogens 2020, 9, 310. [Google Scholar] [CrossRef]
- Nepveu-Traversy, M.-E.; Fausther-Bovendo, H.; Babuadze, G. (Giorgi) Human Tick-Borne Diseases and Advances in Anti-Tick Vaccine Approaches: A Comprehensive Review. Vaccines 2024, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Gourbière, S.; Dorn, P.; Tripet, F.; Dumonteil, E. Genetics and Evolution of Triatomines: From Phylogeny to Vector Control. Heredity 2012, 108, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Operational Manual on Indoor Residual Spraying: Control of Vectors of Malaria, Aedes-Borne Diseases, Chagas Disease, Leishmaniases and Lymphatic Filariasis, 1st ed.; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-008399-8.
- Alphey, L.; Benedict, M.; Bellini, R.; Clark, G.G.; Dame, D.A.; Service, M.W.; Dobson, S.L. Sterile-Insect Methods for Control of Mosquito-Borne Diseases: An Analysis. Vector-Borne Zoonotic Dis. 2010, 10, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Simoni, A.; Tolosana, I.; Bernardini, F. Genetic Control Strategies for Population Suppression in the Anopheles Gambiae Complex: A Review of Current Technologies. Curr. Opin. Insect Sci. 2025, 72, 101430. [Google Scholar] [CrossRef]
- Kefi, M.; Cardoso-Jaime, V.; Saab, S.A.; Dimopoulos, G. Curing Mosquitoes with Genetic Approaches for Malaria Control. Trends Parasitol. 2024, 40, 487–499. [Google Scholar] [CrossRef]
| Delivery Approaches | Tested on (Insects) b | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Injection | Mosquitoes, Ticks, Kissing bugs | High silencing efficiency | Limited scalability, research use only | [74,75,76] |
| Soaking and feeding/oral delivery | Mosquitoes, Ticks, Kissing bugs | Simple application | Low environmental stability, research use only | [76,77,78,79,80,81,82] |
| Nanoparticles | Mosquitoes, Ticks | High stability | Expensive | [84,85,86,87,88,89] |
| Liposomes | Mosquitoes, Ticks | High silencing efficiency | Low environmental stability, costly | [93,94] |
| GMms a Alive | Mosquitoes, Kissing bugs | Low cost, high stability | GMO regulations, constant release | [77,80,82,97,98,99,102,103,104,105] |
| GMms a Heat-killed | Mosquitoes | Low cost, high stability | Constant release required | [77,80,89,98,99,107,108,109,110] |
| GMms a Symbionts (paratransgenesis) | Mosquitoes, Kissing bugs | Low cost, high stability | GMO regulations | [97,113,114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maya-Maldonado, K.; Celestino-Montes, A.; Cardoso-Jaime, V. RNAi-Based Bioinsecticides for Controlling Vector-Borne Diseases. Genes 2025, 16, 1276. https://doi.org/10.3390/genes16111276
Maya-Maldonado K, Celestino-Montes A, Cardoso-Jaime V. RNAi-Based Bioinsecticides for Controlling Vector-Borne Diseases. Genes. 2025; 16(11):1276. https://doi.org/10.3390/genes16111276
Chicago/Turabian StyleMaya-Maldonado, Krystal, Antonio Celestino-Montes, and Victor Cardoso-Jaime. 2025. "RNAi-Based Bioinsecticides for Controlling Vector-Borne Diseases" Genes 16, no. 11: 1276. https://doi.org/10.3390/genes16111276
APA StyleMaya-Maldonado, K., Celestino-Montes, A., & Cardoso-Jaime, V. (2025). RNAi-Based Bioinsecticides for Controlling Vector-Borne Diseases. Genes, 16(11), 1276. https://doi.org/10.3390/genes16111276







