Transcriptomic Differences Related to Neck Pain in Patients with Oropharyngeal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample and Setting
2.2. Sociodemographic and Clinical Characteristics
2.3. Evaluation of Neck Pain
2.4. Peripheral Blood Collection and RNA Extraction
2.5. Library Preparation and Sequencing
2.6. Bioinformatic Processing and Quality Control
2.7. Pathway Enrichment Analysis
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
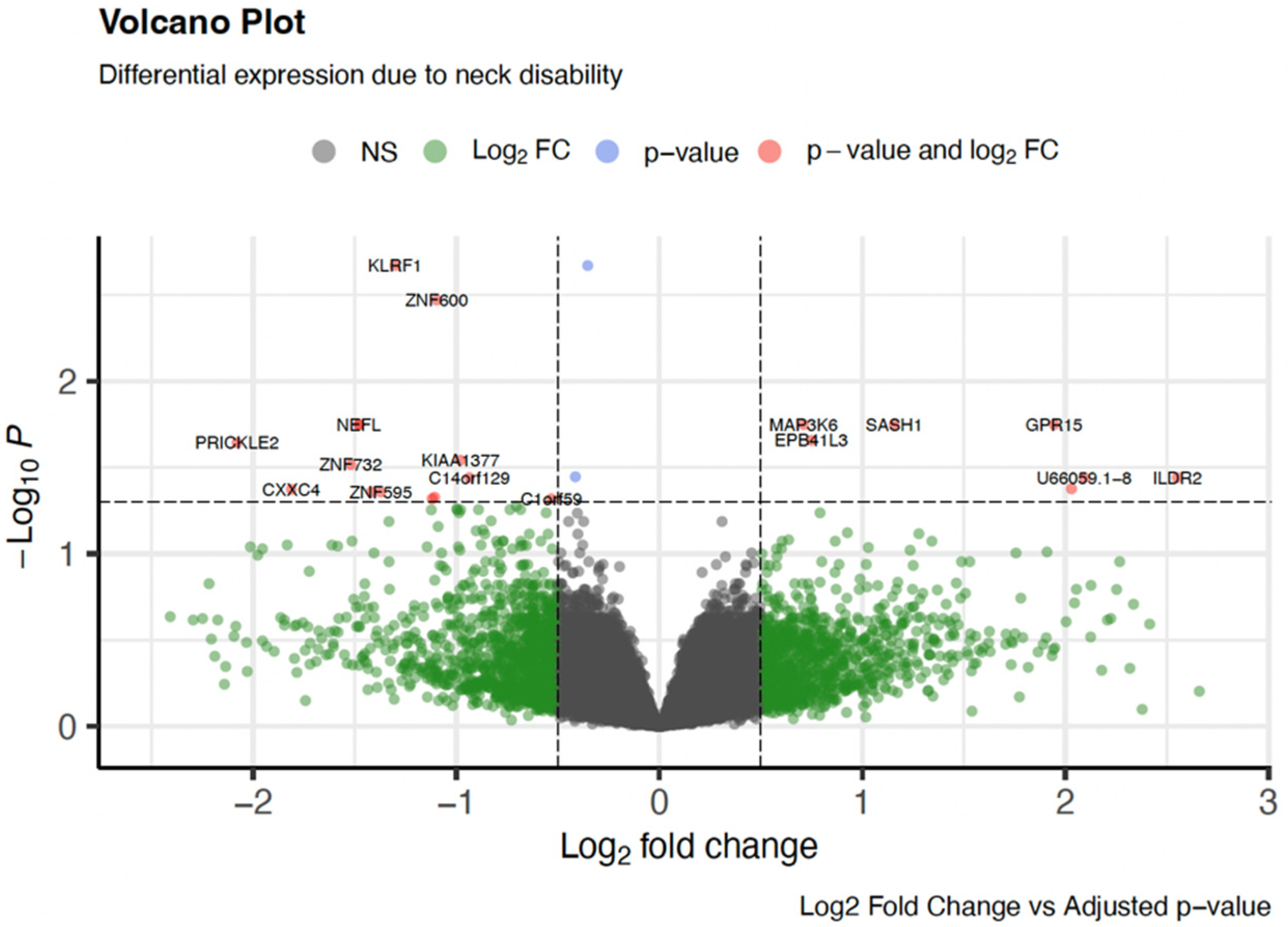
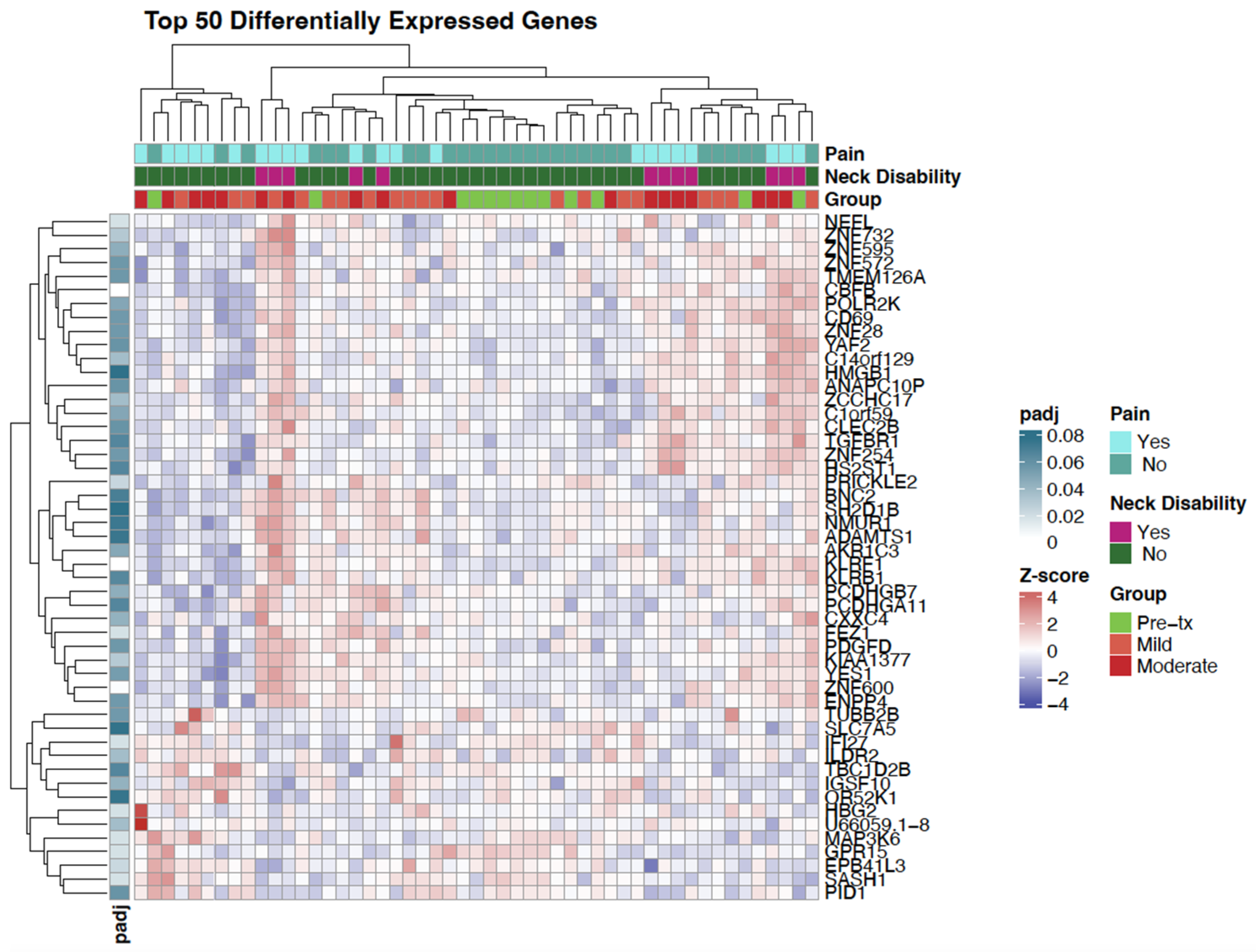
3.2. Differential Gene Expression
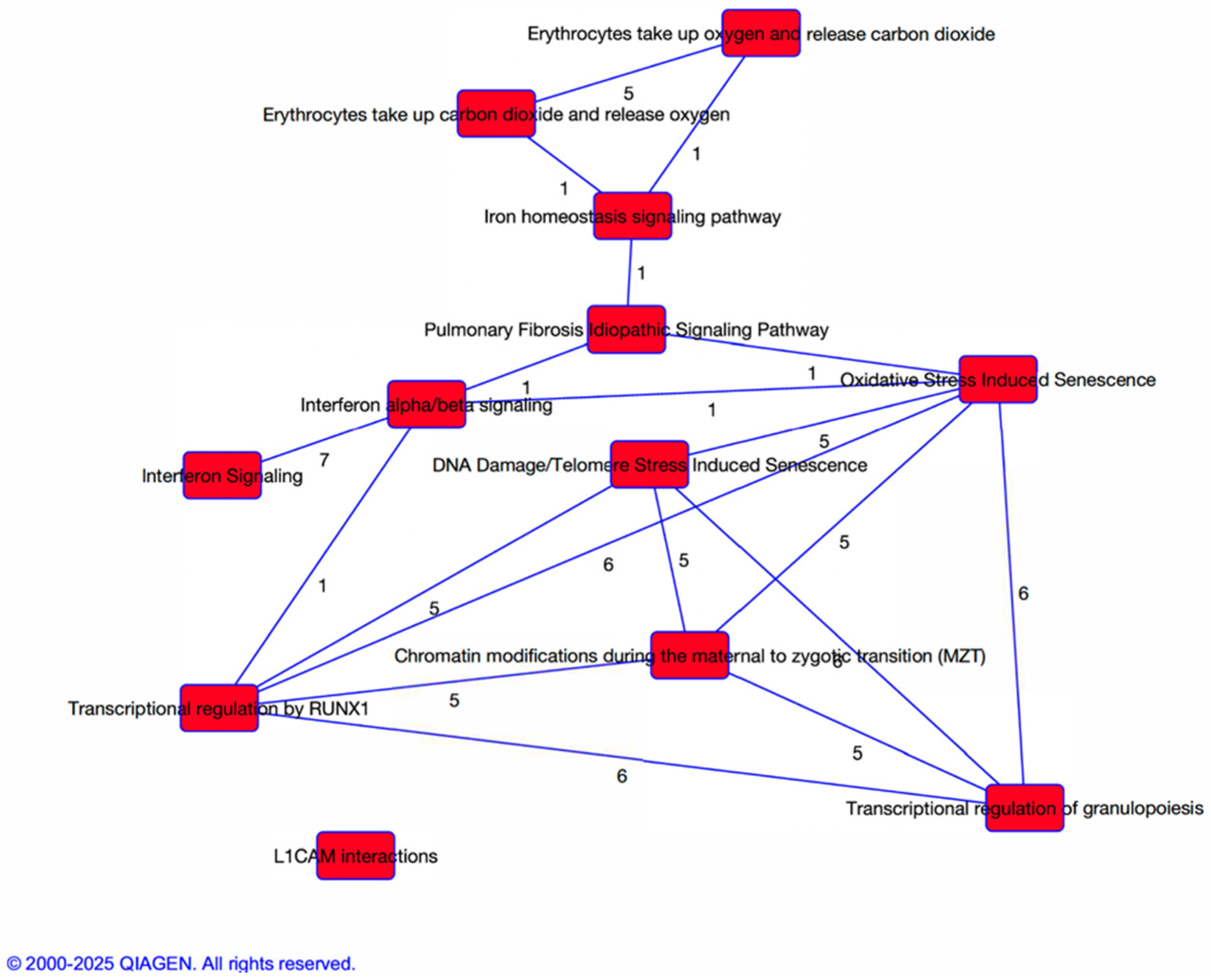
3.3. Pathway and Network Analysis
4. Discussion
4.1. Implications for Clinical Practice
4.2. Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Ellington, T.D.; Henley, S.J.; Senkomago, V.; O’Neil, M.E.; Wilson, R.J.; Singh, S.; Thomas, C.C.; Wu, M.; Richardson, L.C. Trends in Incidence of Cancers of the Oral Cavity and Pharynx—United States 2007–2016. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 433–438. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Fonsêca, T.C.; Jural, L.A.; Marañón-Vásquez, G.A.; Magno, M.B.; Roza, A.L.O.C.; Ferreira, D.M.T.P.; Maia, L.C.; Romañach, M.J.; Agostini, M.; Abrahão, A.C. Global prevalence of human papillomavirus-related oral and oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Clin. Oral. Investig. 2023, 28, 62. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, J.W.; Eun, Y.-G.; Lee, Y.C. A SEER-based analysis of trends in HPV-associated oropharyngeal squamous cell carcinoma. Infect. Agents Cancer 2024, 19, 29. [Google Scholar] [CrossRef]
- Harris, A.; Branstetter, B.; Li, J.; Piva, S.R.; Johnson, J.T.; Nilsen, M.L. Evaluation of Neck Disability Using Computed-Tomography in Head and Neck Cancer Survivors. Front. Pain. Res. 2022, 3, 910247. [Google Scholar] [CrossRef]
- Ghiam, M.K.; Mannion, K.; Dietrich, M.S.; Stevens, K.L.; Gilbert, J.; Murphy, B.A. Assessment of musculoskeletal impairment in head and neck cancer patients. Support. Care Cancer 2017, 25, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. The relationship between physical impairments, quality of life and disability of the neck and upper limb in patients following neck dissection. J. Cancer Surviv. 2018, 12, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gualda, M.Á.; Postigo-Martin, P.; Fernandez-Gonzalez, M.; Martin-Martin, L.; Vargas-Arrabal, P.; Lozano-Lozano, M.; Fernández-Lao, C. Prevalence and characteristics of persistent pain among head and neck cancer survivors: A systematic review and meta-analysis. Pain. Med. 2025, 26, 712–721. [Google Scholar] [CrossRef]
- Shuman, A.G.; Terrell, J.E.; Light, E.; Wolf, G.T.; Bradford, C.R.; Chepeha, D.; Jiang, Y.; McLean, S.; Ghanem, T.A.; Duffy, S.A. Predictors of Pain Among Patients with Head and Neck Cancer. Arch. Otolaryngol.–Head Neck Surg. 2012, 138, 1147. [Google Scholar] [CrossRef] [PubMed]
- Tschiesner, U. Changing the Perspective: Current Trends in the Assessment of Functional Outcome in Patients with Head and Neck Cancer. Curr. Oncol. Rep. 2011, 13, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, M.L.; Lyu, L.; Belsky, M.A.; Mady, L.J.; Zandberg, D.P.; Clump, D.A., 2nd; Skinner, H.D.; Peddada, S.D.; George, S.; Johnson, J.T. Impact of Neck Disability on Health-Related Quality of Life among Head and Neck Cancer Survivors. Otolaryngol. Head Neck Surg. 2020, 162, 64–72. [Google Scholar] [CrossRef]
- Bonsel, J.M.; Itiola, A.J.; Huberts, A.S.; Bonsel, G.J.; Penton, H. The use of patient-reported outcome measures to improve patient-related outcomes—A systematic review. Health Qual. Life Outcomes 2024, 22, 101. [Google Scholar] [CrossRef]
- Zeqiri, E.; Qorri, E.; Todri, J.; Lena, O. Establishing the Neck Disability Index as a Valid Tool for Assessing Persistent Neck Pain in the Albanian Population. Medicina 2025, 61, 955. [Google Scholar] [CrossRef]
- Martinelli, C.; Ercoli, A.; Parisi, S.; Iatì, G.; Pergolizzi, S.; Alfano, L.; Pentimalli, F.; De Laurentiis, M.; Giordano, A.; Cortellino, S. Molecular Mechanisms and Clinical Divergences in HPV-Positive Cervical vs. Oropharyngeal Cancers: A Critical Narrative Review. BMC Med. 2025, 23, 405. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, X.; Shen, H.; Liu, W.; Wang, Y.; Zhang, M.; Yang, T.; Mou, Y.; Ren, C.; Song, X. Cancer neuroscience in head and neck: Interactions, modulation, and therapeutic strategies. Mol. Cancer 2025, 24, 101. [Google Scholar] [CrossRef]
- Wang, W.-L.; Hao, Y.-H.; Pang, X.; Tang, Y.-L. Cancer pain: Molecular mechanisms and management. Mol. Biomed. 2025, 6, 45. [Google Scholar] [CrossRef]
- Anarte-Lazo, E.; Bernal-Utrera, C. A Scoping Review of Clinical Features and Mechanisms of Orofacial Pain and Headache in Patients with Head and Neck Cancer. J. Clin. Med. 2025, 14, 5722. [Google Scholar] [CrossRef]
- Ye, Y.; Cardoso, D.M.; Kayahara, G.M.; Bernabé, D.G. A pilot study to improve pain phenotyping in head and neck cancer patients. Front. Pain Res. 2023, 4, 1146667. [Google Scholar] [CrossRef]
- Mackey, S.; Aghaeepour, N.; Gaudilliere, B.; Kao, M.C.; Kaptan, M.; Lannon, E.; Pfyffer, D.; Weber, K. Innovations in acute and chronic pain biomarkers: Enhancing diagnosis and personalized therapy. Reg. Anesth. Pain. Med. 2025, 50, 110–120. [Google Scholar] [CrossRef]
- Zhi, W.I.; Gentile, D.; Diller, M.; Kinney, A.; Bao, T.; Master, V.; Wang, X.S. Patient-Reported Outcomes of Pain and Related Symptoms in Integrative Oncology Practice and Clinical Research: Evidence and Recommendations. Oncology 2021, 35, 35–41. [Google Scholar] [CrossRef]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Powell, W.R.; Sheehy, A.M.; Kind, A.J. The area deprivation index is the most scientifically validated social exposome tool available for policies advancing health equity. Health Aff. Forefr. 2023. [Google Scholar] [CrossRef]
- Kind, A.J.H.; Buckingham, W.R. Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991–2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef]
- Cleland, J.A.; Fritz, J.M.; Whitman, J.M.; Palmer, J.A. The Reliability and Construct Validity of the Neck Disability Index and Patient Specific Functional Scale in Patients with Cervical Radiculopathy. Spine 2006, 31, 598–602. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; McAlpine, C.; Goldsmith, C.H. Measurement properties of the neck disability index: A systematic review. J. Orthop. Sports Phys. Ther. 2009, 39, 400–417. [Google Scholar] [CrossRef]
- Cleland, J.A.; Childs, J.D.; Whitman, J.M. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch. Phys. Med. Rehabil. 2008, 89, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Vernon, H. Assessment of self-rated disability, impairment, and sincerity of effort in whiplash-associated disorder. J. Musculoskelet. Pain. 2000, 8, 155–167. [Google Scholar] [CrossRef]
- Magaña, L.C.; Murati, S.; Riffitts, M.; Harrison, C.; Harris, A.; Sowa, G.; Johnson, J.T.; Bell, K.; Nilsen, M. Subjective and Objective Measures in Assessing Neck Disability and Pain in Head and Neck Cancer. Laryngoscope 2021, 131, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Network, N.C.C. Adult Cancer Pain (Version 2.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pain.pdf (accessed on 12 September 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Inverso, G.; Mahal, B.A.; Aizer, A.A.; Donoff, R.B.; Chau, N.G.; Haddad, R.I. Marital status and head and neck cancer outcomes. Cancer 2015, 121, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.C.; Challapalli, S.D.; Cass, L.M.; Zahirsha, Z.S.; Adjei Boakye, E.; Massa, S.T.; Osazuwa-Peters, N. Impact of gender on the association between marital status and head and neck cancer outcomes. Oral. Oncol. 2019, 89, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hecht, I.; Toporik, A.; Podojil, J.R.; Vaknin, I.; Cojocaru, G.; Oren, A.; Aizman, E.; Liang, S.C.; Leung, L.; Dicken, Y.; et al. ILDR2 Is a Novel B7-like Protein That Negatively Regulates T Cell Responses. J. Immunol. 2018, 200, 2025–2037. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, Y.C.; Xu, J.K.; Chen, S.Y.; Tseng, L.C.; Huang, J.L.; Lin, C.S. ATM Inhibition-Induced ISG15/IFI27/OASL Is Correlated with Immunotherapy Response and Inflamed Immunophenotype. Cells 2023, 12, 1288. [Google Scholar] [CrossRef]
- Okamoto, Y.; Shikano, S. Emerging roles of a chemoattractant receptor GPR15 and ligands in pathophysiology. Front. Immunol. 2023, 14, 1179456. [Google Scholar] [CrossRef]
- Bayat, A.; Iqbal, S.; Borredy, K.; Amiel, J.; Zweier, C.; Barcia, G.; Kraus, C.; Weyhreter, H.; Bassuk, A.G.; Chopra, M.; et al. PRICKLE2 revisited-further evidence implicating PRICKLE2 in neurodevelopmental disorders. Eur. J. Hum. Genet. 2021, 29, 1235–1244. [Google Scholar] [CrossRef]
- Gehr, N.L.; Mortensen, C.; Stage, T.B.; Pedersen, M.R.V.; Rafaelsen, S.R.; Madsen, J.S.; Olsen, D.A.; Timm, S.; Jensen, L.H.; Hansen, T.F.; et al. Neurofilament light chain as a marker for neuronal damage: Integrating in vitro studies and clinical findings in patients with oxaliplatin-induced neuropathy. Cancer Chemother. Pharmacol. 2025, 95, 53. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Wang, Z.; Tong, V.K.J.; Ming, S.W.S.; Razar, R.; Srimasorn, S.; Ong, W.Y.; Lim, K.L.; Chua, J.J.E. Loss of FEZ1, a gene deleted in Jacobsen syndrome, causes locomotion defects and early mortality by impairing motor neuron development. Hum. Mol. Genet. 2021, 30, 5–20. [Google Scholar] [CrossRef]
- Shi, G.; Zhou, X.; Wang, X.; Zhang, X.; Zhang, P.; Feng, S. Signatures of altered DNA methylation gene expression after central and peripheral nerve injury. J. Cell Physiol. 2020, 235, 5171–5181. [Google Scholar] [CrossRef] [PubMed]
- Hadanny, A.; Forer, R.; Volodarsky, D.; Daniel-Kotovsky, M.; Catalogna, M.; Zemel, Y.; Bechor, Y.; Efrati, S. Hyperbaric oxygen therapy induces transcriptome changes in elderly: A prospective trial. Aging 2021, 13, 24511–24523. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, P.; Ji, K.; Zhang, J.; Yang, S.; Du, B.; Hu, S.; Fan, R. Cyclin-dependent kinase 5 regulates MAPK/ERK signaling in the skin of mice. Acta Histochem. 2018, 120, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, L.; Zhong, W.; Chen, Z.; Chen, J.; Yang, H.; Liu, G. Development and Validation of Epigenetic Signature Predict Survival for Patients with Laryngeal Squamous Cell Carcinoma. DNA Cell Biol. 2021, 40, 247–264. [Google Scholar] [CrossRef]
- Ranaweera, S.S.; Zuniga-Hertz, J.P.; Chitteti, R.; Chernov, A.V.; Patel, H.H. Protective effect of energized structured water on bioenergetic function and oxidative stress in H9c2 cells. EXPLORE 2025, 21, 103232. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Tang, J.; Xu, Y.; Wu, L.; Zhang, J.; Chen, H.; Wang, Z.; Yang, R.; Gao, W.; He, Z. Mitochondrial Damage-induced Ferroptosis: The Molecular Mechanism by Which Psoralen Inhibits the Proliferation and Invasion of Non-small-cell Lung Cancer Cells. Vivo 2025, 39, 2681–2702. [Google Scholar] [CrossRef]
- Shi, C.M.; Xu, G.F.; Yang, L.; Fu, Z.Y.; Chen, L.; Fu, H.L.; Shen, Y.H.; Zhu, L.; Ji, C.B.; Guo, X.R. Overexpression of TFAM protects 3T3-L1 adipocytes from NYGGF4 (PID1) overexpression-induced insulin resistance and mitochondrial dysfunction. Cell Biochem. Biophys. 2013, 66, 489–497. [Google Scholar] [CrossRef]
- Zhang, H.; Qian, Y.; Zhang, Y.; Zhou, X.; Shen, S.; Li, J.; Sun, Z.; Wang, W. Multi-omics analysis deciphers intercellular communication regulating oxidative stress to promote oral squamous cell carcinoma progression. NPJ Precis. Oncol. 2024, 8, 272. [Google Scholar] [CrossRef]
- Cifuentes-Diaz, C.; Chareyre, F.; Garcia, M.; Devaux, J.; Carnaud, M.; Levasseur, G.; Niwa-Kawakita, M.; Harroch, S.; Girault, J.A.; Giovannini, M.; et al. Protein 4.1B contributes to the organization of peripheral myelinated axons. PLoS ONE 2011, 6, e25043. [Google Scholar] [CrossRef]
- Sahin, E.; Depinho, R.A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature 2010, 464, 520–528. [Google Scholar] [CrossRef]
- de Bruijn, M.; Dzierzak, E. Runx transcription factors in the development and function of the definitive hematopoietic system. Blood 2017, 129, 2061–2069. [Google Scholar] [CrossRef]
- Eckersley-Maslin, M.A.; Alda-Catalinas, C.; Reik, W. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat. Rev. Mol. Cell Biol. 2018, 19, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Villamayor, L.; López-García, D.; Rivero, V.; Martínez-Sobrido, L.; Nogales, A.; DeDiego, M.L. The IFN-stimulated gene IFI27 counteracts innate immune responses after viral infections by interfering with RIG-I signaling. Front. Microbiol. 2023, 14, 1176177. [Google Scholar] [CrossRef] [PubMed]
- Kautz, L.; Nemeth, E. Molecular liaisons between erythropoiesis and iron metabolism. Blood 2014, 124, 479–482. [Google Scholar] [CrossRef]
- Morgan, P.K.; Murphy, A.J. Make hematopoiesis great again: Countering oxidative stress! Blood 2025, 145, 1102–1104. [Google Scholar] [CrossRef]
- Dorsey, S.G.; Renn, C.L.; Griffioen, M.; Lassiter, C.B.; Zhu, S.; Huot-Creasy, H.; McCracken, C.; Mahurkar, A.; Shetty, A.C.; Jackson-Cook, C.K.; et al. Whole blood transcriptomic profiles can differentiate vulnerability to chronic low back pain. PLoS ONE 2019, 14, e0216539. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | No Fibrosis (n = 13) | Mild Fibrosis (n = 20) | Moderate/ Severe Fibrosis (n = 18) | p-Value |
|---|---|---|---|---|
| Age (years, mean ± SD) | 63.69 ± 7.04 | 68.80 ± 7.36 | 66.83 ± 10.98 | 0.271 |
| Race: White (count, %) | 12 (92.3%) | 17 (85.0%) | 17 (94.4%) | 0.593 |
| Sex: Male (count, %) | 12 (92.3) | 18 (90.0) | 12 (66.7) | 0.094 |
| Female (count, %) | 1 (7.7) | 2 (10.0) | 6 (33.3) | |
| Marital Status: Married (count, %) | 11 (84.6) | 15 (75.0) | 8 (44.4) | 0.034 * |
| Other a (count, %) | 2 (15.4) | 5 (25.0) | 10 (55.6) | |
| Tobacco Use (ever): Yes (count, %) | 7 (53.8) | 10 (50.0) | 8 (44.4) | 0.870 |
| No (count, %) | 6 (46.2) | 10 (50.0) | 10 (55.6) | |
| Alcohol Use (ever): Yes (count, %) | 11 (84.6) | 18 (90.0) | 12 (66.7) | 0.176 |
| No (count, %) | 2 (15.4) | 2 (10.0) | 6 (33.3) | |
| Tumor Stage: T1/T2 (count, %) | 8 (61.5) | 18 (90.0) | 12 (66.7) | 0.119 |
| T3/T4 (count, %) | 5 (38.5) | 2 (10.0) | 6 (33.3) | |
| HPV Status: Yes (count, %) | 12 (92.3) | 16 (80.0) | 12 (66.7) | 0.225 |
| No (count, %) | 1 (7.6) | 4 (20.0) | 6 (33.3) | |
| Neck Disability: None (count, %) | 12 (92.3) | 19 (95.0) | 8 (44.4) | 0.003 * |
| Any (count, %) | 1 (7.7) | 1 (5.0) | 10 (55.6) | |
| Self-reported Pain: None (count, %) | 12 (92.3) | 13 (65.0%) | 4 (22.2) | <0.001 * |
| Any (count, %) | 1 (7.7) | 7 (35%) | 14 (77.8) | |
| Area Deprivation (mean ± SD) | 59.38 ± 29.37 | 59.05 ± 23.12 | 64.22 ± 18.59 | 0.884 |
| Gene Name | Gene Symbol | Adjusted p-Value | log2 Fold Change | Gene Function |
|---|---|---|---|---|
| Killer Cell Lectin Like Receptor F1 | KLRF1 | 0.002 | −1.30 | Immune Function |
| Core−Binding Factor Subunit Beta | CBFB | 0.002 | −0.35 | Hematopoiesis |
| Zinc Finger Protein 600 | ZNF600 | 0.003 | −1.09 | Transcriptional Regulation |
| Mitogen−Activated Protein 3 Kinase Kinase Kinase 6 | MAP3K6 | 0.017 | 0.71 | Hematopoiesis |
| G Protein−Coupled Receptor 15 | GPR15 | 0.017 | 1.94 | Immune Function |
| SAM And SH3 Domain Containing 1 | SASH1 | 0.017 | 1.16 | Immune Function |
| Neurofilament Light Chain | NEFL | 0.017 | −1.48 | Neuronal Function |
| Hemoglobin Subunit Gamma 2 | HBG2 | 0.017 | 4.21 | Hematopoiesis |
| Fasciculation And Elongation Protein Zeta 1 | FEZ1 | 0.017 | −1.48 | Neuronal Function |
| Interferon Alpha Inducible Protein 27 | IFI27 | 0.018 | 3.01 | Immune Function |
| Erythrocyte Membrane Protein Band 4.1 Like 3 | EPB41L3 | 0.022 | 0.75 | Neuronal Function |
| Prickle Planar Cell Polarity Protein 2 | PRICKLE2 | 0.022 | −2.08 | Neuronal Function |
| Centrosomal Protein 126 | CEP126 | 0.028 | −0.98 | Cellular Function |
| Zinc Finger Protein 732 | ZNF732 | 0.030 | −1.52 | Transcriptional Regulation |
| Zinc Finger CCHC−Type Containing 17 | ZCCHC17 | 0.035 | −0.41 | Transcriptional Regulation |
| Immunoglobulin Like Domain Containing Receptor 2 | ILDR2 | 0.036 | 2.55 | Immune Function |
| GSK3B Interacting Protein | GSKIP | 0.036 | −0.94 | Neuronal Function (neurite outgrowth) |
| Immunoglobulin Superfamily Member 10 | IGSF10 | 0.041 | 2.03 | Neuronal Function |
| CXXC Finger Protein 4 | CXXC4 | 0.042 | −1.81 | Hematopoiesis (Fe) Wnt signaling |
| Zinc Finger Protein 595 | ZNF595 | 0.043 | −1.37 | Transcriptional Regulation |
| Protocadherin Gamma Subfamily B, 7 | PCDHGB7 | 0.043 | −1.42 | Neuronal Function |
| Aldo−Keto Reductase Family 1 Member C3 | AKR1C3 | 0.047 | −1.11 | Cellular Function (tissue distribution) |
| HEN Methyltransferase 1 | HENMT1 | 0.047 | −0.53 | Transcriptional Regulation |
| RNA polymerase II, I and III Subunit K | POLR2K | 0.047 | −1.12 | Transcriptional Regulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, M.A.; Djordjevic, C.; Nilsen, M.L. Transcriptomic Differences Related to Neck Pain in Patients with Oropharyngeal Squamous Cell Carcinoma. Genes 2025, 16, 1277. https://doi.org/10.3390/genes16111277
Wagner MA, Djordjevic C, Nilsen ML. Transcriptomic Differences Related to Neck Pain in Patients with Oropharyngeal Squamous Cell Carcinoma. Genes. 2025; 16(11):1277. https://doi.org/10.3390/genes16111277
Chicago/Turabian StyleWagner, Monica A., Charles Djordjevic, and Marci L. Nilsen. 2025. "Transcriptomic Differences Related to Neck Pain in Patients with Oropharyngeal Squamous Cell Carcinoma" Genes 16, no. 11: 1277. https://doi.org/10.3390/genes16111277
APA StyleWagner, M. A., Djordjevic, C., & Nilsen, M. L. (2025). Transcriptomic Differences Related to Neck Pain in Patients with Oropharyngeal Squamous Cell Carcinoma. Genes, 16(11), 1277. https://doi.org/10.3390/genes16111277






