Immunohistochemical Study of GATA3, c-KIT/CD117, CD56 and CD45 Expression in Proliferative Verrucous Leukoplakia (PVL), PVL-Associated Oral Squamous Cell Carcinoma and Oral Leukoplakia
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Immunohistochemistry
2.3. Immunohistochemical Evaluation
2.4. Statistical Analysis
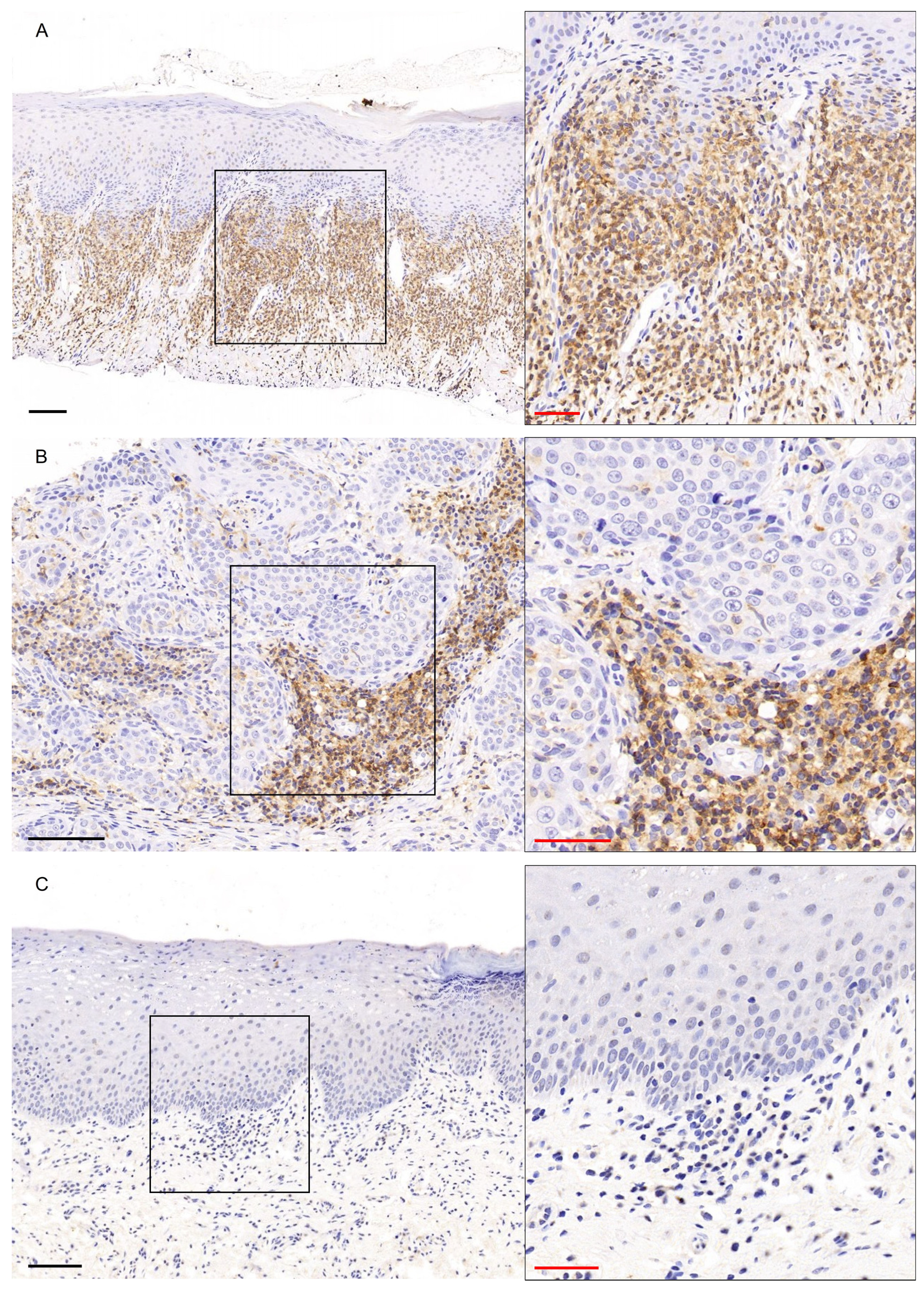
3. Results
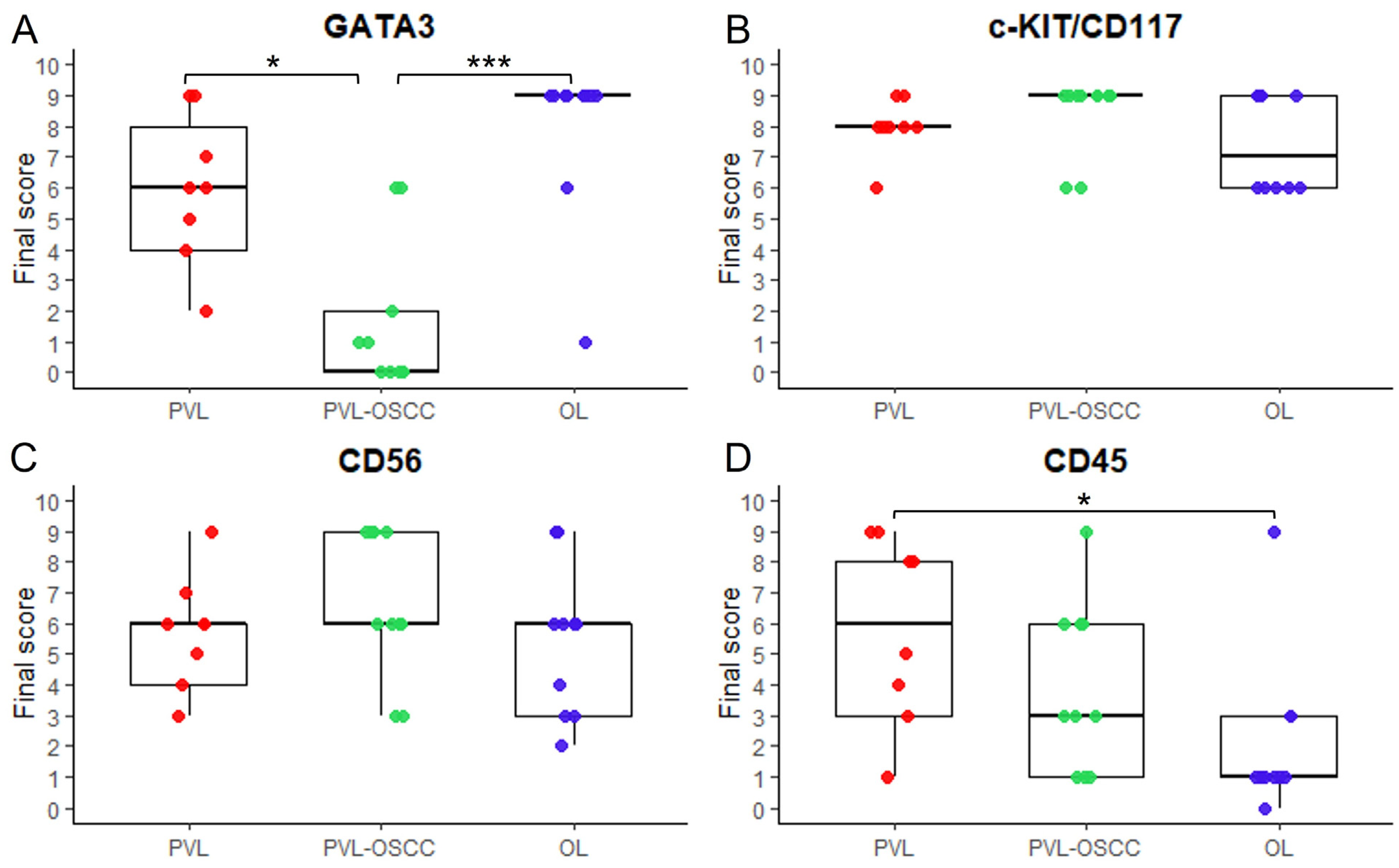
3.1. GATA3 Immunohistochemical Expression
3.2. c-KIT/CD117 Immunohistochemical Expression
3.3. CD56 Immunohistochemical Expression
3.4. CD45 Immunohistochemical Expression


4. Discussion

Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; Gonzalez-Moles, M.A.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef]
- Mello, F.W.; Miguel, A.F.P.; Dutra, K.L.; Porporatti, A.L.; Warnakulasuriya, S.; Guerra, E.N.S.; Rivero, E.R.C. Prevalence of oral potentially malignant disorders: A systematic review and meta-analysis. J. Oral Pathol. Med. 2018, 47, 633–640. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Reichart, P.A.; Philipsen, H.P. Oral erythroplakia—A review. Oral Oncol. 2005, 41, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Zeng, X.; Hu, X.; Hua, H. The global prevalence of oral leukoplakia: A systematic review and meta-analysis from 1996 to 2022. BMC Oral Health 2023, 23, 645. [Google Scholar] [CrossRef]
- Staines, K.; Rogers, H. Oral leukoplakia and proliferative verrucous leukoplakia: A review for dental practitioners. Br. Dent. J. 2017, 223, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Pimenta-Barros, L.A.; Ramos-Garcia, P.; Gonzalez-Moles, M.A.; Aguirre-Urizar, J.M.; Warnakulasuriya, S. Malignant transformation of oral leukoplakia: Systematic review and comprehensive meta-analysis. Oral Dis. 2025, 31, 69–80. [Google Scholar] [CrossRef]
- Paglioni, M.P.; Khurram, S.A.; Ruiz, B.I.I.; Lauby-Secretan, B.; Normando, A.G.; Ribeiro, A.C.P.; Brandao, T.B.; Palmier, N.R.; Lopes, M.A.; da Silva Guerra, E.N.; et al. Clinical predictors of malignant transformation and recurrence in oral potentially malignant disorders: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 573–587. [Google Scholar] [CrossRef]
- Vergier, V.; Czarny, K.; Brau, J.J.; Le Pelletier, F.; Taihi, I. Proliferative Verrucous Leukoplakia Presenting as a Ring Around the Collar and Cancer: A Case Report. Cureus 2024, 16, e56077. [Google Scholar] [CrossRef] [PubMed]
- Borgna, S.C.; Clarke, P.T.; Schache, A.G.; Lowe, D.; Ho, M.W.; McCarthy, C.E.; Adair, S.; Field, E.A.; Field, J.K.; Holt, D.; et al. Management of proliferative verrucous leukoplakia: Justification for a conservative approach. Head. Neck 2017, 39, 1997–2003. [Google Scholar] [CrossRef]
- Staines, K.; Rogers, H. Proliferative verrucous leukoplakia: A general dental practitioner-focused clinical review. Br. Dent. J. 2024, 236, 297–301. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Fitzpatrick, S.G.; Muller, S.; Eisenberg, E.; Upadhyaya, J.D.; Lingen, M.W.; Vigneswaran, N.; Woo, S.B.; Bhattacharyya, I.; Bilodeau, E.A.; et al. Proliferative Verrucous Leukoplakia: An Expert Consensus Guideline for Standardized Assessment and Reporting. Head Neck Pathol. 2021, 15, 572–587. [Google Scholar] [CrossRef]
- Pentenero, M.; Meleti, M.; Vescovi, P.; Gandolfo, S. Oral proliferative verrucous leucoplakia: Are there particular features for such an ambiguous entity? A systematic review. Br. J. Dermatol. 2014, 170, 1039–1047. [Google Scholar] [CrossRef]
- Hansen, L.S.; Olson, J.A.; Silverman, S., Jr. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg. Oral Med. Oral Pathol. 1985, 60, 285–298. [Google Scholar] [CrossRef]
- Cabay, R.J.; Morton, T.H., Jr.; Epstein, J.B. Proliferative verrucous leukoplakia and its progression to oral carcinoma: A review of the literature. J. Oral Pathol. Med. 2007, 36, 255–261. [Google Scholar] [CrossRef]
- Ramos-Garcia, P.; Gonzalez-Moles, M.A.; Mello, F.W.; Bagan, J.V.; Warnakulasuriya, S. Malignant transformation of oral proliferative verrucous leukoplakia: A systematic review and meta-analysis. Oral Dis. 2021, 27, 1896–1907. [Google Scholar] [CrossRef]
- Iocca, O.; Sollecito, T.P.; Alawi, F.; Weinstein, G.S.; Newman, J.G.; De Virgilio, A.; Di Maio, P.; Spriano, G.; Pardinas Lopez, S.; Shanti, R.M. Potentially malignant disorders of the oral cavity and oral dysplasia: A systematic review and meta-analysis of malignant transformation rate by subtype. Head. Neck 2020, 42, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Torrejon-Moya, A.; Jane-Salas, E.; Lopez-Lopez, J. Clinical manifestations of oral proliferative verrucous leukoplakia: A systematic review. J. Oral Pathol. Med. 2020, 49, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Abadie, W.M.; Partington, E.J.; Fowler, C.B.; Schmalbach, C.E. Optimal Management of Proliferative Verrucous Leukoplakia: A Systematic Review of the Literature. Otolaryngol. Head. Neck Surg. 2015, 153, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Palaia, G.; Bellisario, A.; Pampena, R.; Pippi, R.; Romeo, U. Oral Proliferative Verrucous Leukoplakia: Progression to Malignancy and Clinical Implications. Systematic Review and Meta-Analysis. Cancers 2021, 13, 4085. [Google Scholar] [CrossRef]
- Lafuente Ibanez de Mendoza, I.; Lorenzo Pouso, A.I.; Aguirre Urizar, J.M.; Barba Montero, C.; Blanco Carrion, A.; Gandara Vila, P.; Perez Sayans, M. Malignant development of proliferative verrucous/multifocal leukoplakia: A critical systematic review, meta-analysis and proposal of diagnostic criteria. J. Oral Pathol. Med. 2022, 51, 30–38. [Google Scholar] [CrossRef]
- Bagan, J.; Murillo-Cortes, J.; Leopoldo-Rodado, M.; Sanchis-Bielsa, J.M.; Bagan, L. Oral cancer on the gingiva in patients with proliferative leukoplakia: A study of 30 cases. J. Periodontol. 2019, 90, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Menon, R.S.; Kerr, A.R.; De Abreu Alves, F.; Guollo, A.; Ojeda, D.; Woo, S.B. Proliferative leukoplakia: Proposed new clinical diagnostic criteria. Oral Dis. 2018, 24, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.V.; Murillo, J.; Poveda, R.; Gavalda, C.; Jimenez, Y.; Scully, C. Proliferative verrucous leukoplakia: Unusual locations of oral squamous cell carcinomas, and field cancerization as shown by the appearance of multiple OSCCs. Oral Oncol. 2004, 40, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Ai, R.; Tao, Y.; Hao, Y.; Jiang, L.; Dan, H.; Ji, N.; Zeng, X.; Zhou, Y.; Chen, Q. Microenvironmental regulation of the progression of oral potentially malignant disorders towards malignancy. Oncotarget 2017, 8, 81617–81635. [Google Scholar] [CrossRef]
- Deng, S.; Wang, S.; Shi, X.; Zhou, H. Microenvironment in Oral Potentially Malignant Disorders: Multi-Dimensional Characteristics and Mechanisms of Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 8940. [Google Scholar] [CrossRef]
- Caponio, V.C.A.; Zhurakivska, K.; Lo Muzio, L.; Troiano, G.; Cirillo, N. The Immune Cells in the Development of Oral Squamous Cell Carcinoma. Cancers 2023, 15, 3779. [Google Scholar] [CrossRef]
- Rangel, R.; Pickering, C.R.; Sikora, A.G.; Spiotto, M.T. Genetic Changes Driving Immunosuppressive Microenvironments in Oral Premalignancy. Front. Immunol. 2022, 13, 840923. [Google Scholar] [CrossRef]
- Ali, A.; Molska, G.R.; Yeo, H.; Esfandiari, N.; Jeong, W.; Huang, M.; Magalhaes, M. Immune Microenvironment in Oral Potentially Malignant Disorders and Oral Cancer: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 6650. [Google Scholar] [CrossRef]
- Sutera, S.; Furchi, O.A.; Pentenero, M. Investigating Tumor-Infiltrating Lymphocytes in the Microenvironment of Oral Squamous Cell Carcinoma (OSCC) and Oral Potentially Malignant Disorders (OPMDs): Can They Shift Our Perspective? A Scoping Review. J. Clin. Med. 2025, 14, 606. [Google Scholar] [CrossRef]
- Kalogirou, E.M.; Tosios, K.I.; Christopoulos, P.F. The Role of Macrophages in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 611115. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J.; Wu, H.; Huang, J.; Zheng, D.; Wang, S.; Jia, X.; He, Z.; Gong, Y.; Ju, L.; et al. NK cell based immunotherapy against oral squamous cell carcinoma. Front. Immunol. 2024, 15, 1440764. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arriagada, W.A.; Canedo-Marroquin, G.; Adorno-Farias, D.; Fernandez-Ramires, R. New insights into the role of the oral leukoplakia microenvironment in malignant transformation. Front. Oral Health 2024, 5, 1363052. [Google Scholar] [CrossRef]
- Palacon, M.P.; de Oliveira Barbeiro, C.; Fernandes, D.; Biancardi, M.R.; Silveira, H.A.; Ferrisse, T.M.; Leon, J.E.; Kujan, O.; Bufalino, A. Macrophages CD163+ and Factor XIIIa+ Provide a First-Line Defence against Proliferative Verrucous Leukoplakia Antigens. Int. J. Mol. Sci. 2023, 24, 5242. [Google Scholar] [CrossRef]
- Fernandes, D.; Barbeiro, C.O.; Palacon, M.P.; Biancardi, M.R.; Ferrisse, T.M.; Silveira, H.A.; Castilho, R.M.; de Almeida, L.Y.; Leon, J.E.; Bufalino, A. High density of CD8 T cell and immune imbalance of T lymphocytes subsets are associated with proliferative verrucous leukoplakia. Immunology 2023, 168, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Villa, A.; Mistry, N.; Jia, Y.; Quinn, C.T.; Turner, M.M.; Felt, K.D.; Pfaff, K.; Haddad, R.I.; Uppaluri, R.; et al. Comprehensive Immunoprofiling of High-Risk Oral Proliferative and Localized Leukoplakia. Cancer Res. Commun. 2021, 1, 30–40. [Google Scholar] [CrossRef]
- Llorens, C.; Soriano, B.; Trilla-Fuertes, L.; Bagan, L.; Ramos-Ruiz, R.; Gamez-Pozo, A.; Pena, C.; Bagan, J.V. Immune expression profile identification in a group of proliferative verrucous leukoplakia patients: A pre-cancer niche for oral squamous cell carcinoma development. Clin. Oral Investig. 2021, 25, 2645–2657. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, F.; Liu, D.; Kuang, W.; Ji, N.; Li, J.; Zeng, X.; Li, T.; Dan, H.; Chen, Q. Single-cell transcriptomics dissects premalignant progression in proliferative verrucous leukoplakia. Oral Dis. 2024, 30, 172–186. [Google Scholar] [CrossRef]
- Yagi, R.; Zhu, J.; Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 2011, 23, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Ohno, K.; Takata, K.; Gion, Y.; Tachibana, T.; Orita, Y.; Yoshino, T.; Sato, Y. Interleukin 13-positive mast cells are increased in immunoglobulin G4-related sialadenitis. Sci. Rep. 2015, 5, 7696. [Google Scholar] [CrossRef]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- Brooimans, R.A.; Kraan, J.; van Putten, W.; Cornelissen, J.J.; Lowenberg, B.; Gratama, J.W. Flow cytometric differential of leukocyte populations in normal bone marrow: Influence of peripheral blood contamination. Cytom. Part B Clin. Cytom. 2009, 76, 18–26. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022. [Google Scholar]
- Kalogirou, E.M.; Foutadakis, S.; Koutsi, M.A.; Vatsellas, G.; Vlachodimitropoulos, D.; Petsinis, V.; Sklavounou, A.; Agelopoulos, M.; Tosios, K.I. Decoding a gene expression program that accompanies the phenotype of sporadic and basal cell nevus syndrome-associated odontogenic keratocyst. J. Oral Pathol. Med. 2022, 51, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Fishwick, C.; Higgins, J.; Percival-Alwyn, L.; Hustler, A.; Pearson, J.; Bastkowski, S.; Moxon, S.; Swarbreck, D.; Greenman, C.D.; Southgate, J. Heterarchy of transcription factors driving basal and luminal cell phenotypes in human urothelium. Cell Death Differ. 2017, 24, 809–818. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Z.P.; Zhu, Y.; Fu, F.; Tian, L. Contribution of Interstitial Cells of Cajal to Gastrointestinal Stromal Tumor Risk. Med. Sci. Monit. 2021, 27, e929575. [Google Scholar] [CrossRef]
- He, H.; Luthringer, D.J.; Hui, P.; Lau, S.K.; Weiss, L.M.; Chu, P.G. Expression of CD56 and WT1 in ovarian stroma and ovarian stromal tumors. Am. J. Surg. Pathol. 2008, 32, 884–890. [Google Scholar] [CrossRef]
- Poppema, S.; Lai, R.; Visser, L.; Yan, X.J. CD45 (leucocyte common antigen) expression in T and B lymphocyte subsets. Leuk. Lymphoma 1996, 20, 217–222. [Google Scholar] [CrossRef]
- Liang, Y.; Heitzman, J.; Kamat, A.M.; Dinney, C.P.; Czerniak, B.; Guo, C.C. Differential expression of GATA-3 in urothelial carcinoma variants. Hum. Pathol. 2014, 45, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Takai, T.; Fukumoto, T.; Anan, T.; Kimura, T.; Ansai, S.; Oshitani, Y.; Murata, Y.; Sakuma, T.; Hirose, T. CD117 (KIT) is a useful immunohistochemical marker for differentiating porocarcinoma from squamous cell carcinoma. J. Cutan. Pathol. 2016, 43, 219–226. [Google Scholar] [CrossRef]
- Pallavi, K.; Jain, A.; Gulati, N.; Juneja, S.; Shetty, D.C.; Tandon, A.; Aggarwal, D. Neuroectodermal influence in odontogenic cyst and tumor: Evidence through CD56 immunoexpression. Indian. J. Pathol. Microbiol. 2025, 68, 88–94. [Google Scholar] [CrossRef]
- Pantanowitz, J.; Huang, T.; Cantley, R.; Mehra, R.; Pantanowitz, L. Aberrant CD45 Immunoreactivity in Neuroendocrine Neoplasms: A Diagnostic Pitfall-Report of 10 Specimens and Clinical Recommendations. Int. J. Surg. Pathol. 2025, 33, 636–645. [Google Scholar] [CrossRef]
- de Boer, E.; Crane, L.M.; van Oosten, M.; van der Vegt, B.; van der Sluis, T.; Kooijman, P.; Low, P.S.; van der Zee, A.G.; Arts, H.J.; van Dam, G.M.; et al. Folate Receptor-Beta Has Limited Value for Fluorescent Imaging in Ovarian, Breast and Colorectal Cancer. PLoS ONE 2015, 10, e0135012. [Google Scholar] [CrossRef]
- Theelen, W.S.; Mittempergher, L.; Willems, S.M.; Bosma, A.J.; Peters, D.D.; van der Noort, V.; Japenga, E.J.; Peeters, T.; Koole, K.; Sustic, T.; et al. FGFR1, 2 and 3 protein overexpression and molecular aberrations of FGFR3 in early stage non-small cell lung cancer. J. Pathol. Clin. Res. 2016, 2, 223–233. [Google Scholar] [CrossRef]
- Panarelli, N.C. Mast Cell Disorders of the Gastrointestinal Tract: Clarity out of Chaos. Surg. Pathol. Clin. 2023, 16, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Provot, S.; Werb, Z. GATA3 in development and cancer differentiation: Cells GATA have it! J. Cell Physiol. 2010, 222, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Jubber, I.; Lloyd, K.; Cumberbatch, M.; Griffin, J. Gene of the month: GATA3. J. Clin. Pathol. 2023, 76, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.C.; Tai, T.S.; Pai, S.Y. GATA3 and the T-cell lineage: Essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 2009, 9, 125–135. [Google Scholar] [CrossRef]
- Miettinen, M.; McCue, P.A.; Sarlomo-Rikala, M.; Rys, J.; Czapiewski, P.; Wazny, K.; Langfort, R.; Waloszczyk, P.; Biernat, W.; Lasota, J.; et al. GATA3: A multispecific but potentially useful marker in surgical pathology: A systematic analysis of 2500 epithelial and nonepithelial tumors. Am. J. Surg. Pathol. 2014, 38, 13–22. [Google Scholar] [CrossRef]
- Reiswich, V.; Schmidt, C.E.; Lennartz, M.; Hoflmayer, D.; Hube-Magg, C.; Weidemann, S.; Fraune, C.; Buscheck, F.; Moller, K.; Bernreuther, C.; et al. GATA3 Expression in Human Tumors: A Tissue Microarray Study on 16,557 Tumors. Pathobiology 2023, 90, 219–232. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; Llorens, C.; Soriano, B.; Bagan, L.; Moreno, A.; Calabuig-Farinas, S.; Jantus-Lewintre, E.; Bagan, J. Differentially methylated genes in proliferative verrucous leukoplakia reveal potential malignant biomarkers for oral squamous cell carcinoma. Oral Oncol. 2021, 116, 105191. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, S.; Amin, A.; Wang, L. Coexpress of GATA-3 and ER in Anorectal and Head and Neck Squamous Cell Carcinoma Mimicking Metastatic Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 409–413. [Google Scholar] [CrossRef]
- Lin, M.C.; Lin, J.J.; Hsu, C.L.; Juan, H.F.; Lou, P.J.; Huang, M.C. GATA3 interacts with and stabilizes HIF-1alpha to enhance cancer cell invasiveness. Oncogene 2017, 36, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Faustino, I.S.P.; de Pauli Paglioni, M.; de Almeida Mariz, B.A.L.; Normando, A.G.C.; Perez-de-Oliveira, M.E.; Georgaki, M.; Nikitakis, N.G.; Vargas, P.A.; Santos-Silva, A.R.; Lopes, M.A. Prognostic outcomes of oral squamous cell carcinoma derived from proliferative verrucous leukoplakia: A systematic review. Oral Dis. 2023, 29, 1416–1431. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Warnakulasuriya, S.; Ramos-Garcia, P. Prognosis Parameters of Oral Carcinomas Developed in Proliferative Verrucous Leukoplakia: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4843. [Google Scholar] [CrossRef]
- Solus, J.F.; Hassan, K.; Lee, S.J.; Hsi, A.C.; Rosman, I.S.; Dehmeri, S.; Schaffer, A. Cutaneous squamous cell carcinoma progression is associated with decreased GATA-3 immunohistochemical staining. J. Cutan. Pathol. 2016, 43, 347–353. [Google Scholar] [CrossRef]
- Steenbergen, R.D.; OudeEngberink, V.E.; Kramer, D.; Schrijnemakers, H.F.; Verheijen, R.H.; Meijer, C.J.; Snijders, P.J. Down-regulation of GATA-3 expression during human papillomavirus-mediated immortalization and cervical carcinogenesis. Am. J. Pathol. 2002, 160, 1945–1951. [Google Scholar] [CrossRef]
- Goyal, A.; Zhang, G.; Yang, B. Differential expression patterns of GATA3 in usual and differentiated types of vulvar intraepithelial neoplasia: Potential diagnostic implications. Mod. Pathol. 2018, 31, 1131–1140. [Google Scholar] [CrossRef]
- Al Barashdi, M.A.; Ali, A.; McMullin, M.F.; Mills, K. Protein tyrosine phosphatase receptor type C (PTPRC or CD45). J. Clin. Pathol. 2021, 74, 548–552. [Google Scholar] [CrossRef]
- Camacho, M.; Aguero, A.; Sumarroca, A.; Lopez, L.; Pavon, M.A.; Aviles-Jurado, F.X.; Garcia, J.; Quer, M.; Leon, X. Prognostic value of CD45 transcriptional expression in head and neck cancer. Eur. Arch. Otorhinolaryngol. 2018, 275, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.J.; de Ruiter, E.J.; Gielgens, T.; Farshadpour, F.; de Bree, R.; van den Hout, M.; Kremer, B.; Willems, S.M.; Speel, E. Frequent PD-L1 expression in oral squamous cell carcinoma of non-smokers and non-drinkers, and association of tumor infiltrating lymphocytes with favorable prognosis. Transl. Oncol. 2025, 55, 102357. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef]
- Hanna, G.J.; Villa, A.; Nandi, S.P.; Shi, R.; A, O.N.; Liu, M.; Quinn, C.T.; Treister, N.S.; Sroussi, H.Y.; Vacharotayangul, P.; et al. Nivolumab for Patients with High-Risk Oral Leukoplakia: A Nonrandomized Controlled Trial. JAMA Oncol. 2024, 10, 32–41. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. KIT (CD117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 205–220. [Google Scholar] [CrossRef]
- Kitamura, Y.; Hirotab, S. Kit as a human oncogenic tyrosine kinase. Cell Mol. Life Sci. 2004, 61, 2924–2931. [Google Scholar] [CrossRef]
- Oliveira-Neto, H.H.; Leite, A.F.; Costa, N.L.; Alencar, R.C.; Lara, V.S.; Silva, T.A.; Leles, C.R.; Mendonca, F.E.; Batista, A.C. Decrease in mast cells in oral squamous cell carcinoma: Possible failure in the migration of these cells. Oral Oncol. 2007, 43, 484–490. [Google Scholar] [CrossRef]
- Fernandez, A.; Marshall, M.; Santibanez, N.; Martínez, C.; Fernandez, J.; Martínez, R.; Briceño, J.; Zumaran, C.; Peñaloza, J.; Fuentevilla, I. Comparing the immuno-expression of endothelial markers in normal oral mucosa, oral epithelial dysplasia and oral squamous cell carcinoma: Relationship between E-cadherin, vimentin, CD31, CD117 and epithelial-mesenchymal transition. MOJ Anat. Physiol. 2017, 4, 301–306. [Google Scholar]
- Sathiyamoorthy, J.; Shyamsundar, V.; Shanmugam, S.; Mani, J.G.; Hari, R. Immunohistochemical expression of c-kit in oral squamous cell carcinoma patients in south Indian populations. Asian J. Pharm. Clin. Res. 2018, 11, 247–250. [Google Scholar] [CrossRef]
- Ansari, F.M.; Asif, M.; Kiani, M.N.; Ara, N.; Ishaque, M.; Khan, R. Evaluation of Mast Cell Density using CD117 antibody and Microvessel Density Using CD34 Antibody in Different Grades of Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 3533–3538. [Google Scholar] [CrossRef]
- Attramadal, C.G.; Kumar, S.; Gao, J.; Boysen, M.E.; Halstensen, T.S.; Bryne, M. Low Mast Cell Density Predicts Poor Prognosis in Oral Squamous Cell Carcinoma and Reduces Survival in Head and Neck Squamous Cell Carcinoma. Anticancer. Res. 2016, 36, 5499–5506. [Google Scholar] [CrossRef]
- Brockmeyer, P.; Kling, A.; Schulz, X.; Perske, C.; Schliephake, H.; Hemmerlein, B. High mast cell density indicates a longer overall survival in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 14677. [Google Scholar] [CrossRef]
- Tegginamani, A.S.; Shivakumar, V.H.; Ismail, S.M.B.; Abraham, M.T.; Fernandes, B.A.; Zamzuri, A.T.B. C-kit Expression in Oral Leukoplakia. J. Coll. Physicians Surg. Pak. 2022, 32, 256–258. [Google Scholar] [CrossRef]
- Ongkeko, W.M.; Altuna, X.; Weisman, R.A.; Wang-Rodriguez, J. Expression of protein tyrosine kinases in head and neck squamous cell carcinomas. Am. J. Clin. Pathol. 2005, 124, 71–76. [Google Scholar] [CrossRef]
- Gudiseva, S.; Santosh, A.B.R.; Chitturi, R.; Anumula, V.; Poosarla, C.; Baddam, V.R.R. The role of mast cells in oral squamous cell carcinoma. Contemp. Oncol. 2017, 21, 21–29. [Google Scholar] [CrossRef]
- Martinez, A.L.; Shannon, M.J.; Sloan, T.; Mace, E.M. CD56/NCAM mediates cell migration of human NK cells by promoting integrin-mediated adhesion turnover. Mol. Biol. Cell 2024, 35, ar64. [Google Scholar] [CrossRef]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 2023, 23, 90–105. [Google Scholar] [CrossRef]
- Caruntu, A.; Moraru, L.; Lupu, M.; Vasilescu, F.; Dumitrescu, M.; Cioplea, M.; Popp, C.; Dragusin, A.; Caruntu, C.; Zurac, S. Prognostic Potential of Tumor-Infiltrating Immune Cells in Resectable Oral Squamous Cell Carcinoma. Cancers 2021, 13, 2268. [Google Scholar] [CrossRef]
- Wagner, S.; Wittekindt, C.; Reuschenbach, M.; Hennig, B.; Thevarajah, M.; Wurdemann, N.; Prigge, E.S.; von Knebel Doeberitz, M.; Dreyer, T.; Gattenlohner, S.; et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer 2016, 138, 2263–2273. [Google Scholar] [CrossRef]
- Stangl, S.; Tontcheva, N.; Sievert, W.; Shevtsov, M.; Niu, M.; Schmid, T.E.; Pigorsch, S.; Combs, S.E.; Haller, B.; Balermpas, P.; et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2018, 142, 1911–1925. [Google Scholar] [CrossRef]

| Protein Marker | Applied Scoring System | Extent Score 1 | Intensity Score |
|---|---|---|---|
| GATA3 | Liang et al. 2014 [49] | 0 (0%), 1 (1–10%), 2 (11–50%), 3 (51–80%), 4 (>80%) | 0 (negative), 1 (weak), 2 (moderate), 3 (strong) |
| c-KIT/CD117 | Goto et al. 2016 [50] | 0 (<1%), 1 (1–4%), 2 (5–29%), 3 (≥30%) | 0 (negative), 1 (weak), 2 (moderate), 3 (strong) |
| CD56 | Pallavi et al. 2025 [51] | 0 (0%), 1 (1–9%), 2 (10–20%), 3 (21–50%), 4 (>50%) | 0 (negative), 1 (weak), 2 (moderate), 3 (strong) |
| CD45 | Pantanowitz et al. 2025 [52] | 0 (0%), 1 (1–24%), 2 (25–49%), 3 (50–74%), 4 (75–99%), 5 (100%) | 0 (negative), 1 (weak), 2 (moderate), 3 (strong) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, E.-M.; Katsoulas, N.; Goutas, D.; Vasili, K.; Mikoglou, E.; Tzanavari, T.; Tosios, K.I. Immunohistochemical Study of GATA3, c-KIT/CD117, CD56 and CD45 Expression in Proliferative Verrucous Leukoplakia (PVL), PVL-Associated Oral Squamous Cell Carcinoma and Oral Leukoplakia. Genes 2025, 16, 1275. https://doi.org/10.3390/genes16111275
Kalogirou E-M, Katsoulas N, Goutas D, Vasili K, Mikoglou E, Tzanavari T, Tosios KI. Immunohistochemical Study of GATA3, c-KIT/CD117, CD56 and CD45 Expression in Proliferative Verrucous Leukoplakia (PVL), PVL-Associated Oral Squamous Cell Carcinoma and Oral Leukoplakia. Genes. 2025; 16(11):1275. https://doi.org/10.3390/genes16111275
Chicago/Turabian StyleKalogirou, Eleni-Marina, Nikolaos Katsoulas, Dimitrios Goutas, Konstantina Vasili, Eleni Mikoglou, Theodora Tzanavari, and Konstantinos I. Tosios. 2025. "Immunohistochemical Study of GATA3, c-KIT/CD117, CD56 and CD45 Expression in Proliferative Verrucous Leukoplakia (PVL), PVL-Associated Oral Squamous Cell Carcinoma and Oral Leukoplakia" Genes 16, no. 11: 1275. https://doi.org/10.3390/genes16111275
APA StyleKalogirou, E.-M., Katsoulas, N., Goutas, D., Vasili, K., Mikoglou, E., Tzanavari, T., & Tosios, K. I. (2025). Immunohistochemical Study of GATA3, c-KIT/CD117, CD56 and CD45 Expression in Proliferative Verrucous Leukoplakia (PVL), PVL-Associated Oral Squamous Cell Carcinoma and Oral Leukoplakia. Genes, 16(11), 1275. https://doi.org/10.3390/genes16111275






