Glycolytic Reprogramming in Uterine Fibroids: Genetic, Transcriptomic, Proteomic, and Metabolomic Insights
Abstract
1. Introduction
2. Overview of Glycolytic Reprogramming
3. Role of Glycolytic Reprogramming in Uterine Fibroids: What Is Currently Known?
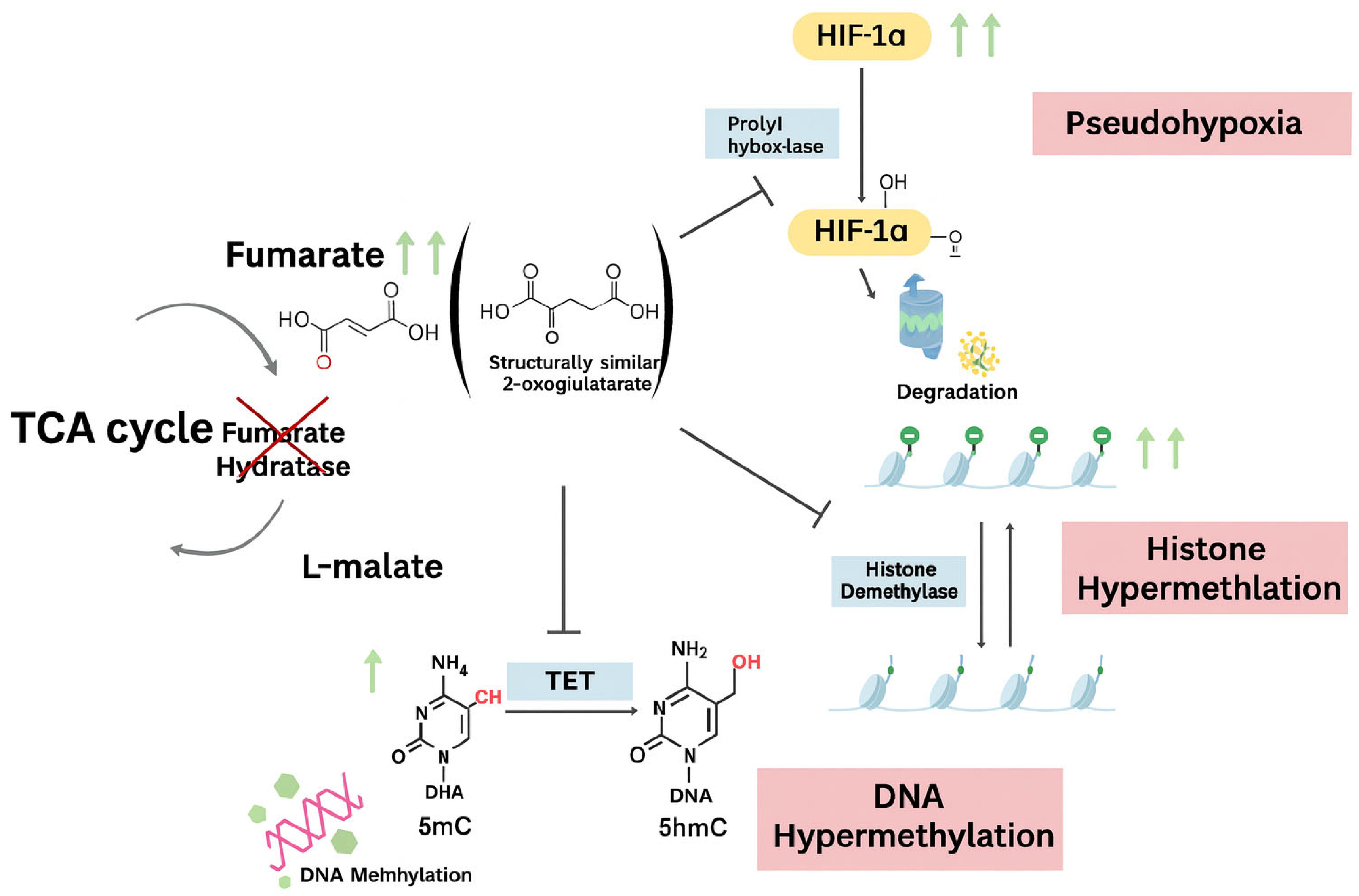
3.1. Genetic Insights
3.2. Transcriptomic Insights
3.3. Proteomic Insights
3.4. Metabolomic Insights
4. Mechanistic Links Between Glycolytic Reprogramming and Fibroid Pathogenesis
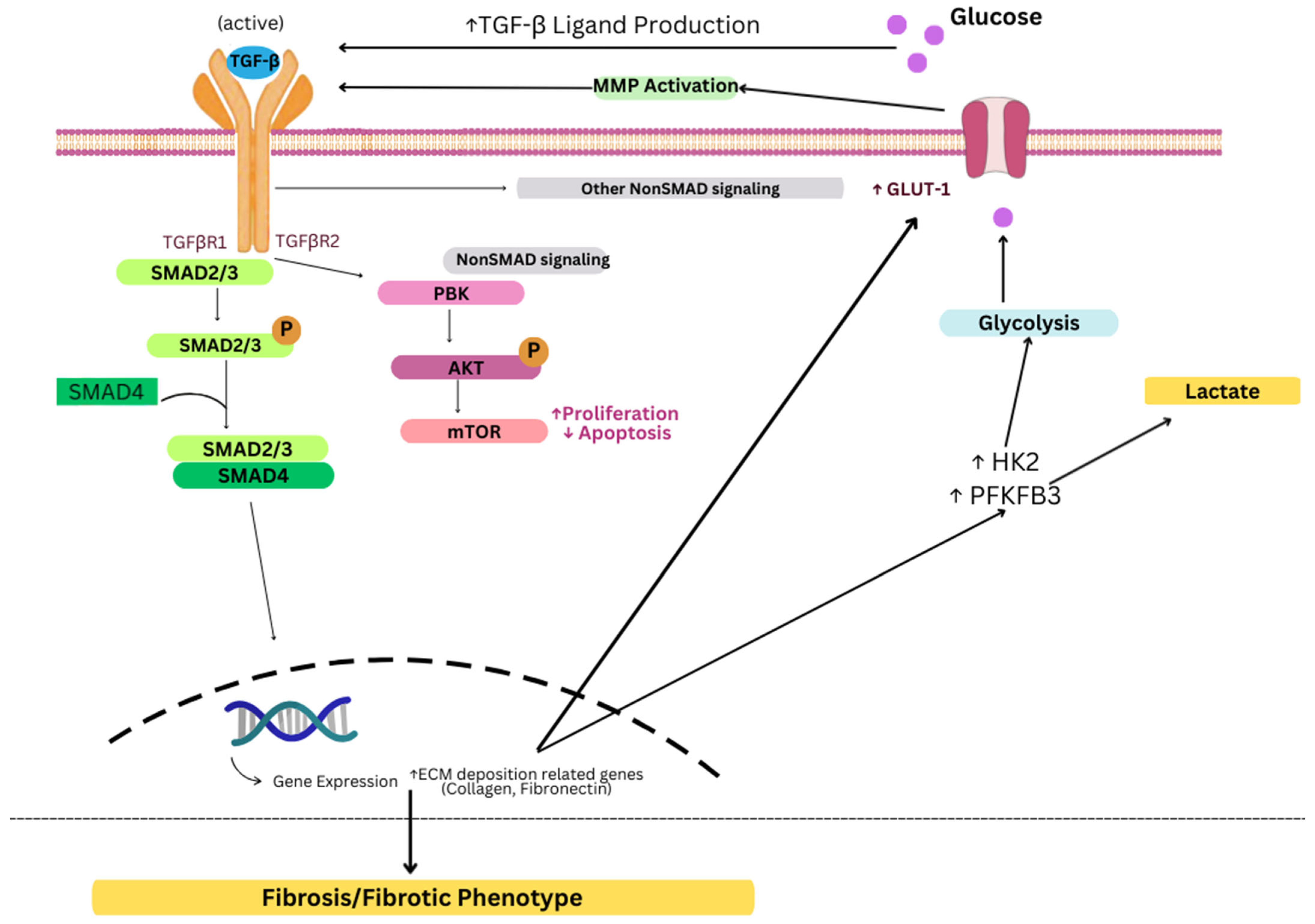
4.1. Transforming Growth Factor—Beta (TGFβ) Activation
4.2. Wnt/β Catenin Signaling
4.3. Extracellular Matrix Stiffness and Mechanotransduction
4.4. Pathway Crosstalk and Synergistic Effects
5. Clinical and Therapeutic Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Wang, F.; Chen, L.; Tong, H. Global Epidemiological Characteristics of Uterine Fibroids. Arch. Med. Sci. 2023, 19, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.E.; Al-Hendy, A.; Kappus, D.; Galitsky, A.; Stewart, E.A.; Kerolous, M. Burden, Prevalence, and Treatment of Uterine Fibroids: A Survey of U.S. Women. J. Womens Health 2018, 27, 1359–1367. [Google Scholar] [CrossRef]
- Katon, J.G.; Plowden, T.C.; Marsh, E.E. Racial Disparities in Uterine Fibroids and Endometriosis: A Systematic Review and Application of Social, Structural, and Political Context. Fertil. Steril. 2023, 119, 355–363. [Google Scholar] [CrossRef]
- Epidemiology and Management of Uterine Fibroids-Giuliani-2020-International Journal of Gynecology & Obstetrics-Wiley Online Library. Available online: https://obgyn.onlinelibrary.wiley.com/doi/10.1002/ijgo.13102 (accessed on 29 August 2025).
- Neumann, B.; Singh, B.; Brennan, J.; Blanck, J.; Segars, J.H. The Impact of Fibroid Treatments on Quality of Life and Mental Health: A Systematic Review. Fertil. Steril. 2024, 121, 400–425. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ciebiera, M.; Wlodarczyk, M.; Alkhrait, S.; Maajid, E.; Yang, Q.; Hsia, S.-M.; Al-Hendy, A. Current and Emerging Treatment Options for Uterine Fibroids. Drugs 2023, 83, 1649–1675. [Google Scholar] [CrossRef]
- Szkodziak, P.; Szkodziak, F.; Trzeciak, K.; Czuczwar, P. Minimally Invasive Procedures in the Management of Uterine Fibroids. Prz. Menopauzalny 2017, 16, 122–125. [Google Scholar] [CrossRef]
- Cianci, S.; Gulino, F.A.; Palmara, V.; La Verde, M.; Ronsini, C.; Romeo, P.; Occhipinti, S.; Incognito, G.G.; Capozzi, V.A.; Restaino, S.; et al. Exploring Surgical Strategies for Uterine Fibroid Treatment: A Comprehensive Review of Literature on Open and Minimally Invasive Approaches. Medicina 2024, 60, 64. [Google Scholar] [CrossRef]
- Al-Hendy, A.; Myers, E.R.; Stewart, E. Uterine Fibroids: Burden and Unmet Medical Need. Semin. Reprod. Med. 2017, 35, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hazimeh, D.; Coco, A.; Casubhoy, I.; Segars, J.; Singh, B. The Annual Economic Burden of Uterine Fibroids in the United States (2010 Versus 2022): A Comparative Cost-Analysis. Reprod. Sci. 2024, 31, 3743–3756. [Google Scholar] [CrossRef]
- Medikare, V.; Kandukuri, L.R.; Ananthapur, V.; Deenadayal, M.; Nallari, P. The Genetic Bases of Uterine Fibroids; A Review. J. Reprod. Infertil. 2011, 12, 181–191. [Google Scholar] [PubMed]
- Ali, M.; Ciebiera, M.; Vafaei, S.; Alkhrait, S.; Chen, H.-Y.; Chiang, Y.-F.; Huang, K.-C.; Feduniw, S.; Hsia, S.-M.; Al-Hendy, A. Progesterone Signaling and Uterine Fibroid Pathogenesis; Molecular Mechanisms and Potential Therapeutics. Cells 2023, 12, 1117. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.M.; Bloise, E.; Ortiga-Carvalho, T.M. Hormones and Pathogenesis of Uterine Fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 34, 13–24. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 Mediates Metabolic Responses to Intratumoral Hypoxia and Oncogenic Mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Tan, Z.; Banerjee, S.; Cui, H.; Ge, J.; Liu, R.-M.; Bernard, K.; Thannickal, V.J.; Liu, G. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.-A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective Fatty Acid Oxidation in Renal Tubular Epithelial Cells Has a Key Role in Kidney Fibrosis Development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Weeks, K.L.; Pretorius, L.; McMullen, J.R. Molecular Distinction between Physiological and Pathological Cardiac Hypertrophy: Experimental Findings and Therapeutic Strategies. Pharmacol. Ther. 2010, 128, 191–227. [Google Scholar] [CrossRef]
- Khanal, S.; Liu, Y.; Bamidele, A.O.; Wixom, A.Q.; Washington, A.M.; Jalan-Sakrikar, N.; Cooper, S.A.; Vuckovic, I.; Zhang, S.; Zhong, J.; et al. Glycolysis in Hepatic Stellate Cells Coordinates Fibrogenic Extracellular Vesicle Release Spatially to Amplify Liver Fibrosis. Sci. Adv. 2024, 10, eadn5228. [Google Scholar] [CrossRef]
- Ruan, Y.; Feng, W.; Yang, C. A Novel Nonsense Mutation in the Fumarate Hydratase Gene in a Chinese Patient with Recurrent Leiomyomas. FS Rep. 2023, 4, 410–415. [Google Scholar] [CrossRef]
- Miettinen, M.; Felisiak-Golabek, A.; Wasag, B.; Chmara, M.; Wang, Z.; Butzow, R.; Lasota, J. Fumarase-Deficient Uterine Leiomyomas: An Immunohistochemical, Molecular Genetic, and Clinicopathologic Study of 86 Cases. Am. J. Surg. Pathol. 2016, 40, 1661. [Google Scholar] [CrossRef] [PubMed]
- Harrison, W.J.; Andrici, J.; Maclean, F.; Madadi-Ghahan, R.; Farzin, M.; Sioson, L.; Toon, C.W.; Clarkson, A.; Watson, N.; Pickett, J.; et al. Fumarate Hydratase–Deficient Uterine Leiomyomas Occur in Both the Syndromic and Sporadic Settings. Am. J. Surg. Pathol. 2016, 40, 599. [Google Scholar] [CrossRef] [PubMed]
- Siegler, L.; Erber, R.; Burghaus, S.; Brodkorb, T.; Wachter, D.; Wilkinson, N.; Bolton, J.; Stringfellow, H.; Haller, F.; Beckmann, M.W.; et al. Fumarate Hydratase (FH) Deficiency in Uterine Leiomyomas: Recognition by Histological Features versus Blind Immunoscreening. Virchows Arch. 2018, 472, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Popp, B.; Erber, R.; Kraus, C.; Vasileiou, G.; Hoyer, J.; Burghaus, S.; Hartmann, A.; Beckmann, M.W.; Reis, A.; Agaimy, A. Targeted Sequencing of FH-Deficient Uterine Leiomyomas Reveals Biallelic Inactivating Somatic Fumarase Variants and Allows Characterization of Missense Variants. Mod. Pathol. 2020, 33, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Menko, F.H.; Maher, E.; Schmidt, L.S.; Middelton, L.A.; Aittomäki, K.; Tomlinson, I.; Richard, S.; Linehan, W.M. Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC). Renal Cancer Risk, Surveillance and Treatment. Fam. Cancer 2014, 13, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Sciacovelli, M.; Gonçalves, E.; Johnson, T.I.; Zecchini, V.R.; da Costa, A.S.H.; Gaude, E.; Drubbel, A.V.; Theobald, S.J.; Abbo, S.R.; Tran, M.G.B.; et al. Fumarate Is an Epigenetic Modifier That Elicits Epithelial-to-Mesenchymal Transition. Nature 2016, 537, 544–547. [Google Scholar] [CrossRef]
- O’Flaherty, L.; Adam, J.; Heather, L.C.; Zhdanov, A.V.; Chung, Y.-L.; Miranda, M.X.; Croft, J.; Olpin, S.; Clarke, K.; Pugh, C.W.; et al. Dysregulation of Hypoxia Pathways in Fumarate Hydratase-Deficient Cells Is Independent of Defective Mitochondrial Metabolism. Hum. Mol. Genet. 2010, 19, 3844–3851. [Google Scholar] [CrossRef]
- Vanharanta, S.; Pollard, P.J.; Lehtonen, H.J.; Laiho, P.; Sjöberg, J.; Leminen, A.; Aittomäki, K.; Arola, J.; Kruhoffer, M.; Ørntoft, T.F.; et al. Distinct Expression Profile in Fumarate-Hydratase-Deficient Uterine Fibroids. Hum. Mol. Genet. 2006, 15, 97–103. [Google Scholar] [CrossRef]
- Catherino, W.H.; Mayers, C.M.; Mantzouris, T.; Armstrong, A.Y.; Linehan, W.M.; Segars, J.H. Compensatory Alterations in Energy Homeostasis Characterized in Uterine Tumors from Hereditary Leiomyomatosis and Renal Cell Cancer. Fertil. Steril. 2007, 88, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of α-KG-Dependent Histone and DNA Demethylases by Fumarate and Succinate That Are Accumulated in Mutations of FH and SDH Tumor Suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Cho, C.-H.; Cha, S.-D.; Back, W.-K.; Kim, M.-K.; Kim, J.-C. Gene Expression Analysis between Uterine Leiomyoma and Normal Myometrial Tissues by DNA Chip. Korean J. Obstet. Gynecol. 2003, 46, 701–706. [Google Scholar]
- SRI 72nd Annual Meeting: Scientific Program & Abstracts. Reprod. Sci. 2025, 32, 1–324. [CrossRef]
- Ura, B.; Scrimin, F.; Arrigoni, G.; Franchin, C.; Monasta, L.; Ricci, G. A Proteomic Approach for the Identification of Up-Regulated Proteins Involved in the Metabolic Process of the Leiomyoma. Int. J. Mol. Sci. 2016, 17, 540. [Google Scholar] [CrossRef] [PubMed]
- Düz, S.A.; Mumcu, A.; Doğan, B.; Sarıdoğan, E.; Tuncay, G.; Onat, T.; Karaer, A. Metabolomics Approach Using HR-MAS NMR Spectroscopy for the Assessment of Metabolic Profiles of Uterine Fibroids. Anal. Biochem. 2025, 704, 115885. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, H.-R.; Mehine, M.; Mäkinen, N.; Pasanen, A.; Pitkänen, E.; Karhu, A.; Sarvilinna, N.S.; Sjöberg, J.; Heikinheimo, O.; Bützow, R.; et al. Global Metabolomic Profiling of Uterine Leiomyomas. Br. J. Cancer 2017, 117, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, S.; Yao, Y.; Zhang, G. Role of Human Monocarboxylate Transporter 1 (hMCT1) and 4 (hMCT4) in Tumor Cells and the Tumor Microenvironment. Cancer Manag. Res. 2023, 15, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, L.; Fu, T.; Zhou, Q.; Zhou, D.; Xiao, L.; Liu, J.; Kong, Y.; Xie, H.; Yi, F.; et al. Exercise Inducible Lactate Dehydrogenase B Regulates Mitochondrial Function in Skeletal Muscle. J. Biol. Chem. 2016, 291, 25306–25318. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the Regulation of Protein Abundance from Proteomic and Transcriptomic Analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Hanse, E.A.; Ruan, C.; Kachman, M.; Wang, D.; Lowman, X.H.; Kelekar, A. Cytosolic Malate Dehydrogenase Activity Helps Support Glycolysis in Actively Proliferating Cells and Cancer. Oncogene 2017, 36, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; O’Leary, E.M.; Witt, L.J.; Tian, Y.; Gökalp, G.A.; Meliton, A.Y.; Dulin, N.O.; Mutlu, G.M. Glutamine Metabolism Is Required for Collagen Protein Synthesis in Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2019, 61, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.J.; Paterson, K.; Riera-Domingo, C.; Sumpton, D.; Däbritz, J.H.M.; Tardito, S.; Boldrini, C.; Hernandez-Fernaud, J.R.; Athineos, D.; Dhayade, S.; et al. Cancer-Associated Fibroblasts Require Proline Synthesis by PYCR1 for the Deposition of pro-Tumorigenic Extracellular Matrix. Nat. Metab. 2022, 4, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Genetic Mechanisms Driving Uterine Leiomyoma Pathobiology, Epidemiology, and Treatment. Available online: https://www.mdpi.com/2073-4425/15/5/558 (accessed on 29 August 2025).
- Kietzmann, T. Vitamin C: From Nutrition to Oxygen Sensing and Epigenetics. Redox Biol. 2023, 63, 102753. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Regulation of Collagen Biosynthesis by Ascorbic Acid: A Review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar] [PubMed]
- Möbius, K.; Arias-Cartin, R.; Breckau, D.; Hännig, A.-L.; Riedmann, K.; Biedendieck, R.; Schröder, S.; Becher, D.; Magalon, A.; Moser, J.; et al. Heme Biosynthesis Is Coupled to Electron Transport Chains for Energy Generation. Proc. Natl. Acad. Sci. USA 2010, 107, 10436–10441. [Google Scholar] [CrossRef]
- Frezza, C.; Zheng, L.; Folger, O.; Rajagopalan, K.N.; MacKenzie, E.D.; Jerby, L.; Micaroni, M.; Chaneton, B.; Adam, J.; Hedley, A.; et al. Haem Oxygenase Is Synthetically Lethal with the Tumour Suppressor Fumarate Hydratase. Nature 2011, 477, 225–228. [Google Scholar] [CrossRef]
- Giarratana, A.O.; Prendergast, C.M.; Salvatore, M.M.; Capaccione, K.M. TGF-β Signaling: Critical Nexus of Fibrogenesis and Cancer. J. Transl. Med. 2024, 22, 594. [Google Scholar] [CrossRef]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef]
- Hariyanto, N.I.; Yo, E.C.; Wanandi, S.I. Regulation and Signaling of TGF-β Autoinduction. Int. J. Mol. Cell. Med. 2021, 10, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Hinz, B. The Extracellular Matrix and Transforming Growth Factor-Β1: Tale of a Strained Relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef]
- Nishimura, S.L. Integrin-Mediated Transforming Growth Factor-β Activation, a Potential Therapeutic Target in Fibrogenic Disorders. Am. J. Pathol. 2009, 175, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kim, H.; Liu, X.; Sugiura, H.; Kohyama, T.; Fang, Q.; Wen, F.-Q.; Abe, S.; Wang, X.; Atkinson, J.J.; et al. Matrix Metalloproteinase-9 Activates TGF-β and Stimulates Fibroblast Contraction of Collagen Gels. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L1006–L1015. [Google Scholar] [CrossRef] [PubMed]
- TGF-β Signaling in Health, Disease and Therapeutics|Signal Transduction and Targeted Therapy. Available online: https://www.nature.com/articles/s41392-024-01764-w (accessed on 29 August 2025).
- Chia, Z.-J.; Cao, Y.; Little, P.J.; Kamato, D. Transforming Growth Factor-β Receptors: Versatile Mechanisms of Ligand Activation. Acta Pharmacol. Sin. 2024, 45, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Saadat, S.; Noureddini, M.; Mahjoubin-Tehran, M.; Nazemi, S.; Shojaie, L.; Aschner, M.; Maleki, B.; Abbasi-kolli, M.; Rajabi Moghadam, H.; Alani, B.; et al. Pivotal Role of TGF-β/Smad Signaling in Cardiac Fibrosis: Non-Coding RNAs as Effectual Players. Front. Cardiovasc. Med. 2021, 7, 588347. [Google Scholar] [CrossRef]
- Isaka, Y. Targeting TGF-β Signaling in Kidney Fibrosis. Int. J. Mol. Sci. 2018, 19, 2532. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Lee, B.S.; Nowak, R.A. Human Leiomyoma Smooth Muscle Cells Show Increased Expression of Transforming Growth Factor-Beta 3 (TGF Beta 3) and Altered Responses to the Antiproliferative Effects of TGF Beta. J. Clin. Endocrinol. Metab. 2001, 86, 913–920. [Google Scholar] [CrossRef]
- Norian, J.M.; Malik, M.; Parker, C.Y.; Joseph, D.; Leppert, P.C.; Segars, J.H.; Catherino, W.H. Transforming Growth Factor Β3 Regulates the Versican Variants in the Extracellular Matrix-Rich Uterine Leiomyomas. Reprod. Sci. 2009, 16, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.S.; Malik, M.; Nurudeen, S.; Catherino, W.H. Myometrial Cells Undergo Fibrotic Transformation under the Influence of Transforming Growth Factor Beta-3. Fertil. Steril. 2010, 93, 1500–1508. [Google Scholar] [CrossRef]
- Ciebiera, M.; Włodarczyk, M.; Słabuszewska-Jóźwiak, A.; Nowicka, G.; Jakiel, G. Influence of Vitamin D and Transforming Growth Factor Β3 Serum Concentrations, Obesity, and Family History on the Risk for Uterine Fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef]
- Kitagawa, T.; Masumi, A.; Akamatsu, Y. Transforming Growth Factor-Beta 1 Stimulates Glucose Uptake and the Expression of Glucose Transporter mRNA in Quiescent Swiss Mouse 3T3 Cells. J. Biol. Chem. 1991, 266, 18066–18071. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Derynck, R. Essential Role of TGF-β Signaling in Glucose-Induced Cell Hypertrophy. Dev. Cell 2009, 17, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Masin, M.; Vazquez, J.; Rossi, S.; Groeneveld, S.; Samson, N.; Schwalie, P.C.; Deplancke, B.; Frawley, L.E.; Gouttenoire, J.; Moradpour, D.; et al. GLUT3 Is Induced during Epithelial-Mesenchymal Transition and Promotes Tumor Cell Proliferation in Non-Small Cell Lung Cancer. Cancer Metab. 2014, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β Signaling in the Tumor Metabolic Microenvironment and Targeted Therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Guido, C.; Whitaker-Menezes, D.; Capparelli, C.; Balliet, R.; Lin, Z.; Pestell, R.G.; Howell, A.; Aquila, S.; Andò, S.; Martinez-Outschoorn, U.; et al. Metabolic Reprogramming of Cancer-Associated Fibroblasts by TGF-β Drives Tumor Growth: Connecting TGF-β Signaling with “Warburg-like” Cancer Metabolism and L-Lactate Production. Cell Cycle 2012, 11, 3019–3035. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.-G. The Interplay Between TGF-β Signaling and Cell Metabolism. Front. Cell Dev. Biol. 2022, 10, 846723. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Choi, M.E.; Ding, Y.; Kim, S.I. TGF-β Signaling via TAK1 Pathway: Role in Kidney Fibrosis. Semin. Nephrol. 2012, 32, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Blyszczuk, P.; Müller-Edenborn, B.; Valenta, T.; Osto, E.; Stellato, M.; Behnke, S.; Glatz, K.; Basler, K.; Lüscher, T.F.; Distler, O.; et al. Transforming Growth Factor-β-Dependent Wnt Secretion Controls Myofibroblast Formation and Myocardial Fibrosis Progression in Experimental Autoimmune Myocarditis. Eur. Heart J. 2017, 38, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of Canonical Wnt Signalling Is Required for TGF-β-Mediated Fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Polkinghorne, M.; Antoniades, C. Non-Canonical WNT Signalling in Cardiovascular Disease: Mechanisms and Therapeutic Implications. Nat. Rev. Cardiol. 2022, 19, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-Catenin Signaling in Cancers and Targeted Therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Działo, E.; Czepiel, M.; Tkacz, K.; Siedlar, M.; Kania, G.; Błyszczuk, P. WNT/β-Catenin Signaling Promotes TGF-β-Mediated Activation of Human Cardiac Fibroblasts by Enhancing IL-11 Production. Int. J. Mol. Sci. 2021, 22, 10072. [Google Scholar] [CrossRef]
- Shi, J.; Li, F.; Luo, M.; Wei, J.; Liu, X. Distinct Roles of Wnt/β-Catenin Signaling in the Pathogenesis of Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Mediat. Inflamm. 2017, 2017, 3520581. [Google Scholar] [CrossRef]
- Cao, H.; Wang, C.; Chen, X.; Hou, J.; Xiang, Z.; Shen, Y.; Han, X. Inhibition of Wnt/β-Catenin Signaling Suppresses Myofibroblast Differentiation of Lung Resident Mesenchymal Stem Cells and Pulmonary Fibrosis. Sci. Rep. 2018, 8, 13644. [Google Scholar] [CrossRef]
- Tan, R.J.; Zhou, D.; Zhou, L.; Liu, Y. Wnt/β-Catenin Signaling and Kidney Fibrosis. Kidney Int. Suppl. (2011) 2014, 4, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mangioni, S.; Viganò, P.; Lattuada, D.; Abbiati, A.; Vignali, M.; Di Blasio, A.M. Overexpression of the Wnt5b Gene in Leiomyoma Cells: Implications for a Role of the Wnt Signaling Pathway in the Uterine Benign Tumor. J. Clin. Endocrinol. Metab. 2005, 90, 5349–5355. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yin, P.; Navarro, A.; Moravek, M.B.; Coon, J.S.; Druschitz, S.A.; Serna, V.A.; Qiang, W.; Brooks, D.C.; Malpani, S.S.; et al. Paracrine Activation of WNT/β-Catenin Pathway in Uterine Leiomyoma Stem Cells Promotes Tumor Growth. Proc. Natl. Acad. Sci. USA 2013, 110, 17053–17058. [Google Scholar] [CrossRef] [PubMed]
- Sabeh, M.E.; Saha, S.K.; Afrin, S.; Islam, M.S.; Borahay, M.A. Wnt/β-Catenin Signalling Pathway in Uterine Leiomyoma: Role in Tumor Biology and Targeting Opportunities. Mol. Cell. Biochem. 2021, 476, 3513–3536. [Google Scholar] [CrossRef]
- Pate, K.T.; Stringari, C.; Sprowl-Tanio, S.; Wang, K.; TeSlaa, T.; Hoverter, N.P.; McQuade, M.M.; Garner, C.; Digman, M.A.; Teitell, M.A.; et al. Wnt Signaling Directs a Metabolic Program of Glycolysis and Angiogenesis in Colon Cancer. EMBO J. 2014, 33, 1454–1473. [Google Scholar] [CrossRef]
- Esen, E.; Chen, J.; Karner, C.M.; Okunade, A.L.; Patterson, B.W.; Long, F. WNT-LRP5 Signaling Induces Warburg Effect through mTORC2 Activation during Osteoblast Differentiation. Cell Metab. 2013, 17, 745–755. [Google Scholar] [CrossRef]
- Kim, J.; Gao, P.; Liu, Y.-C.; Semenza, G.L.; Dang, C.V. Hypoxia-Inducible Factor 1 and Dysregulated c-Myc Cooperatively Induce Vascular Endothelial Growth Factor and Metabolic Switches Hexokinase 2 and Pyruvate Dehydrogenase Kinase 1. Mol. Cell. Biol. 2007, 27, 7381–7393. [Google Scholar] [CrossRef]
- Yang, Q.; Al-Hendy, A. Update on the Role and Regulatory Mechanism of Extracellular Matrix in the Pathogenesis of Uterine Fibroids. Int. J. Mol. Sci. 2023, 24, 5778. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular Matrix in Uterine Leiomyoma Pathogenesis: A Potential Target for Future Therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef]
- Jamaluddin, M.F.B.; Nahar, P.; Tanwar, P.S. Proteomic Characterization of the Extracellular Matrix of Human Uterine Fibroids. Endocrinology 2018, 159, 2656–2669. [Google Scholar] [CrossRef] [PubMed]
- Leppert, P.C.; Jayes, F.L.; Segars, J.H. The Extracellular Matrix Contributes to Mechanotransduction in Uterine Fibroids. Obs. Gynecol. Int. 2014, 2014, 783289. [Google Scholar] [CrossRef]
- Ge, H.; Tian, M.; Pei, Q.; Tan, F.; Pei, H. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front. Oncol. 2021, 11, 631991. [Google Scholar] [CrossRef] [PubMed]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B. Extracellular Matrix Stiffness: Mechanisms in Tumor Progression and Therapeutic Potential in Cancer. Exp. Hematol. Oncol. 2025, 14, 54. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.-Y.; et al. Vascular Stiffness Mechanoactivates YAP/TAZ-Dependent Glutaminolysis to Drive Pulmonary Hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef]
- Islam, M.S.; Afrin, S.; Singh, B.; Jayes, F.L.; Brennan, J.T.; Borahay, M.A.; Leppert, P.C.; Segars, J.H. Extracellular Matrix and Hippo Signaling as Therapeutic Targets of Antifibrotic Compounds for Uterine Fibroids. Clin. Transl. Med. 2021, 11, e475. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Petraglia, F.; Catherino, W.H.; Donnez, J. Pathogenesis of Uterine Fibroids: Current Understanding and Future Directions. Fertil. Steril. 2024, 122, 6–11. [Google Scholar] [CrossRef]
- Navarro, A.; Bariani, M.V.; Yang, Q.; Al-Hendy, A. Understanding the Impact of Uterine Fibroids on Human Endometrium Function. Front. Cell Dev. Biol. 2021, 9, 633180. [Google Scholar] [CrossRef] [PubMed]
- Mension, E.; Calaf, J.; Chapron, C.; Dolmans, M.M.; Donnez, J.; Marcellin, L.; Petraglia, F.; Vannuccini, S.; Carmona, F. An Update on the Management of Uterine Fibroids: Personalized Medicine or Guidelines? J. Endometr. Uterine Disord. 2024, 7, 100080. [Google Scholar] [CrossRef]
- Hussein, S.; Khanna, P.; Yunus, N.; Gatza, M.L. Nuclear Receptor-Mediated Metabolic Reprogramming and the Impact on HR+ Breast Cancer. Cancers 2021, 13, 4808. [Google Scholar] [CrossRef]
- Ahn, S.; Park, J.H.; Grimm, S.L.; Piyarathna, D.W.B.; Samanta, T.; Putluri, V.; Mezquita, D.; Fuqua, S.A.W.; Putluri, N.; Coarfa, C.; et al. Metabolomic Rewiring Promotes Endocrine Therapy Resistance in Breast Cancer. Cancer Res. 2024, 84, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (HIF): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.F.; Kimura, K.; Bernhardt, W.M.; Shrimanker, N.; Akai, Y.; Hohenstein, B.; Saito, Y.; Johnson, R.S.; Kretzler, M.; Cohen, C.D.; et al. Hypoxia Promotes Fibrogenesis in Vivo via HIF-1 Stimulation of Epithelial-to-Mesenchymal Transition. J. Clin. Investig. 2007, 117, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.; Williams, R.; Kirkpatrick, L.; Paine-Murrieta, G.; Powis, G. Antitumor Activity and Pharmacodynamic Properties of PX-478, an Inhibitor of Hypoxia-Inducible Factor-1alpha. Mol. Cancer Ther. 2004, 3, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Palayoor, S.T.; Mitchell, J.B.; Cerna, D.; Degraff, W.; John-Aryankalayil, M.; Coleman, C.N. PX-478, an Inhibitor of Hypoxia-Inducible Factor-1alpha, Enhances Radiosensitivity of Prostate Carcinoma Cells. Int. J. Cancer 2008, 123, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Arastu, S.; Lam, J.; Kim, H.; Wang, W.; Wang, S.; Bhatt, V.; Lopes, E.C.; Hu, Z.; Sun, M.; et al. Glucose-6-Phosphate Dehydrogenase Maintains Redox Homeostasis and Biosynthesis in LKB1-Deficient KRAS-Driven Lung Cancer. Nat. Commun. 2024, 15, 5857. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, F.; Zhang, Y.; Lin, Z.; Yang, J.; Han, X.; Feng, Y.; Pei, X.; Li, F.; Liu, Q.; et al. Targeting Glucose-6-Phosphate Dehydrogenase by 6-AN Induces ROS-Mediated Autophagic Cell Death in Breast Cancer. FEBS J. 2023, 290, 763–779. [Google Scholar] [CrossRef] [PubMed]


| Approach | Study (Year) | Objective | Biological Model | Analytic Methods | Results |
|---|---|---|---|---|---|
| Genetic | Vanharanta et al. (2006) [31] | To determine the differences in global gene expression in FH mutant fibroids vs. wild-type fibroids | Fresh-frozen myometrium (n = 11) Fresh-frozen fibroids (n = 22):
| Microarray (GeneChip® HG-U133A, TIGR MeV); validation with qRT-PCR | ↑ Expression of all ten differentially expressed genes involved in glycolysis in FH mutants compared FH wild-type fibroids and myometrial tissue |
| Catherino et al. (2007) [32] | To determine gene expression of glycolysis and TCA cycle enzymes in HLRCC fibroids vs. non-syndromic fibroids | Matched human fibroid- myometrium pairs from patients with HLRCC (n = 1; 3 samples) and without HLRCC (n = 11) | Microarray (Affymetrix U-133 chip) | Non-syndromic fibroids No differential expression in glycolysis or TCA cycle enzymes compared to matched myometrium HLRCC fibroids ↓ Fumarate hydratase ↑ Glycolysis enzymes compared to matched myometrium | |
| Transcriptomic | Kwon et al. (2003) [34] | To investigate differential gene expression between fibroid and myometrial tissue | Matched fibroid-myometrium pairs (n = 5) | DNA microarray/chip; validation with RT-PCR | ↑ Hexokinase 1 and ↑ Hexokinase 2 expression (>3 fold) in fibroid tissue vs. myometrial tissue |
| Alsamraae et al. (2025) [35] | To investigate differential global genomic expression profiles gene expression between fibroid and myometrial tissue | Matched fibroid-myometrium pairs (n = 5) | Whole-genome RNA sequencing (RNA-seq), pathway enrichment; validation with IHC | ↑ glycolysis pathway activity (>50-fold enrichment) IHC ↑ MCT1 expression ↓ LDHB expression in fibroid tissue compared to myometrium | |
| Proteomic | Ura et al. (2016) [36] | To | Matched fibroid-myometrium pairs (n = 5) | 2-DE + mass spectrometry (MS); validation with Western blotting | Upregulation of LDH-B, MDH-1, and GOT-1 |
| Metabolomic | Duz et al. (2025) [37] | To determine dysregulated metabolites in fibroid vs. myometrial tissues | Matched fibroid-myometrium pairs (n = 5) Control myometrium samples (n = 14) | HR-MAS NMR; multivariate analysis (PCA, PLS-DA); validation with univariate analysis | Fibroids vs. adjacent myometrium ↑ Lactate, alanine, glutamate, glutamine, methionine, isocitrate, choline, GPC, PC, o-phosphoethanolamine, taurine, myo-inositol, phenylacetate, ascorbate, glucose, methylhistidine Fibroid-adjacent myometrium vs. control myometrium ↓ Valine, leucine, isoleucine, ethanol, arginine, N-acetyl tyrosine, acetone, p-methylhistidine, glucose, phenylacetate, myo-inositol, α-glucose |
| Heinonen et al. (2017) [38] | To determine dysregulated metabolites in fibroid subtypes (MED12, HMGA2, FH) | Fibroid samples (n = 25; from 17 patients) FH-deficient (n = 7) MED12 (n = 7) HMGA2 overexpression (n = 2) Triple wild-type fibroids (n = 9) Myometrium samples (n = 17) Matched controls | LC-MS/MS; pathway enrichment | Shared across subtypes ↓ heme and homocarnosine FH-deficient subtype ↑ TCA intermediates (fumarate, malate, succinate, α-KG), PPP activation (↑ G6PD/PGD/TKT), unique ↑ N6-succinyladenosine and argininosuccinate. MED12 subtype ↓ vitamin A, ↓ vitamin C metabolites, ↓ multiple amino acids and sphingolipids; altered methionine/cysteine/SAM/taurine metabolism. HMGA2 overexpression subtype Distinct but less pronounced lipid/amino acid changes; separate clustering from FH/MED12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sayed, S.; Pan, A.; Vanos, V.; Michel, R.; Borahay, M. Glycolytic Reprogramming in Uterine Fibroids: Genetic, Transcriptomic, Proteomic, and Metabolomic Insights. Genes 2025, 16, 1268. https://doi.org/10.3390/genes16111268
El Sayed S, Pan A, Vanos V, Michel R, Borahay M. Glycolytic Reprogramming in Uterine Fibroids: Genetic, Transcriptomic, Proteomic, and Metabolomic Insights. Genes. 2025; 16(11):1268. https://doi.org/10.3390/genes16111268
Chicago/Turabian StyleEl Sayed, Samya, Alvina Pan, Valentina Vanos, Rachel Michel, and Mostafa Borahay. 2025. "Glycolytic Reprogramming in Uterine Fibroids: Genetic, Transcriptomic, Proteomic, and Metabolomic Insights" Genes 16, no. 11: 1268. https://doi.org/10.3390/genes16111268
APA StyleEl Sayed, S., Pan, A., Vanos, V., Michel, R., & Borahay, M. (2025). Glycolytic Reprogramming in Uterine Fibroids: Genetic, Transcriptomic, Proteomic, and Metabolomic Insights. Genes, 16(11), 1268. https://doi.org/10.3390/genes16111268





