Secondary Mitochondrial Dysfunction in Gaucher Disease Type I, II and III—Review of the Experimental and Clinical Evidence
Abstract
1. Introduction
2. Methods
3. Neurological Manifestations and GD
Pathophysiology of Neurological Complications in GD
4. Mechanism of Mitochondrial Dysfunction in GD
4.1. Mouse Models/Experimental Studies
4.2. Human Studies
5. Clinical Evidence
6. Advances in the Treatment of GD
6.1. Pharmacological Chaperone Therapy
6.2. Substrate Reduction Therapy
6.3. Gene Therapy
7. Adjunct Therapies
8. Limitations
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine Triphosphate |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| GCase | Glucocerebrosidase |
| GlcCer | Glucosylceramide |
| GlcSph | Glucosylsphingosine |
| ETC | Electron transport chain |
| ER | Endothelial reticulum |
| ERT | Enzyme replacement therapy |
| GD | Gaucher disease |
| LSD | Lysosomal storage disorder |
| MRC | Mitochondrial respiratory chain |
| ROS | Reactive oxygen species |
| SNGP | Supranuclear gaze palsy |
| PD | Parkinson disease |
| SRT | Substrate reduction therapy |
| SSI | Severity score index |
References
- Fuller, M.; Meikle, P.J.; Hopwood, J.J. Epidemiology of Lysosomal Storage Diseases: An Overview. In Fabry Disease: Perspectives from 5 Years of FOS; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis: Oxford, UK, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11603/ (accessed on 5 September 2025).
- Dandana, A.; Ben Khelifa, S.; Chahed, H.; Miled, A.; Ferchichi, S. Gaucher Disease: Clinical, Biological and Therapeutic Aspects. Pathobiol. J. Immunopath Mol. Cellul. Biol. 2016, 83, 13–23. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, Z.; Pan, H.; Ma, S.; Xiong, F.; He, F. The molecular mechanism of Gaucher disease caused by compound heterozygous mutations in GBA1 gene. Front. Pediatr. 2023, 11, 1092645. [Google Scholar] [CrossRef]
- Boer, D.E.C.; van Smeden, J.; Bouwstra, J.A.; Aerts, J.M.F.G. Glucocerebrosidase: Functions in and Beyond the Lysosome. J. Clin. Med. 2020, 9, 736. [Google Scholar] [CrossRef]
- de la Mata, M.; Cotán, D.; Villanueva-Paz, M.; de Lavera, I.; Álvarez-Córdoba, M.; Luzón-Hidalgo, R.; Suárez-Rivero, J.M.; Tiscornia, G.; Oropesa-Ávila, M. Mitochondrial Dysfunction in Lysosomal Storage Disorders. Diseases 2016, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Baixauli, F.; Acín-Pérez, R.; Villarroya-Beltrí, C.; Mazzeo, C.; Nuñez-Andrade, N.; Gabandé-Rodriguez, E.; Ledesma, M.D.; Blázquez, A.; Martin, M.A.; Falcón-Pérez, J.M.; et al. Mitochondrial Respiration Controls Lysosomal Function during Inflammatory T Cell Responses. Cell Metab. 2015, 22, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.A.; Heales, S.; Rahman, K.; Sexton, D.W.; Hargreaves, I. The effect of cellular coenzyme Q10 deficiency on lysosomal acidification. J. Clin. Med. 2020, 9, 1923. [Google Scholar] [CrossRef] [PubMed]
- D’aMore, S.; Page, K.; Donald, A.; Taiyari, K.; Tom, B.; Deegan, P.; Tan, C.Y.; Poole, K.; Jones, S.A.; Mehta, A.; et al. In-depth phenotyping for clinical stratification of Gaucher disease. Orphanet J. Rare Dis. 2021, 16, 431. [Google Scholar] [CrossRef]
- Bennett, L.L.; Mohan, D. Gaucher disease and its treatment options. Ann. Pharmacother. 2013, 47, 1182–1193. [Google Scholar] [CrossRef]
- Weiss, K.; Gonzalez, A.; Lopez, G.; Pedoeim, L.; Groden, C.; Sidransky, E. The clinical management of Type 2 Gaucher disease. Mol. Genet. Metab. 2015, 114, 110–122. [Google Scholar] [CrossRef]
- Donald, A.; Tan, C.Y.; Chakrapani, A.; Hughes, D.A.; Sharma, R.; Cole, D.; Bardins, S.; Gorges, M.; Jones, S.A.; Schneider, E. Eye movement biomarkers allow for the definition of phenotypes in Gaucher Disease. Orphanet J. Rare Dis. 2020, 15, 349. [Google Scholar] [CrossRef]
- Charrow, J.; Andersson, H.C.; Kaplan, P.; Kolodny, E.H.; Mistry, P.; Pastores, G.; Rosenbloom, B.E.; Scott, C.R.; Wappner, R.S.; Weinreb, N.J.; et al. The Gaucher registry: Demographics and disease characteristics of 1698 patients with Gaucher disease. Arch. Intern. Med. 2000, 160, 2835–2843. [Google Scholar] [CrossRef]
- Weinreb, N.J.; Deegan, P.; Kacena, K.A.; Mistry, P.; Pastores, G.M.; Velentgas, P.; vom Dahl, S. Life expectancy in Gaucher disease type 1. Am. J. Hematol. 2008, 83, 896–900. [Google Scholar] [CrossRef]
- Kałużna, M.; Trzeciak, I.; Ziemnicka, K.; Machaczka, M.; Ruchała, M. Endocrine and metabolic disorders in patients with Gaucher disease type 1: A review. Orphanet J. Rare Dis. 2019, 14, 275. [Google Scholar] [CrossRef]
- Erikson, A.; Bembi, B.; Schiffmann, R. 5 Neuronopathic forms of Gaucher’s disease. Baillière’s Clin. Haematol. 1997, 10, 711–723. [Google Scholar] [CrossRef]
- Finkbeiner, S. The Autophagy Lysosomal Pathway and Neurodegeneration. Cold Spring Harb. Perspect. Biol. 2020, 12, a033993. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; Boland, B.; van der Spoel, A.C. The cell biology of disease: Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012, 199, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Demers-Lamarche, J.; Guillebaud, G.; Tlili, M.; Todkar, K.; Bélanger, N.; Grondin, M.; Nguyen, A.P.; Michel, J.; Germain, M. Loss of Mitochondrial Function Impairs Lysosomes. J. Biol. Chem. 2016, 291, 10263–10276. [Google Scholar] [CrossRef] [PubMed]
- Linari, S.; Castaman, G. Clinical manifestations and management of Gaucher disease. Clinical cases in mineral and bone metabolism: The official journal of the Italian Society of Osteoporosis. Miner. Metab. Skelet. Dis. 2015, 12, 157–164. [Google Scholar] [CrossRef]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Jia, L.; Quinn, B.; Zamzow, M.; Stringer, K.; Aronow, B.; Sun, Y.; Zhang, W.; Setchell, K.D.; Grabowski, G.A. Global gene expression profile progression in Gaucher disease mouse models. BMC Genom. 2011, 12, 20. [Google Scholar] [CrossRef]
- Aflaki, E.; Westbroek, W.; Sidransky, E. The Complicated Relationship between Gaucher Disease and Parkinsonism: Insights from a Rare Disease. Neuron 2017, 93, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, H.; Tang, B.; Guo, J. Clinical, mechanistic, biomarker, and therapeutic advances in GBA1-associated Parkinson’s disease. Transl. Neurodegener. 2014, 13, 48. [Google Scholar] [CrossRef]
- Adly, A.A.M.; Ismail, E.A.R.; Ibrahim, F.A.; Atef, M.; El Sayed, K.A.; Aly, N.H. A 6-month randomized controlled trial for vitamin E supplementation in pediatric patients with Gaucher disease: Effect on oxidative stress, disease severity and hepatic complications. J. Inherit. Metab. Dis. 2025, 48, e12792. [Google Scholar] [CrossRef]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Skrahin, A.; Horowitz, M.; Istaiti, M.; Skrahina, V.; Lukas, J.; Yahalom, G.; Cohen, M.E.; Revel-Vilk, S.; Goker-Alpan, O.; Becker-Cohen, M.; et al. GBA1-Associated Parkinson’s Disease Is a Distinct Entity. Int. J. Mol. Sci. 2024, 25, 7102. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Krainc, D. Mechanisms of glucocerebrosidase dysfunction in Parkinson’s disease. J. Mol. Biol. 2023, 435, 168023. [Google Scholar] [CrossRef]
- Marano, M.; Zizzo, C.; Malaguti, M.C.; Bacchin, R.; Cavallieri, F.; De Micco, R.; Spagnolo, F.; Bentivoglio, A.R.; Schirinzi, T.; Bovenzi, R.; et al. Increased glucosylsphingosine levels and Gaucher disease in GBA1-associated Parkinson’s disease. Park. Relat. Disord. 2024, 124, 107023. [Google Scholar] [CrossRef]
- Xu, Y.H.; Xu, K.; Sun, Y.; Liou, B.; Quinn, B.; Li, R.H.; Xue, L.; Zhang, W.; Setchell, K.D.R.; Witte, D.; et al. Multiple pathogenic proteins implicated in neuronopathic Gaucher disease mice. Hum. Mol. Genet. 2014, 23, 3943–3957. [Google Scholar] [CrossRef]
- Luettel, D.M.; Terluk, M.R.; Roh, J.; Weinreb, N.J.; Kartha, R.V. Emerging biomarkers in Gaucher disease. Adv. Clin. Chem. 2025, 124, 1–56. [Google Scholar]
- Stepien, K.M.; Cufflin, N.; Donald, A.; Jones, S.; Church, H.; Hargreaves, I.P. Secondary Mitochondrial Dysfunction as a Cause of Neurodegenerative Dysfunction in Lysosomal Storage Diseases and an Overview of Potential Therapies. Int. J. Mol. Sci. 2022, 23, 10573. [Google Scholar] [CrossRef]
- Gegg, M.E.; Burke, D.; Heales, S.J.; Cooper, J.M.; Hardy, J.; Wood, N.W.; Schapira, A.H. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol. 2012, 72, 455–463. [Google Scholar] [CrossRef]
- Kadowaki, H.; Nishitoh, H. Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes 2013, 4, 306–333. [Google Scholar] [CrossRef]
- Davidson, B.A.; Hassan, S.; Garcia, E.J.; Tayebi, N.; Sidransky, E. Exploring genetic modifiers of Gaucher disease: The next horizon. Hum. Mutat. 2018, 39, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.E.; Usnich, T.; Scherzer, C.R.; Klein, C.; Chung, S.J. GBA1 Variants and Parkinson’s Disease: Paving the Way for Targeted Therapy. J. Mov. Disord. 2023, 16, 261–278. [Google Scholar] [CrossRef]
- Zhao, J.F.; Shapiro, N.; Sathe, G.; Brewer, A.; Macartney, T.J.; Wood, N.T.; Negoita, F.; Sakamoto, K.; Sapkota, G.P. Targeted dephosphorylation of TFEB promotes its nuclear translocation. iScience 2024, 27, 110432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baden, P.; Perez, M.J.; Raji, H.; Bertoli, F.; Kalb, S.; Illescas, M.; Spanos, F.; Giuliano, C.; Calogero, A.M.; Oldrati, M.; et al. Glucocerebrosidase is imported into mitochondria and preserves complex I integrity and energy metabolism. Nat. Commun. 2023, 14, 1930. [Google Scholar] [CrossRef]
- Ducatez, F.; Berger, M.G.; Pilon, C.; Plichet, T.; Lesueur, C.; Berger, J.; Belmatoug, N.; Marret, S.; Bekri, S.; Tebani, A. Deciphering metabolic shifts in Gaucher disease type 1: A multi-omics study. J. Mol. Med. 2025, 103, 187–203. [Google Scholar] [CrossRef]
- Osellame, L.D.; Rahim, A.A.; Hargreaves, I.P.; Gegg, M.E.; Richard-Londt, A.; Brandner, S.; Waddington, S.N.; Schapira, A.H.V.; Duchen, M.R. Mitochondria and quality control defects in a mouse model of Gaucher disease–links to Parkinson’s disease. Cell Metab. 2013, 17, 941–953. [Google Scholar] [CrossRef]
- Cleeter, M.W.; Chau, K.Y.; Gluck, C.; Mehta, A.; Hughes, D.A.; Duchen, M.; Wood, N.W.; Hardy, J.; Mark Cooper, J.; Schapira, A.H. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem. Int. 2013, 62, 1–7. [Google Scholar] [CrossRef]
- Plotegher, N.; Perocheau, D.; Ferrazza, R.; Massaro, G.; Bhosale, G.; Zambon, F.; Rahim, A.A.; Guella, G.; Waddington, S.N.; Szabadkai, G.; et al. Impaired cellular bioenergetics caused by GBA1 depletion sensitizes neurons to calcium overload. Cell Death Differ. 2020, 27, 1588–1603. [Google Scholar] [CrossRef]
- Luth, E.S.; Stavrovskaya, I.G.; Bartels, T.; Kristal, B.S.; Selkoe, D.J. Soluble, prefibrillar alpha-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J. Biol. Chem. 2014, 289, 21490–21507. [Google Scholar] [CrossRef]
- de la Mata, M.; Cotán, D.; Oropesa-Ávila, M.; Garrido-Maraver, J.; Cordero, M.D.; Villanueva Paz, M.; Delgado Pavón, A.; Alcocer-Gómez, E.; de Lavera, I.; Ybot-González, P.; et al. Pharmacological Chaperones and Coenzyme Q10 Treatment Improves Mutant β-Glucocerebrosidase Activity and Mitochondrial Function in Neuronopathic Forms of Gaucher Disease. Sci. Rep. 2015, 5, 10903. [Google Scholar] [CrossRef]
- Rubilar, J.C.; Outeiro, T.F.; Klein, A.D. The lysosomal β-glucocerebrosidase strikes mitochondria: Implications for Parkinson’s therapeutics. Brain 2024, 147, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Deganuto, M.; Pittis, M.G.; Pines, A.; Dominissini, S.; Kelley, M.R.; Garcia, R.; Quadrifoglio, F.; Bembi, B.; Tell, G. Altered intracellular redox status in Gaucher disease fibroblasts and impairment of adaptive response against oxidative stress. J. Cell. Physiol. 2007, 212, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Roversi, F.M.; Galdieri, L.C.; Grego, B.H.; Souza, F.G.; Micheletti, C.; Martins, A.M.; D’ALmeida, V. Blood oxidative stress markers in Gaucher disease patients. Clin. Chim. Acta 2016, 364, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Kartha, R.V.; Terluk, M.R.; Brown, R.; Travis, A.; Mishra, U.R.; Rudser, K.; Lau, H.; Jarnes, J.R.; Cloyd, J.C.; Weinreb, N.J. Patients with Gaucher disease display systemic oxidative stress dependent on therapy status. Mol. Genet. Metab. Rep. 2020, 25, 100667. [Google Scholar] [CrossRef]
- Mello, A.S.; da Silva Garcia, C.; de Souza Machado, F.; da Silva Medeiros, N.; Wohlenberg, M.F.; Marinho, J.P.; Dani, C.; Funchal, F.D.S.; Coelho, J.C. Oxidative stress parameters of Gaucher disease type I patients. Mol. Genet. Metab. Rep. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Stepien, K.M.; Roncaroli, F.; Turton, N.; Hendriksz, C.J.; Roberts, M.; Heaton, R.A.; Hargreaves, I. Mechanisms of Mitochondrial Dysfunction in Lysosomal Storage Disorders: A Review. J. Clin. Med. 2020, 9, 2596. [Google Scholar] [CrossRef]
- Dopeso-Reyes, I.G.; Sucunza, D.; Rico, A.J.; Pignataro, D.; Marín-Ramos, D.; Roda, E.; Rodríguez-Pérez, A.I.; Labandeira-García, J.L.; Lanciego, J.L. Glucocerebrosidase expression patterns in the non-human primate brain. Brain Struct. Funct. 2018, 223, 343–355. [Google Scholar] [CrossRef]
- Chung, E.; Mo, H.; Wang, S.; Zu, Y.; Elfakhani, M.; Rios, S.R.; Chyu, M.-C.; Yang, R.-S.; Shen, C.-L. Potential roles of vitamin E in age-related changes in skeletal muscle health. Nutr. Res. 2018, 49, 23–36. [Google Scholar] [CrossRef]
- Bousvaros, A.; Zurakowski, D.; Duggan, C.; Law, T.; Rifai, N.; Goldberg, N.E. Vitamins A and E serum levels in children and young adults with inflammatory bowel disease: Effect of disease activity. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 129–135. [Google Scholar]
- Mozafari, H.; Khatami, S.; Kiani, A.; Rahimi, Z.; Vaisi-Raygani, A.; Afsharnaderi, A.; Alaei, M.R. oxidative stress parameters, trace elements, and lipid profile in Iranian patients with Gaucher disease. Biol. Trace Element Res. 2020, 193, 130–137. [Google Scholar] [CrossRef]
- de la Mata, M.; Cotán, D.; Oropesa-Ávila, M.; Villanueva-Paz, M.; de Lavera, I.; Álvarez-Córdoba, M.; Luzón-Hidalgo, R.; Suárez-Rivero, J.M.; Tiscornia, G.; Sánchez-Alcázar, J.A. Coenzyme Q10 partially restores pathological alterations in a macrophage model of Gaucher disease. Orphanet J. Rare Dis. 2017, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Kantar, A.; Klimek, L.; Cazan, D.; Sperl, A.; Sent, U.; Mesquita, M. An overview of efficacy and safety of ambroxol for the treatment of acute and chronic respiratory diseases with a special regard to children. Multidiscip. Respir. Med. 2020, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Bendikov-Bar, I.; Maor, G.; Filocamo, M.; Horowitz, M. Ambroxol as a pharmacological chaperone for mutant glucocerebrosidase. Blood Cells Mol. Dis. 2013, 50, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liou, B.; Lin, Y.; Fannin, V.; Zhang, W.; Feldman, R.A.; Setchell, K.D.R.; Grabowski, G.A.; Sun, Y. Substrate Reduction Therapy Reverses Mitochondrial, mTOR, and Autophagy Alterations in a Cell Model of Gaucher Disease. Cells 2021, 10, 2286. [Google Scholar] [CrossRef]
- Deng, Y.; Dong, G.; Meng, Y.; Noinaj, N.; Huang, R. Structure–Activity Relationship Studies of Venglustat on NTMT1 Inhibition. J. Med. Chem. 2023, 66, 1601–1615. [Google Scholar] [CrossRef]
- Peterschmitt, M.J.; Crawford, N.P.S.; Gaemers, S.J.M.; Ji, A.J.; Sharma, J.; Pham, T.T. Pharmacokinetics, Pharmacodynamics, Safety, and Tolerability of Oral Venglustat in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2021, 10, 86–98. [Google Scholar] [CrossRef]
- Harai, N.; Ichijo, M.; Uchinuma, H.; Hanihara, M.; Kawaguchi, Y.; Ichikawa, D.; Tsuchiya, K. Gaucher Disease Types I and III Responded Well to Substrate Reduction Therapy Using Eliglustat. Intern. Med. 2023, 62, 3005–3011. [Google Scholar] [CrossRef]
- Kulkarni, A.; Chen, T.; Sidransky, E.; Han, T.-U. Advancements in Viral Gene Therapy for Gaucher Disease. Genes 2024, 15, 364. [Google Scholar] [CrossRef]
- Goker-Alpan, O.; Whittaker, A.; Ferrante, F. A Phase 1, Open-Label, Safety, Tolerability, and Efficacy Study of FLT201 in Adult Patients with Gaucher Disease Type 1 (GALILEO-1) Spur Therapeutics. Study Details. A Gene Therapy Study in Patients with Gaucher Disease Type 1. 2024. Available online: https://ClinicalTrials.gov (accessed on 5 September 2025).
- Kopytova, A.E.; Rychkov, G.N.; Nikolaev, M.A.; Baydakova, G.V.; Cheblokov, A.A.; Senkevich, K.A.; Bogdanova, D.A.; Bolshakova, O.I.; Miliukhina, I.V.; Bezrukikh, V.A.; et al. Ambroxol increases glucocerebrosidase (GCase) activity and restores GCase translocation in primary patient-derived macrophages in Gaucher disease and Parkinsonism. Parkinsonism Relat. Disord. 2021, 84, 112–121. [Google Scholar] [CrossRef]
- Concolino, D.; Deodato, F.; Parini, R. Enzyme replacement therapy: Efficacy and limitations. Ital. J. Pediatr. 2018, 44 (Suppl. S2), 117–126. [Google Scholar] [CrossRef]
- Mullin, S.; Smith, L.; Lee, K.; D’Souza, G.; Woodgate, P.; Elflein, J.; Hällqvist, J.; Toffoli, M.; Streeter, A.; Hosking, J.; et al. Ambroxol for the treatment of patients with Parkinson disease with and without glucocerebrosidase gene mutations: A nonrandomized, noncontrolled trial. JAMA Neurol. 2020, 77, 427–434. [Google Scholar] [CrossRef]
- Schiffmann, R.; Sevigny, J.; Rolfs, A.; Davies, E.H.; Goker-Alpan, O.; Abdelwahab, M.; Vellodi, A.; Mengel, E.; Lukina, E.; Yoo, H.W.; et al. The definition of neuronopathic Gaucher disease. J. Inherit. Metab. Dis. 2020, 43, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Charkhand, B.; Scantlebury, M.H.; Narita, A.; Zimran, A.; Al-Hertani, W. Effect of Ambroxol chaperone therapy on Glucosylsphingosine (Lyso-Gb1) levels in two Canadian patients with type 3 Gaucher disease. Mol. Genet. Metab. Rep. 2019, 20, 100476. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.W.; Ong, D.S.; Wang, Y.J.; Balch, W.E.; Yates, J.R.; Segatori, L.; Kelly, J.W. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell 2008, 134, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Drelichman, G.; Castañeda-Hernández, G.; Cem Ar, M.; Dragosky, M.; Garcia, R.; Lee, H.; Moiseev, S.; Naderi, M.; Rosenbaum, H.; Žnidar, I.; et al. The road to biosimilars in rare diseases—Ongoing lessons from Gaucher disease. Am. J. Hematol. 2020, 95, 233–237. [Google Scholar] [CrossRef]
- Gegg, M.E.; Schapira, A.H. Mitochondrial Dysfunction Associated with Glucocerebrosidase Deficiency. Neurobiol. Dis. 2016, 90, 43–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hargreaves, I.P. Coenzyme Q10 as a Therapy for Mitochondrial Disease. Int. J. Biochem. Cell Biol. 2014, 49, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and immune function: An overview. Antioxidants 2021, 10, 759. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I.P. Efficacy and Safety of Coenzyme Q10 Supplementation in Neonates, Infants and Children: An Overview. Antioxidants 2024, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Rigat, B.; Mahuran, D. Diltiazem, a L-type Ca2+ channel blocker, also acts as a pharmacological chaperone in Gaucher patient cells. Mol. Genet. Metab. 2009, 96, 225–232. [Google Scholar] [CrossRef]
- Hatamian, S.; Abdi, A.; Asl, F.S.S.; Tafazolimoghadam, A.; Tavasol, A.; Nejad, S.A.M.; Madadi, R.; Tajabadi, Z.; Dehghani, M.; Ahmadpoor, N.; et al. Examining the therapeutic potential and side effects of calcium channel blockers in mortality and morbidity of patients with stroke: A systematic review of pre-clinical and clinical studies. IBRO Neurosci. Rep. 2025, 18, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, E.A.; Kornberg, A.; Acker, M. Vitamin E deficiency due to increased consumption in beta-thalassemia and in Gaucher’s disease. Ann. N. Y. Acad. Sci. 1982, 393, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Thomassen, A.S.; Mashaw, S.A.; MacDonald, E.M.; Waguespack, A.; Hickey, L.; Singh, A.; Gungor, D.; Kallurkar, A.; Kaye, A.M.; et al. Vitamin E (α-Tocopherol): Emerging Clinical Role and Adverse Risks of Supplementation in Adults. Cureus 2025, 17, e78679. [Google Scholar] [CrossRef]
- Zesiewicz, T.; Salemi, J.L.; Perlman, S.; Sullivan, K.L.; Shaw, J.D.; Huang, Y.; Isaacs, C.; Gooch, C.; Lynch, D.R.; Klein, M.B. Double-blind, randomized and controlled trial of EPI-743 in Friedreich’s ataxia. Neurodegener. Dis. Manag. 2018, 8, 233–242. [Google Scholar] [CrossRef]
- Szegő, É.M.; Dominguez-Meijide, A.; Gerhardt, E.; König, A.; Koss, D.J.; Li, W.; Pinho, R.; Fahlbusch, C.; Johnson, M.; Santos, P.; et al. Cytosolic trapping of a mitochondrial heat shock protein is an early pathological event in synucleinopathies. Cell Rep. 2019, 28, 65–77. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Klein, A.D.; Outeiro, T.F. Glucocerebrosidase mutations disrupt the lysosome and now the mitochondria. Nat. Commun. 2023, 14, 6383. [Google Scholar] [CrossRef]


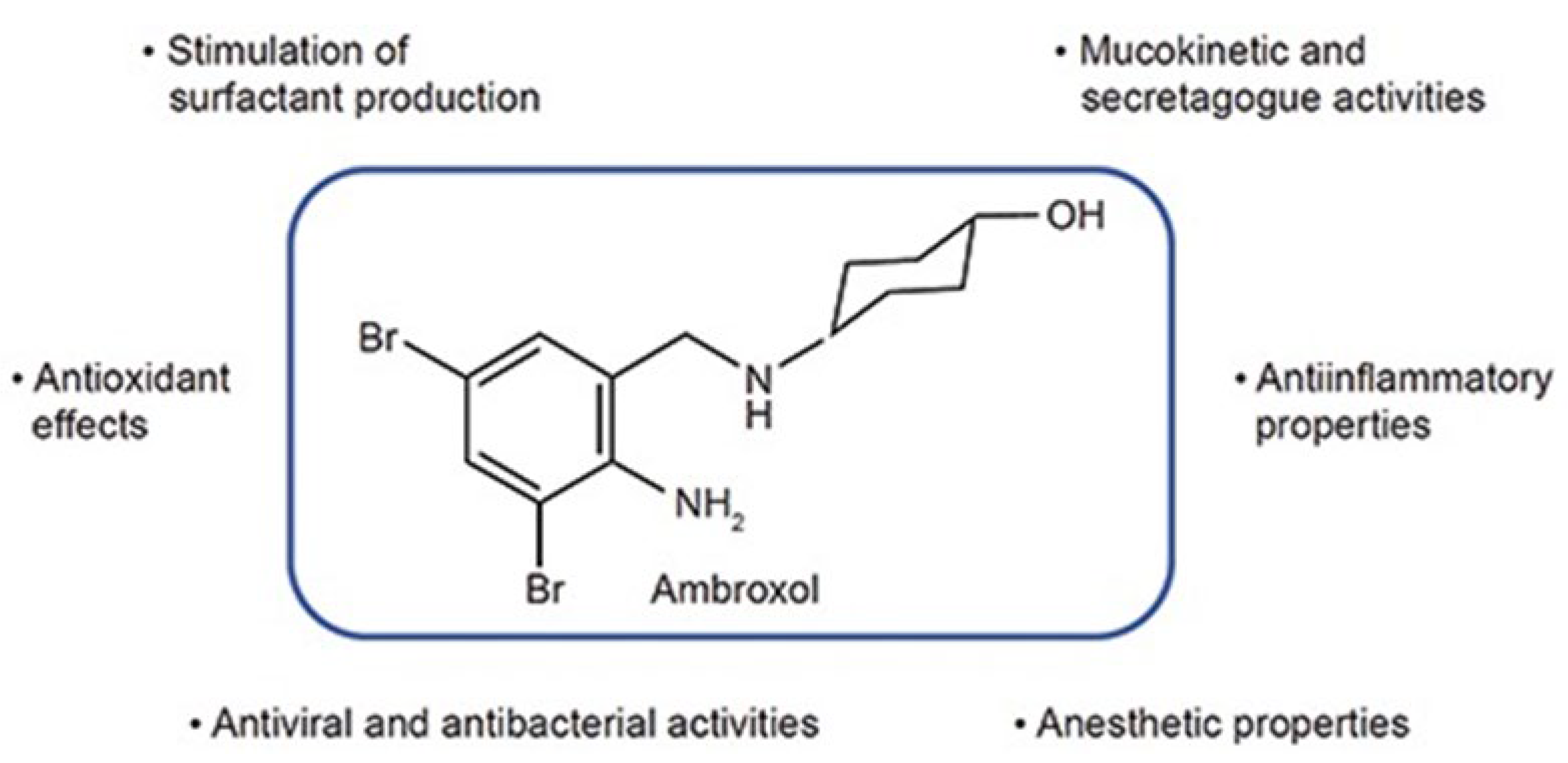
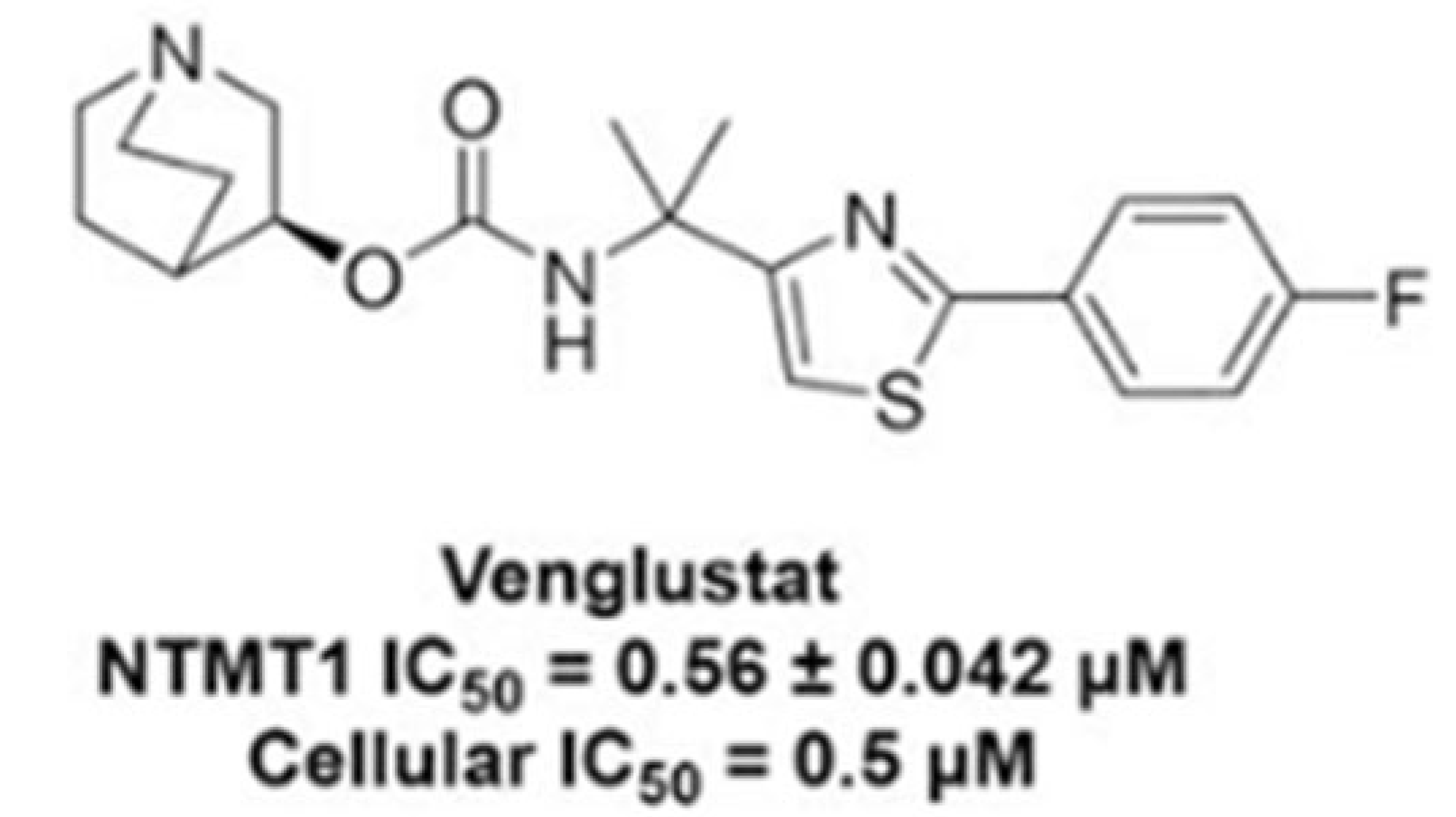
| Non-Neuronopathic (Type 1) | Acute-Neuronopathic (Type 2) | Chronic-Neuronopathic (Type 3) | |
|---|---|---|---|
| Prevalence | 1:40,000–1:60,000 | <1:100,000 | <1:50,000–1:100,000 |
| CNS involvement | None | Severe | Mild to severe; (progressive) |
| Clinical manifestations | Skeletal abnormalities; anaemia; thrombocytopaenia; hepatosplenomegaly (all progressive) | Supranuclear gaze palsy (SNGP); hydrops fetalis; ichthyosis [10]; hepatosplenomegaly (moderate); thrombocytopaenia (severe). | Skeletal abnormalities; anaemia; thrombocytopaenia; hepatosplenomegaly (all progressive) |
| Neurological symptoms | - | Lethal neurological impairment; strabismus opisthotonus, trismus. | Abnormal eye movements (i.e., VIth nerva palsy); ataxia; seizures; dementia (usually appearing later in life) [11] |
| Age of onset | Any age [12] | Infancy [9] | Childhood to adolescence [10] |
| Life expectancy | >60 years [13] | Up to 2 years [14] | ~30–40 years [15], but clinical experience shows the clinical experience is much longer (up to the seventh decade) in patients with milder clinical phenotypes. |
| Drug | Mechanism of Action | Reference | Trial Number | CNS Target Organ Y/N |
|---|---|---|---|---|
| Crossing BBB | ||||
| Ambroxol (clinical trial) | Iminosugar, GCase chaperone; binds to GCase, facilitating its trafficking to the lysosome, and in a low pH it is released, increasing GCase activity in cultured macrophages derived from GD and GBA1-PD patients by ∼3.5-fold, while reducing substrate levels by ∼2-fold when compared to untreated cells; in neuronopathic GD patients with N188S, G193W, F213I/RecNciI and D409H/IVS10-1G > A genotypes | [63,65] [66,67] | NCT02941822, NCT05778617, NCT05830396, NCT05287503, NCT02914366, NCT04388969, NCT0458825 | Y [38] |
| Gene therapy (Clinical trial) | Augmentation of residual GCase expression, and its potential to improve the clinical phenotype by reduction and prevention of cellular accumulation of GCase substrate. | - | NCT05324943 | Y |
| GZ452 (Venglustat analogue) (Mouse model) Venglustat (clinical trial) | SRT | [58] [59] | NCT02906020 NCT02843035 | Inhibited GlcCer production and normalised GlcCer levels in GD neurons Y, but no significant reduction in GlcCer in the CNS of GD patients |
| Not crossing BBB | ||||
| SRT: Eliglustat Miglustat (clinical trial) | Inhibiting UDP-GlcCer synthase, an enzyme that catalyses GlcCer biosynthesis, reducing GlcCer influx load into the lysosome. Inhibitors of GlcCer synthase. | [68] | - - | N N |
| ERTs: Imiglycarase Veraglucerase alpha Taliglucerase alpha And their Biosimilars (clinical trial) | Delivers a functional enzyme to break down the accumulated GlcCer. Reduces hepatosplenomegaly, improves anaemia and thrombocytopenia and ameliorates skeletal damage like bone pain and crises. | [64] [69] | - | N N |
| Drug | Mechanism of Action | [Reference] or NCT | Study Population | Outcome Measures/CV% | Effect Size | Biochemical Parameters and Safety Considerations |
|---|---|---|---|---|---|---|
| Co-enzyme Q10 (experiemental studies) | Reduce GlcCer accumulation, mitochondrial dysfunction and oxidative stress in chemically induced GD macrophages model | [7,39,44] | - | - | - | The safety of supplemental Co-enzyme Q10 is well established in more than 200 randomised controlled clinical trials in a wide range of disorders, with CoQ10 at doses of 200–300 mg/day for 3–6 months typically utilised, although some studies have used much higher daily doses (2700 mg/day) No serious adverse effects were reported [73]. |
| Inhibitors of Ca2+ channels, such as diltiazem and verapamil (experimental studies) | By increasing ER calcium concentrations and activity of Ca2+-dependent endogenous molecular chaperones, were able to partially restore lysosomal enzyme folding, trafficking and activity | [70,74] | - | - | - | The potential side effects of calcium channel blockers were assessed in the systematic review by Hatamian et al., 2025 [75] which concluded that the effectiveness of these drugs many vary depending on the dosage and patient population. |
| Vitamin E (tocopherol) (clinical trial) EPI-743 | Targets oxidoreductase enzymes essential for redox control of metabolism | [24]-NCT06211478 [76] [72] | 6 GD1 14 GD3 | SSI decreased from 9.5 (8–13) to 5.5 (3–10) lyso GL1 decreased from 178 (120–208) ng/mL to 146.7 (89.8–184.6) ng/mL SSI decreased from 14 (11–16) to 8.5 (7–11) lyso GL1 decreased from 247.55 (179.8–473.0) ng/mL to 203.5 (152–301) ng/mL | p
= 0.017 p = 0.001 p < 0.001 | MDA and antioxidant markers including peroxiredoxin 2, Glutathione peroxidase, superoxide dismutase and reduced glutathione. High-dose vitamin E supplementation may affect normal cellular processes including immunity and cell growth, contribute to oxidative stress and also amplify the risk of bleeding [77]. EPI-743 treatment has been demonstrated to be safe and well tolerated [78]. |
| HSP10 (Experiemental study) | Chaperonin protein | [79] | - | - | - | Mitochondrial GCase promotes the maintenance of mitochondrial complex I measured as the ratio of NADH oxidase/co-enzyme Q reductase activities. More clinical studies are required to assess the potential side effects associated with HSP10. |
| N
-acetylcysteine (NAC; 7200 mg/day) (clinical trials) | Anti-inflammatory properties | NCT02583672 NCT02437396 | 20 subjects | - | - | Concentration of glutathione in brain (μmol/g) after months, measured by MRI. Complement 5A and Hepcidin NAC is well tolerated in oral doses below 1200 mg/day and has anticoagulant and platelet-inhibiting properties [80]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewsbury, M.; Purcell, T.; Hughes, D.; Donald, A.; Hargreaves, I.P.; Stepien, K.M. Secondary Mitochondrial Dysfunction in Gaucher Disease Type I, II and III—Review of the Experimental and Clinical Evidence. Genes 2025, 16, 1269. https://doi.org/10.3390/genes16111269
Dewsbury M, Purcell T, Hughes D, Donald A, Hargreaves IP, Stepien KM. Secondary Mitochondrial Dysfunction in Gaucher Disease Type I, II and III—Review of the Experimental and Clinical Evidence. Genes. 2025; 16(11):1269. https://doi.org/10.3390/genes16111269
Chicago/Turabian StyleDewsbury, Mollie, Tyler Purcell, Derralynn Hughes, Aimee Donald, Iain P. Hargreaves, and Karolina M. Stepien. 2025. "Secondary Mitochondrial Dysfunction in Gaucher Disease Type I, II and III—Review of the Experimental and Clinical Evidence" Genes 16, no. 11: 1269. https://doi.org/10.3390/genes16111269
APA StyleDewsbury, M., Purcell, T., Hughes, D., Donald, A., Hargreaves, I. P., & Stepien, K. M. (2025). Secondary Mitochondrial Dysfunction in Gaucher Disease Type I, II and III—Review of the Experimental and Clinical Evidence. Genes, 16(11), 1269. https://doi.org/10.3390/genes16111269







