The Enhancement of Fungal Disease Resistance in Major Staple Crops Using CRISPR-Cas Technology
Abstract
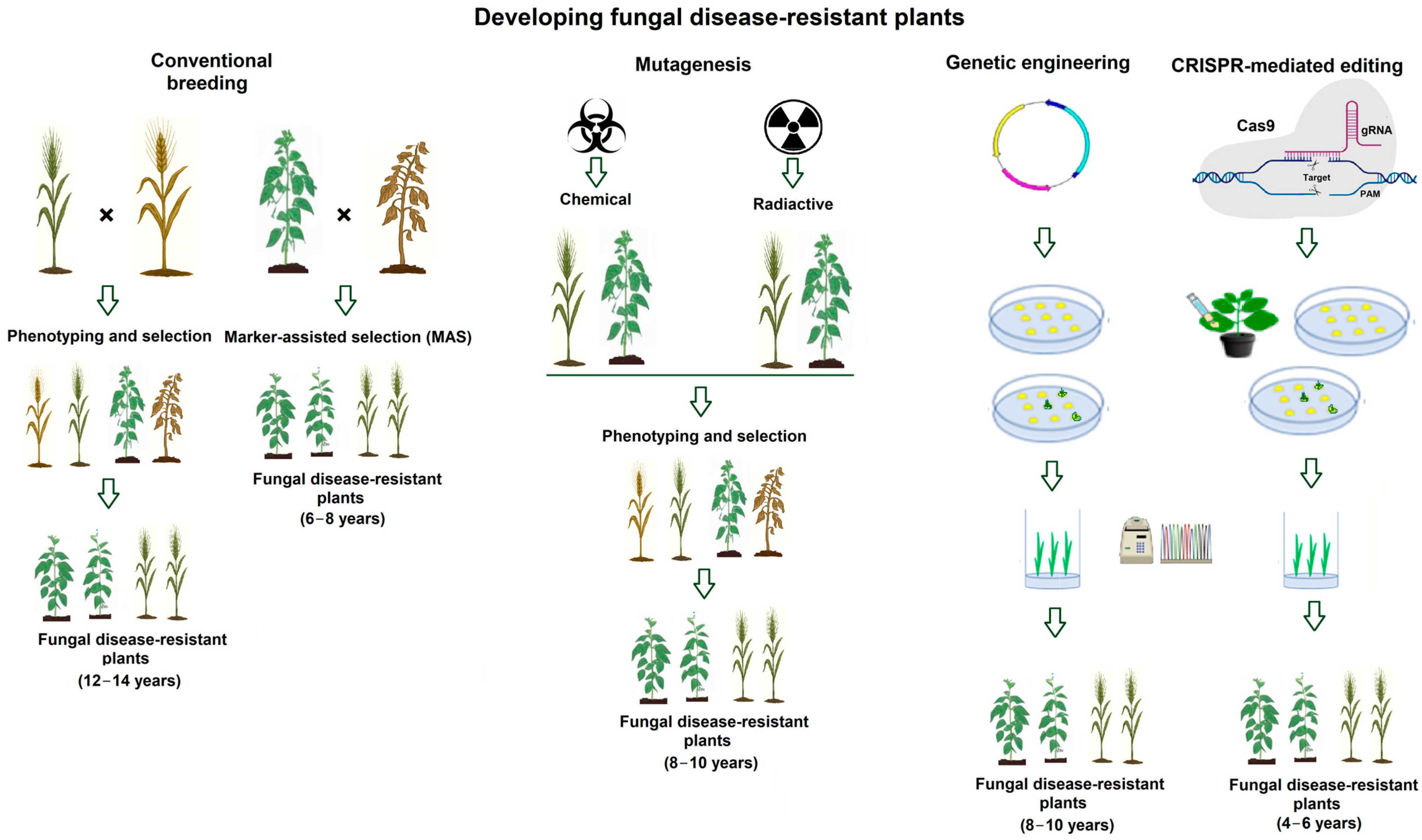
1. Introduction
2. CRISPR–Cas Genome Editing to Improve Fungal-Disease Resistance in Main Cereal Crops
2.1. Wheat
2.2. Rice
| Pathogen | Target Gene | Delivery Method | Type of Editing | References |
|---|---|---|---|---|
| Wheat | ||||
| F. graminearum | Fhb1 | Particle bombardment | Deletion mutation | [41] |
| F. graminearum | TaHRC | Particle bombardment transformation for Cas9 and viral mediated transformation for gRNA | Knockout | [42] |
| F. graminearum | TaHRC | Agrobacterium- mediated transformation | Knockout | [43] |
| P. nodorum | Tsn1 and Snn5 | Cas9-RNP-mediated editing | Knockout | [46] |
| P. nodorum | Tox3 | RNP complex targeting | Knockout | [47] |
| B. graminis f. sp. tritici | MLO-B1 | Cas9-RNP mediated editing | Deletion | [49] |
| B. graminis f.sp. tritici | TaMLO | Particle bombardment | Knockout | [51] |
| B. graminis f. sp. tritici | TaEDR1 | Particle bombardment | Knockout | [50] |
| B. graminis f. sp. tritici P. striiformis f. sp. tritici | TaMKP1 | Agrobacterium- mediated transformation | Knockout | [55] |
| P. striiformis f. sp. tritici | TaPsIPK1 | Agrobacterium-mediated transformation | Knockout | [56] |
| P. triticina Erikss | TaGW2 | Agrobacterium-mediated transformation | Knockout | [57] |
| Rice | ||||
| M. oryzae | ALB1 and RSY1 | Specific base pair edits | Base editing | [59] |
| M. oryzae | OsERF922 | Protoplast transformation | Mutation | [60] |
| M. oryzae | OsSEC3A | Protoplast transfection | Mutation | [61] |
| M. oryzae | Rei1, Ppg1, Bip1, Bip2, Dbf2 | Protoplast transformation | Mutation | [62] |
| M. oryzae | Pi21 | Agrobacterium- mediated transformation | Knockout | [63] |
| M. oryzae | Pita, Pi21 and ERF922 | Agrobacterium- mediated transformation | Knockout | [64] |
| M. oryzae | TMS5, Pi21 u Xa13 | Agrobacterium- mediated transformation | Mutation | [65] |
| M. oryzae | Pi21 u OsSULTR3;6 | Agrobacterium- mediated transformation | Knockout | [66] |
| M. oryzae | Bsr-d1, Pi21 u ERF922 | Agrobacterium- mediated transformation | Knockout | [67] |
| M. oryzae | Pi21 | Agrobacterium- mediated transformation | Knockout | [68] |
| M. oryzae | Pid3 | Agrobacterium- mediated transformation | Knockout | [69] |
| M. oryzae | Bsr-d1, Perox3 | Agrobacterium- mediated transformation | Knockout | [70] |
| M. oryzae, U. virens | MoATG3, MoATG7 u UvPal1 | Agrobacterium- mediated transformation | Knockout | [71] |
| M. oryzae | rBE5 | Agrobacterium- mediated transformation | Base editing | [72] |
2.3. Barley
3. CRISPR-Cas Genome Editing for Improvement Fungal Disease Resistance of Legumes
| Pathogen | Target Gene | Delivery Method | Type of Editing | References |
|---|---|---|---|---|
| Soybean | ||||
| E. diffusa | GmMLO02, GmMLO19, GmMLO20, GmMLO23 | Agrobacterium-mediated transformation | Knockout | [79] |
| P. sojae | RXLR Avr4/6 | (PEG)-mediated protoplast transformations | Knockout | [81] |
4. CRISPR-Cas Genome Editing for Improvement Fungal Disease Resistance of Vegetables
4.1. Tomato
| Pathogen | Target Gene | Delivery Method | Type of Editing | References |
|---|---|---|---|---|
| Tomato | ||||
| Oidium neolycopersici | SlMlo1 | Agrobacterium- mediated transformation | Knockout | [86] |
| O. neolycopersici | SlMlo1, SlPelo | Agrobacterium- mediated transformation | Knockout | [87] |
| O. neolycopersici | PMR4 | Agrobacterium- mediated transformation | Knockout | [88] |
| O. neolycopersici | SlDND1 | Agrobacterium- mediated transformation | Knockout | [89] |
| Phytophthora infestans | SlPMR4 | Agrobacterium- mediated transformation | Knockout | [90] |
| P. infestans | miR482b, miR482c | Agrobacterium- mediated transformation | Knockout | [91] |
| Phytophthora capsici | SlDMR6-1 | Agrobacterium- mediated transformation | Knockout | [92] |
| Fusarium oxysporum | XSP10, SlSAMT | Agrobacterium- mediated transformation | Knockout | [93] |
| F. oxysporum | SlPUB21, SlPUB17/SlPUB21 | Agrobacterium- mediated transformation | Knockout | [94] |
| Botrytis cinerea | SlPLC2 | Agrobacterium- mediated transformation | Knockout | [95] |
| B. cinerea | SlPG2a, SlPL | Agrobacterium- mediated transformation | Knockout | [96] |
| Verticillium dahliae | SlWAT | Agrobacterium- mediated transformation | Knockout | [97] |
| Pepper | ||||
| Leveillula taurica | CaMLO2 | RNP | Knockout | [98] |
| L. taurica | CaMLO2 | RNP | Knockout | [99] |
| Colletotrichum truncatum | CaERF28 | Agrobacterium-mediated transformation | Knockout | [100] |
| Eggplant | ||||
| P. infestans, P. capsici | SmDMR6 | Agrobacterium-mediated transformation | Knockout | [101] |
4.2. Pepper
4.3. Eggplant
5. Challenges, Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| Cas | CRISPR-associated protein |
| DSBs | Double-stranded breaks |
| NHEJ | Non-homologous end joining |
| HDR | Homology-directed repair |
| FHB | Fusarium head blight |
| BSMV | Barley mosaic virus |
| VIGS | Virus-induced gene silencing |
| RNP | Ribonucleoprotein |
| SNB | Septoria nodorum blotch |
| MLO | Powdery mildew locus O |
| CRISPRi | CRISPR interference |
| BYDV | Barley yellow dwarf virus |
| FAO | Food and Agriculture Organization of the United Nations |
| DMR6 | Downy mildew resistance 6 |
| DND1 | Defense no death 1 |
| DCL1 | Dicer-like1 |
| NBS-LRR | Nucleotide-binding site |
| LRR | Leucine-rich repeat |
| XSP10 | Xylem sap protein 10 |
| SlSAMT | Salicylic acid methyltransferase |
| LTP | Lipid transfer protein |
| SA | Salicylic acid |
| MeSA | Methyl salicylate |
| SAM | S-adenosyl-L-methionine |
| SlPG2a | Polygalacturonase |
| SlPL | Pectate lyase |
| GMOs | Genetically modified organisms |
References
- The United Nations. World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 2 October 2025).
- Food and Agriculture Organization. Plant Production and Protection. Available online: https://www.fao.org/plant-production-protection/about/en. (accessed on 2 October 2025).
- Mikaberidze, A.; Gokhale, C.S.; Bargués-Ribera, M.; Verma, P. The Cost of Fungicide Resistance Evolution in Multi-Field Plant Epidemics. PLoS Sustain. Transform. 2025, 4, e0000178. [Google Scholar] [CrossRef]
- Wang, N.; Sundin, G.W.; De La Fuente, L.; Cubero, J.; Tatineni, S.; Brewer, M.T.; Zeng, Q.; Bock, C.H.; Cunniffe, N.J.; Wang, C.; et al. Key Challenges in Plant Pathology in the Next Decade. Phytopathology 2024, 114, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Sbai, H.; Hajib, A.; Msairi, S.; Amalich, S.; Bouyahya, A.; Lee, L.-H.; Wen Goh, K.; Tabyaoui, M.; Harhar, H. Fungal Infections of Legume Crops: Challenges and Management Approaches. J. Agric. Food Res. 2024, 18, 101447. [Google Scholar] [CrossRef]
- Tripathi, A.N.; Maurya, S.; Pandey, K.K.; Behera, T.K. Global Scenario of Vegetable Fungal Diseases. Veg. Sci. 2024, 51, 54–65. [Google Scholar] [CrossRef]
- El Afandi, G.; Irfan, M. Pesticides Risk Assessment Review: Status, Modeling Approaches, and Future Perspectives. Agronomy 2024, 14, 2299. [Google Scholar] [CrossRef]
- Islam, T.; Danishuddin; Tamanna, N.T.; Matin, M.N.; Barai, H.R.; Haque, M.A. Resistance Mechanisms of Plant Pathogenic Fungi to Fungicide, Environmental Impacts of Fungicides, and Sustainable Solutions. Plants 2024, 13, 2737. [Google Scholar] [CrossRef]
- Morgounov, A.; Akin, B.; Demir, L.; Keser, M.; Kokhmetova, A.; Martynov, S.; Orhan, Ş.; Özdemir, F.; Özseven, I.; Sapakhova, Z.; et al. Yield Gain Due to Fungicide Application in Varieties of Winter Wheat (Triticum Aestivum) Resistant and Susceptible to Leaf Rust. Crop Pasture Sci. 2015, 66, 649–659. [Google Scholar] [CrossRef]
- Madenova, A.; Sapakhova, Z.; Bakirov, S.; Galymbek, K.; Yernazarova, G.; Kokhmetova, A.; Keishilov, Z. Screening of Wheat Genotypes for the Presence of Common Bunt Resistance Genes. Saudi J. Biol. Sci. 2021, 28, 2816–2823. [Google Scholar] [CrossRef]
- Sun, L.; Lai, M.; Ghouri, F.; Nawaz, M.A.; Ali, F.; Baloch, F.S.; Nadeem, M.A.; Aasim, M.; Shahid, M.Q. Modern Plant Breeding Techniques in Crop Improvement and Genetic Diversity: From Molecular Markers and Gene Editing to Artificial Intelligence—A Critical Review. Plants 2024, 13, 2676. [Google Scholar] [CrossRef]
- Gao, M.; Hao, Z.; Ning, Y.; He, Z. Revisiting Growth–Defence Trade-Offs and Breeding Strategies in Crops. Plant Biotechnol. J. 2024, 22, 1198–1205. [Google Scholar] [CrossRef]
- Ahmad, S.; Wei, X.; Sheng, Z.; Hu, P.; Tang, S. CRISPR/Cas9 for Development of Disease Resistance in Plants: Recent Progress, Limitations and Future Prospects. Brief. Funct. Genom. 2018, 19, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kong, F.; Sun, K.; Wang, T.; Guo, T. From Classical Radiation to Modern Radiation: Past, Present, and Future of Radiation Mutation Breeding. Front. Public. Health 2021, 9, 768071. [Google Scholar] [CrossRef] [PubMed]
- Permyakova, N.V.; Deineko, E.V. Crop Improvement: Comparison of Transgenesis and Gene Editing. Horticulturae 2024, 10, 57. [Google Scholar] [CrossRef]
- Ahmad, S.; Rana, I.A.; Folta, K.M.; Lorenzo, C.D.; Khan, S.H. Editorial: Gene Editing to Achieve Zero Hunger. Front. Genome Ed. 2025, 7, 1632120. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; He, Y.; Li, J.; Xia, L. An Update on Precision Genome Editing by Homology-Directed Repair in Plants. Plant. Physiol. 2022, 188, 1780–1794. [Google Scholar] [CrossRef]
- Chaplin, A.K.; Hardwick, S.W.; Stavridi, A.K.; Buehl, C.J.; Goff, N.J.; Ropars, V.; Liang, S.; De Oliveira, T.M.; Chirgadze, D.Y.; Meek, K.; et al. Cryo-EM of NHEJ Supercomplexes Provides Insights into DNA Repair. Mol. Cell 2021, 81, 3400–3409.e3. [Google Scholar] [CrossRef]
- Fugger, K.; West, S.C. Keeping Homologous Recombination in Check. Cell Res. 2016, 26, 397–398. [Google Scholar] [CrossRef]
- Schiml, S.; Fauser, F.; Puchta, H. The CRISPR/Cas System Can Be Used as Nuclease for in Planta Gene Targeting and as Paired Nickases for Directed Mutagenesis in Arabidopsis Resulting in Heritable Progeny. Plant J. 2014, 80, 1139–1150. [Google Scholar] [CrossRef]
- Shintani, S.; Mihara, M.; Li, C.; Nakahara, Y.; Hino, S.; Nakashiro, K.I.; Hamakawa, H. Up-Regulation of DNA-Dependent Protein Kinase Correlates with Radiation Resistance in Oral Squamous Cell Carcinoma. Cancer Sci. 2003, 94, 894–900. [Google Scholar] [CrossRef]
- Srivastava, M.; Nambiar, M.; Sharma, S.; Karki, S.S.; Goldsmith, G.; Hegde, M.; Kumar, S.; Pandey, M.; Singh, R.K.; Ray, P.; et al. An Inhibitor of Nonhomologous End-Joining Abrogates Double-Strand Break Repair and Impedes Cancer Progression. Cell 2012, 151, 1474–1487. [Google Scholar] [CrossRef]
- Jayathilaka, K.; Sheridan, S.D.; Bold, T.D.; Bochenska, K.; Logan, H.L.; Weichselbaum, R.R.; Bishop, D.K.; Connell, P.P. A Chemical Compound That Stimulates the Human Homologous Recombination Protein RAD51. Proc. Natl. Acad. Sci. USA 2008, 105, 15848–15853. [Google Scholar] [CrossRef]
- Song, J.; Yang, D.; Xu, J.; Zhu, T.; Chen, Y.E.; Zhang, J. RS-1 Enhances CRISPR/Cas9- and TALEN-Mediated Knock-in Efficiency. Nat. Commun. 2016, 7, 10548. [Google Scholar] [CrossRef] [PubMed]
- Even-Faitelson, L.; Samach, A.; Melamed-Bessudo, C.; Avivi-Ragolsky, N.; Levy, A.A. Localized Egg-Cell Expression of Effector Proteins for Targeted Modification of the Arabidopsis Genome. Plant J. 2011, 68, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A.; Sretenovic, S.; Bansal, K.C.; Qi, Y. Precise Plant Genome Editing Using Base Editors and Prime Editors. Nat. Plants 2021, 7, 1166–1187. [Google Scholar] [CrossRef] [PubMed]
- Rozov, S.M.; Permyakova, N.V.; Deineko, E.V. The Problem of the Low Rates of CRISPR/Cas9-Mediated Knock-Ins in Plants: Approaches and Solutions. Int. J. Mol. Sci. 2019, 20, 3371. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Liu, D.R. Prime Editing for Precise and Highly Versatile Genome Manipulation. Nat. Rev. Genet. 2023, 24, 161–177. [Google Scholar] [CrossRef]
- He, Z.; Xie, Y.; Huang, H.; Zhang, Z.; Feng, S.; Xu, R.; Chen, X.; Gao, F.; Li, P.; Zhu, M.; et al. A Base Editor Facilitates Simultaneous Purine and Pyrimidine Substitutions for Ex Vivo And In Vivo Mutagenesis Screens. Technology 2025, 32, 1183–1196. [Google Scholar] [CrossRef]
- Peng, H.; Li, J.; Sun, K.; Tang, H.; Huang, W.; Li, X.; Wang, S.; Ding, K.; Han, Z.; Li, Z.; et al. Advances and Applications of Plant Base Editing Technologies. Int. J. Mol. Sci. 2025, 26, 9452. [Google Scholar] [CrossRef]
- Waites, J.; Achary, V.M.M.; Syombua, E.D.; Hearne, S.J.; Bandyopadhyay, A. CRISPR-Mediated Genome Editing of Wheat for Enhancing Disease Resistance. Front. Genome Ed. 2025, 7, 1542487. [Google Scholar] [CrossRef]
- Lau, C.-H.; Tin, C.; Suh, Y. CRISPR-Based Strategies for Targeted Transgene Knock-in and Gene Correction. Fac. Rev. 2020, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Sapakhova, Z.; Kanat, R.; Choi, K.; Daurov, D.; Daurova, A.; Zhambakin, K.; Shamekova, M. CRISPR-Cas Gene Editing Technology in Potato. Int. J. Mol. Sci. 2025, 26, 7496. [Google Scholar] [CrossRef]
- Rynjah, D.; Sandhanam, K.; Bhattacharjee, B.; Deka, B.; Newar, A.; Kalita, T.; Nath, J.; Ahmed, A.B.; Sahu, R.K.; Das, T. CRISPR/Cas9 Gene Editing Systems for Enhancing Secondary Metabolite Biosynthesis via Reproductive Tissue Modification. Discov. Plants 2025, 2, 245. [Google Scholar] [CrossRef]
- Kokhmetova, A.; Kremneva, O.; Volkova, G.; Atishova, M.; Sapakhova, Z. Evaluation of Wheat Cultivars Growing in Kazakhstan and Russia for Resistance to Tan Spot. J. Plant Pathol. 2017, 99, 161–167. [Google Scholar] [CrossRef]
- Kokhmetova, A.M.; Ali, S.; Sapakhova, Z.; Atishova, M.N. Identification of Genotypes-Carriers of Resistance to Tan Spot Ptr ToxA and Ptr ToxB of Pyrenophora Tritici-Repentis in Common Wheat Collection. Vavilovskii Zhurnal Genet. Sel. 2018, 22, 978–986. [Google Scholar] [CrossRef]
- Madenova, A.; Kokhmetova, A.; Sapakhova, Z.; Galymbek, K.; Keishilov, Z.; Akan, K.; Yesserkenov, A. Effect of Common Bunt [Tilletia Caries (DC) Tul] Infection on Agronomic Traits and Resistance of Wheat Entries. Res. Crops 2020, 21, 791–797. [Google Scholar] [CrossRef]
- Taj, M.; Sajjad, M.; Li, M.; Yasmeen, A.; Mubarik, M.S.; Kaniganti, S.; He, C. Potential Targets for CRISPR/Cas Knockdowns to Enhance Genetic Resistance Against Some Diseases in Wheat (Triticum aestivum L.). Front. Genet. 2022, 13, 926955. [Google Scholar] [CrossRef]
- Mu, K.; Ren, X.; Yang, H.; Zhang, T.; Yan, W.; Yuan, F.; Wu, J.; Kang, Z.; Han, D.; Deng, R.; et al. CRISPR-Cas12a-Based Diagnostics of Wheat Fungal Diseases. J. Agric. Food Chem. 2022, 70, 7240–7247. [Google Scholar] [CrossRef]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A Deletion Mutation in TaHRC Confers Fhb1 Resistance to Fusarium Head Blight in Wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Chen, H.; Su, Z.; Tian, B.; Liu, Y.; Pang, Y.; Kavetskyi, V.; Trick, H.N.; Bai, G. Development and Optimization of a Barley Stripe Mosaic Virus-Mediated Gene Editing System to Improve Fusarium Head Blight Resistance in Wheat. Plant Biotechnol. J. 2022, 20, 1018–1020. [Google Scholar] [CrossRef]
- Karmacharya, A.; Li, D.; Leng, Y.; Shi, G.; Liu, Z.; Yang, S.; Du, Y.; Dai, W.; Zhong, S. Targeting Disease Susceptibility Genes in Wheat Through Wide Hybridization with Maize Expressing Cas9 and Guide RNA. Mol. Plant-Microbe Interact. 2023, 36, 554–557. [Google Scholar] [CrossRef]
- Brauer, E.K.; Balcerzak, M.; Rocheleau, H.; Leung, W.; Schernthaner, J.; Subramaniam, R.; Ouellet, T. Genome Editing of a Deoxynivalenol-Induced Transcription Factor Confers Resistance to Fusarium graminearum in Wheat. Mol. Plant Microbe Interact. 2020, 33, 553–560. [Google Scholar] [CrossRef]
- Jones, D.A.B.; Rybak, K.; Hossain, M.; Bertazzoni, S.; Williams, A.; Tan, K.C.; Phan, H.T.T.; Hane, J.K. Repeat-Induced Point Mutations Driving Parastagonospora Nodorum Genomic Diversity Are Balanced by Selection against Non-Synonymous Mutations. Commun. Biol. 2024, 7, 1614. [Google Scholar] [CrossRef] [PubMed]
- Poddar, S.; Tanaka, J.; Running, K.L.D.; Kariyawasam, G.K.; Faris, J.D.; Friesen, T.L.; Cho, M.J.; Cate, J.H.D.; Staskawicz, B. Optimization of Highly Efficient Exogenous-DNA-Free Cas9-Ribonucleoprotein Mediated Gene Editing in Disease Susceptibility Loci in Wheat (Triticum aestivum L.). Front. Plant Sci. 2023, 13, 1084700. [Google Scholar] [CrossRef]
- Khan, H.; McDonald, M.C.; Williams, S.J.; Solomon, P.S. Assessing the Efficacy of CRISPR/Cas9 Genome Editing in the Wheat Pathogen Parastagonspora Nodorum. Fungal Biol. Biotechnol. 2020, 7, 4. [Google Scholar] [CrossRef]
- Zou, S.; Xu, Y.; Li, Q.; Wei, Y.; Zhang, Y.; Tang, D. Wheat Powdery Mildew Resistance: From Gene Identification to Immunity Deployment. Front. Plant Sci. 2023, 14, 1269498. [Google Scholar] [CrossRef]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-Edited Powdery Mildew Resistance in Wheat without Growth Penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous Modification of Three Homoeologs of TaEDR1 by Genome Editing Enhances Powdery Mildew Resistance in Wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous Editing of Three Homoeoalleles in Hexaploid Bread Wheat Confers Heritable Resistance to Powdery Mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Kokhmetova, A.; Rathan, N.D.; Sehgal, D.; Malysheva, A.; Kumarbayeva, M.; Nurzhuma, M.; Bolatbekova, A.; Krishnappa, G.; Gultyaeva, E.; Kokhmetova, A.; et al. QTL Mapping for Seedling and Adult Plant Resistance to Stripe and Leaf Rust in Two Winter Wheat Populations. Front. Genet. 2023, 14, 1265859. [Google Scholar] [CrossRef]
- Ochilov, B.O.; Turakulov, K.S.; Meliev, S.K.; Melikuziev, F.A.; Aytenov, I.S.; Murodova, S.M.; Khalillaeva, G.O.; Chinikulov, B.K.; Azimova, L.A.; Urinov, A.M.; et al. Development of Yellow Rust-Resistant and High-Yielding Bread Wheat (Triticum aestivum L.) Lines Using Marker-Assisted Backcrossing Strategies. Int. J. Mol. Sci. 2025, 26, 7603. [Google Scholar] [CrossRef]
- Malysheva, A.; Kokhmetova, A.; Urazaliev, R.; Kumarbayeva, M.; Keishilov, Z.; Nurzhuma, M.; Bolatbekova, A.; Kokhmetova, A. Phenotyping and Identification of Molecular Markers Associated with Leaf Rust Resistance in the Wheat Germplasm from Kazakhstan, CIMMYT and ICARDA. Plants 2023, 12, 2786. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, F.; Su, J.; Fang, A.; Tian, B.; Yu, Y.; Bi, C.; Ma, D.; Xiao, S.; Yang, Y. CRISPR-Targeted Mutagenesis of Mitogen-Activated Protein Kinase Phosphatase 1 Improves Both Immunity and Yield in Wheat. Plant Biotechnol. J. 2024, 22, 1929–1941. [Google Scholar] [CrossRef]
- Wang, N.; Tang, C.; Fan, X.; He, M.; Gan, P.; Zhang, S.; Hu, Z.; Wang, X.; Yan, T.; Shu, W.; et al. Inactivation of a Wheat Protein Kinase Gene Confers Broad-Spectrum Resistance to Rust Fungi. Cell 2022, 185, 2961–2974.e19. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Guo, M.; Pan, Y.; Hao, C.; Hou, J.; Yan, L.; Zhang, X.; Chen, X.; Li, T. Knockout of GRAIN WIDTH2 Has a Dual Effect on Enhancing Leaf Rust Resistance and Increasing Grain Weight in Wheat. Plant Biotechnol. J. 2024, 22, 2007–2009. [Google Scholar] [CrossRef]
- Conde, S.; Catarino, S.; Ferreira, S.; Temudo, M.P.; Monteiro, F. Rice Pests and Diseases Around the World: Literature-Based Assessment with Emphasis on Africa and Asia. Agriculture 2025, 15, 667. [Google Scholar] [CrossRef]
- Foster, A.J.; Martin-Urdiroz, M.; Yan, X.; Wright, S.; Soanes, D.M.; Talbot, N.J. CRISPR-Cas9 Ribonucleoprotein-Mediated Co-Editing and Counterselection in the Rice Blast Fungus. Sci. Rep. 2018, 8, 14355. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.G.; Zhao, K. Enhanced Rice Blast Resistance by CRISPR/ Cas9-Targeted Mutagenesis of the ERF Transcription Factor Gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, J.; Wang, M.; Ren, Y.; Wang, S.; Lei, C.; Cheng, Z. Sodmergen Disruption of OsSEC3A Increases the Content of Salicylic Acid and Induces Plant Defense Responses in Rice. J. Exp. Bot. 2018, 69, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Zhao, J.; Bhadauria, V.; Peng, Y.L. Efficient Gene Editing with an Arg-TRNA Promoter-Driven CRISPR/Cas9 in the Rice Blast Fungus Pyricularia Oryzae. Phytopathol. Res. 2024, 6, 56. [Google Scholar] [CrossRef]
- Wang, F.-Q.; Fan, F.-J.; Li, W.-Q.; Zhu, J.-Y.; Wang, J.; Zhong, W.-G.; Yang, J. Knock-out Efficiency Analysis of Pi21 Gene Using CRISPR/Cas9 in Rice. Chin. J. Off. Rice Sci. 2016, 30, 469–478. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Tu, R.; Liu, Q.; Wu, W.; Fu, X.; Cao, L.; Shen, X. Orientation improvement of blast resistance in rice via CRISPR/Cas9 system. Chin. J. Rice Sci. 2019, 33, 313–322. [Google Scholar] [CrossRef]
- Li, S.; Shen, L.; Hu, P.; Liu, Q.; Zhu, X.; Qian, Q.; Wang, K.; Wang, Y. Developing Disease-Resistant Thermosensitive Male Sterile Rice by Multiplex Gene Editing. J. Integr. Plant Biol. 2019, 61, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fang, Y.; Wu, H.; Zhao, N.; Guo, X.; Mackon, E.; Peng, H.; Huang, S.; He, Y.; Qin, B.; et al. Improvement of Resistance to Rice Blast and Bacterial Leaf Streak by CRISPR/Cas9-Mediated Mutagenesis of Pi21 and OsSULTR3;6 in Rice (Oryza sativa L.). Front. Plant Sci. 2023, 14, 1209384. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, S.; Jiang, N.; Zhao, X.; Bai, Z.; Liu, J.; Yao, W.; Tang, Q.; Xiao, G.; Lv, C.; et al. Engineering of Rice Varieties with Enhanced Resistances to Both Blast and Bacterial Blight Diseases via CRISPR/Cas9. Plant Biotechnol. J. 2022, 20, 876–885. [Google Scholar] [CrossRef]
- Peng, Q.; Li, J.; Xu, H.; Zhang, X.; Zhang, D.; Zhu, S. Editing Pi21 Gene to Improve the Blast Resistance of Cultivated Rice dalixiang by Using CRISPR/Cas9 System. Mol. Plant Breed. 2022. Available online: https://kns.cnki.net/kcms/detail/46.1068.S.20220221.1756.019.html (accessed on 15 September 2025).
- Wu, X.; Li, J.-L.; Ceng, Q.-H.; Zhang, D.-S.; Xu, H.-F.; Jiang, X.; Song, L.; Peng, Q.; Zhu, S.-S. Improvement of Blast Resistance of Rice Variety Dalixiang by CRISPR/Cas9 Gene Editing Technology. Seed 2021, 40, 50–55. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, J.; Chern, M.; Zhu, X.; Yang, C.; He, K.; Liu, Y.; He, M.; Wang, J.; Song, L.; et al. New Insights into Bsr-D1-Mediated Broad-Spectrum Resistance to Rice Blast. Mol. Plant Pathol. 2020, 21, 951–960. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Xie, K. CRISPR/DCas9-Mediated Gene Silencing in Two Plant Fungal Pathogens. mSphere 2023, 8, e0059422. [Google Scholar] [CrossRef]
- Ren, B.; Yan, F.; Kuang, Y.; Li, N.; Zhang, D.; Zhou, X.; Lin, H.; Zhou, H. Improved Base Editor for Efficiently Inducing Genetic Variations in Rice with CRISPR/Cas9-Guided Hyperactive HAID Mutant. Mol. Plant 2018, 11, 623–626. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.; Solís, I.; Igartua, E. Barley History and Breeding in Spain. Agriculture 2024, 14, 1674. [Google Scholar] [CrossRef]
- Çelik Oğuz, A.; Karakaya, A. Genetic Diversity of Barley Foliar Fungal Pathogens. Agronomy 2021, 11, 434. [Google Scholar] [CrossRef]
- Poursafar, A.; Leng, Y.; Zhong, S. Development of a CRISPR/Cas9-Mediated Gene Knockout Method for Functional Genomics of the Barley Spot Blotch Pathogen Bipolaris Sorokiniana. PhytoFrontiersTM 2024, 4, 682–689. [Google Scholar] [CrossRef]
- Ge, C.; Moolhuijzen, P.; Hickey, L.; Wentzel, E.; Deng, W.; Dinglasan, E.G.; Ellwood, S.R. Physiological Changes in Barley Mlo-11 Powdery Mildew Resistance Conditioned by Tandem Repeat Copy Number. Int. J. Mol. Sci. 2020, 21, 8769. [Google Scholar] [CrossRef]
- Mishra, R.; Tripathi, M.K.; Sikarwar, R.S.; Singh, Y.; Tripathi, N. Soybean (Glycine max L. Merrill): A Multipurpose Legume Shaping Our World. Plant Cell Biotechnol. Mol. Biol. 2024, 25, 17–37. [Google Scholar] [CrossRef]
- McCaghey, M.; Willbur, J.; Smith, D.L.; Kabbage, M. The Complexity of the Sclerotinia Sclerotiorum Pathosystem in Soybean: Virulence Factors, Resistance Mechanisms, and Their Exploitation to Control Sclerotinia Stem Rot. Trop. Plant Pathol. 2019, 44, 12–22. [Google Scholar] [CrossRef]
- Bui, T.P.; Le, H.; Ta, D.T.; Nguyen, C.X.; Le, N.T.; Tran, T.T.; Van Nguyen, P.; Stacey, G.; Stacey, M.G.; Pham, N.B.; et al. Enhancing Powdery Mildew Resistance in Soybean by Targeted Mutation of MLO Genes Using the CRISPR/Cas9 System. BMC Plant Biol. 2023, 23, 533. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Kale, S.D.; Liu, T.; Tang, Q.; Wang, X.; Arredondo, F.D.; Basnayake, S.; Whisson, S.; Drenth, A.; Maclean, D.; et al. Different Domains of Phytophthora Sojae Effector Avr4/6 Are Recognized by Soybean Resistance Genes Rps4 and Rps6. Mol. Plant-Microbe Interact. 2010, 23, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Tyler, B.M. Efficient Disruption and Replacement of an Effector Gene in the Oomycete Phytophthora Sojae Using CRISPR/Cas9. Mol. Plant Pathol. 2016, 17, 127–139. [Google Scholar] [CrossRef]
- Pavan, S.; Jacobsen, E.; Visser, R.G.F.; Bai, Y. Loss of Susceptibility as a Novel Breeding Strategy for Durable and Broad-Spectrum Resistance. Mol. Breed. 2010, 25, 1–12. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Bü, R.; Hollricher, K. The Barley Mlo Gene: A Novel Control Element of Plant Pathogen Resistance. Cell 1997, 88, 695–705. [Google Scholar] [CrossRef]
- Acevedo-Garcia, J.; Kusch, S.; Panstruga, R. Magical Mystery Tour: MLO Proteins in Plant Immunity and Beyond. J. Physiol. 2014, 204, 273–281. [Google Scholar] [CrossRef]
- Nekrasov, V.; Wang, C.; Win, J.; Lanz, C.; Weigel, D.; Kamoun, S. Rapid Generation of a Transgene-Free Powdery Mildew Resistant Tomato by Genome Deletion. Sci. Rep. 2017, 7, 482. [Google Scholar] [CrossRef]
- Pramanik, D.; Shelake, R.M.; Park, J.; Kim, M.J.; Hwang, I.; Park, Y.; Kim, J.Y. CRISPR/Cas9-Mediated Generation of Pathogen-Resistant Tomato against Tomato Yellow Leaf Curl Virus and Powdery Mildew. Int. J. Mol. Sci. 2021, 22, 1878. [Google Scholar] [CrossRef]
- Santillán Martínez, M.I.; Bracuto, V.; Koseoglou, E.; Appiano, M.; Jacobsen, E.; Visser, R.G.F.; Wolters, A.M.A.; Bai, Y. CRISPR/Cas9-Targeted Mutagenesis of the Tomato Susceptibility Gene PMR4 for Resistance against Powdery Mildew. BMC Plant Biol. 2020, 20, 284. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cui, L.; Martina, M.; Bracuto, V.; Meijer-Dekens, F.; Wolters, A.M.A.; Moglia, A.; Bai, Y.; Acquadro, A. Less Is More: CRISPR/Cas9-Based Mutations in DND1 Gene Enhance Tomato Resistance to Powdery Mildew with Low Fitness Costs. BMC Plant Biol. 2024, 24, 763. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Maioli, A.; Yan, Z.; Bai, Y.; Valentino, D.; Milani, A.M.; Pompili, V.; Comino, C.; Lanteri, S.; Moglia, A.; et al. CRISPR/Cas9-Based Knock-Out of the PMR4 Gene Reduces Susceptibility to Late Blight in Two Tomato Cultivars. Int. J. Mol. Sci. 2022, 23, 14542. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Meng, J.; He, X.; Zhang, Y.; Liu, Y.; Zhang, C.; Qi, H.; Luan, Y. Editing Mir482b and Mir482c Simultaneously by Crispr/Cas9 Enhanced Tomato Resistance to Phytophthora Infestans. Phytopathology 2021, 111, 1008–1016. [Google Scholar] [CrossRef]
- Paula de Toledo Thomazella, D.; Seong, K.; Mackelprang, R.; Dahlbeck, D.; Geng, Y.; Gill, U.S.; Qi, T.; Pham, J.; Giuseppe, P.; Lee, C.Y.; et al. Loss of Function of a DMR6 Ortholog in Tomato Confers Broad-Spectrum Disease Resistance. Proc. Natl. Acad. Sci. USA 2021, 118, e2026152118. [Google Scholar] [CrossRef]
- Debbarma, J.; Saikia, B.; Singha, D.L.; Das, D.; Keot, A.K.; Maharana, J.; Velmurugan, N.; Arunkumar, K.P.; Reddy, P.S.; Chikkaputtaiah, C. CRISPR/Cas9-Mediated Mutation in XSP10 and SlSAMT Genes Impart Genetic Tolerance to Fusarium Wilt Disease of Tomato (Solanum lycopersicum L.). Genes 2023, 14, 488. [Google Scholar] [CrossRef]
- Ramirez Gaona, M.; van Tuinen, A.; Schipper, D.; Kano, A.; Wolters, P.J.; Visser, R.G.F.; van Kan, J.A.L.; Wolters, A.A.; Bai, Y. Mutation of PUB17 in Tomato Leads to Reduced Susceptibility to Necrotrophic Fungi. Plant Biotechnol. J. 2023, 21, 2157–2159. [Google Scholar] [CrossRef] [PubMed]
- Perk, E.A.; D’Ambrosio, J.M.; Cerrudo, I.; Robuschi, L.; Juárez, M.; Vélez, P.; Mary, V.; Theumer, M.; Segretin, M.E.; Laxalt, A.M. Phosphoinositide-Specific Phospholipase C 2 (SlPLC2) Facilitates Vesicle Formation and Modulates Immune Signaling in Tomato Phytophthora Infestans Interactions. bioRxiv 2025. [Google Scholar] [CrossRef]
- Ortega-Salazar, I.; Crum, D.; Sbodio, A.O.; Sugiyama, Y.; Adaskaveg, A.; Wang, D.; Seymour, G.B.; Li, X.; Wang, S.C.; Blanco-Ulate, B. Double CRISPR Knockout of Pectin Degrading Enzymes Improves Tomato Shelf-Life While Ensuring Fruit Quality. Plants People Planet. 2024, 6, 330–340. [Google Scholar] [CrossRef]
- Hanika, K.; Schipper, D.; Chinnappa, S.; Oortwijn, M.; Schouten, H.J.; Thomma, B.P.H.J.; Bai, Y. Impairment of Tomato WAT1 Enhances Resistance to Vascular Wilt Fungi Despite Severe Growth Defects. Front. Plant Sci. 2021, 12, 721674. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H. Harnessing CRISPR/Cas9 for Enhanced Disease Resistance in Hot Peppers: A Comparative Study on CaMLO2-Gene-Editing Efficiency across Six Cultivars. Int. J. Mol. Sci. 2023, 24, 16775. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J.; Won, K.H. A Stable DNA-Free Screening System for CRISPR/RNPs-Mediated Gene Editing in Hot and Sweet Cultivars of Capsicum Annuum. BMC Plant Biol. 2020, 20, 449. [Google Scholar] [CrossRef]
- Mishra, R.; Mohanty, J.N.; Mahanty, B.; Joshi, R.K. A Single Transcript CRISPR/Cas9 Mediated Mutagenesis of CaERF28 Confers Anthracnose Resistance in Chilli Pepper (Capsicum annuum L.). Planta 2021, 254, 5. [Google Scholar] [CrossRef]
- Ferrero, M.; Valentino, D.; Milani, A.M.; Comino, C.; Lanteri, S.; Acquadro, A.; Moglia, A. Enhancing Tolerance to Phytophthora Spp. in Eggplant through DMR6–1 CRISPR/Cas9 Knockout. Plant Stress 2024, 14, 100691. [Google Scholar] [CrossRef]
- Vogel, J.; Somerville, S. Isolation and Characterization of Powdery Mildew-Resistant Arabidopsis Mutants. Proc. Natl. Acad. Sci. USA 1999, 97, 1897–1902. [Google Scholar] [CrossRef]
- Sun, K.; Wolters, A.M.A.; Loonen, A.E.H.M.; Huibers, R.P.; van der Vlugt, R.; Goverse, A.; Jacobsen, E.; Visser, R.G.F.; Bai, Y. Down-Regulation of Arabidopsis DND1 Orthologs in Potato and Tomato Leads to Broad-Spectrum Resistance to Late Blight and Powdery Mildew. Transgenic Res. 2016, 25, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.H.; Fan, L.; Liu, Y.; Xu, H.; Llewellyn, D.; Wilson, I. MiR482 Regulation of NBS-LRR Defense Genes during Fungal Pathogen Infection in Cotton. PLoS ONE 2013, 8, e84390. [Google Scholar] [CrossRef]
- McHale, L.; Tan, X.; Koehl, P.; Michelmore, R.W. Plant NBS-LRR Proteins: Adaptable Guards. Genome Biol. 2006, 7, 212. [Google Scholar] [CrossRef]
- Zeilmaker, T.; Ludwig, N.R.; Elberse, J.; Seidl, M.F.; Berke, L.; Van Doorn, A.; Schuurink, R.C.; Snel, B.; Van Den Ackerveken, G. Downy Mildew Resistant 6 and DMR6-like Oxygenase 1 Are Partially Redundant but Distinct Suppressors of Immunity in Arabidopsis. Plant J. 2015, 81, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Krasikov, V.; Dekker, H.L.; Rep, M.; Takken, F.L.W. The Tomato Xylem Sap Protein XSP10 Is Required for Full Susceptibility to Fusarium Wilt Disease. J. Exp. Bot. 2011, 62, 963–973. [Google Scholar] [CrossRef]
- Koo, Y.J.; Kim, M.A.; Kim, E.H.; Song, J.T.; Jung, C.; Moon, J.-K.; Kim, J.-H.; Seo, H.S.; Song, S.I.; Kim, J.-K.; et al. Overexpression of Salicylic Acid Carboxyl Methyltransferase Reduces Salicylic Acid-Mediated Pathogen Resistance in Arabidopsis thaliana. Plant Mol. Biol. 2007, 64, 1–15. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium Oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R. A Review of Fusarium Oxysporum on Its Plant Interaction and Industrial Use. J. Med. Plants Stud. 2018, 6, 112–115. [Google Scholar] [CrossRef]
- Ramírez Gaona, M.; van Tuinen, A.; Schipper, D.; Ramos Peregrina, Á.; Visser, R.G.F.; van Kan, J.A.L.; Bai, Y.; Wolters, A.-M.A. Mutation of PUB21 in Tomato Leads to Reduced Susceptibility to Necrotrophic Fungi. BMC Plant Biol. 2025, 25, 1038. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.J.; Van Den Abeele, C.; Ortega-Salazar, I.; Papin, V.; Adaskaveg, J.A.; Wang, D.; Casteel, C.L.; Seymour, G.B.; Blanco-Ulate, B. Host Susceptibility Factors Render Ripe Tomato Fruit Vulnerable to Fungal Disease despite Active Immune Responses. J. Exp. Bot. 2021, 72, 2696–2709. [Google Scholar] [CrossRef]
- Denancé, N.; Ranocha, P.; Oria, N.; Barlet, X.; Rivière, M.P.; Yadeta, K.A.; Hoffmann, L.; Perreau, F.; Clément, G.; Maia-Grondard, A.; et al. Arabidopsis Wat1 (Walls Are Thin1)-Mediated Resistance to the Bacterial Vascular Pathogen, Ralstonia Solanacearum, Is Accompanied by Cross-Regulation of Salicylic Acid and Tryptophan Metabolism. Plant J. 2013, 73, 225–239. [Google Scholar] [CrossRef]
- Ranocha, P.; Dima, O.; Nagy, R.; Felten, J.; Corratge-Faillie, C.; Novak, O.; Morreel, K.; Lacombe, B.; Martinez, Y.; Pfrunder, S.; et al. Arabidopsis WAT1 Is a Vacuolar Auxin Transport Facilitator Required for Auxin Homoeostasis. Nat. Commun. 2013, 4, 2625. [Google Scholar] [CrossRef]
- Cui, L.; van den Munckhof, M.C.; Bai, Y.; Voorrips, R.E. Resistance to Anthracnose Rot Disease in Capsicum. Agronomy 2023, 13, 1434. [Google Scholar] [CrossRef]
- Sanati, S.; Razavi, B.M.; Hosseinzadeh, H. A Review of the Effects of Capsicum Annuum L. And Its Constituent, Capsaicin, in Metabolic Syndrome. Iran. J. Basic. Med. Sci. 2018, 21, 439–448. [Google Scholar] [CrossRef]
- Mieslerová, B.; Cook, R.T.A.; Wheater, C.P.; Lebeda, A. Ecology of Powdery Mildews—Influence of Abiotic Factors on their Development and Epidemiology. Crit. Rev. Plant Sci. 2022, 41, 365–390. [Google Scholar] [CrossRef]
- Mishra, R.; Rout, E.; Joshi, R.K. Identification of Resistant Sources Against Anthracnose Disease Caused by Colletotrichum truncatum and Colletotrichum gloeosporioides in Capsicum annuum L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 517–524. [Google Scholar] [CrossRef]
- Oo, M.M.; Oh, S.-K. Chilli Anthracnose (Colletotrichum Spp.) Disease and Its Management Approach. Korean J. Agric. Sci. 2016, 43, 153–162. [Google Scholar] [CrossRef]
- Taher, D.; Solberg, S.; Prohens, J.; Chou, Y.Y.; Rakha, M.; Wu, T.H. World Vegetable Center Eggplant Collection: Origin, Composition, Seed Dissemination and Utilization in Breeding. Front. Plant Sci. 2017, 8, 1484. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, F.; Zhang, J.; Liu, H.; Rahman, S.; Islam, S.; Ma, W.; She, M. Application of Crispr/Cas9 in Crop Quality Improvement. Int. J. Mol. Sci. 2021, 22, 4206. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Tang, L.; Shahzad, R.; Mawia, A.M.; Rao, G.S.; Jamil, S.; Wei, C.; Sheng, Z.; Shao, G.; Wei, X.; et al. CRISPR-Based Crop Improvements: A Way Forward to Achieve Zero Hunger. J. Agric. Food Chem. 2021, 69, 8307–8323. [Google Scholar] [CrossRef]
- Mandal, S.; Ghorai, M.; Anand, U.; Roy, D.; Kant, N.; Mishra, T.; Mane, A.B.; Jha, N.K.; Lal, M.K.; Tiwari, R.K.; et al. Cytokinins: A Genetic Target for Increasing Yield Potential in the CRISPR Era. Front. Genet. 2022, 13, 883930. [Google Scholar] [CrossRef]
- Des Marais, D.L.; Juenger, T.E. Pleiotropy, Plasticity, and the Evolution of Plant Abiotic Stress Tolerance. Ann. N. Y. Acad. Sci. 2010, 1206, 56–79. [Google Scholar] [CrossRef]
- Ferriera Neres, D.; Wright, R.C. Pleiotropy, a Feature or a Bug? Toward Co-Ordinating Plant Growth, Development, and Environmental Responses through Engineering Plant Hormone Signaling. Curr. Opin. Biotechnol. 2024, 88, 103151. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; He, F.; Akhunova, A.; Chao, S.; Trick, H.; Akhunov, E. Transgenerational CRISPR-Cas9 Activity Facilitates Multiplex Gene Editing in Allopolyploid Wheat. Cris. J. 2018, 1, 65–74. [Google Scholar] [CrossRef]
- Lu, H.P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.Y.; Gatehouse, A.M.R.; Lou, Y.G.; et al. Resistance of Rice to Insect Pests Mediated by Suppression of Serotonin Biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef]
- Borrelli, V.M.G.; Brambilla, V.; Rogowsky, P.; Marocco, A.; Lanubile, A. The Enhancement of Plant Disease Resistance Using Crispr/Cas9 Technology. Front. Plant Sci. 2018, 9, 1245. [Google Scholar] [CrossRef]
- Schenke, D.; Cai, D. Applications of CRISPR/Cas to Improve Crop Disease Resistance: Beyond Inactivation of Susceptibility Factors. iScience 2020, 23, 101478. [Google Scholar] [CrossRef] [PubMed]
- Piergentili, R.; Del Rio, A.; Signore, F.; Umani Ronchi, F.; Marinelli, E.; Zaami, S. Crispr-Cas and Its Wide-Ranging Applications: From Human Genome Editing to Environmental Implications, Technical Limitations, Hazards and Bioethical Issues. Cells 2021, 10, 969. [Google Scholar] [CrossRef] [PubMed]
- Verdezoto-Prado, J.; Chicaiza-Ortiz, C.; Mejía-Pérez, A.B.; Freire-Torres, C.; Viteri-Yánez, M.; Deng, L.; Barba-Ostria, C.; Guamán, L.P. Advances in Environmental Biotechnology with CRISPR/Cas9: Bibliometric Review and Cutting-Edge Applications. Discov. Appl. Sci. 2025, 7, 167. [Google Scholar] [CrossRef]
- Phelps, M.P.; Seeb, L.W.; Seeb, J.E. Transforming Ecology and Conservation Biology through Genome Editing. Conserv. Biol. 2020, 34, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Phelps, A.; Godbehere, A.; Evans, B.; Takizawa, C.; Chinen, G.; Singh, H.; Fang, Z.; Du, Z.Y. Revolutionizing Agriculture with CRISPR Technology: Applications, Challenges, and Future Perspectives. Biotechnol. J. 2025, 20, e70113. [Google Scholar] [CrossRef]
- Saini, H.; Thakur, R.; Gill, R.; Tyagi, K.; Goswami, M. CRISPR/Cas9-Gene Editing Approaches in Plant Breeding. GM Crops Food 2023, 14, 1–17. [Google Scholar] [CrossRef]
- Kusch, S.; Panstruga, R. Mlo-Based Resistance: An Apparently Universal “Weapon” to Defeat Powdery Mildew Disease. Mol. Plant-Microbe Interact. 2017, 30, 179–189. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, H.; Cui, Y.; Cong, L.; Zhang, D. Application of Different Types of CRISPR/Cas-Based Systems in Bacteria. Microb. Cell Fact. 2020, 19, 172. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, Classification and Evolution of CRISPR-Cas Systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, L.; Zhang, Y.; Yan, T.; Kong, G.; Kong, L.; Guo, B.; Qiu, M.; Wang, Y.; Jing, M.; et al. An Oomycete Plant Pathogen Reprograms Host Pre-MRNA Splicing to Subvert Immunity. Nat. Commun. 2017, 8, 2051. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhu, T.; Tian, Z.; Li, C.; Zhang, W.; Song, R. High-Efficiency CRISPR/Cas9 Multiplex Gene Editing Using the Glycine TRNA-Processing System-Based Strategy in Maize. BMC Biotechnol. 2016, 16, 58. [Google Scholar] [CrossRef]
- Xie, K.; Minkenberg, B.; Yang, Y. Boosting CRISPR/Cas9 Multiplex Editing Capability with the Endogenous TRNA-Processing System. Proc. Natl. Acad. Sci. USA 2015, 112, 3570–3575. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Xu, J.; Wang, N. Development of Multiplex Genome Editing Toolkits for Citrus with High Efficacy in Biallelic and Homozygous Mutations. Plant Mol. Biol. 2020, 104, 297–307. [Google Scholar] [CrossRef]
- Sagawa, C.H.D.; Thomson, G.; Mermaz, B.; Vernon, C.; Liu, S.; Jacob, Y.; Irish, V.F. An Efficient Multiplex Approach to CRISPR/Cas9 Gene Editing in Citrus. Plant Methods 2024, 20, 148. [Google Scholar] [CrossRef]
- Rasul, I.; Zafar, F.; Ali, M.A.; Nadeem, H.; Siddique, M.H.; Shahid, M.; Ashfaq, U.A.; Azeem, F. Genetic Basis for Biotic Stress Resistance in Plants from Solanaceae Family: A Review. Int. J. Agric. Biol. 2019, 22, 178–194. [Google Scholar]
- Pottinger, S.E.; Innes, R.W. RPS5-Mediated Disease Resistance: Fundamental Insights and Translational Applications. Annu. Rev. Phytopathol. 2020, 58, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hua, L.; Gupta, A.; Tricoli, D.; Edwards, K.J.; Yang, B.; Li, W. Development of an Agrobacterium-Delivered CRISPR/Cas9 System for Wheat Genome Editing. Plant Biotechnol. J. 2019, 17, 1623–1635. [Google Scholar] [CrossRef]
- Gan, W.C.; Ling, A.P.K. CRISPR/Cas9 in Plant Biotechnology: Applications and Challenges. Biotechnologia 2022, 103, 81–93. [Google Scholar] [CrossRef]
- European Commission. Study on the Status of New Genomic Techniques Under Union Law and in Light of the Court of Justice Ruling in Case C-528/16. SWD(2021) 92 Final. 2021. Available online: https://food.ec.europa.eu/system/files/2021-04/gmo_mod-bio_ngt_eu-study.pdf (accessed on 4 July 2025).
- Molitorisová, A.; Clemens, S.; Fresco, L.; Hubar-Kołodziejczyk, A.; Tosun, J.; Niggli, U.; Qaim, M.; Visser, R.G.F.; Weber, A.P.M.; Wesseler, J.; et al. New Genomic Techniques in Organic Production: Considerations for Science-Based, Effective, and Acceptable EU Regulation. Cell Rep. Sustain. 2025, 2, 100405. [Google Scholar] [CrossRef]
- Puchta, H. Regulation of Gene-Edited Plants in Europe: From the Valley of Tears into the Shining Sun? aBIOTECH 2024, 5, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hussain, A.; Manghwar, H.; Xie, K.; Xie, S.; Zhao, S.; Larkin, R.M.; Qing, P.; Jin, S.; Ding, F. Genome Editing with the CRISPR-Cas System: An Art, Ethics and Global Regulatory Perspective. Plant Biotechnol. J. 2020, 18, 1651–1669. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nature 2016, 532, 293. [Google Scholar] [CrossRef]
- Lema, M.A. Regulatory Aspects of Gene Editing in Argentina. Transgenic Res. 2019, 28, 147–150. [Google Scholar] [CrossRef]
- da Cunha, N.B.; Silva Junior, J.J.d.; Araújo, A.M.M.; de Souza, L.R.; Leite, M.L.; Medina, G.d.S.; Rodriguez, G.R.; dos Anjos, R.M.; Rodrigues, J.C.M.; Costa, F.F.; et al. Updates on the Regulatory Framework of Edited Organisms in Brazil: A Molecular Rev-olution in Brazilian Agribusiness. Genes 2025, 16, 553. [Google Scholar] [CrossRef]
- Sánchez, M.A. The Global Advance of Genome-Edited Plants to the Market: The Key Role of Chile in Its Development. Plants 2024, 13, 3597. [Google Scholar] [CrossRef]
- Gatica-Arias, A. The regulatory current status of plant breeding technologies in some Latin American and the Caribbean countries. Plant Cell Tissue Organ Cult. 2020, 141, 229–242. [Google Scholar] [CrossRef]
- Mou, T.; Song, Q.; Liu, Y.; Song, J. Initiating the commercialization of genetically modified staple crops in China: Domestic bi-otechnological advancements, regulatory milestones, and governance frameworks. GM Crops Food 2025, 16, 450–481. [Google Scholar] [CrossRef] [PubMed]
- USDA. Biotechnology and Other New Production Technologies, Biotechnology and Other New Production Technologies Addendum, Bio-Technology-Plants and Animals, Cloning; USDA: Washington, DC, USA, 2025. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapakhova, Z.; Kanat, R.; Daurov, D.; Daurova, A.; Shamekova, M.; Zhambakin, K. The Enhancement of Fungal Disease Resistance in Major Staple Crops Using CRISPR-Cas Technology. Genes 2025, 16, 1263. https://doi.org/10.3390/genes16111263
Sapakhova Z, Kanat R, Daurov D, Daurova A, Shamekova M, Zhambakin K. The Enhancement of Fungal Disease Resistance in Major Staple Crops Using CRISPR-Cas Technology. Genes. 2025; 16(11):1263. https://doi.org/10.3390/genes16111263
Chicago/Turabian StyleSapakhova, Zagipa, Rakhim Kanat, Dias Daurov, Ainash Daurova, Malika Shamekova, and Kabyl Zhambakin. 2025. "The Enhancement of Fungal Disease Resistance in Major Staple Crops Using CRISPR-Cas Technology" Genes 16, no. 11: 1263. https://doi.org/10.3390/genes16111263
APA StyleSapakhova, Z., Kanat, R., Daurov, D., Daurova, A., Shamekova, M., & Zhambakin, K. (2025). The Enhancement of Fungal Disease Resistance in Major Staple Crops Using CRISPR-Cas Technology. Genes, 16(11), 1263. https://doi.org/10.3390/genes16111263






