Computational Identification of Genetic Background of Infertility and Calculating Inbreeding Coefficient in Dromedary Camel Herds
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Phenotype
2.2. Sample Collection and DNA Isolation
2.3. SNP Genotyping and Quality Control
2.4. Population Structure Analysis
2.5. Relatedness and Inbreeding Assessment
2.6. Genetic Diversity Metrics
2.7. Genome-Wide Association Analysis
2.8. Statistical Power and Limitations
3. Results
3.1. Inbreeding and Genetic Diversity
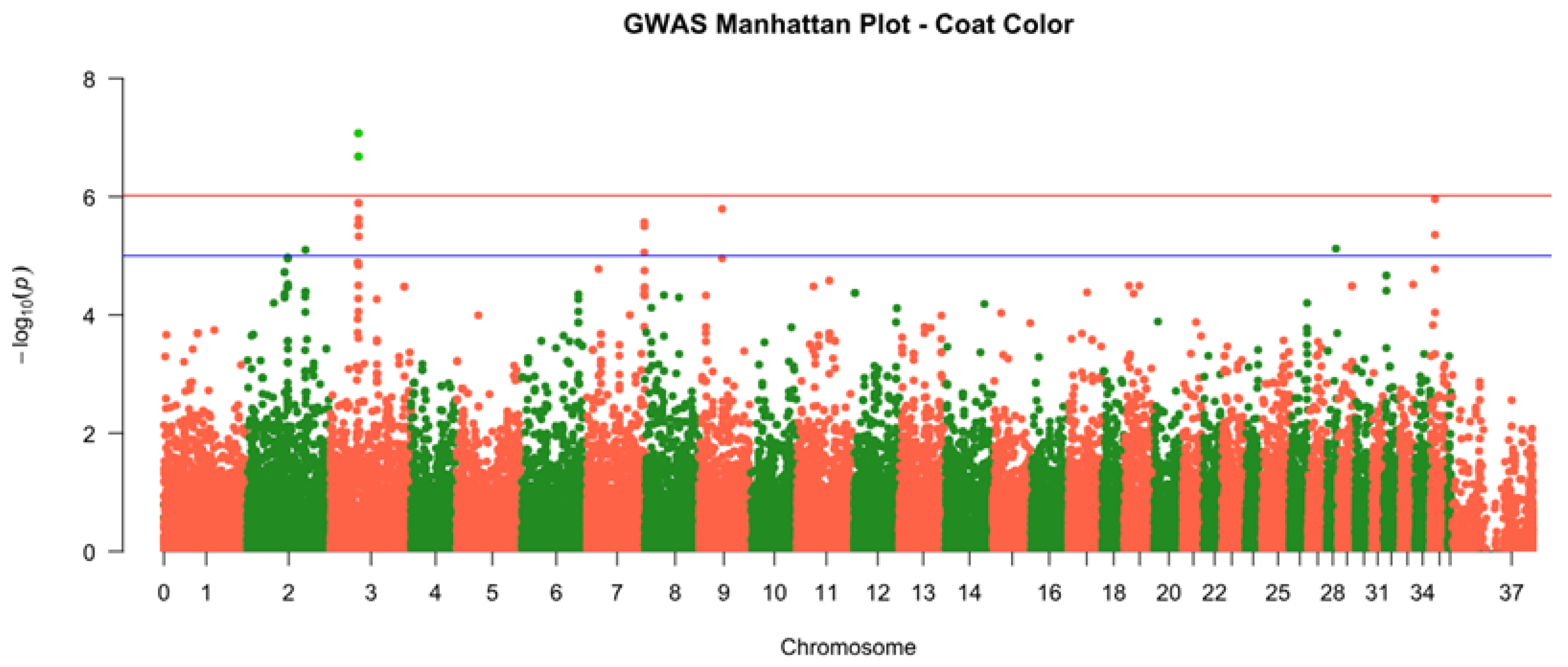
3.2. Association Testing
3.3. Trait-Specific Analyses
3.4. Differentiation Between Groups
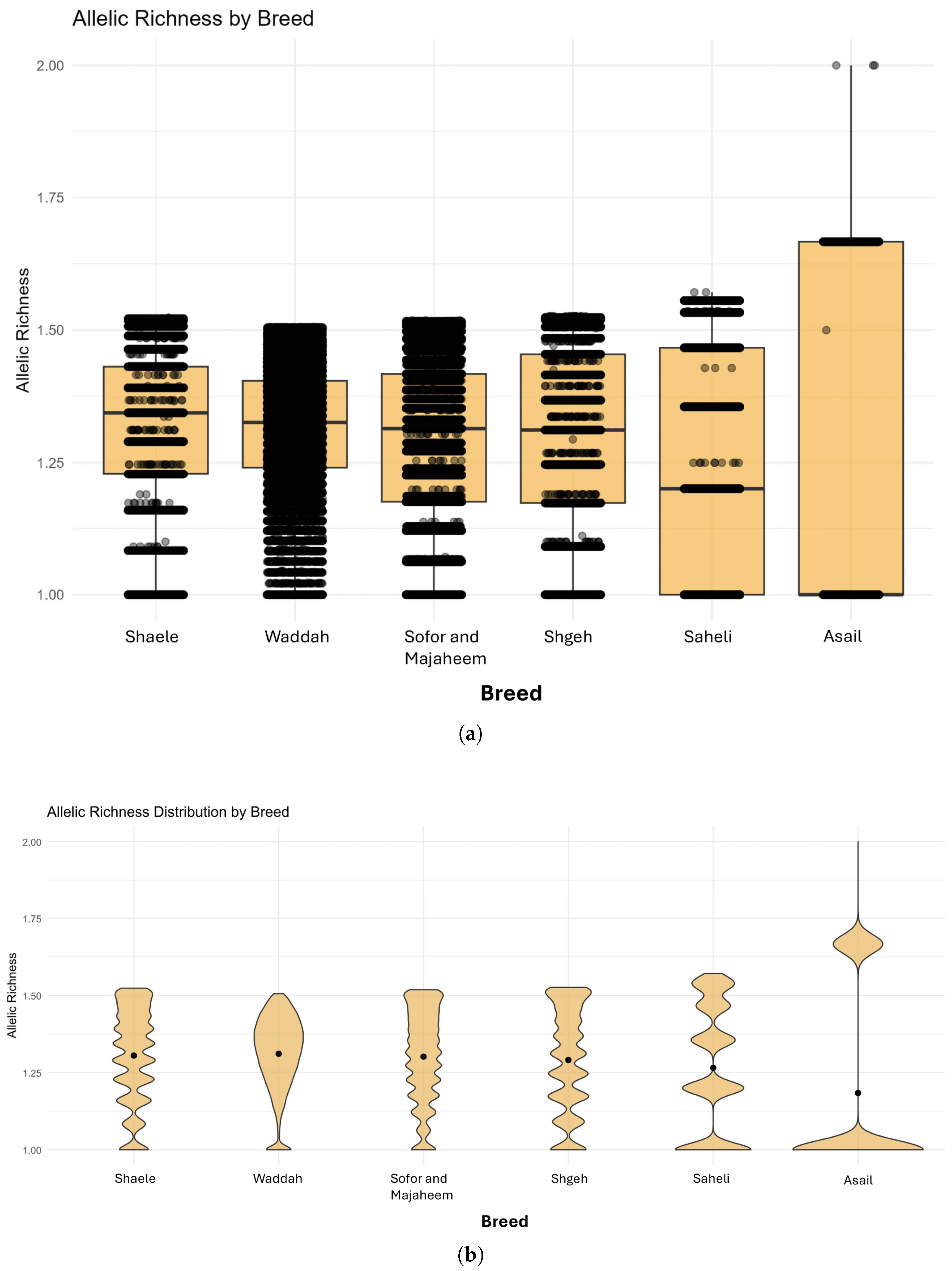
3.5. Allelic Richness by Breed
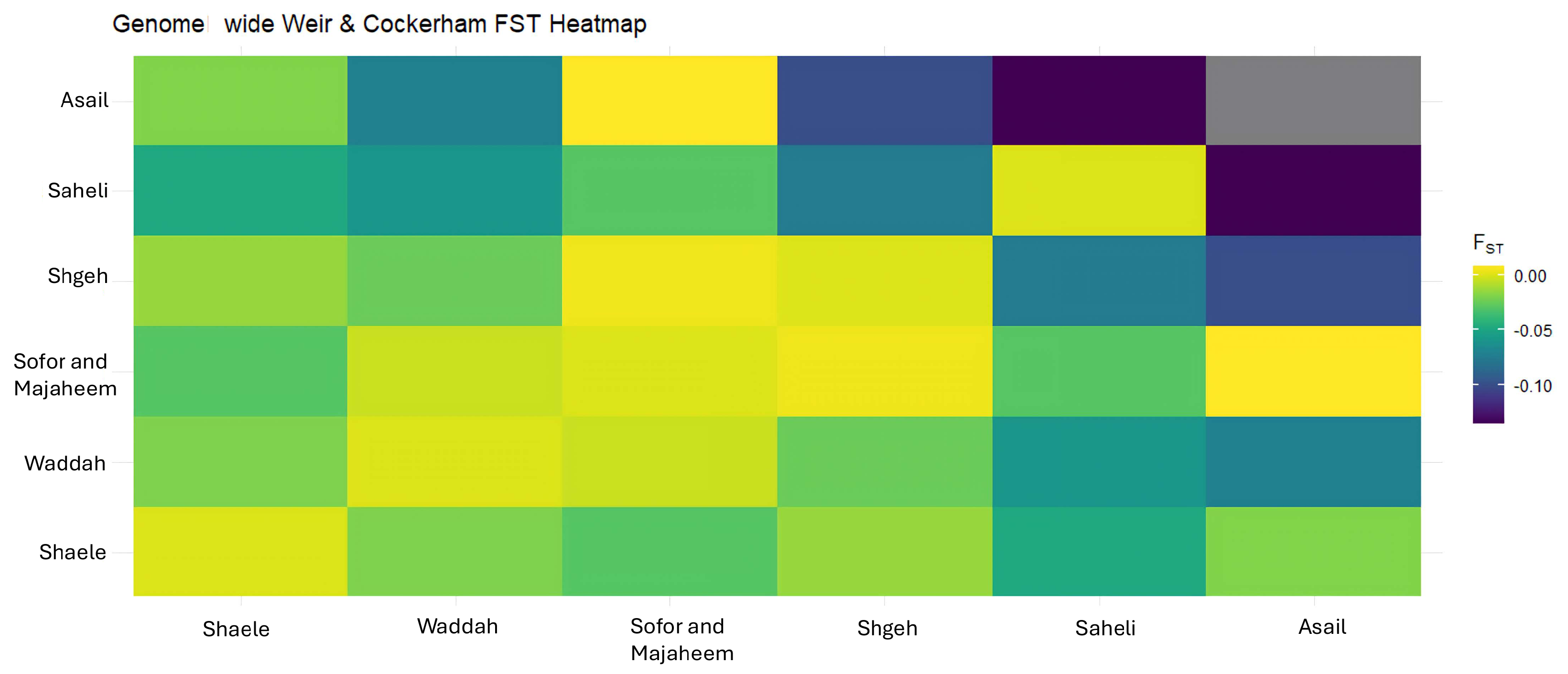
3.6. Genetic Differentiation (FST)
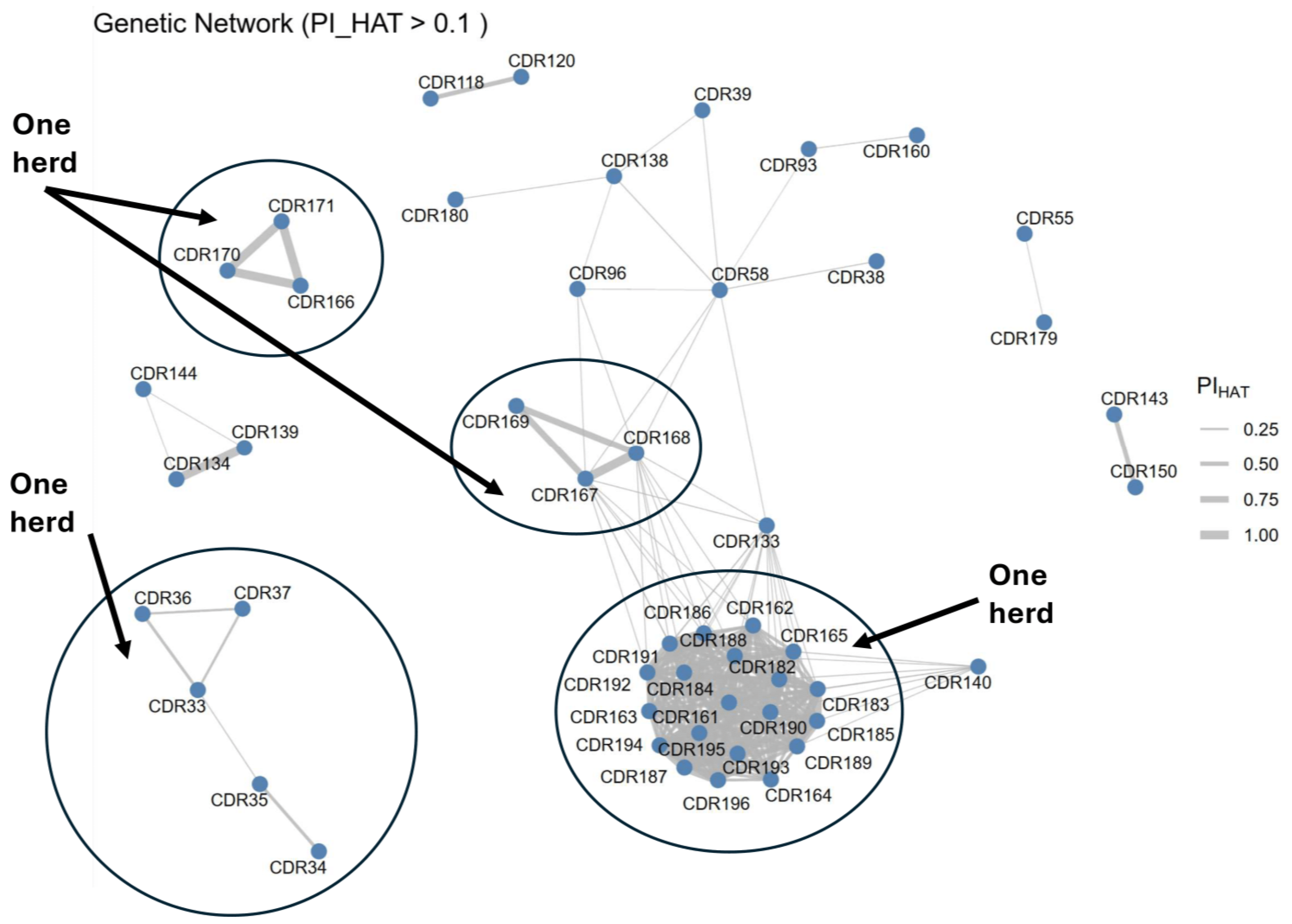
3.7. Genetic Relatedness Network
3.8. Inbreeding Coefficient by Breed and Sex
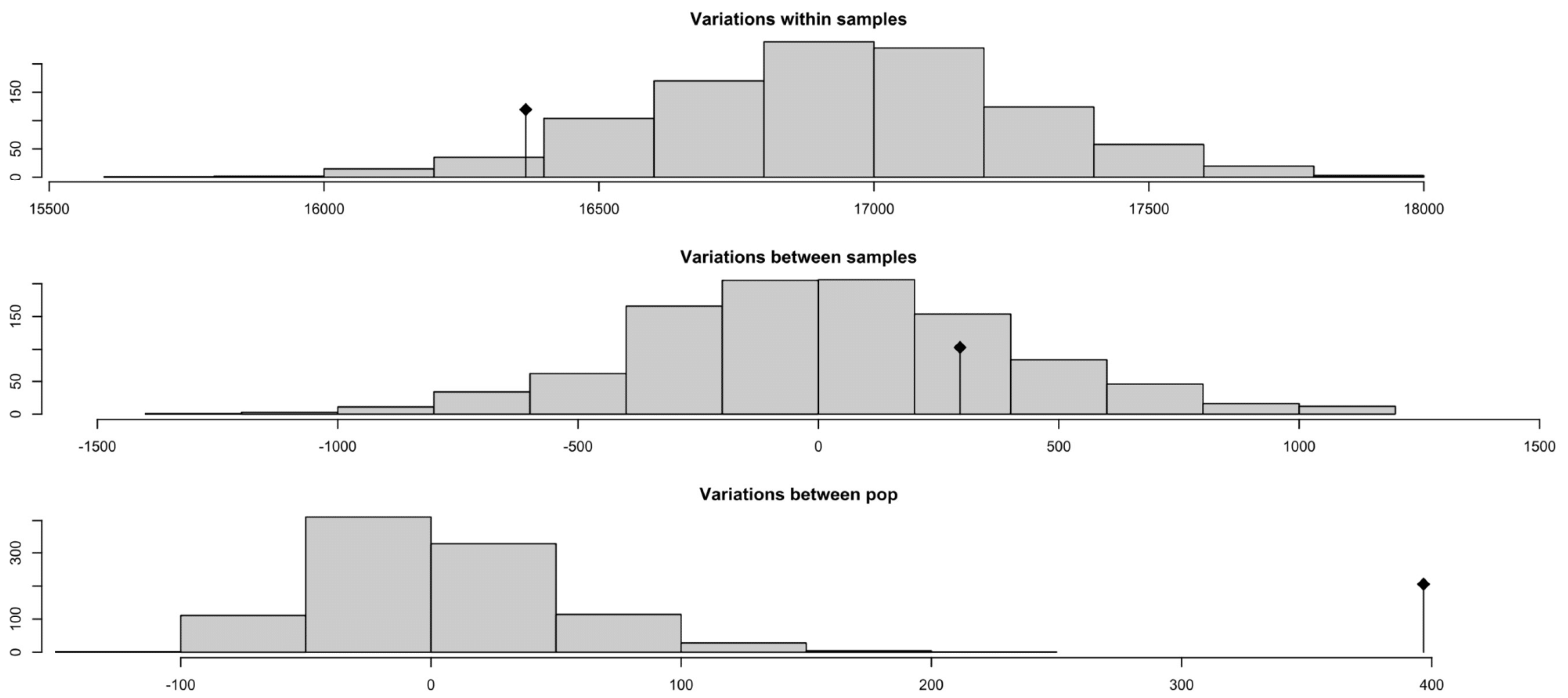
3.9. Analysis of Molecular Variance (AMOVA)
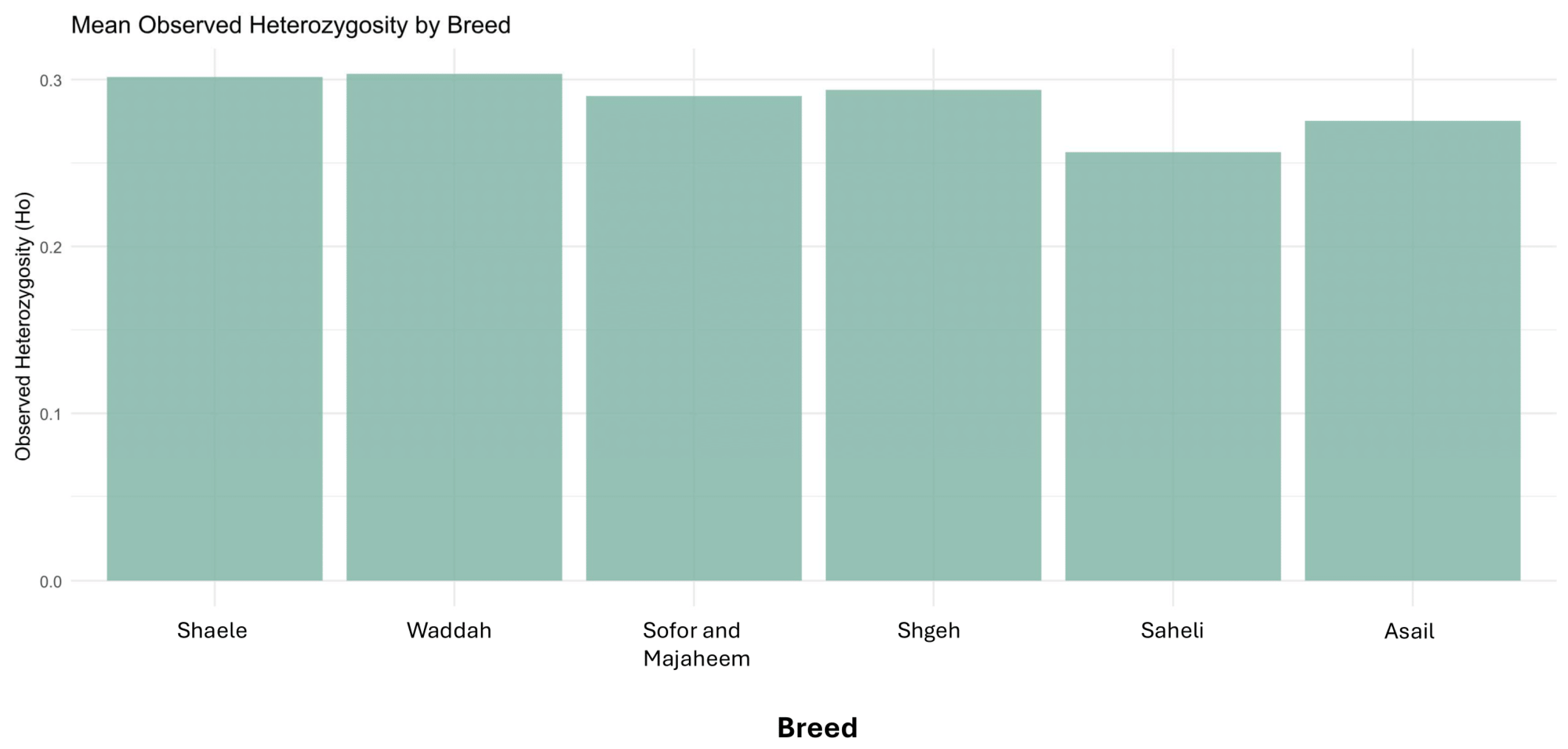
3.10. Observed Heterozygosity by Breed
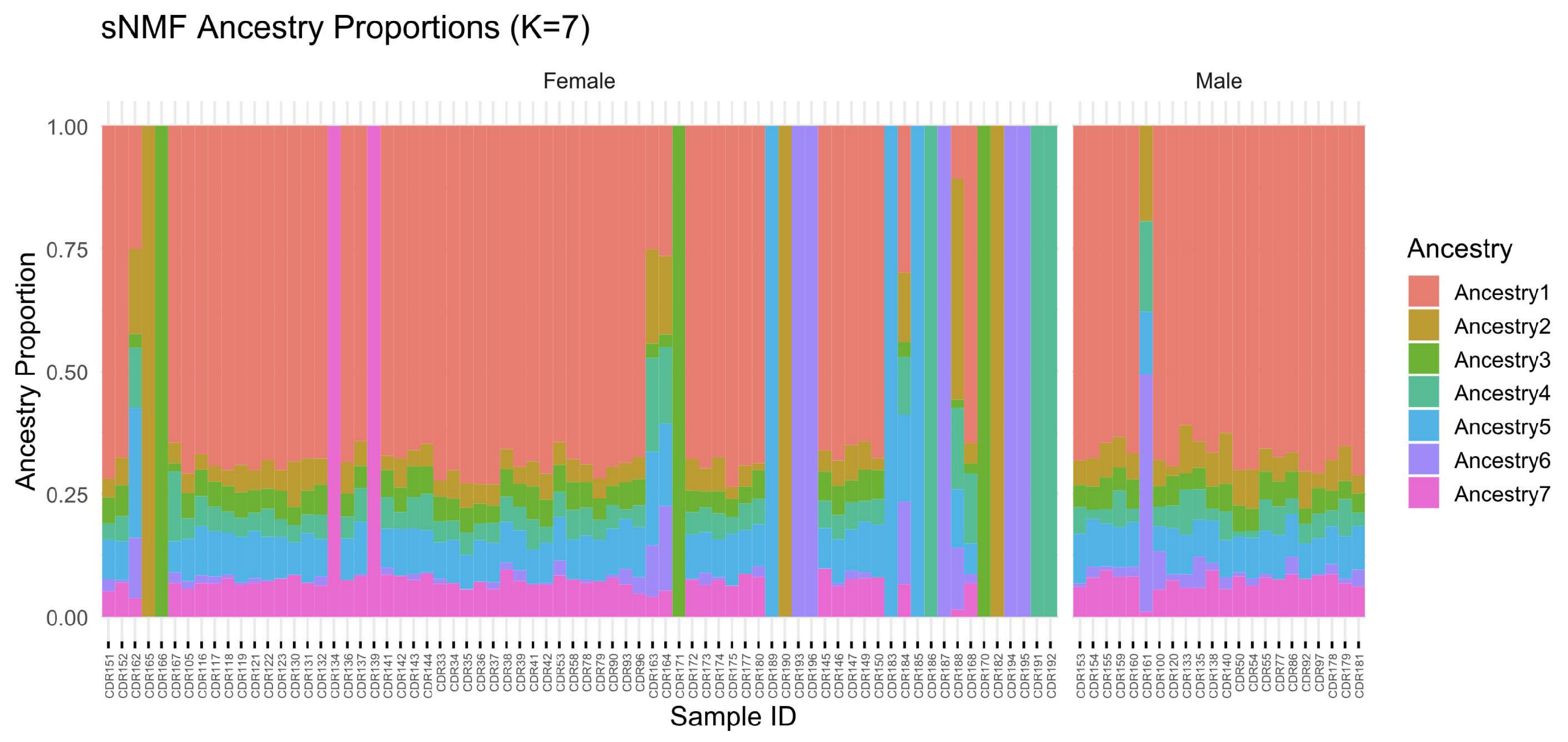
3.11. Ancestry Proportions by Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMOVA | Analysis of Molecular Variance |
| F | Inbreeding coefficient |
| FST | Fixation index |
| GWAS | Genome-wide association study |
| IBD | Identity-by-descent |
| PCA | Principal component analysis |
| ROH | Runs of homozygosity |
| SNP | Single-nucleotide polymorphism |
References
- Al-Bulushi, S.; Manjunatha, B.; De Graaf, S.; Rickard, J. Reproductive seasonality of male dromedary camels. Anim. Reprod. Sci. 2019, 202, 10–20. [Google Scholar] [CrossRef]
- Monaco, D. Breeding soundness evaluation, a tool for improving the sustainability of dromedary camel (Camelus dromedarius) breeding systems in arid and semi-arid lands. Anim. Reprod. Sci. 2025, 277, 107854. [Google Scholar] [CrossRef]
- Bahbahani, H.; Mohammad, Z.; Alfoudari, A.; Al Abri, M. Genomic insights into racing camels: Inbreeding levels and positive selection linked to athletic traits. Animal 2025, 19, 101467. [Google Scholar] [CrossRef]
- Wright, S. Coefficients of inbreeding and relationship. Am. Nat. 1922, 56, 330–338. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H.; Zhang, W.; Jiang, J.; Qian, H.; Man, C.; Gao, H.; Chen, Q.; Du, L.; Chen, S.; et al. Development and validation of a 5K low-density SNP chip for Hainan cattle. BMC Genom. 2024, 25, 873. [Google Scholar] [CrossRef]
- Mekonnen, K.T.; Lee, D.H.; Cho, Y.G.; Son, A.Y.; Seo, K.S. Genomic and Conventional Inbreeding Coefficient Estimation Using Different Estimator Models in Korean Duroc, Landrace, and Yorkshire Breeds Using 70K Porcine SNP BeadChip. Animals 2024, 14, 2621. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Han, Z.; Yang, R.; Zhou, W.; Peng, Y.; He, J.; Liu, S. Population structure and selective signature analysis of local sheep breeds in Xinjiang, China based on high-density SNP chip. Sci. Rep. 2024, 14, 28133. [Google Scholar] [CrossRef]
- Deniskova, T.E.; Dotsev, A.V.; Abdelmanova, A.S.; Petrov, S.N.; Frolov, A.N.; Platonov, S.A.; Gladyr, E.A.; Gusev, I.V.; Selionova, M.I.; Rodionov, A.N.; et al. Genetic diversity in the Orenburg goat breed revealed by single-nucleotide polymorphism (SNP) analysis: Initial steps in saving a threatened population. Genes 2024, 15, 1375. [Google Scholar] [CrossRef]
- Iglesias Pastrana, C.; Navas González, F.J.; Macri, M.; Martínez Martínez, M.d.A.; Ciani, E.; Delgado Bermejo, J.V. Identification of novel genetic loci related to dromedary camel (Camelus dromedarius) morphometrics, biomechanics, and behavior by genome-wide association studies. BMC Vet. Res. 2024, 20, 418. [Google Scholar] [CrossRef]
- Browning, S.; Browning, B. Identity by descent between distant relatives: Detection and applications. Annu. Rev. Genet. 2012, 46, 617–633. [Google Scholar] [CrossRef]
- McQuillan, R.; Leutenegger, A.-L.; Rahman, R.A.; Franklin, C.S.; Pericic, M.; Barac-Lauc, L.; Smolej-Narancic, N.; Janicijevik, B.; Polasek, O. Runs of homozygosity in European populations. Am. J. Hum. Genet. 2008, 83, 359–372. [Google Scholar] [CrossRef]
- Visscher, P.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 Years of GWAS discovery: Biology, function, and translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef]
- Chen, Z.; Boehnke, M.; Wen, X.; Mukherjee, B. Revisiting the genome-wide significance threshold for common variant GWAS. G3 2021, 11, jkaa056. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Uyenoyama, M.K. Wright’s hierarchical F-statistics. Mol. Biol. Evol. 2024, 41, msae083. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Almathen, F.; Elbir, H.; Bahbahani, H.; Mwacharo, J.; Hanotte, O. Polymorphisms in MC1R and ASIP genes are associated with coat colour variation in the Arabian camel. J. Hered. 2018, 109, 700–706. [Google Scholar] [CrossRef]
- Alexander, D.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- AlAskar, H.; Alhajeri, B.H.; Almathen, F.; Alhaddad, H. Genetic diversity and population structure of dromedary camel-types. J. Hered. 2020, 111, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Almathen, F.; Bahbahani, H.; Elbir, H.; Alfattah, M.; Sheikh, A.; Hanotte, O. Genetic structure of Arabian Peninsula dromedary camels revealed three geographic groups. Saudi J. Biol. Sci. 2022, 29, 1422–1427. [Google Scholar] [CrossRef]
- Pacheco, H.A.; Rossoni, A.; Cecchinato, A.; Peñagaricano, F. Identification of runs of homozygosity associated with male fertility in Italian Brown Swiss cattle. Front. Genet. 2023, 14, 1227310. [Google Scholar] [CrossRef] [PubMed]
- Laseca, N.; Molina, A.; Ramón, M.; Valera, M.; Azcona, F.; Encina, A.; Demyda-Peyrás, S. Fine-scale analysis of runs of homozygosity islands affecting fertility in mares. Front. Vet. Sci. 2022, 9, 754028. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Tolone, M.; Barbato, M.; Alsubaie, F.M.; Alrefaei, A.F.; Almutairi, M. Geographical distribution, genetic diversity, and environmental adaptations of dromedary camel breeds in Saudi Arabia. Front. Vet. Sci. 2025, 11, 1490186. [Google Scholar] [CrossRef]
- Alshanbari, F.A.; Ibrahim, Z.H. Genetic Variants on SLC45A2 Gene Associated with White Coat Color in the Dromedary Camel (Camelus dromedarius). Indian J. Anim. Res. 2025, 1, 8. [Google Scholar] [CrossRef]

| Breed | n | Coat Color | Body Size | Purpose | Region |
|---|---|---|---|---|---|
| Asail | 2 | Brown | Small | Racing | Coast |
| Saheli | 5 | Brown body with dark brown to black wool | Small to medium | Meat/milk | West coast |
| Majaheem | 17 | Black | Large | Meat/milk | Desert |
| Sofor | 12 | Dark brown with black wool | Medium to large | Meat/milk | Desert |
| Shaele | 14 | Brown | Medium to large | Meat/milk | Desert |
| Shageh | 18 | Light brown | Medium to large | Meat/milk | Desert |
| Waddah | 28 | White | Medium to large | Meat/milk | Desert |
| Breed | Male | Female | Total |
|---|---|---|---|
| Asail | 2 | 0 | 2 |
| Saheli | 0 | 5 | 5 |
| Majaheem | 2 | 15 | 17 |
| Sofor | 4 | 8 | 12 |
| Shaele | 6 | 8 | 14 |
| Shageh | 3 | 15 | 18 |
| Waddah | 3 | 25 | 28 |
| Total | 20 | 76 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshanbari, F.A.; Aloraini, A. Computational Identification of Genetic Background of Infertility and Calculating Inbreeding Coefficient in Dromedary Camel Herds. Genes 2025, 16, 1238. https://doi.org/10.3390/genes16101238
Alshanbari FA, Aloraini A. Computational Identification of Genetic Background of Infertility and Calculating Inbreeding Coefficient in Dromedary Camel Herds. Genes. 2025; 16(10):1238. https://doi.org/10.3390/genes16101238
Chicago/Turabian StyleAlshanbari, Fahad A., and Abdulrahman Aloraini. 2025. "Computational Identification of Genetic Background of Infertility and Calculating Inbreeding Coefficient in Dromedary Camel Herds" Genes 16, no. 10: 1238. https://doi.org/10.3390/genes16101238
APA StyleAlshanbari, F. A., & Aloraini, A. (2025). Computational Identification of Genetic Background of Infertility and Calculating Inbreeding Coefficient in Dromedary Camel Herds. Genes, 16(10), 1238. https://doi.org/10.3390/genes16101238






