HOXA10 and HOXA11 Methylation: Epigenetic Barriers to Endometrial Receptivity in ART
Abstract
1. Successes and Failures of Assisted Reproductive Technology
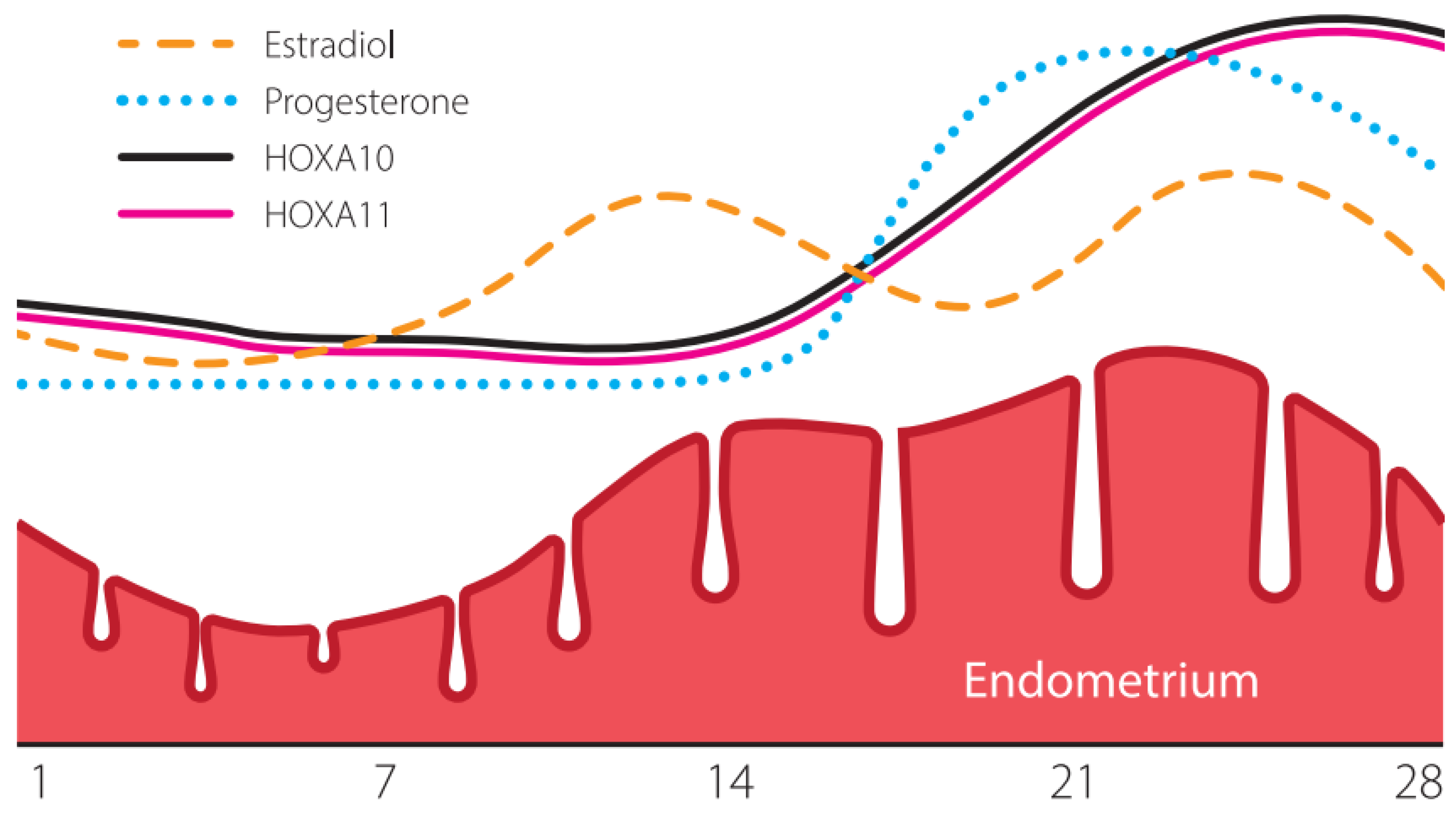
2. Endometrial Receptivity: Genetics and Epigenetics
3. Endometrial Receptivity Disorders as a Reason for Implantation Failure
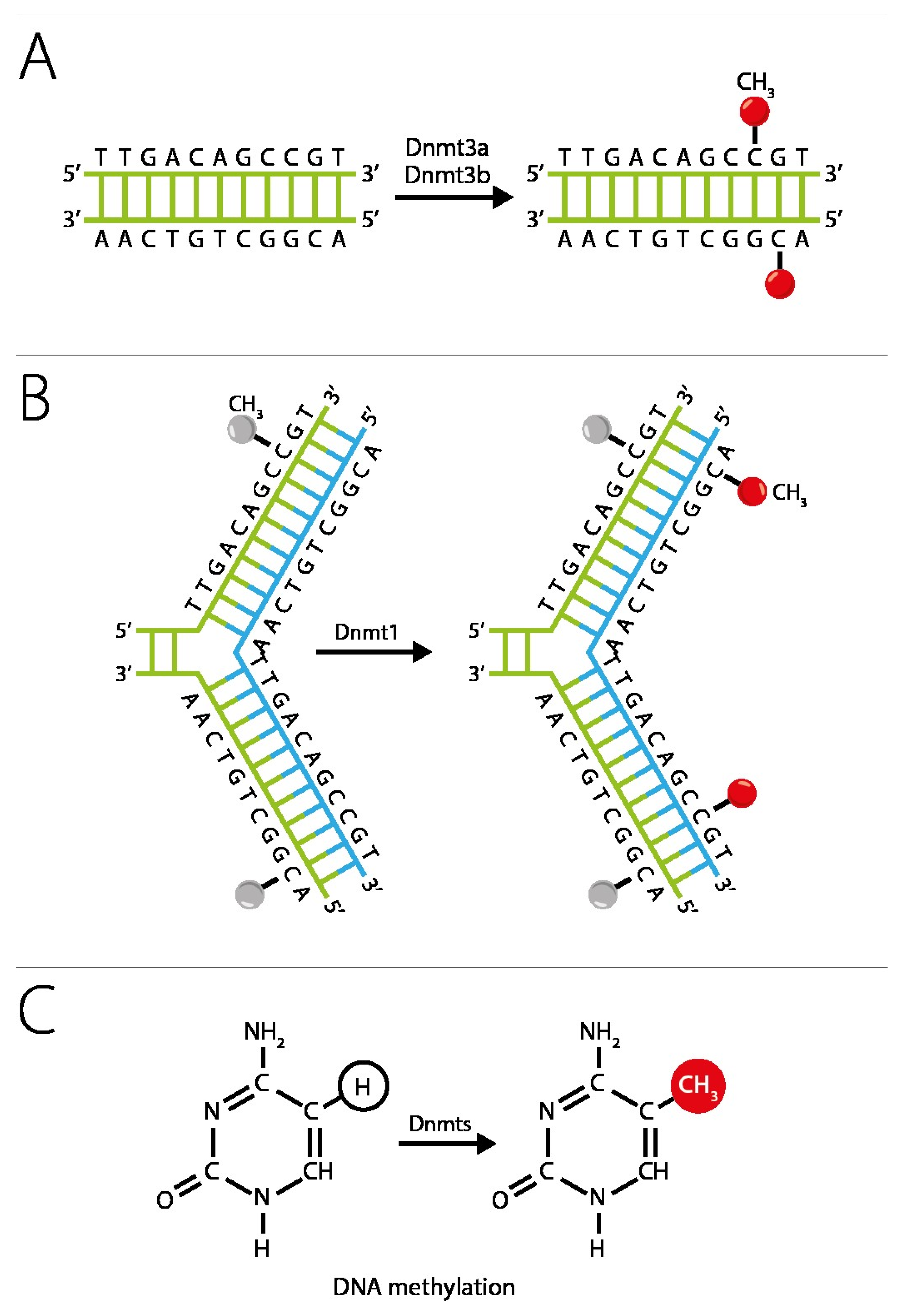
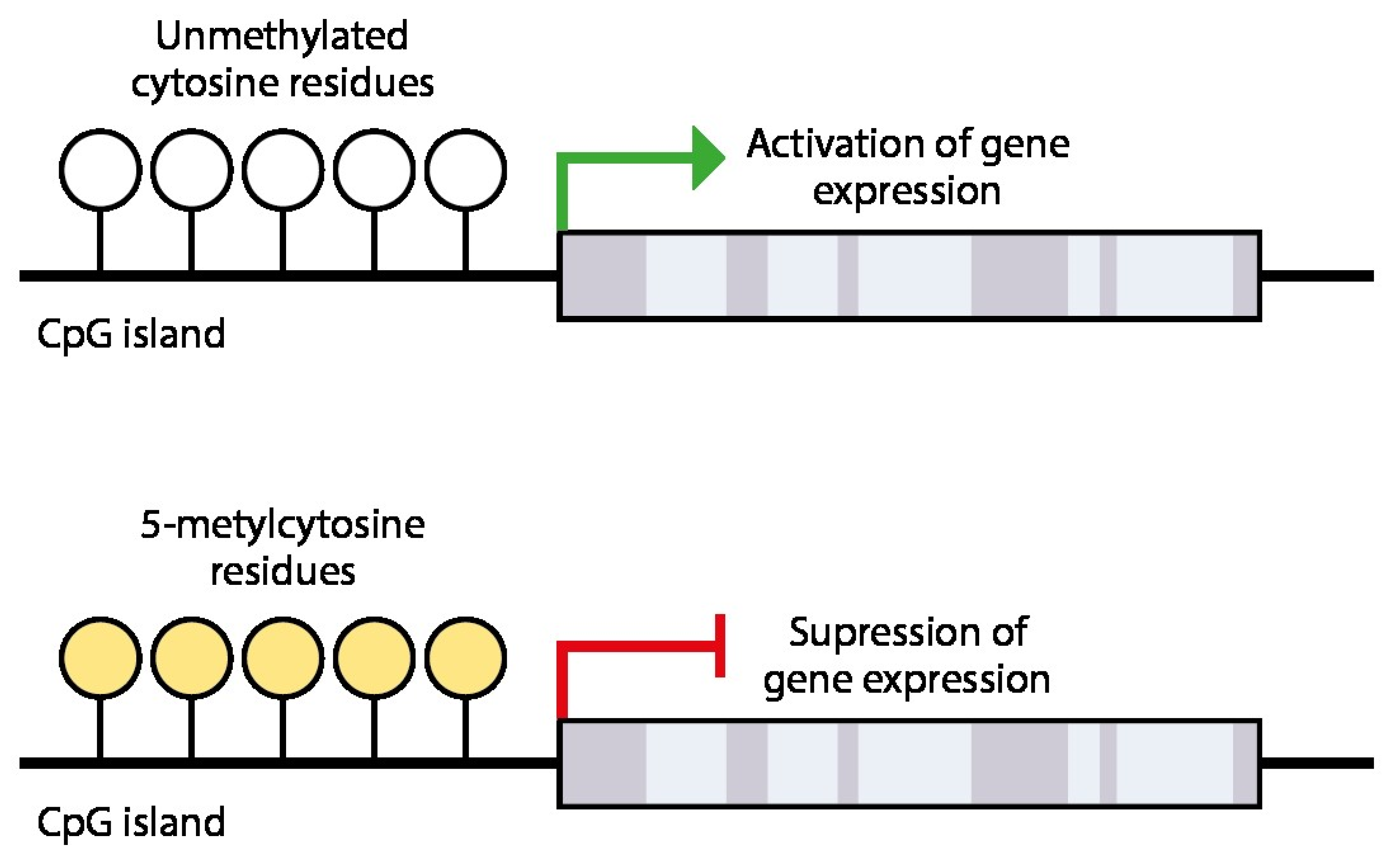
4. Epigenetic Silencing of Receptivity Regulating Genes
5. Molecular Genetic Methods for Assessing Endometrial Receptivity
- Ultrasound markers: endometrial thickness; trilinear pattern; endometrial volume; uterine artery pulsatility index; resistance parameters in the uterine, arcuate, radial, basal, and spiral arteries—assessed on the day of the ovulation trigger injection and the day of embryo transfer;
- Markers in endometrium biopsy samples and the method of evaluation:
- BLC6 (B-cell lymphoma 6 protein)—immunocytohistochemistry [78];
- Inhibin A—quantitative polymerase chain reaction (PCR) [79];
- Integrins αvβ3, α4 β1, α1β1—immunocytohistochemistry [80];
- Ligand of L-selectin—immunohistochemistry and Western blotting [81];
- Aromatase P450—quantitative PCR [82];
- Vascular endothelial growth factor (VEGF)—immunocytohistochemical study [83];
- Human chorionic gonadotrophin/luteinizing hormone receptor (HCG/LH-R)—streptavidin-biotin-peroxidase complex technique for immunohistochemicstry [84];
- Macrophage colony-stimulating factor (M-CSF) Macrophage colony-stimulating factor M-CSF—streptavidin-biotin-peroxidase complex technique for immunohistochemistry [84];
- HOXA10—streptavidin-biotin-peroxidase complex technique for immunohistochemistry [84];
- Endometrial receptivity array (ERA)—gene expression profile [24].
- Hysteroscopy: ring type of gland arrangement and the presence of well-developed varicose-like vessels; assessment of endometrial blood flow (>29 mL/min/100 g) [77];
- Pinopods count by electron microscopy (minimum 60 fields at ×2000 magnification) [77].
6. Methods for Improving Endometrial Receptivity
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, Regional, and Global Trends in Infertility Prevalence since 1990: A Systematic Analysis of 277 Health Surveys. PLoS Med. 2012, 9, e1001356. [Google Scholar] [CrossRef]
- Naqvi, H.; Ilagan, Y.; Krikun, G.; Taylor, H.S. Altered Genome-Wide Methylation in Endometriosis. Reprod. Sci. 2014, 21, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International Estimates of Infertility Prevalence and Treatment-Seeking: Potential Need and Demand for Infertility Medical Care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Health of the Russian Federation. Clinical Guidelines. Female Infertility. 2021. Available online: https://cr.minzdrav.gov.ru/view-cr/641_2?ysclid=mgqq40fslv87761482 (accessed on 28 January 2025).
- Accounts Chamber of the Russian Federation. Report on the Results of the Expert Analysis of the Efficiency of Spending Compulsory Health Insurance Funds in 2019 and the Expired Period of 2020 on In Vitro Fertilization. Available online: https://ach.gov.ru/upload/iblock/9b0/9b06bc06ddedb49a807822ad7cd21621.pdf (accessed on 28 January 2025).
- Korsak, V.S.; Smirnova, A.A.; Shurygina, O.V. ART Register of RAHR, 2020. Probl. Reprod. 2022, 28, 12–27. [Google Scholar] [CrossRef]
- Federal State Statistics Service. Statistical Compilation. In Health Care in Russia 2019; Statistical Compilation/Russian Statistics Service: Moscow, Russia, 2019; p. 170. Available online: https://rosstat.gov.ru/storage/mediabank/Zdravoohran-2019.pdf (accessed on 28 January 2025).
- Safronova, A.S.; Buralkina, N.A.; Burduli, A.G.; Arakelyan, A.S.; Chuprynin, V.D.; Adamyan, L.V. Reproductive Potential of Patients with Various Forms of Endometriosis (Literature Review). Probl. reprod. 2021, 27, 24–32. [Google Scholar] [CrossRef]
- Choe, J.; Shanks, A.L. In Vitro Fertilization; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/32965937/ (accessed on 28 January 2025). [PubMed]
- Sunderam, S.; Kissin, D.M.; Crawford, S.B.; Folger, S.G.; Boulet, S.L.; Warner, L.; Barfield, W.D. Assisted Reproductive Technology Surveillance—United States, 2015. MMWR Surveill Summ 2018, 67, 1–28. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M.; Haaf, T. In Vitro Fertilization Technology and Child Health. Dtsch. Arztebl. Int. 2020, 117, 23–30. [Google Scholar] [CrossRef]
- Shishkina, K.Y.; Biryukova, N.V. Risks of abnormalities in children conceived using IVF methods: A literature review. Sci. Herit. 2021, 56–59. [Google Scholar]
- Lysenko, O.V.; Smirnova, I.V. Medical and social characteristics of women referred for IVF and failure analysis. Bull. Vitebsk. State Med. Univ. 2010, 9, 1–7. [Google Scholar]
- Kushnir, V.A.; Smith, G.D.; Adashi, E.Y. The Future of IVF: The New Normal in Human Reproduction. Reprod. Sci. 2022, 29, 849–856. [Google Scholar] [CrossRef]
- Polovneva, M.I.; Korneeva, I.E.; Bourmenskaya, O.V. Modern methods of influence at endometrial receptivity in patients with recurrent implantation failure (review). Gynecology 2018, 20, 66–70. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Makrygiannakis, F.; Vrekoussis, T. Approaches to Improve Endometrial Receptivity in Case of Repeated Implantation Failures. Front. Cell Dev. Biol. 2021, 9, 613227. [Google Scholar] [CrossRef]
- Khabarov, S.V.; Khadartseva, K.A. The effect of age on the failures of assisted reproductive technology programs. J. New Med. Technol. 2018, 12, 74–79. [Google Scholar] [CrossRef]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-Update Overview on Etiology, Diagnosis, Treatment and Future Directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent Implantation Failure: Definition and Management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Holzer, H.E.G. Recurrent Implantation Failure: Gamete and Embryo Factors. Fertil. Steril. 2012, 97, 1021–1027. [Google Scholar] [CrossRef]
- Demiral, İ.; Doğan, M.; Baştu, E.; Buyru, F. Genomic, Proteomic and Lipidomic Evaluation of Endometrial Receptivity. Turk. J. Obstet. Gynecol. 2015, 12, 237–243. [Google Scholar] [CrossRef]
- Busnelli, A.; Reschini, M.; Cardellicchio, L.; Vegetti, W.; Somigliana, E.; Vercellini, P. How Common Is Real Repeated Implantation Failure? An Indirect Estimate of the Prevalence. Reprod. Biomed. Online 2020, 40, 91–97. [Google Scholar] [CrossRef]
- Knyazeva, E.A.; Kuznetsova, M.V.; Burmenskaya, O.V.; Donnikov, A.E.; Kalinina, E.A. Expression of MSX1, HOXA11 and TP53I3 in the endometrium associated with the onset of pregnancy after repeated failed IVF attempts in patients with tubo-peritoneal factor infertility. Gynecology 2020, 22, 23–28. [Google Scholar] [CrossRef]
- Ruiz-Alonso, M.; Galindo, N.; Pellicer, A.; Simón, C. What a Difference Two Days Make: “Personalized” Embryo Transfer (pET) Paradigm: A Case Report and Pilot Study. Hum. Reprod. 2014, 29, 1244–1247. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and Molecular Determinants of Embryo Implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef] [PubMed]
- Altmäe, S.; Koel, M.; Võsa, U.; Adler, P.; Suhorutšenko, M.; Laisk-Podar, T.; Kukushkina, V.; Saare, M.; Velthut-Meikas, A.; Krjutškov, K.; et al. Meta-Signature of Human Endometrial Receptivity: A Meta-Analysis and Validation Study of Transcriptomic Biomarkers. Sci. Rep. 2017, 7, 10077. [Google Scholar] [CrossRef]
- He, A.; Wu, H.; Zou, Y.; Wan, C.; Zhao, J.; Zhang, Q.; Liu, N.; Liu, D.; Li, Y.; Fu, J.; et al. Can Biomarkers Identified from the Uterine Fluid Transcriptome Be Used to Establish a Noninvasive Endometrial Receptivity Prediction Tool? A Proof-of-Concept Study. Reprod. Biol. Endocrinol. 2023, 21, 20. [Google Scholar] [CrossRef]
- Prapas, Y.; Prapas, N.; Jones, E.E.; Duleba, A.J.; Olive, D.L.; Chatziparasidou, A.; Vlassis, G. The Window for Embryo Transfer in Oocyte Donation Cycles Depends on the Duration of Progesterone Therapy. Hum. Reprod. 1998, 13, 720–723. [Google Scholar] [CrossRef]
- Behjati, S.; Tarpey, P.S. What Is next Generation Sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Liu, S.; Bai, X.; Zou, B.; Li, Y. Transcriptome Sequencing of Endometrium Revealed Alterations in mRNAs and lncRNAs after Ovarian Stimulation. J. Assist. Reprod. Genet. 2020, 37, 21–32. [Google Scholar] [CrossRef]
- Retis-Resendiz, A.M.; González-García, I.N.; León-Juárez, M.; Camacho-Arroyo, I.; Cerbón, M.; Vázquez-Martínez, E.R. The Role of Epigenetic Mechanisms in the Regulation of Gene Expression in the Cyclical Endometrium. Clin. Epigenetics 2021, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.R.; Chandrashekar, D.S.; Kakar, R.; Davuluri, S.; Bajpai, A.K.; Nayak, S.; Bhutada, S.; Acharya, K.; Sachdeva, G. Endometrial Receptivity: A Revisit to Functional Genomics Studies on Human Endometrium and Creation of HGEx-ERdb. PLoS ONE 2013, 8, e58419. [Google Scholar] [CrossRef]
- Vitiello, D.; Kodaman, P.H.; Taylor, H.S. HOX Genes in Implantation. Semin. Reprod. Med. 2007, 25, 431–436. [Google Scholar] [CrossRef]
- Carroll, S.B. Homeotic Genes and the Evolution of Arthropods and Chordates. Nature 1995, 376, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bagot, C.N.; Kliman, H.J.; Taylor, H.S. Maternal Hoxa10 Is Required for Pinopod Formation in the Development of Mouse Uterine Receptivity to Embryo Implantation. Dev. Dyn. 2001, 222, 538–544. [Google Scholar] [CrossRef]
- Daftary, G.S.; Taylor, H.S. Pleiotropic Effects of Hoxa10 on the Functional Development of Peri-Implantation Endometrium. Mol. Reprod. Dev. 2004, 67, 8–14. [Google Scholar] [CrossRef]
- Maltseva, L.I.; Kiselev, V.I.; Poloznikov, A.A.; Sharipova, R.I.; Zhelezova, M.E. Efficacy of epigallocatechin-3-gallate as a treatment for endometritis in women with reproductive impairment. Pract. Med. 2019, 17, 62–67. [Google Scholar] [CrossRef]
- Sukhikh, G.T.; Osipyants, A.I.; Maltseva, L.I.; Smolina, G.R.; Poloznikov, A.A.; Muyzhnek, E.L.; Kiselev, V.I. Abnormal Hypermethylation of the HOXA10 and HOXA11 Genes in Infertility Associated with Chronic Endometritis. Obstet. Gynecol. 2015, 12, 69–74. [Google Scholar]
- Taylor, H.S. The Role of HOX Genes in Human Implantation. Hum. Reprod. Update 2000, 6, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Taylor, H.S. The Role of HOX Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef]
- Cakmak, H.; Taylor, H.S. Molecular Mechanisms of Treatment Resistance in Endometriosis: The Role of Progesterone-Hox Gene Interactions. Semin. Reprod. Med. 2010, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, T.A.; Kalinina, E.A.; Knyazeva, E.A.; Kiselev, V.I.; Smolnikova, V.Y.; Sukhikh, G.T. The Role of Abnormal Hypermethylation of the HOXA10 and HOXA11 Promoters in Implantation Failures in IVF Programs. Gynecol. Endocrinol. 2019, 35, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Geerts, D.; Bu, Z.; Ai, J.; Jin, L.; Li, Y.; Zhang, H.; Zhu, G. Regulation of Endometrial Receptivity by the Highly Expressed HOXA9, HOXA11 and HOXD10 HOX-Class Homeobox Genes. Hum. Reprod. 2014, 29, 781–790. [Google Scholar] [CrossRef]
- Zaydiyeva, Z.S.; Uruymagova, A.T. Polycystic ovarian syndrome: Current understanding of pathogenesis, diagnosis and treatment. Meditsinskiy Sov. = Med. Counc. 2021, 13, 102–111. [Google Scholar] [CrossRef]
- Horsthemke, B.; Ludwig, M. Assisted Reproduction: The Epigenetic Perspective. Hum. Reprod. Update 2005, 11, 473–482. [Google Scholar] [CrossRef]
- Кoзлoв, B.A. Метилирoвание Днк Клетки И Патoлoгия Организма. Медицинская Иммунoлoгия 2008, 10, 307–318. [Google Scholar]
- Koukoura, O.; Sifakis, S.; Spandidos, D.A. DNA Methylation in Endometriosis (Review). Mol. Med. Rep. 2016, 13, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Li, D.-J.; Wang, X.-Q. Towards a Better Understanding of Endometriosis-Related Infertility: A Review on How Endometriosis Affects Endometrial Receptivity. Biomolecules 2023, 13, 430. [Google Scholar] [CrossRef]
- Shukla, A.; Sehgal, M.; Singh, T.R. Hydroxymethylation and Its Potential Implication in DNA Repair System: A Review and Future Perspectives. Gene 2015, 564, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, Y.; Yang, D.; Peng, H. Advances in Endometrial Receptivity and Embryo Implantation by Multi-Omics Techniques. Anim. Zoonoses 2025, 1, 286–294. [Google Scholar] [CrossRef]
- Bommarito, P.A.; Fry, R.C. The Role of DNA Methylation in Gene Regulation. In Toxicoepigenetics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 127–151. [Google Scholar]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Wang, L.; Tan, Y.J.; Wang, M.; Chen, Y.F.; Li, X.Y. DNA Methylation Inhibitor 5-Aza-2’-Deoxycytidine Modulates Endometrial Receptivity Through Upregulating HOXA10 Expression. Reprod. Sci. 2019, 26, 839–846. [Google Scholar] [CrossRef]
- Pîrlog, L.-M.; Pătrășcanu, A.-A.; Ona, M.-D.; Cătană, A.; Rotar, I.C. HOXA10 and HOXA11 in Human Endometrial Benign Disorders: Unraveling Molecular Pathways and Their Impact on Reproduction. Biomolecules 2025, 15, 563. [Google Scholar] [CrossRef]
- Messaoudi, S.; El Kasmi, I.; Bourdiec, A.; Crespo, K.; Bissonnette, L.; Le Saint, C.; Bissonnette, F.; Kadoch, I.-J. 15 Years of Transcriptomic Analysis on Endometrial Receptivity: What Have We Learnt? Fertil. Res. Pract. 2019, 5, 9. [Google Scholar] [CrossRef]
- Ovarian Stimulation, T.E.G.G.O.; Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; et al. ESHRE Guideline: Ovarian Stimulation for IVF/ICSI†. Hum. Reprod. Open 2020, 2020, hoaa009. [Google Scholar] [CrossRef]
- Macklon, N.S.; Stouffer, R.L.; Giudice, L.C.; Fauser, B.C.J.M. The Science behind 25 Years of Ovarian Stimulation for in Vitro Fertilization. Endocr. Rev. 2006, 27, 170–207. [Google Scholar] [CrossRef]
- Andersson, K.L.; Bussani, C.; Fambrini, M.; Polverino, V.; Taddei, G.L.; Gemzell-Danielsson, K.; Scarselli, G. DNA Methylation of HOXA10 in Eutopic and Ectopic Endometrium. Hum. Reprod. 2014, 29, 1906–1911. [Google Scholar] [CrossRef]
- Barnhart, K.; Dunsmoor-Su, R.; Coutifaris, C. Effect of Endometriosis on in Vitro Fertilization. Fertil. Steril. 2002, 77, 1148–1155. [Google Scholar] [CrossRef]
- Taylor, H.S.; Bagot, C.; Kardana, A.; Olive, D.; Arici, A. HOX Gene Expression Is Altered in the Endometrium of Women with Endometriosis. Hum. Reprod. 1999, 14, 1328–1331. [Google Scholar] [CrossRef]
- Kim, J.J.; Taylor, H.S.; Lu, Z.; Ladhani, O.; Hastings, J.M.; Jackson, K.S.; Wu, Y.; Guo, S.W.; Fazleabas, A.T. Altered Expression of HOXA10 in Endometriosis: Potential Role in Decidualization. Mol. Hum. Reprod. 2007, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Health of the Russian Federation. Clinical Guidelines. Polycystic Ovarian Syndrome. 2021. Available online: https://cr.minzdrav.gov.ru/preview-cr/258_2?ysclid=mgqror193y438349344 (accessed on 30 January 2025).
- Neven, A.C.H.; Laven, J.; Teede, H.J.; Boyle, J.A. A Summary on Polycystic Ovary Syndrome: Diagnostic Criteria, Prevalence, Clinical Manifestations, and Management According to the Latest International Guidelines. Semin. Reprod. Med. 2018, 36, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Endometrium in PCOS: Implantation and Predisposition to Endocrine CA. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Cermik, D.; Selam, B.; Taylor, H.S. Regulation of HOXA-10 Expression by Testosterone in Vitro and in the Endometrium of Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 238–243. [Google Scholar] [CrossRef]
- The Ministry of Health of the Russian Federation. Clinical Guidelines. Uterine Fibroids. 2021. Available online: https://cr.minzdrav.gov.ru/view-cr/257_2?ysclid=mgqrpkuh6c628955574 (accessed on 30 January 2025).
- Sinclair, D.C.; Mastroyannis, A.; Taylor, H.S. Leiomyoma Simultaneously Impair Endometrial BMP-2-Mediated Decidualization and Anticoagulant Expression through Secretion of TGF-Β3. J. Clin. Endocrinol. Metab. 2011, 96, 412–421. [Google Scholar] [CrossRef]
- Fomchenko, N.E.; Voropayev, E.V. biological aspects of DNA methylation (Literature Review). Health Ecol. Issues 2012, 55–59. [Google Scholar] [CrossRef]
- Fessele, K.L.; Wright, F. Primer in Genetics and Genomics, Article 6: Basics of Epigenetic Control. Biol. Res. Nurs. 2018, 20, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.F.; Plass, C. Methylation Matters. J. Med. Genet. 2001, 38, 285–303. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Helin, K. Role of TET Enzymes in DNA Methylation, Development, and Cancer. Genes. Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef]
- Williams, K.; Christensen, J.; Helin, K. DNA Methylation: TET Proteins-Guardians of CpG Islands? EMBO Rep. 2011, 13, 28–35. [Google Scholar] [CrossRef]
- Huang, Y.; Rao, A. Connections between TET Proteins and Aberrant DNA Modification in Cancer. Trends Genet. 2014, 30, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, M.; Wirstlein, P.; Zawadzka, M.; Wender-Ożegowska, E.; Jagodziński, P.P. Alternation of Ten-Eleven Translocation 1, 2, and 3 Expression in Eutopic Endometrium of Women with Endometriosis-Associated Infertility. Gynecol. Endocrinol. 2018, 34, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Rao, C.M. Epigenetic Tools (The Writers, The Readers and The Erasers) and Their Implications in Cancer Therapy. Eur. J. Pharmacol. 2018, 837, 8–24. [Google Scholar] [CrossRef]
- Kibanov, M.V.; Makhmudova, G.M.; Gokhberg, Y.A. In search for an ideal marker of endometrial receptivity: From histology to comprehensive molecular genetics-based approaches. Alm. Clin. Med. 2019, 47, 12–25. [Google Scholar] [CrossRef]
- Craciunas, L.; Gallos, I.; Chu, J.; Bourne, T.; Quenby, S.; Brosens, J.J.; Coomarasamy, A. Conventional and Modern Markers of Endometrial Receptivity: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2019, 25, 202–223. [Google Scholar] [CrossRef]
- Almquist, L.D.; Likes, C.E.; Stone, B.; Brown, K.R.; Savaris, R.; Forstein, D.A.; Miller, P.B.; Lessey, B.A. Endometrial BCL6 Testing for the Prediction of in Vitro Fertilization Outcomes: A Cohort Study. Fertil. Steril. 2017, 108, 1063–1069. [Google Scholar] [CrossRef]
- Silveira, C.O.; Rezende, C.P.; Ferreira, M.C.; Del Puerto, H.L.; Reis, F.M. Implantation Failure Is Associated With Increased α-Inhibin and β-Glycan Gene Expression in Secretory Phase Endometrium: Nested Case-Control Study of Infertile Women Undergoing IVF/Fresh Embryo Transfer. Reprod. Sci. 2017, 24, 720–725. [Google Scholar] [CrossRef]
- Thomas, K.; Thomson, A.; Wood, S.; Kingsland, C.; Vince, G.; Lewis-Jones, I. Endometrial Integrin Expression in Women Undergoing in Vitro Fertilization and the Association with Subsequent Treatment Outcome. Fertil. Steril. 2003, 80, 502–507. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, J.-Z.; He, R.-H.; Qian, Y.-L.; Jin, F.; Huang, H.-F. High Expression of L-Selectin Ligand in Secretory Endometrium Is Associated with Better Endometrial Receptivity and Facilitates Embryo Implantation in Human Being. Am. J. Reprod. Immunol. 2008, 60, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Brosens, J.; Verhoeven, H.; Campo, R.; Gianaroli, L.; Gordts, S.; Hazekamp, J.; Hägglund, L.; Mardesic, T.; Varila, E.; Zech, J.; et al. High Endometrial Aromatase P450 mRNA Expression Is Associated with Poor IVF Outcome. Hum. Reprod. 2004, 19, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Jinno, M.; Ozaki, T.; Iwashita, M.; Nakamura, Y.; Kudo, A.; Hirano, H. Measurement of Endometrial Tissue Blood Flow: A Novel Way to Assess Uterine Receptivity for Implantation. Fertil. Steril. 2001, 76, 1168–1174. [Google Scholar] [CrossRef]
- Seo, W.S.; Jee, B.C.; Moon, S.Y. Expression of Endometrial Protein Markers in Infertile Women and the Association with Subsequent in Vitro Fertilization Outcome. Fertil. Steril. 2011, 95, 2707–2710. [Google Scholar] [CrossRef]
- Liu, W.; Tal, R.; Chao, H.; Liu, M.; Liu, Y. Effect of Local Endometrial Injury in Proliferative vs. Luteal Phase on IVF Outcomes in Unselected Subfertile Women Undergoing in Vitro Fertilization. Reprod. Biol. Endocrinol. 2017, 15, 75. [Google Scholar] [CrossRef]
- Szczepańska, M.; Wirstlein, P.; Skrzypczak, J.; Jagodziński, P.P. Expression of HOXA11 in the Mid-Luteal Endometrium from Women with Endometriosis-Associated Infertility. Reprod. Biol. Endocrinol. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gimeno, P.; Horcajadas, J.A.; Martínez-Conejero, J.A.; Esteban, F.J.; Alamá, P.; Pellicer, A.; Simón, C. A Genomic Diagnostic Tool for Human Endometrial Receptivity Based on the Transcriptomic Signature. Fertil. Steril. 2011, 95, 50–60.e15. [Google Scholar] [CrossRef]
- Rubin, S.C.; Abdulkadir, M.; Lewis, J.; Harutyunyan, A.; Hirani, R.; Grimes, C.L. Review of Endometrial Receptivity Array: A Personalized Approach to Embryo Transfer and Its Clinical Applications. J. Pers. Med. 2023, 13, 749. [Google Scholar] [CrossRef] [PubMed]
- Maziotis, E.; Kalampokas, T.; Giannelou, P.; Grigoriadis, S.; Rapani, A.; Anifantakis, M.; Kotsifaki, A.; Pantou, A.; Triantafyllidou, O.; Tzanakaki, D.; et al. Commercially Available Molecular Approaches to Evaluate Endometrial Receptivity: A Systematic Review and Critical Analysis of the Literature. Diagnostics 2022, 12, 2611. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, Y.; Ke, X.; Liang, X.; Lin, Y.; Wang, F. Does Endometrial Receptivity Array Improve Reproductive Outcomes in Euploid Embryo Transfer Cycles? A Systematic Review. Front. Endocrinol. 2023, 14, 1251699. [Google Scholar] [CrossRef]
- Ye, L.; Dimitriadis, E. Endometrial Receptivity-Lessons from “Omics”. Biomolecules 2025, 15, 106. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Lim, J.; Xing, H.; Chen, Y.; Yang, S.; Fu, X. Molecular and Biological Markers for Assessing Endometrial Receptivity in Infertile Women: A Narrative Review. J. Int. Med. Res. 2025, 53, 3000605251328893. [Google Scholar] [CrossRef]
- He, A.; Zou, Y.; Wan, C.; Zhao, J.; Zhang, Q.; Yao, Z.; Tian, F.; Wu, H.; Huang, X.; Fu, J.; et al. The Role of Transcriptomic Biomarkers of Endometrial Receptivity in Personalized Embryo Transfer for Patients with Repeated Implantation Failure. J. Transl. Med. 2021, 19, 176. [Google Scholar] [CrossRef]
- Chen, J.; He, A.; Zhang, Q.; Zhao, J.; Fu, J.; Li, H.; Li, Y. The RNA-Seq Based Endometrial Receptivity Test (rsERT) Compared to Pinopode: A Better Diagnostic Tool for Endometrial Receptivity for Patients with Recurrent Implantation Failure in Chinese Population. Front. Endocrinol. 2022, 13, 1009161. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Y.; Li, R.; Chen, Y.; Huang, L.; Tan, Z.; Ban, X.; Zhou, L.; Xu, C.; Qiu, Y.; et al. Personalized Embryo Transfer Guided by rsERT Improves Pregnancy Outcomes in Patients with Repeated Implantation Failure. Front. Med. 2024, 11, 1369317. [Google Scholar] [CrossRef]
- Liouta, G.; Adamaki, M.; Tsintarakis, A.; Zoumpourlis, P.; Liouta, A.; Agelaki, S.; Zoumpourlis, V. DNA Methylation as a Diagnostic, Prognostic, and Predictive Biomarker in Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 2996. [Google Scholar] [CrossRef] [PubMed]
- Taryma-Leśniak, O.; Sokolowska, K.E.; Wojdacz, T.K. Current Status of Development of Methylation Biomarkers for in Vitro Diagnostic IVD Applications. Clin. Epigenetics 2020, 12, 100. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Mena-Mollá, S.; García-Giménez, J.L. Epigenetic IVD Tests for Personalized Precision Medicine in Cancer. Front. Genet. 2019, 10, 621. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Seco-Cervera, M.; Tollefsbol, T.O.; Romá-Mateo, C.; Peiró-Chova, L.; Lapunzina, P.; Pallardó, F.V. Epigenetic Biomarkers: Current Strategies and Future Challenges for Their Use in the Clinical Laboratory. Crit. Rev. Clin. Lab. Sci. 2017, 54, 529–550. [Google Scholar] [CrossRef]
- Lee, Y.-X.; Su, P.-H.; Do, A.Q.; Tzeng, C.-R.; Hu, Y.-M.; Chen, C.-H.; Chen, C.-W.; Liao, C.-C.; Chen, L.-Y.; Weng, Y.-C.; et al. Cervical Secretion Methylation Is Associated with the Pregnancy Outcome of Frozen-Thawed Embryo Transfer. Int. J. Mol. Sci. 2023, 24, 1726. [Google Scholar] [CrossRef]
- Pathare, A.D.S.; Hinduja, I. Aberrant DNA Methylation Profiling Affecting the Endometrial Receptivity in Recurrent Implantation Failure Patients Undergoing in Vitro Fertilization. Am. J. Reprod. Immunol. 2020, 83, e13196. [Google Scholar] [CrossRef]
- Xue, P.; Zhou, W.; Fan, W.; Jiang, J.; Kong, C.; Zhou, W.; Zhou, J.; Huang, X.; Yang, H.; Han, Q.; et al. Increased METTL3-Mediated m6A Methylation Inhibits Embryo Implantation by Repressing HOXA10 Expression in Recurrent Implantation Failure. Reprod. Biol. Endocrinol. 2021, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Han, M.; Zhou, M.; Liu, M.; Li, Y.; Xu, B.; Zhang, A. Down-Regulation of S100P Induces Apoptosis in Endometrial Epithelial Cell during GnRH Antagonist Protocol. Reprod. Biol. Endocrinol. 2021, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kodithuwakku, S.P.; Chan, R.W.S.; Yeung, W.S.B.; Yao, Y.; Ng, E.H.Y.; Chiu, P.C.N.; Lee, C.-L. Three-Dimensional Culture Models of Human Endometrium for Studying Trophoblast-Endometrium Interaction during Implantation. Reprod. Biol. Endocrinol. 2022, 20, 120. [Google Scholar] [CrossRef]
- Xu, S.; Hu, D.; Ye, Y.; Mu, Y.; Xiong, Y.; Zhang, Y. Identification of Serum Small Non-Coding RNA as Biomarkers for Endometrial Receptivity. Genomics 2025, 117, 111002. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Chen, Y.; Jiang, Q.; Li, W.; Lu, M.; Zhou, J.; Lin, L.; Xing, J.; Zhang, M.; Zhao, S.; et al. The Long Noncoding RNA LUCAT1 Regulates Endometrial Receptivity via the miR-495-3p/S100P Axis. Commun. Biol. 2025, 8, 318. [Google Scholar] [CrossRef]
- von Grothusen, C.; Lalitkumar, S.; Boggavarapu, N.R.; Gemzell-Danielsson, K.; Lalitkumar, P.G. Recent Advances in Understanding Endometrial Receptivity: Molecular Basis and Clinical Applications. Am. J. Reprod. Immunol. 2014, 72, 148–157. [Google Scholar] [CrossRef]
- Sun, B.; Yeh, J. Non-Invasive and Mechanism-Based Molecular Assessment of Endometrial Receptivity During the Window of Implantation: Current Concepts and Future Prospective Testing Directions. Front. Reprod. Health 2022, 4, 863173. [Google Scholar] [CrossRef]
- Barash, A.; Dekel, N.; Fieldust, S.; Segal, I.; Schechtman, E.; Granot, I. Local Injury to the Endometrium Doubles the Incidence of Successful Pregnancies in Patients Undergoing in Vitro Fertilization. Fertil. Steril. 2003, 79, 1317–1322. [Google Scholar] [CrossRef]
- Krasnopolskaya, K.V.; Nazarenko, T.A.; Fedorov, A.A. Clinical Outcomes of IVF Programs with Donor Oocytes Using Endometrial Scratching Technology in Patients with Extreme Moderate Endometrial Development Delay. Med. Alph. 2017, 3, 46–50. [Google Scholar]
- Li, J.; Mo, S.; Chen, Y. The Effect of G-CSF on Infertile Women Undergoing IVF Treatment: A Meta-Analysis. Syst. Biol. Reprod. Med. 2017, 63, 239–247. [Google Scholar] [CrossRef]
- Miralaei, S.; Ashrafi, M.; Arabipoor, A.; Zolfaghari, Z.; Taghvaei, S. The Incidence Rate of Unresponsive Thin Endometrium in Frozen Embryo Transfer Cycles: A Case-Series of Therapy with Granulocyte Colony Stimulating Factor. Int. J. Reprod. Biomed. 2019, 17, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Guedez, L.; Stetler-Stevenson, W.G.; Wolff, L.; Wang, J.; Fukushima, P.; Mansoor, A.; Stetler-Stevenson, M. In Vitro Suppression of Programmed Cell Death of B Cells by Tissue Inhibitor of Metalloproteinases-1. J. Clin. Investig. 1998, 102, 2002–2010. [Google Scholar] [CrossRef]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea Polyphenol (-)-Epigallocatechin-3-Gallate Inhibits DNA Methyltransferase and Reactivates Methylation-Silenced Genes in Cancer Cell Lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar] [PubMed]
- Jeong, W.-S.; Kim, I.-W.; Hu, R.; Kong, A.-N.T. Modulatory Properties of Various Natural Chemopreventive Agents on the Activation of NF-kappaB Signaling Pathway. Pharm. Res. 2004, 21, 661–670. [Google Scholar] [CrossRef]
- Bebneva, T.N.; Muizhnek, E.L.; Rogovskaya, S.I.; Kiselev, V.; Ashrafyan, L.A.; Hamoshin, B.B. Pathogenetic treatment of neoplastic processes of the cervix: New approaches. Doctor. Ru. 2016, 3, 9–14. [Google Scholar]
- Centofanti, F.; Buono, A.; Verboni, M.; Tomino, C.; Lucarini, S.; Duranti, A.; Pandolfi, P.P.; Novelli, G. Synthetic Methodologies and Therapeutic Potential of Indole-3-Carbinol (I3C) and Its Derivatives. Pharmaceuticals 2023, 16, 240. [Google Scholar] [CrossRef]
- Lyn-Cook, B.D.; Mohammed, S.I.; Davis, C.; Word, B.; Haefele, A.; Wang, H.; Hammons, G. Gender Differences in Gemcitabine (Gemzar) Efficacy in Cancer Cells: Effect of Indole-3-Carbinol. Anticancer Res. 2010, 30, 4907–4913. [Google Scholar]
- Thomson, C.A.; Chow, H.H.S.; Wertheim, B.C.; Roe, D.J.; Stopeck, A.; Maskarinec, G.; Altbach, M.; Chalasani, P.; Huang, C.; Strom, M.B.; et al. A Randomized, Placebo-Controlled Trial of Diindolylmethane for Breast Cancer Biomarker Modulation in Patients Taking Tamoxifen. Breast Cancer Res. Treat. 2017, 165, 97–107. [Google Scholar] [CrossRef]
- Smetnik, S.A.; Smetnik, S.V.; Kiselev, K.V. Experience of using indole-3-carbinol in the treatment of breast diseases and the prevention of breast cancer. Akusherstvo i Ginekol. 2017, 2, 106–112. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Zhang, S.; Akbari, M.; Salmena, L.; Llacuachaqui, M.; Zeligs, M.; Sun, P.; Narod, S.A. BRCA1 mRNA Levels Following a 4-6-Week Intervention with Oral 3,3’-Diindolylmethane. Br. J. Cancer 2014, 111, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Kadesnikova Yu, A.; Tikhonovskaya, O.A.; Petrov, I.A.; Okorokov, A.O.; Logvinov, S.V. The effect of indole-3-carbinol on the reproductive health of women with functional ovarian cysts. Sib. J. Clin. Exp. Med. 2010, 25, 123–125. [Google Scholar]
- Tayukina, I.P.; Mustafina, L.M.; Tikhonovskaya, O.A.; Logvinov, S.V. Expression of steroid hormones receptors and morphofunctional condition endometrium at patients with anomalous uterine bleedings. Bull. Sib. Med. 2009, 8, 50–55. [Google Scholar] [CrossRef]
- Tayukina, I.P.; Mustafina, L.М.; Tikhonovskaya, O.A.; Logvinov, S.V. Morphology and function of the endometrium and expression of sex steroid hormone receptors in patients with infertility. Sib. J. Clin. Exp. Med. 2010, 25, 110–112. [Google Scholar]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Pricl, S.; Townley, H.; Thorat, N. Novel Epigenetic Therapeutic Strategies and Targets in Cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166552. [Google Scholar] [CrossRef]
- Griazeva, E.D.; Fedoseeva, D.M.; Radion, E.I.; Ershov, P.V.; Meshkov, I.O.; Semyanihina, A.V.; Makarova, A.S.; Makarov, V.V.; Yudin, V.S.; Keskinov, A.A.; et al. Current Approaches to Epigenetic Therapy. Epigenomes 2023, 7, 23. [Google Scholar] [CrossRef]
- Dai, W.; Qiao, X.; Fang, Y.; Guo, R.; Bai, P.; Liu, S.; Li, T.; Jiang, Y.; Wei, S.; Na, Z.; et al. Epigenetics-Targeted Drugs: Current Paradigms and Future Challenges. Signal Transduct. Target. Ther. 2024, 9, 332. [Google Scholar] [CrossRef]
- Oronsky, B.T.; Oronsky, A.L.; Lybeck, M.; Oronsky, N.C.; Scicinski, J.J.; Carter, C.; Day, R.M.; Rodriguez Orengo, J.F.; Rodriguez-Torres, M.; Fanger, G.F.; et al. Episensitization: Defying Time’s Arrow. Front. Oncol. 2015, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, P.; Chen, C.; Ding, W.; Tillement, O.; Bai, H.; Zhang, S. Targeting Epigenetic Regulators as a Promising Avenue to Overcome Cancer Therapy Resistance. Signal Transduct. Target. Ther. 2025, 10, 219. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Method | Brief Description/Biomaterial | Advantages | Disadvantages |
|---|---|---|---|
| ERA (Endometrial Receptivity Array) (Transcriptomic Analysis) | Analysis of 238+ gene expression to determine endometrial receptivity status and personalize the embryo transfer day (pWOI). Biomaterial: Endometrial biopsy. |
|
|
| rsERT (nirsERT) (Non-Invasive Transcriptomic Test) | RNA sequencing to assess the transcriptomic profile. Biomaterial: Uterine fluid. |
|
|
| Tests for Gene Methylation Levels (HOXA10, HOXA11) (Epigenetic Analysis) | Assessment of methylation levels in gene promoter regions using PCR. Biomaterial: Endometrial biopsy. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudlay, D.; Kiselev, V.; Sukhikh, G. HOXA10 and HOXA11 Methylation: Epigenetic Barriers to Endometrial Receptivity in ART. Genes 2025, 16, 1230. https://doi.org/10.3390/genes16101230
Kudlay D, Kiselev V, Sukhikh G. HOXA10 and HOXA11 Methylation: Epigenetic Barriers to Endometrial Receptivity in ART. Genes. 2025; 16(10):1230. https://doi.org/10.3390/genes16101230
Chicago/Turabian StyleKudlay, Dmitry, Vsevolod Kiselev, and Gennady Sukhikh. 2025. "HOXA10 and HOXA11 Methylation: Epigenetic Barriers to Endometrial Receptivity in ART" Genes 16, no. 10: 1230. https://doi.org/10.3390/genes16101230
APA StyleKudlay, D., Kiselev, V., & Sukhikh, G. (2025). HOXA10 and HOXA11 Methylation: Epigenetic Barriers to Endometrial Receptivity in ART. Genes, 16(10), 1230. https://doi.org/10.3390/genes16101230







