Genetic Prediction of Eye, Hair, and Skin Color: Forensic Applications and Challenges in Latin American Populations
Abstract
1. Introduction
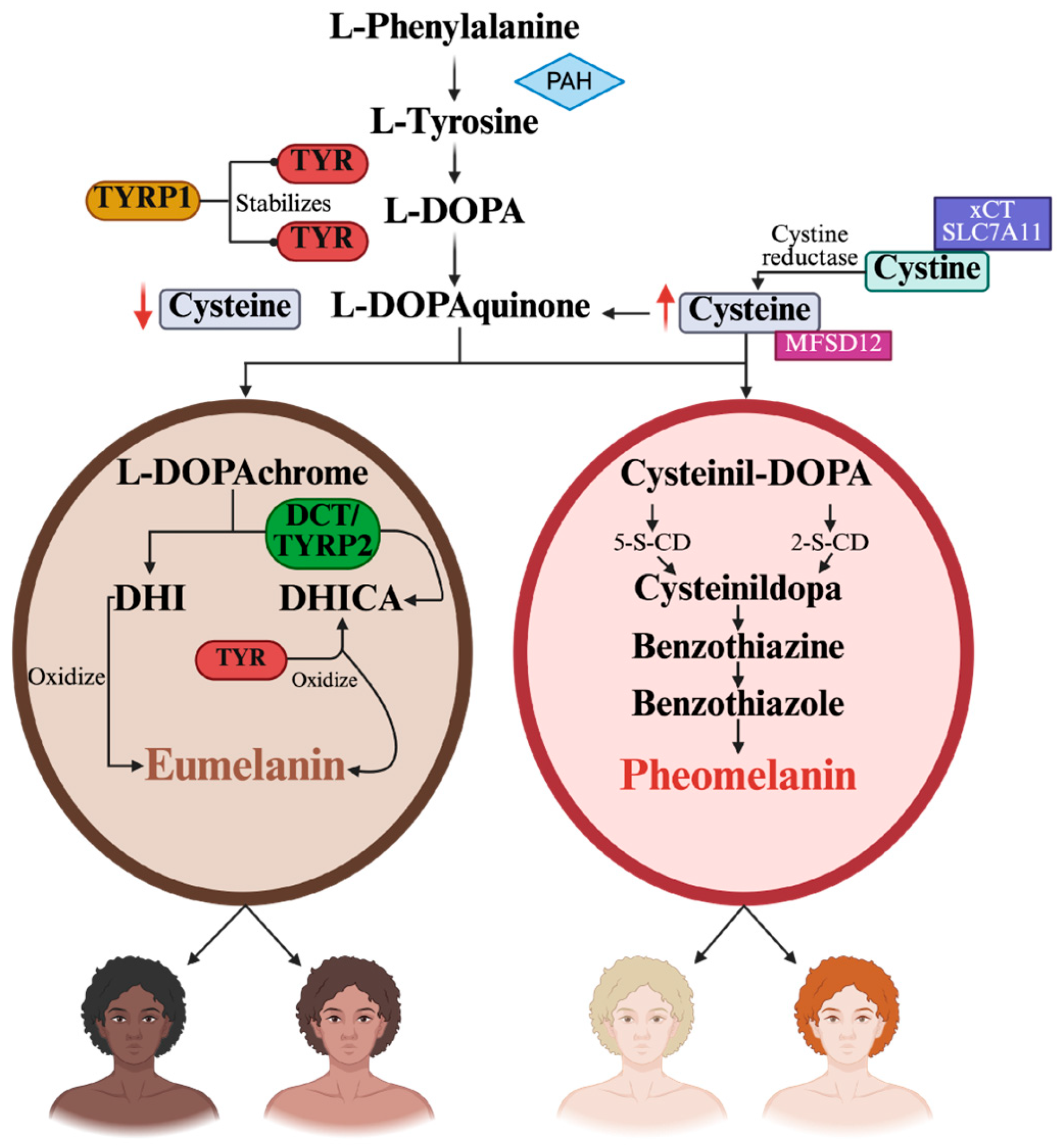
2. Genetic Basis of Eye, Hair, and Skin Color
3. Phenotype Prediction Tools
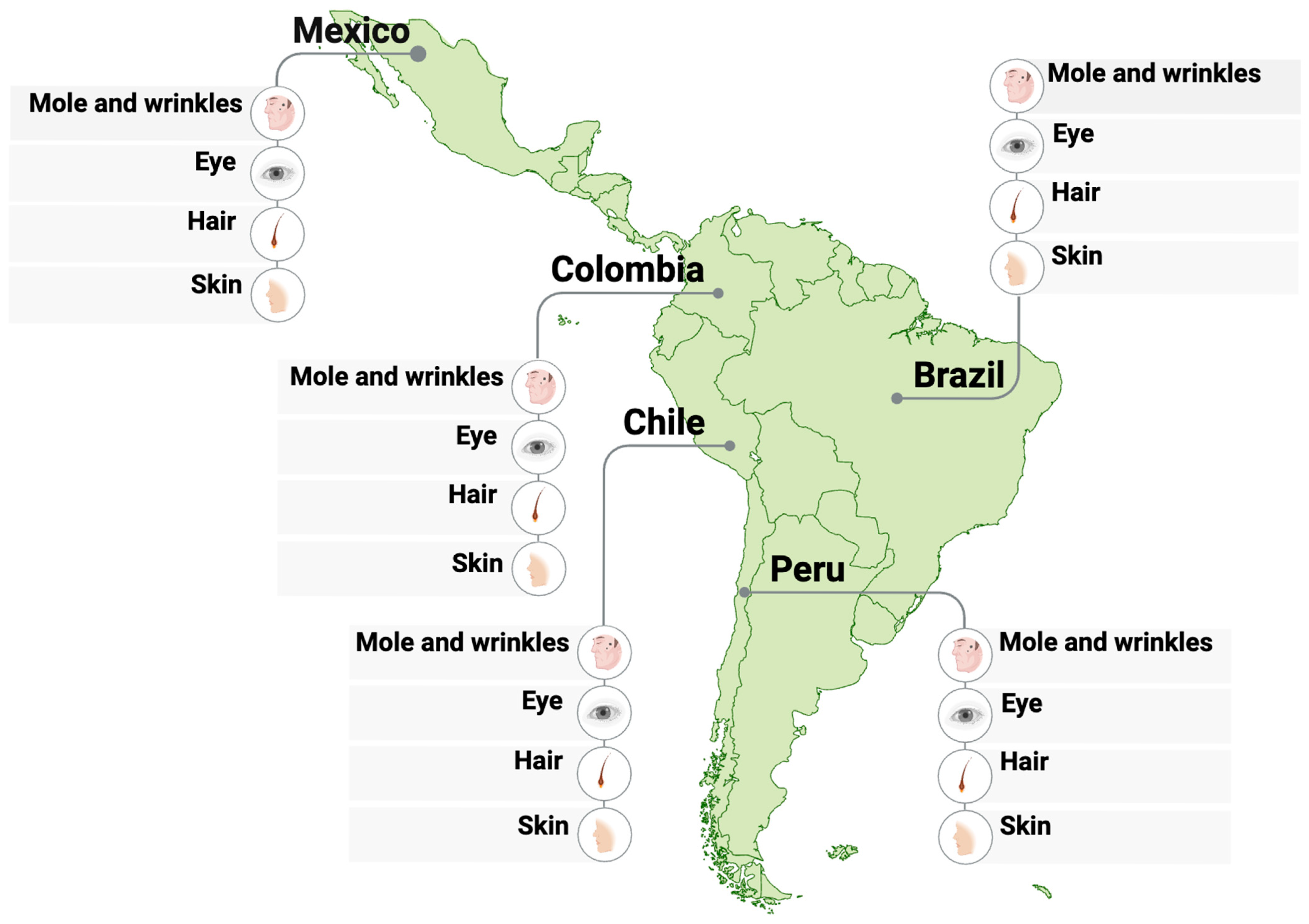
4. Forensic Applications in Latin America
5. Ethical and Technical Challenges
6. Discussion: Future Directions and Recommendations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lemos, Y.V.; Furtado, A.N.; Lima, A.Z.; Dionísio, A.S.; Araújo, R.M.; Cunha, E. Human identification by medical findings in a forensic anthropology context. Forensic Sci. Res. 2024, 9, owae041. [Google Scholar] [CrossRef]
- Rathnayake, R.W.R.K.; Marasinghe, E.; Perera, L.R.T.; Bandaranayake, V.J. Exploring forensic challenges and insights: Implications of genetic analyses on degraded DNA Samples. Int. J. Forensic Sci. 2024, 9, 1–9. [Google Scholar] [CrossRef]
- Zubakov, D.; Chamier-Ciemińska, J.; Kokmeijer, I.; Maciejewska, A.; Martínez, P.; Pawłowski, R.; Kayser, M. Introducing novel type of human DNA markers for forensic tissue identification: DNA copy number variation allows the detection of blood and semen. Forensic Sci. Int. Genet. 2018, 36, 112–118. [Google Scholar] [CrossRef]
- Fabbri, M.; Alfieri, L.; Mazdai, L.; Frisoni, P.; Gaudio, R.M.; Neri, M. Application of forensic DNA phenotyping for prediction of eye, hair and skin colour in highly decomposed bodies. Healthcare 2023, 11, 647. [Google Scholar] [CrossRef]
- Tozzo, P.; Politi, C.; Delicati, A.; Gabbin, A.; Caenazzo, L. External visible characteristics prediction through SNPs analysis in the forensic setting: A review. Front. Biosci. 2021, 26, 828–850. [Google Scholar] [CrossRef]
- Chaitanya, L.; Breslin, K.; Zuñiga, S.; Wirken, L.; Pośpiech, E.; Kukla-Bartoszek, M.; Walsh, S. The HIrisPlex-S system for eye, hair and skin colour prediction from DNA: Introduction and forensic developmental validation. Forensic Sci. Int. Genet. 2018, 35, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Brancato, D.; Coniglio, E.; Bruno, F.; Agostini, V.; Saccone, S.; Federico, C. Forensic DNA phenotyping: Genes and genetic variants for Eye Color Prediction. Genes 2023, 14, 1604. [Google Scholar] [CrossRef] [PubMed]
- Abbatangelo, C.L.; Lona Durazo, F.; Wendt, F.R.; Parra, E.J.; Novroski, N.M. From genetic association to forensic prediction: Computational methods and tools for identifying phenotypically informative single nucleotide polymorphisms. Forensic Genom. 2023, 3, 47–68. [Google Scholar] [CrossRef]
- Walsh, S.; Chaitanya, L.; Clarisse, L.; Wirken, L.; Draus-Barini, J.; Kovatsi, L.; Maeda, H.; Ishikawa, T.; Sijen, T.; de Knijff, P.; et al. Developmental validation of the HIrisPlex system: DNA-based eye and hair colour prediction for forensic and anthropological usage. Forensic Sci. Int. Genet. 2014, 9, 150–161. [Google Scholar] [CrossRef]
- Peng, F.; Zhu, G.; Hysi, P.G.; Eller, R.J.; Chen, Y.; Li, Y.; Hamer, M.A.; Zeng, C.; Hopkins, R.L.; Jacobus, C.L.; et al. genome-wide Association studies identify multiple genetic loci influencing eyebrow color variation in Europeans. J. Investig. Dermatol. 2019, 139, 1601–1605. [Google Scholar] [CrossRef]
- Hernando, B.; Ibanez, M.V.; Deserio-Cuesta, J.A.; Soria-Navarro, R.; Vilar-Sastre, I.; Martinez-Cadenas, C. Genetic determinants of freckle occurrence in the Spanish population: Towards ephelides prediction from human DNA samples. Forensic Sci. Int. Genet. 2018, 33, 38–47. [Google Scholar] [CrossRef]
- Pośpiech, E.; Karłowska-Pik, J.; Marcinska, M.; Abidi, S.; Andersen, J.D.; Berge, M.V.D.; Carracedo, A.; Eduardoff, M.; Freire-Aradas, A.; Morling, N.; et al. Evaluation of the predictive capacity of DNA variants associated with straight hair in Europeans. Forensic Sci. Int. Genet. 2015, 19, 280–288. [Google Scholar] [CrossRef]
- Marcinska, M.; Pospiech, E.; Abidi, S.; Andersen, J.D.; van den Berge, M.; Carracedo, A.; Eduardoff, M.; Marczakiewicz-Lustig, A.; Morling, N.; Sijen, T.; et al. Evaluation of DNA variants associated with androgenetic alopecia and their potential to predict male pattern baldness. PLoS ONE 2015, 10, e0127852. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhong, K.; Jing, X.; Uitterlinden, A.G.; Hendriks, A.E.J.; Drop, S.L.S.; Kayser, M. Update on the predictability of tall stature from DNA markers in Europeans. Forensic Sci. Int. Genet. 2019, 42, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dabas, P.; Jain, S.; Khajuria, H.; Nayak, B.P. Forensic DNA phenotyping: Inferring phenotypic traits from crime scene DNA. J. Forensic Leg. Med. 2022, 88, 102351. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.C. Forensic DNA phenotyping: A promising tool to aid forensic investigation. Current situation. Span. J. Leg. Med. 2020, 46, 183–190. [Google Scholar] [CrossRef]
- Adhikari, K.; Mendoza-Revilla, J.; Chacón-Duque, J.C.; Fuentes-Guajardo, M.; Ruiz-Linares, A. Admixture in Latin America. Curr. Opin. Genet. Dev. 2016, 41, 106–114. [Google Scholar] [CrossRef]
- Linares, A.; Adhikari, K.; Acuña-Alonzo, V.; Quinto-Sanchez, M.; Jaramillo, C.; Arias, W.; Fuentes, M.; Pizarro, M.; Everardo, P.; de Avila, F.; et al. Admixture in Latin America: Geographic structure, phenotypic diversity and self-perception of ancestry based on 7342 individuals. PLoS Genet. 2014, 10, e1004572. [Google Scholar] [CrossRef]
- Suarez-Kurtz, G.; Parra, E.J. Population diversity in pharmacogenetics: A Latin American perspective. Adv. Pharmacol. 2018, 83, 133–154. [Google Scholar] [CrossRef]
- Manga, P.; Loftus, S. Genetics of skin, hair, and eye color in human pigmentation disorders. Ann. Hum. Genet. 2025, 89, e70003. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, melanin, and melanogenesis: The yin and yang relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Snyman, M.; Walsdorf, R.E.; Wix, S.N.; Gill, J.G. The metabolism of melanin synthesis—From melanocytes to melanoma. Pigment. Cell Melanoma Res. 2024, 37, 438–452. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The biochemistry of melanogenesis: An insight into the function and mechanism of melanogenesis-related proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef]
- Branicki, W.; Brudnik, U.; Wojas-Pelc, A. Interactions between HERC2, OCA2 and MC1R may influence human pigmentation phenotype. Ann. Hum. Genet. 2009, 73, 160–170. [Google Scholar] [CrossRef]
- Parra, E.J. Human pigmentation variation: Evolution, genetic basis, and implications for public health. Am. J. Phys. Anthropol. 2007, 134 (Suppl. S45), 85–105. [Google Scholar] [CrossRef]
- Brancato, D.; Bruno, F.; Coniglio, E.; Sturiale, V.; Saccone, S.; Federico, C. The chromatin organization close to SNP rs12913832, involved in eye color variation, is evolutionary conserved in vertebrates. Int. J. Mol. Sci. 2024, 25, 6602. [Google Scholar] [CrossRef]
- Visser, M.; Kayser, M.; Palstra, R.J. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012, 22, 446–455. [Google Scholar] [CrossRef]
- Sturm, R.A.; Larsson, M. Genetics of human iris colour and patterns. Pigment. Cell Melanoma Res. 2009, 22, 544–562. [Google Scholar] [CrossRef]
- Hohl, D.M.; Bezus, B.; Ratowiecki, J.; Catanesi, C.I. Genetic and phenotypic variability of iris color in Buenos Aires population. Genet. Mol. Biol. 2018, 41, 50–58. [Google Scholar] [CrossRef]
- Salvo, N.M.; Andersen, J.D.; Janssen, K.; Meyer, O.L.; Berg, T.; Børsting, C.; Olsen, G.H. Association between variants in the OCA2-HERC2 region and blue eye colour in HERC2 rs12913832 AA and AG individuals. Genes 2023, 14, 698. [Google Scholar] [CrossRef]
- Beaumont, K.A.; Shekar, S.N.; Newton, R.A.; James, M.R.; Stow, J.L.; Duffy, D.L.; Sturm, R.A. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum. Mol. Genet. 2007, 16, 2249–2260, Erratum in Hum. Mol. Genet. 2007, 16, 2988. [Google Scholar] [CrossRef]
- Le, L.; Sirés-Campos, J.; Raposo, G.; Delevoye, C.; Marks, M.S. Melanosome biogenesis in the pigmentation of mammalian skin. Integr. Comp. Biol. 2021, 61, 1517–1545. [Google Scholar] [CrossRef]
- Stokowski, R.P.; Pant, P.V.; Dadd, T.; Fereday, A.; Hinds, D.A.; Jarman, C.; Filsell, W.; Ginger, R.S.; Green, M.R.; van der Ouderaa, F.J.; et al. A genomewide association study of skin pigmentation in a South Asian population. Am. J. Hum. Genet. 2007, 81, 1119–1132. [Google Scholar] [CrossRef]
- Bastian, B.C.; Pinkel, D. Expanding the genetic spectrum of pigmentation. Pigment. Cell Melanoma Res. 2008, 21, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R.A. Molecular genetics of human pigmentation diversity. Hum. Mol. Genet. 2009, 18, R9–R17. [Google Scholar] [CrossRef] [PubMed]
- Mallick, C.B.; Iliescu, F.M.; Möls, M.; Hill, S.; Tamang, R.; Chaubey, G.; Goto, R.; Ho, S.Y.; Gallego Romero, I.; Crivellaro, F.; et al. The light skin allele of SLC24A5 in South Asians and Europeans shares identity by descent. PLoS Genet. 2013, 9, e1003912. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Ginger, R.S.; Dadd, T.; Gunn, D.; Lim, F.L.; Sawicka, M.; Sandel, M.; Schnetkamp, P.P.; Green, M.R. NCKX5, a natural regulator of human skin colour variation, regulates the expression of key pigment genes MC1R and alpha-MSH and alters cholesterol homeostasis in normal human melanocytes. Adv. Exp. Med. Biol. 2013, 961, 95–107. [Google Scholar] [CrossRef]
- White, D.; Rabago-Smith, M. Genotype–phenotype associations and human eye color. J. Hum. Genet. 2011, 56, 5–7. [Google Scholar] [CrossRef]
- Pośpiech, E.; Wojas-Pelc, A.; Walsh, S.; Liu, F.; Maeda, H.; Ishikawa, T.; Skowron, M.; Kayser, M.; Branicki, W. The common occurrence of epistasis in the determination of human pigmentation and its impact on DNA-based pigmentation phenotype prediction. Forensic Sci. Int. Genet. 2014, 11, 64–72. [Google Scholar] [CrossRef]
- Beleza, S.; Johnson, N.A.; Candille, S.I.; Absher, D.M.; Coram, M.A.; Lopes, J.; Campos, J.; Araújo, I.I.; Anderson, T.M.; Vilhjálmsson, B.J.; et al. Genetic architecture of skin and eye color in an African-European admixed population. PLoS Genet. 2013, 9, e1003372. [Google Scholar] [CrossRef]
- Pośpiech, E.; Draus-Barini, J.; Kupiec, T.; Wojas-Pelc, A.; Branicki, W. Gene-gene interactions contribute to eye colour variation in humans. J. Hum. Genet. 2011, 56, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, K.; Mendoza-Revilla, J.; Sohail, A.; Fuentes-Guajardo, M.; Lampert, J.; Chacón-Duque, J.C.; Hurtado, M.; Villegas, V.; Granja, V.; Acuña-Alonzo, V.; et al. A GWAS in Latin Americans highlights the convergent evolution of lighter skin pigmentation in Eurasia. Nat. Commun. 2019, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.; Kittles, R.; Shriver, M. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat. Genet. 2004, 36 (Suppl. S11), S54–S60. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Douville, C.; Wang, C.; Niknafs, N.; Yeo, G.; Beleva-Guthrie, V.; Carter, H.; Stenson, P.D.; Cooper, D.N.; Li, B. A probabilistic model to predict clinical phenotypic traits from genome sequencing. PLoS Comput. Biol. 2014, 10, e1003825. [Google Scholar] [CrossRef]
- Kwok, P. Approaches to allele frequency determination. Pharmacogenomics 2000, 1, 231–235. [Google Scholar] [CrossRef]
- Krishnan, V.G.; Westhead, D.R. A comparative study of machine-learning methods to predict the effects of single nucleotide polymorphisms on protein function. Bioinformatics 2003, 19, 2199–2209. [Google Scholar] [CrossRef]
- Medvedev, A.; Sharma, S.M.; Tsatsorin, E.; Nabieva, E.; Yarotsky, D. Human genotype-to-phenotype predictions: Boosting accuracy with nonlinear models. PLoS ONE 2022, 17, e0273293. [Google Scholar] [CrossRef]
- Palmal, S.; Adhikari, K.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; De Cerqueira, C.C.S.; Bonfante, B.; Chacón-Duque, J.C.; Sohail, A.; Hurtado, M.; Villegas, V.; et al. Prediction of eye, hair and skin colour in Latin Americans. Forensic Sci. Int. Genet. 2021, 53, 102517. [Google Scholar] [CrossRef]
- Carratto, T.M.; Marcorin, L.; Debortoli, G.; Silva, G.; Fracasso, N.; Oliveira, M.; Pereira, A.; Silva, A.; Donadi, E.; Simões, A.; et al. Evaluation of the HIrisPlex-S system in a Brazilian population sample. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 794–796. [Google Scholar] [CrossRef]
- Pereira, J.L.; De Souza, C.A.; Neyra, J.E.; Leite, J.M.; Cerqueira, A.; Mingroni-Netto, R.C.; Soler, J.M.; Rogero, M.M.; Sarti, F.M.; Fisberg, R.M. Genetic ancestry and self-reported “skin color/race” in the urban admixed population of São Paulo city, Brazil. Genes 2024, 15, 917. [Google Scholar] [CrossRef]
- Zou, J.; Schiebinger, L. Ensuring that biomedical AI benefits diverse populations. EBioMedicine 2021, 67, 103358. [Google Scholar] [CrossRef]
- MacLean, C.; Lamparello, A. Forensic DNA phenotyping in criminal investigations and criminal courts: Assessing and mitigating the dilemmas inherent in the science. Recent Adv. DNA Gene Seq. (Former. Recent Pat. DNA Gene Seq.) 2015, 8, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Simcoe, M.; Valdes, A.; Liu, F.; Furlotte, N.A.; Evans, D.M.; Hemani, G.; Ring, S.M.; Smith, G.D.; Duffy, D.L.; Zhu, G. Genome-wide association study in almost 195,000 individuals identifies 50 previously unidentified genetic loci for eye color. Sci. Adv. 2021, 7, eabd1239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Keating, B.J. Trans-ethnic genome-wide association studies: Advantages and challenges of mapping in diverse populations. Genome Med. 2014, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Granja, R.; Machado, H. Forensic DNA phenotyping and its politics of legitimation and contestation: Views of forensic geneticists in Europe. Soc. Stud. Sci. 2020, 53, 850–868. [Google Scholar] [CrossRef]
- Toom, V.; Wienroth, M.; M’charek, A.; Prainsack, B.; Williams, R.; Duster, T.; Heinemann, T.; Kruse, C.; Machado, H.; Murphy, E. Approaching ethical, legal and social issues of emerging forensic DNA phenotyping (FDP) technologies comprehensively: Reply to ‘Forensic DNA phenotyping: Predicting human appearance from crime scene material for investigative purposes’ by Manfred Kayser. Forensic Sci. Int. Genet. 2016, 22, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Van Laan, M.C. The genetic witness: Forensic DNA phenotyping. J. Emerg. Forensic Sci. Res. 2017, 2, 33–52. Available online: https://jefsr.uwindsor.ca/index.php/jefsr/article/download/5005/4303 (accessed on 29 September 2025).
- Kushwaha, N.P.; Ahmed, N.U.; Rana, N.M. Forensic DNA phenotyping: A promising tool to predict human appearance for forensic purposes. GSC Biol. Pharm. Sci. 2023, 23, 34–41. [Google Scholar] [CrossRef]
- Kayser, M.; Branicki, W.; Parson, W.; Phillips, C. Recent advances in Forensic DNA Phenotyping of appearance, ancestry and age. Forensic Sci. Int. Genet. 2023, 65, 102870. [Google Scholar] [CrossRef]
- Marin, E.S.; Cruz-Santiago, A. Antigone’s forensic DNA database: Forensic technologies and the search for the disappeared in Mexico. Athenea Digit. Rev. Pensam. E Investig. Soc. 2018, 18, 129. [Google Scholar] [CrossRef]
- Nyberg Sørensen, N.; Huttunen, L. Missing migrants and the politics of disappearance in armed conflicts and migratory contexts. Ethnos 2020, 87, 321–337. [Google Scholar] [CrossRef]
- Aguilar-Velázquez, J.A.; Llamas-de-Dios, B.J.; Córdova-Mercado, M.F.; Coronado-Ávila, C.E.; Salas-Salas, O.; López-Quintero, A.; Ramos-González, B.; Rangel-Villalobos, H. Accuracy of eye and hair color prediction in Mexican mestizos from Monterrey city based on ForenSeqTM DNA signature prep. Genes 2023, 14, 1120. [Google Scholar] [CrossRef]
- Aguilar-Velázquez, J.A.; García-Aceves, M.E.; Córdova-Mercado, M.F.; Guardado-Estrada, M.; Peña-Durán, E.; Villavicencio-Queijeiro, A.; Salas-Salas, O.; Coronado-Ávila, C.E.; Cárdenas-Monroy, C.A.; Ramos-González, B.; et al. Choice between DNA primer sets (A or B) of the ForenSeq kit: Forensic evaluation in a Mexican admixed population sample. Int. J. Leg. Med. 2025, 139, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Carratto, T.M.T. Análise da Acurácia de Diferentes Sistemas Genéticos de Predição de Fenótipos de Pigmentação em Amostra de Indivíduos Miscigenados da População Brasileira. Master’s Thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, São Paulo, Brazil, 2020. [Google Scholar] [CrossRef]
- Egaña, S.; Turner, S.; Doretti, M.; Bernardi, P.; Ginarte, A. Commingled remains and human rights investigations. In Recovery, Analysis, and Identification of Commingled Human Remains; Humana Press: Totowa, NJ, USA, 2008; pp. 57–80. [Google Scholar] [CrossRef]
- Navarro-López, B.; Baeta, M.; Suárez-Ulloa, V.; Martos-Fernández, R.; Moreno-López, O.; Martínez-Jarreta, B.; Jiménez, S.; Olalde, I.; De Pancorbo, M.M. Exploring eye, hair, and skin pigmentation in a Spanish population: Insights from Hirisplex-S predictions. Genes 2024, 15, 1330. [Google Scholar] [CrossRef] [PubMed]
- Galanter, J.M.; Fernandez-Lopez, J.C.; Gignoux, C.R.; Barnholtz-Sloan, J.; Fernandez-Rozadilla, C.; Via, M.; Hidalgo-Miranda, A.; Contreras, A.V.; Figueroa, L.U.; Raska, P.; et al. Development of a panel of genome-wide ancestry informative markers to study admixture throughout the Americas. PLoS Genet. 2012, 8, e1002554. [Google Scholar] [CrossRef] [PubMed]
- Boldo, A.B. Fenotipado Forense de ADN: Desafíos jurídicos en la investigación criminal y su aplicación en Europa. Rev. Derecho Genoma Hum. Genética Biotecnol. Med. Av. 2005, in press. [Google Scholar] [CrossRef]
- Chen, Y.; André, M.; Adhikari, K.; Blin, M.; Bonfante, B.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Palmal, S.; Chacón-Duque, J.C.; Hurtado, M.; et al. A genome-wide association study identifies novel gene associations with facial skin wrinkling and mole count in Latin Americans. Br. J. Dermatol. 2021, 185, 988–998. [Google Scholar] [CrossRef]
- Adhikari, K.; Fontanil, T.; Cal, S.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Chacón-Duque, J.C.; Al-Saadi, F.; Johansson, J.A.; Quinto-Sanchez, M.; Acuña-Alonzo, V.; et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nat. Commun. 2016, 7, 10815. [Google Scholar] [CrossRef]
- Fracasso, N.C.d.A.; de Andrade, E.S.; Wiezel, C.E.V.; Andrade, C.C.F.; Zanão, L.R.; da Silva, M.S.; Marano, L.A.; Donadi, E.A.C.; Castelli, E.; Simões, A.L.; et al. Haplotypes from the SLC45A2 gene are associated with the presence of freckles and eye, hair and skin pigmentation in Brazil. Leg. Med. 2017, 25, 43–51. [Google Scholar] [CrossRef]
- Carratto, T.M.T.; Marcorin, L.; do Valle-Silva, G.; de Oliveira, M.L.G.; Donadi, E.A.; Simões, A.L.; Castelli, E.C.; Mendes-Junior, C.T. Prediction of eye and hair pigmentation phenotypes using the HIrisPlex system in a Brazilian admixed population sample. Int. J. Leg. Med. 2021, 135, 1329–1339. [Google Scholar] [CrossRef]
- Zhou, G.; Yolou, I.; Xie, Y.; Zhao, H. Leveraging local ancestry and cross-ancestry genetic architecture to improve genetic prediction of complex traits in admixed populations. Am. J. Hum. Genet. 2025, 112, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Gemenet, D.C.; De Boeck, B.; Da Silva Pereira, G.; Kitavi, M.N.; Ssali, R.T.; Utoblo, O.; Swanckaert, J.; Carey, E.; Gruneberg, W.; Yada, B.; et al. When a phenotype is not the genotype: Implications of phenotype misclassification and pedigree errors in genomics-assisted breeding of sweetpotato (Ipomoea batatas (L.) Lam.). bioRxiv 2019. preprint. [Google Scholar] [CrossRef]
- Raji, I.D.; Gebru, T.; Mitchell, M.; Buolamwini, J.; Lee, J.; Denton, E. Saving face: Investigating the ethical concerns of facial recognition auditing. In AIES ‘20: Proceedings of the AAAI/ACM Conference on AI, Ethics, and Society, New York NY USA, 7–9 February 2020; Association for Computing Machinery: New York, NY, USA, 2020; pp. 145–151. [Google Scholar] [CrossRef]
- Bartram, I.; Plümecke, T.; Schultz, S. Genetic racial profiling: Extended DNA analyses and entangled processes of discrimination. Sci. Technol. Stud. 2022, 35, 44–69. [Google Scholar] [CrossRef]
- Hopman, R. Opening up forensic DNA phenotyping: The logics of accuracy, commonality and valuing. New Genet. Soc. 2020, 39, 424–440. [Google Scholar] [CrossRef]
- Coquet, M.; Terrado-Ortuño, N. Forensic DNA phenotyping: Privacy breach, bias reification and the pitfalls of abstract assessments of rights. Int. J. Police Sci. Manag. 2023, 25, 262–279. [Google Scholar] [CrossRef]
- Wright, G.E.; Adeyemo, A.A.; Tiffin, N. Informed consent and ethical re-use of African genomic data. Hum. Genom. 2014, 8, 18. [Google Scholar] [CrossRef]
- Pina, E.; Ramos, J.; Jorge, H.; Váz, P.; Silva, J.; Wanzeller, C.; Abbasi, M.; Martins, P. Data Privacy and Ethical Considerations in Database Management. J. Cybersecur. Priv. 2024, 4, 494–517. [Google Scholar] [CrossRef]
- Burke, W.; Beskow, L.M.; Trinidad, S.B.; Fullerton, S.M.; Brelsford, K. Informed consent in translational genomics: Insufficient without trustworthy governance. J. Law Med. Ethics 2018, 46, 79–86. [Google Scholar] [CrossRef]
- O’Doherty, J.E.; Lebedev, M.A.; Ifft, P.J.; Zhuang, K.Z.; Shokur, S.; Bleuler, H.; Nicolelis, M.A.L. Active tactile exploration using a brain–machine–brain interface. Nature 2011, 479, 228–231. [Google Scholar] [CrossRef]
- Samuel, G.; Prainsack, B. Civil society stakeholder views on forensic DNA phenotyping: Balancing risks and benefits. Forensic Sci. Int. Genet. 2019, 43, 102157. [Google Scholar] [CrossRef] [PubMed]
- Bodner, M.; Parson, W. Informed consent for forensic genetic population studies: Status quo and a call for harmonization. Forensic Sci. Int. Genet. 2025, 78, 103298. [Google Scholar] [CrossRef] [PubMed]
- Zieger, M.; Scudder, N. Ethical and legal reflections on secondary research using genetic data acquired for criminal investigation purposes. Forensic Sci. Int. Genet. 2025, 75, 103178. [Google Scholar] [CrossRef]
- Salzano, F.M.; Sans, M. Interethnic admixture and the evolution of Latin American populations. Genet. Mol. Biol. 2014, 37, 151–170. [Google Scholar] [CrossRef]
- Marano, L.A.; Andersen, J.D.; Goncalves, F.T.; Garcia, A.L.O.; Fridman, C. Evaluation of HIrisplex-S system markers for eye, skin and hair color prediction in an admixed Brazilian population. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 427–428. [Google Scholar] [CrossRef]
- Shriver, M.D.; Smith, M.W.; Jin, L.; Marcini, A.; Akey, J.M.; Deka, R.; Ferrell, R.E. Ethnic-affiliation estimation by use of population-specific DNA markers. Am. J. Hum. Genet. 1997, 60, 957–964. [Google Scholar]
- Becher, D.; Jmel, H.; Kheriji, N.; Sarno, S.; Kefi, R. Genetic landscape of forensic DNA phenotyping markers among Mediterranean populations. Forensic Sci. Int. 2024, 354, 111906. [Google Scholar] [CrossRef]
- Campos, A.H.; De Dávila, M.T. Biobanks in Latin America: Insights from a multinational survey conducted in 2021 during the COVID-19 era. In Biopreservation and Biobanking; Mary Ann Liebert Inc.: New Rochelle, NY, USA, 2025. [Google Scholar] [CrossRef]
- Saluja, S.; Anderson, S.G. Opportunities for promoting open data in the Caribbean through biobanks. Rev. Panam. Salud Pública 2025, 49, e11. [Google Scholar] [CrossRef]
- Lee, Y.S.; Garrido, N.L.B.; Lord, G.; Maggio, Z.A.; Khomtchouk, B.B. Ethical considerations for biobanks serving underrepresented populations. Bioethics 2024, 39, 240–249. [Google Scholar] [CrossRef]
- Rivera-Paredez, B.; Hidalgo-Bravo, A.; López-Montoya, P.; Becerra Cervera, A.; Patiño, N.; Denova-Gutiérrez, E.; Salmerón, J.; Velázquez-Cruz, R. Skin pigmentation related variants in Mexican population and interaction effects on serum 25(OH)D concentration and vitamin D deficiency. Sci. Rep. 2024, 14, 17378. [Google Scholar] [CrossRef]
- Terrado-Ortuño, N.; May, P. Forensic DNA phenotyping: A review on SNP panels, genotyping techniques, and prediction models. Forensic Sci. Res. 2025, 10, owae013. [Google Scholar] [CrossRef]
- Atwood, L.; Raymond, J.; Sears, A.; Bell, M.; Daniel, R. From identification to intelligence: An assessment of the suitability of forensic DNA phenotyping service providers for use in Australian law enforcement casework. Front. Genet. 2021, 11, 56701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-López, B.A.; López-Ceballos, A.G.; López-Aguilar, C.A.; Rico-Méndez, M.A.; Acosta-Ramírez, K.L.; Cano-Ravell, A.; Gembe-Olivarez, G.; López-Quintero, A.; Aguilar-Velázquez, J.A.; Ramírez-de-Arellano Sánchez, J.A.; et al. Genetic Prediction of Eye, Hair, and Skin Color: Forensic Applications and Challenges in Latin American Populations. Genes 2025, 16, 1227. https://doi.org/10.3390/genes16101227
Flores-López BA, López-Ceballos AG, López-Aguilar CA, Rico-Méndez MA, Acosta-Ramírez KL, Cano-Ravell A, Gembe-Olivarez G, López-Quintero A, Aguilar-Velázquez JA, Ramírez-de-Arellano Sánchez JA, et al. Genetic Prediction of Eye, Hair, and Skin Color: Forensic Applications and Challenges in Latin American Populations. Genes. 2025; 16(10):1227. https://doi.org/10.3390/genes16101227
Chicago/Turabian StyleFlores-López, Beatriz Armida, Anna Guadalupe López-Ceballos, Cristal Azucena López-Aguilar, Manuel Alejandro Rico-Méndez, Kesia Lyvier Acosta-Ramírez, Alan Cano-Ravell, Gildardo Gembe-Olivarez, Andres López-Quintero, José Alonso Aguilar-Velázquez, Jorge Adrian Ramírez-de-Arellano Sánchez, and et al. 2025. "Genetic Prediction of Eye, Hair, and Skin Color: Forensic Applications and Challenges in Latin American Populations" Genes 16, no. 10: 1227. https://doi.org/10.3390/genes16101227
APA StyleFlores-López, B. A., López-Ceballos, A. G., López-Aguilar, C. A., Rico-Méndez, M. A., Acosta-Ramírez, K. L., Cano-Ravell, A., Gembe-Olivarez, G., López-Quintero, A., Aguilar-Velázquez, J. A., Ramírez-de-Arellano Sánchez, J. A., & Moreno-Ortiz, J. M. (2025). Genetic Prediction of Eye, Hair, and Skin Color: Forensic Applications and Challenges in Latin American Populations. Genes, 16(10), 1227. https://doi.org/10.3390/genes16101227







