Short Inverted Repeats as Mutational Hotspots and Putative Drivers of Genome Instability in Osteosarcoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Characterization of SIRs
2.2. COSMIC Datasets
2.3. Whole Genome Sequencing Analysis
2.4. SIR Mutation Analysis
2.5. Somatic Mutation Analysis
2.6. Statistics and Visualization
3. Results
3.1. Identification and Characterization of SIRs in the Human Genome
3.2. Distribution of SIRs in Functional Genomic Regions
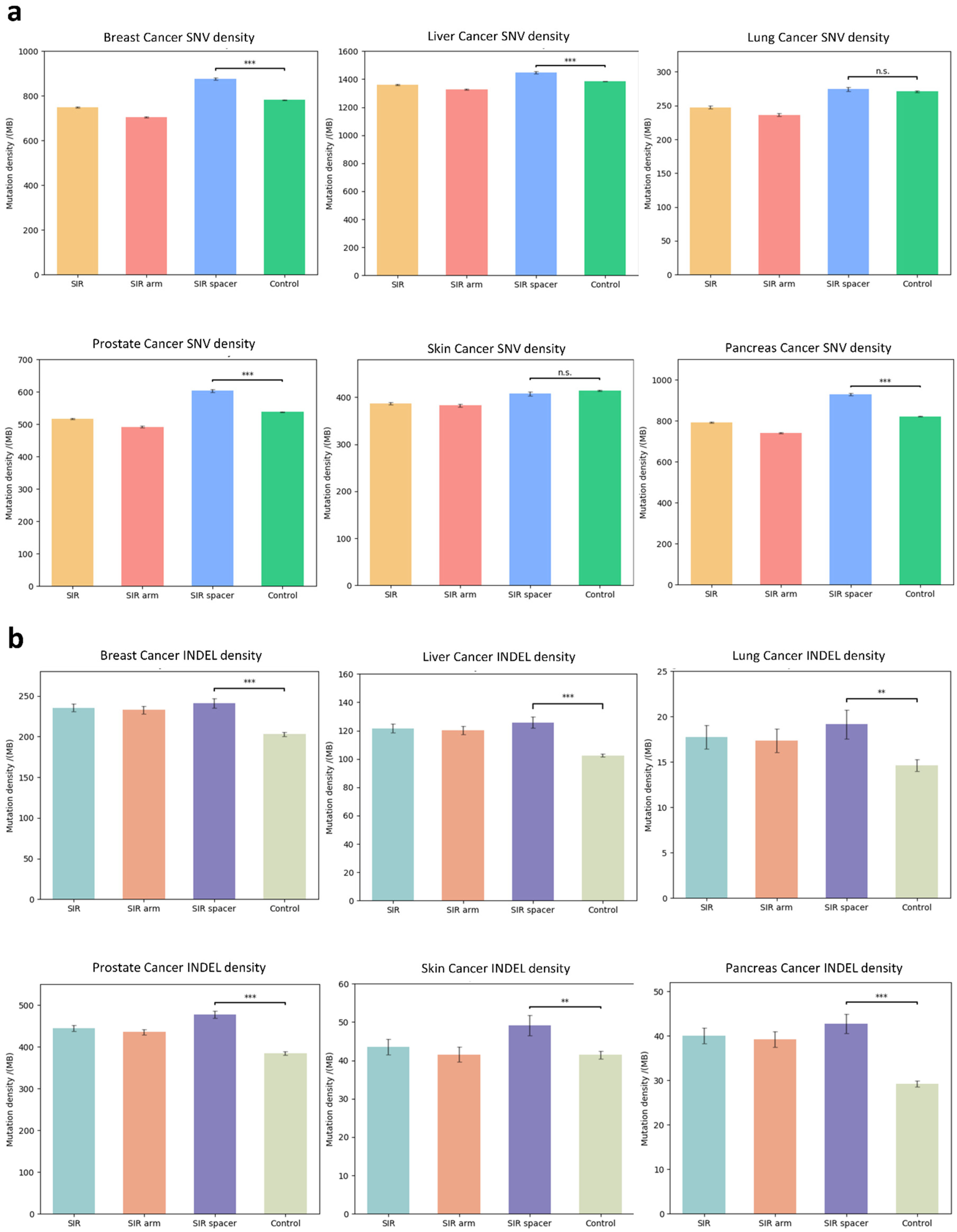
3.3. SIR-Associated Mutational Patterns Across Multiple Cancer Types
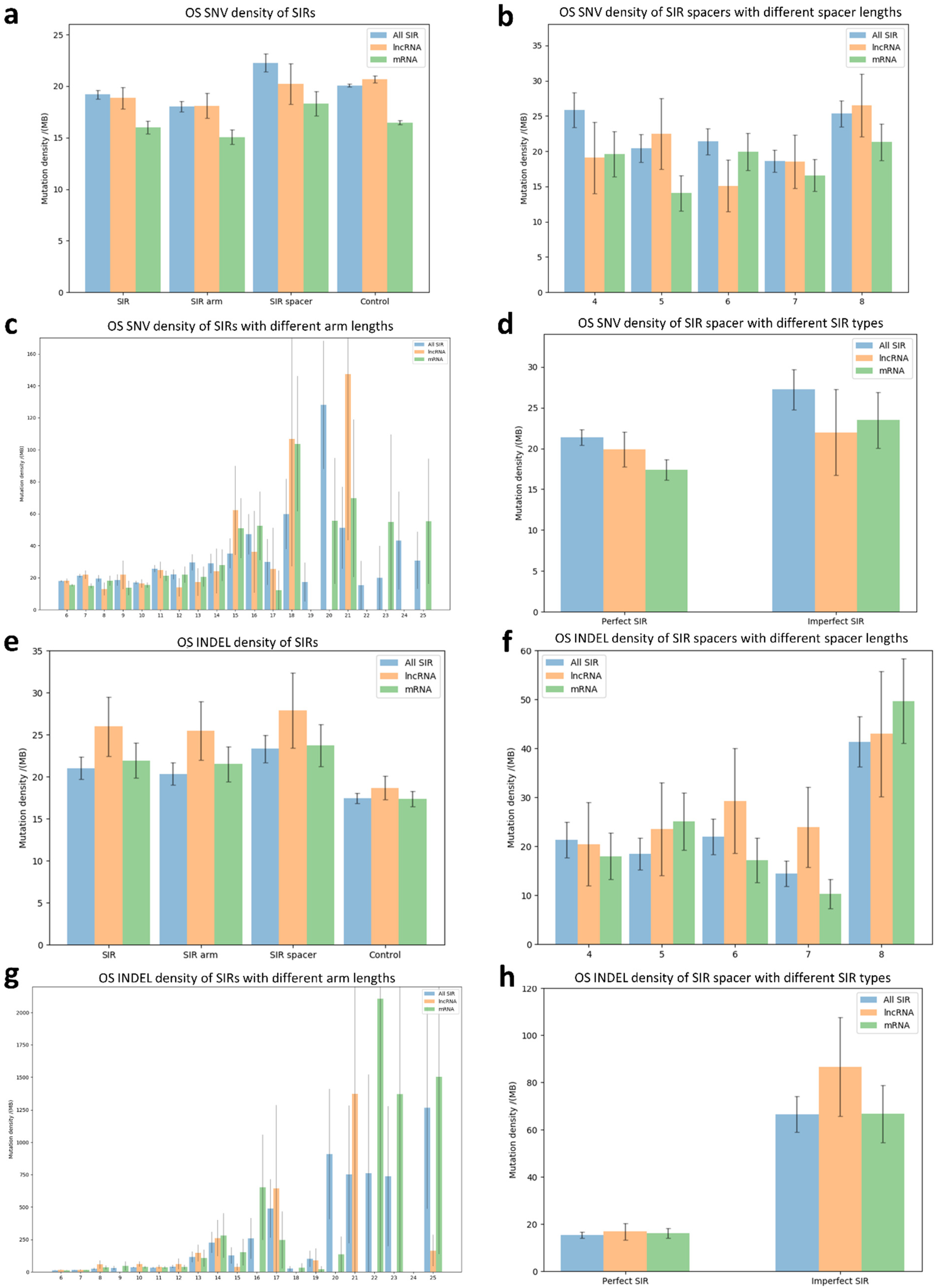
3.4. Mutation Profiles of SNVs and INDELs Within SIRs in Osteosarcoma
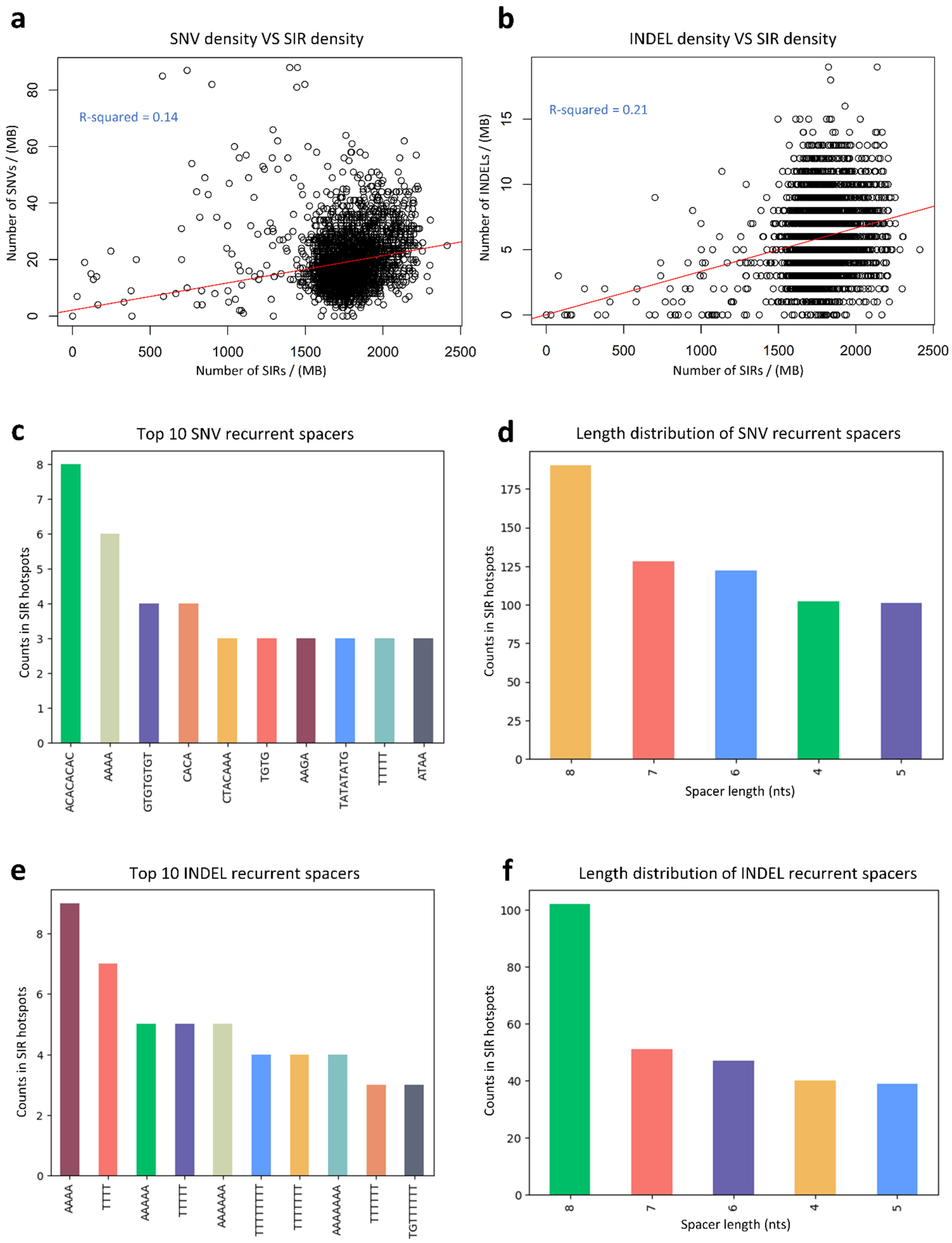
3.5. Relationship Between SNV Density, INDEL Density, and SIR Density
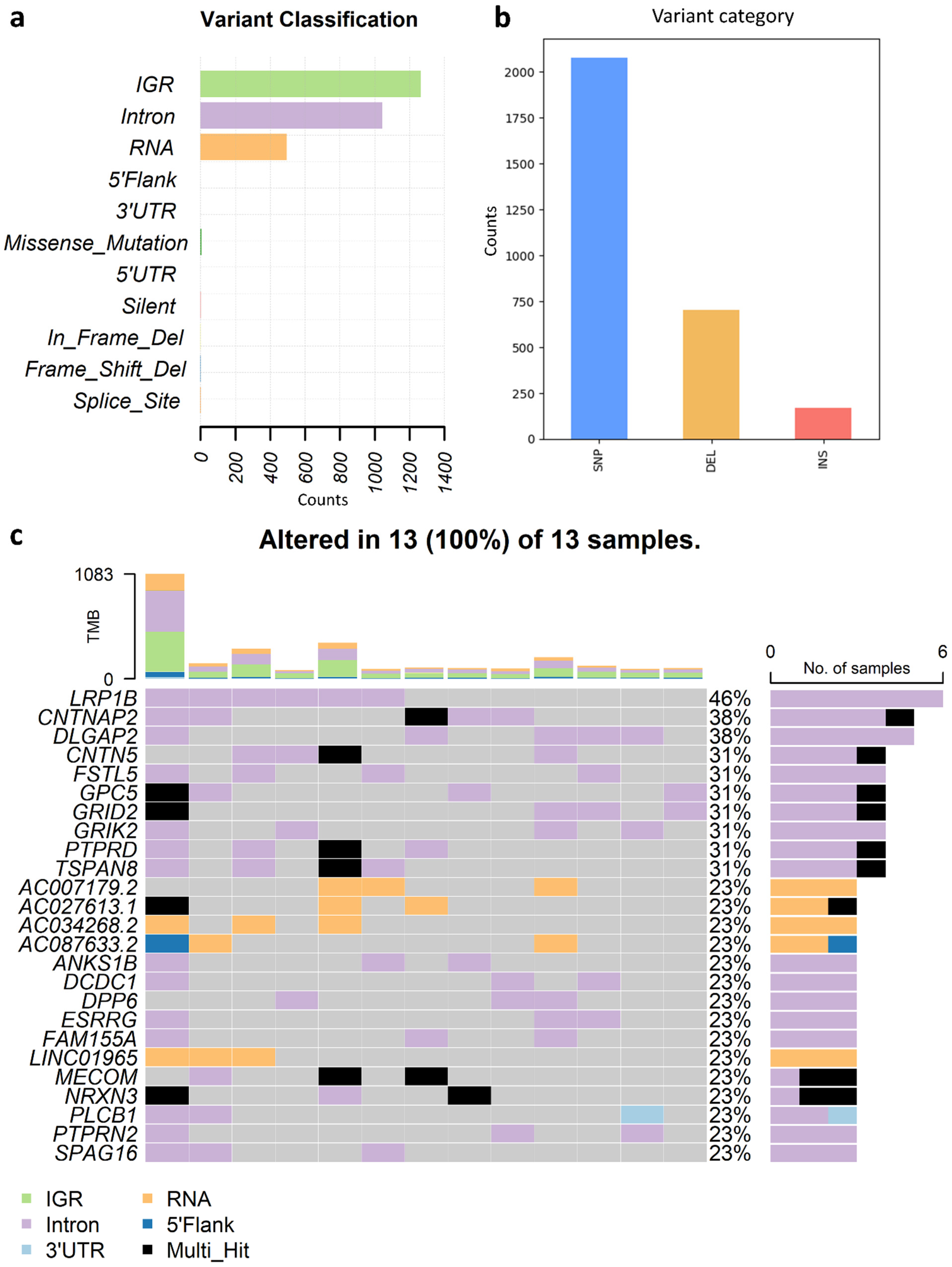
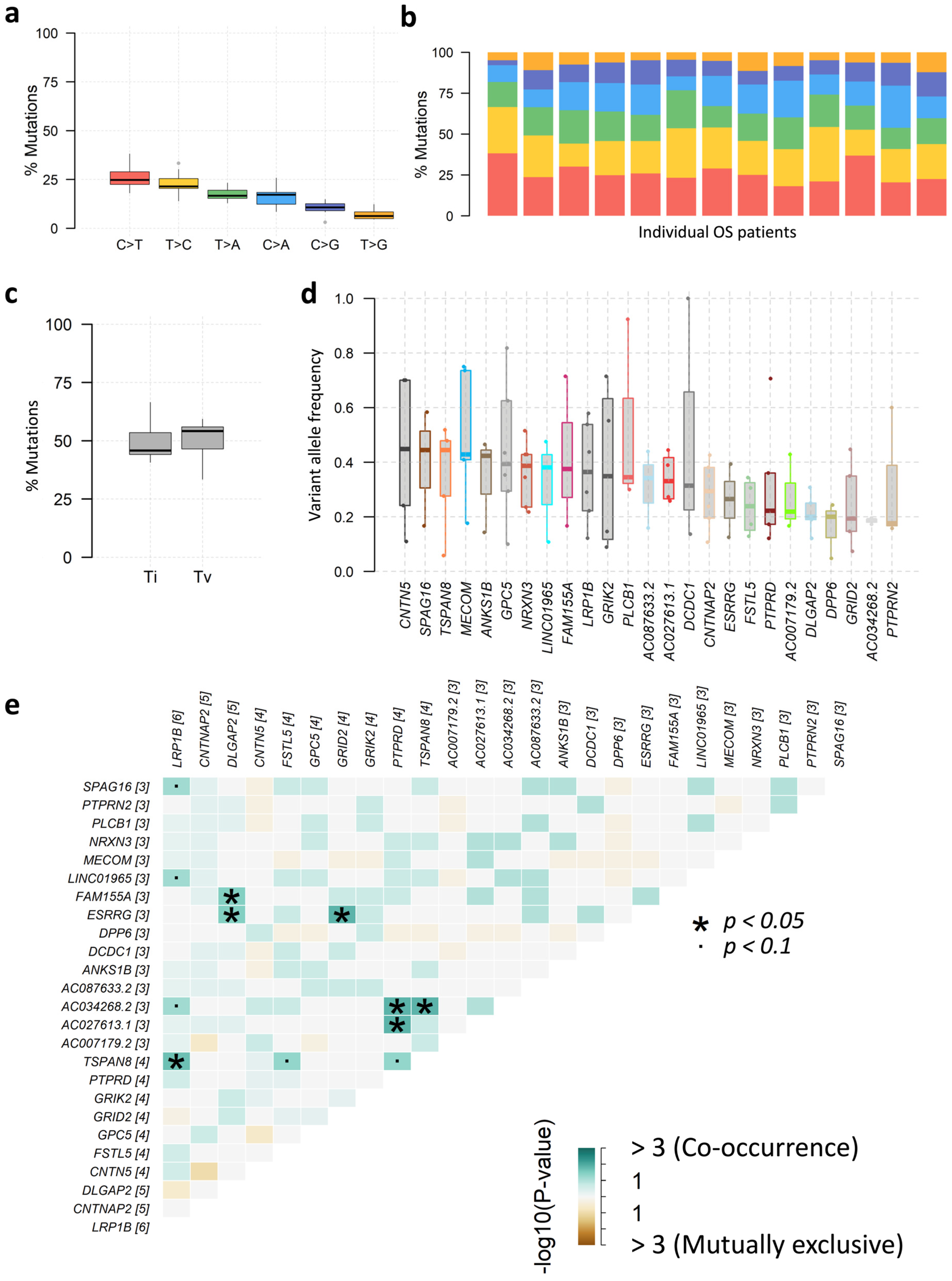
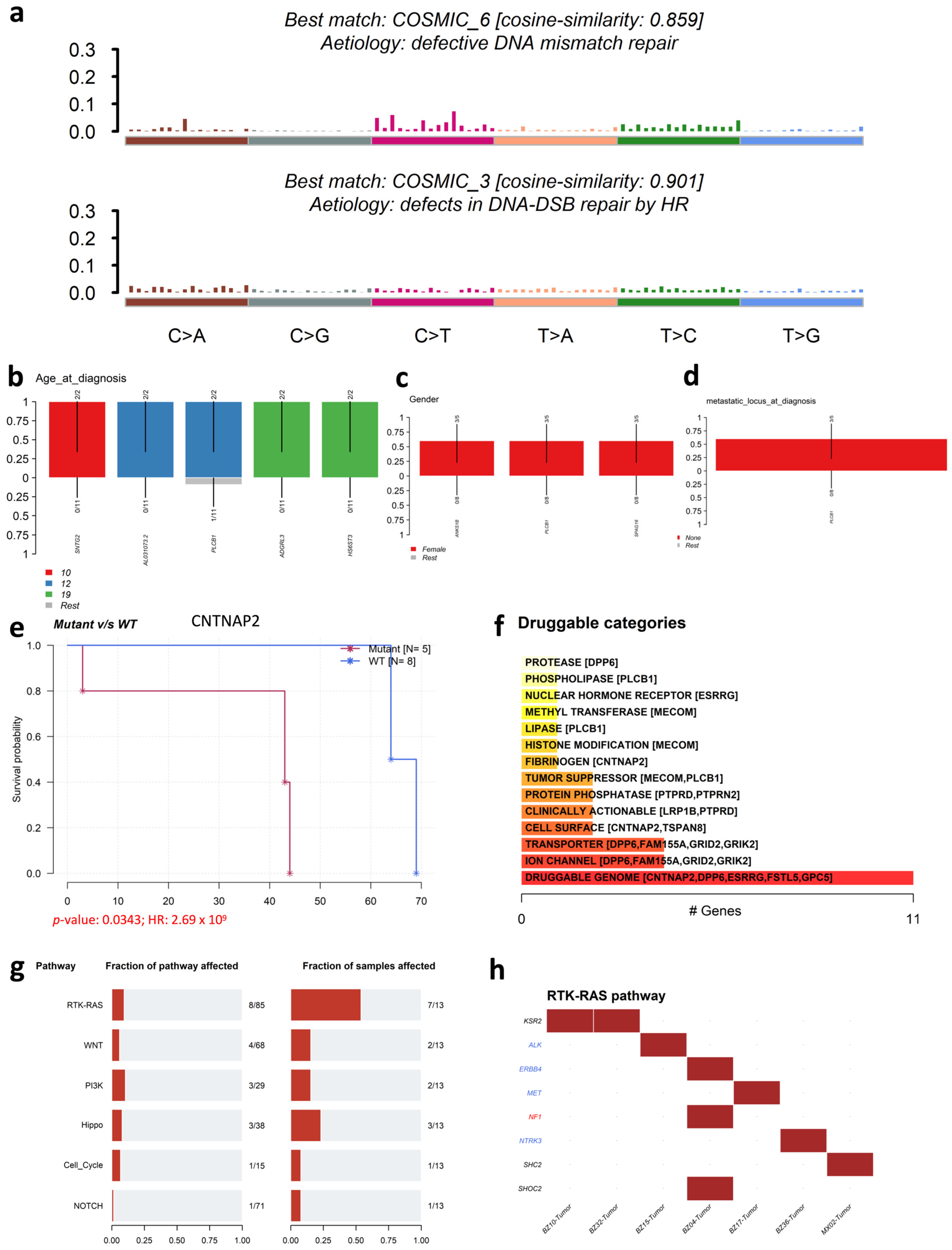
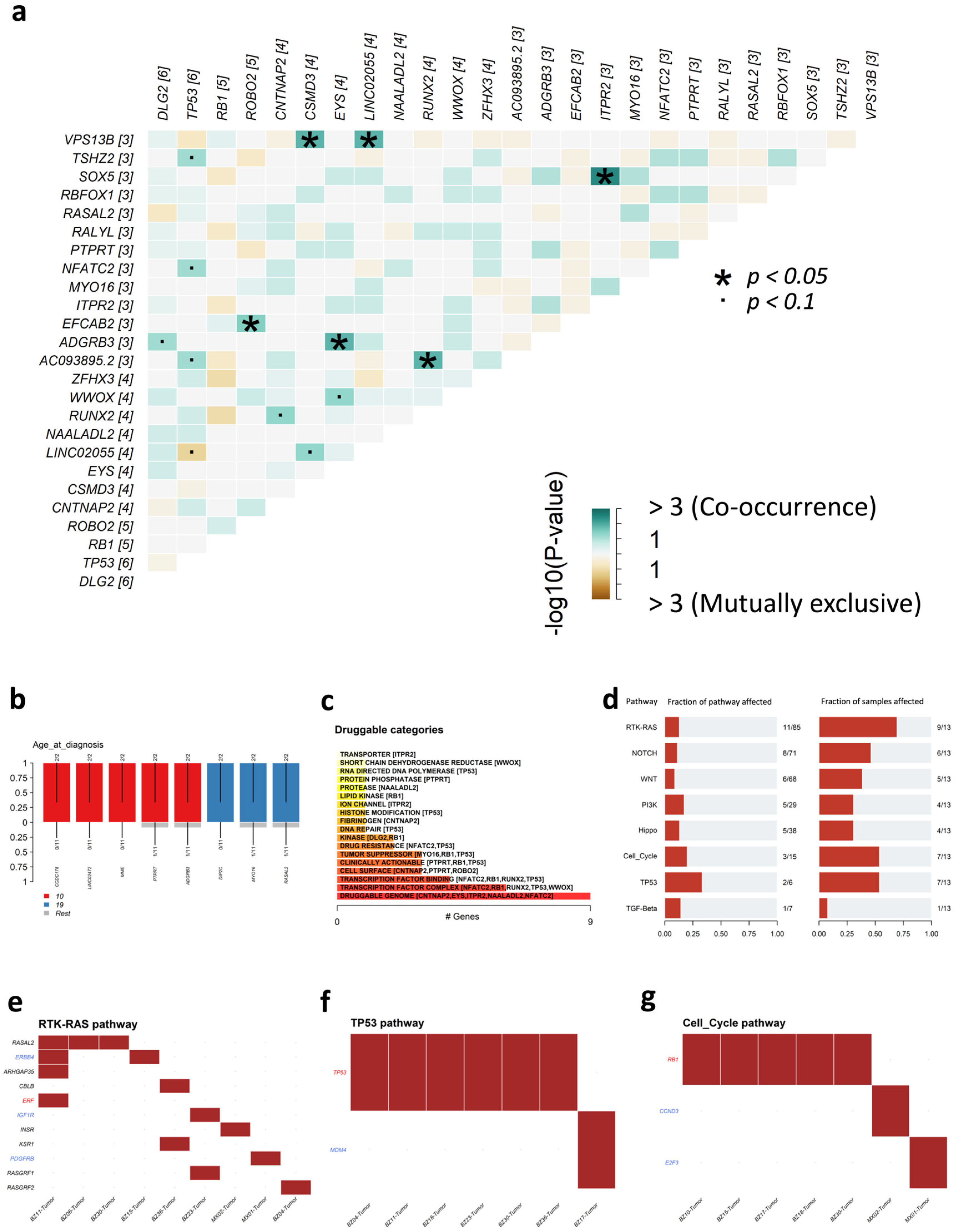
3.6. Analysis of SIR-Associated SNVs and INDELs in Osteosarcoma
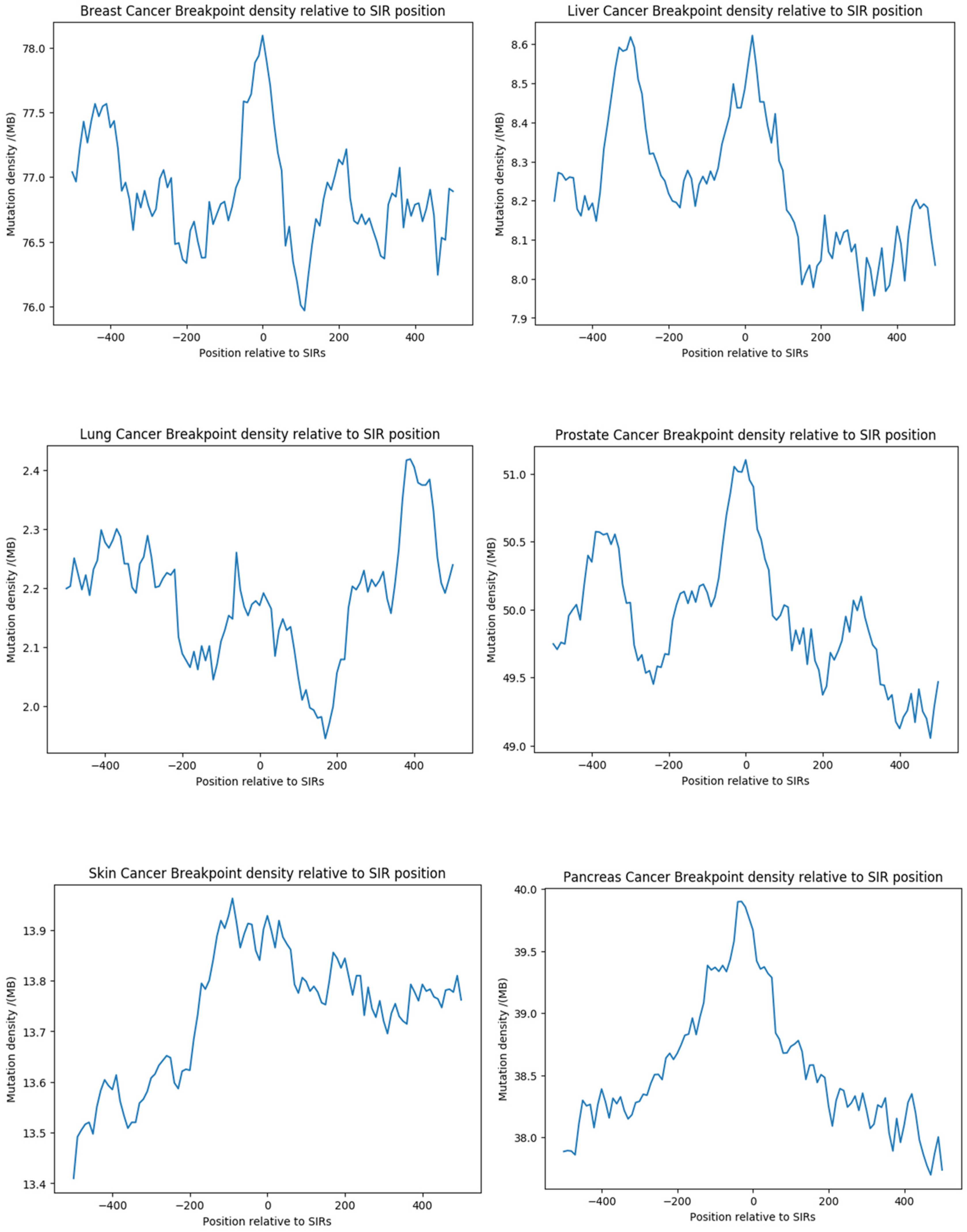
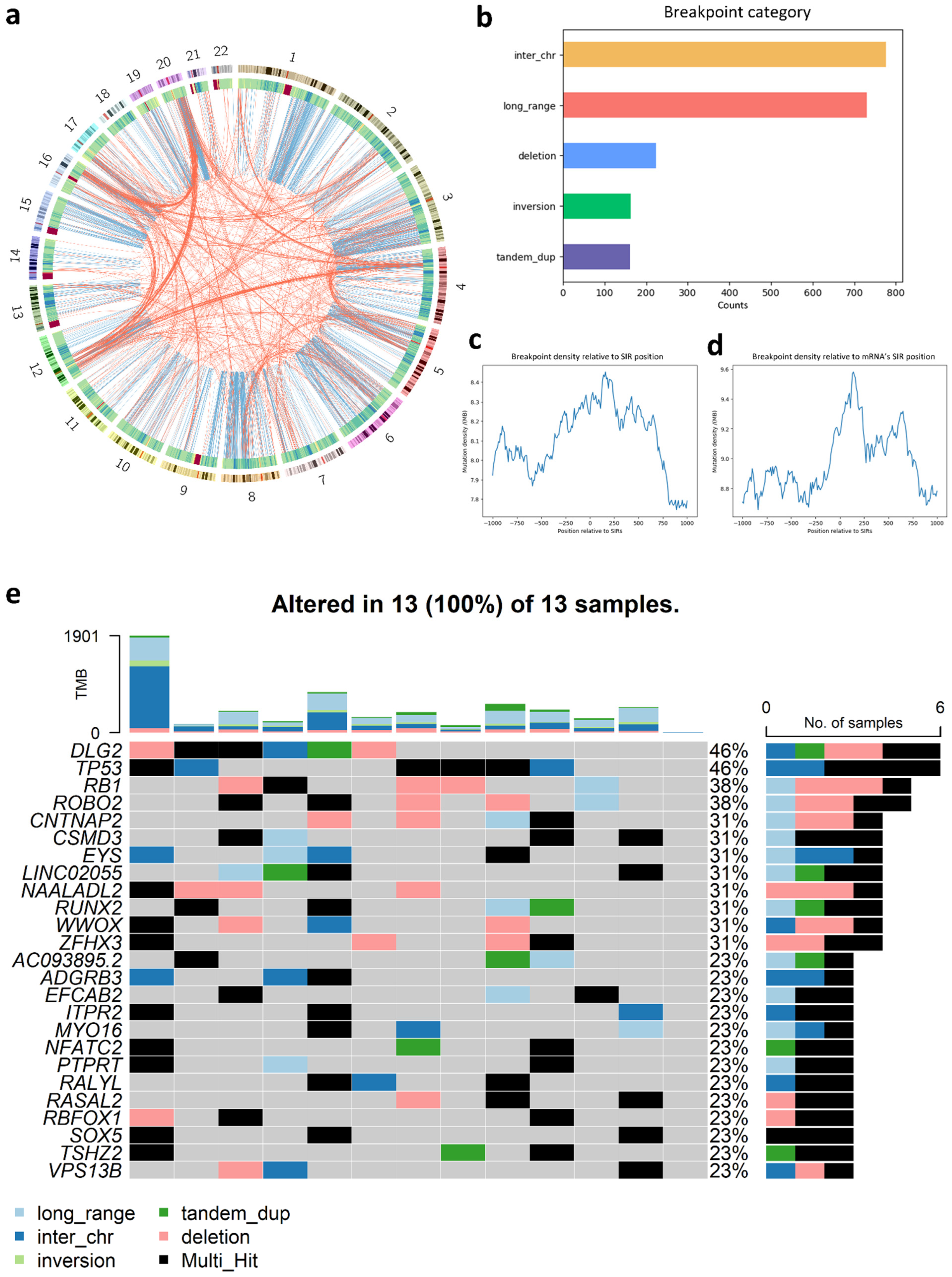
3.7. Analysis of SIR-Associated Breakpoints in Osteosarcoma
4. Discussion
4.1. General Mutational Hotspot Properties of SIRs
4.2. Osteosarcoma-Specific Patterns and Potential Driver Roles
4.3. Clinical and Therapeutic Relevance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIR | Short Inverted Repeat |
| OS | Osteosarcoma |
| SNV | Single Nucleotide Variant |
| INDEL | Small Insertion and Deletion |
| DSB | Double-Strand Break |
| HR | Homologous Recombination |
| NHEJ | Non-Homologous End-Joining |
| WGS | Whole Genome Sequencing |
References
- Padeken, J.; Zeller, P.; Gasser, S.M. Repeat DNA in genome organization and stability. Curr. Opin. Genet. Dev. 2015, 31, 12–19. [Google Scholar] [CrossRef] [PubMed]
- de Koning, A.P.J.; Gu, W.; Castoe, T.A.; Batzer, M.A.; Pollock, D.D. Repetitive Elements May Comprise Over Two-Thirds of the Human Genome. PLoS Genet. 2011, 7, e1002384. [Google Scholar] [CrossRef] [PubMed]
- Warburton, P.E.; Giordano, J.; Cheung, F.; Gelfand, Y.; Benson, G. Inverted Repeat Structure of the Human Genome: The X-Chromosome Contains a Preponderance of Large, Highly Homologous Inverted Repeats That Contain Testes Genes. Genome Res. 2004, 14, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, G.; Bacolla, A.; Zhao, J.; Spitser, S.; Vasquez, K.M. Short Inverted Repeats Are Hotspots for Genetic Instability: Relevance to Cancer Genomes. Cell Rep. 2015, 10, 1674–1680. [Google Scholar] [CrossRef]
- Shi, J.; Liang, C. Generic Repeat Finder: A High-Sensitivity Tool for Genome-Wide De Novo Repeat Detection. Plant Physiol. 2019, 180, 1803–1815. [Google Scholar] [CrossRef]
- Wang, Y.; Leung, F.C.C. Long inverted repeats in eukaryotic genomes: Recombinogenic motifs determine genomic plasticity. FEBS Lett. 2006, 580, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Brázda, V.; Laister, R.C.; Jagelská, E.B.; Arrowsmith, C. Cruciform structures are a common DNA feature important for regulating biological processes. BMC Mol. Biol. 2011, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Bikard, D.; Loot, C.; Baharoglu, Z.; Mazel, D. Folded DNA in Action: Hairpin Formation and Biological Functions in Prokaryotes. Microbiol. Mol. Biol. Rev. 2010, 74, 570–588. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.R. Meeting DNA palindromes head-to-head. Genes Dev. 2008, 22, 2612–2620. [Google Scholar] [CrossRef]
- Zou, X.; Morganella, S.; Glodzik, D.; Davies, H.; Li, Y.; Stratton, M.R.; Nik-Zainal, S. Short inverted repeats contribute to localized mutability in human somatic cells. Nucleic Acids Res. 2017, 45, 11213–11221. [Google Scholar] [CrossRef]
- Nag, D.K.; Petes, T.D. Seven-base-pair inverted repeats in DNA form stable hairpins in vivo in Saccharomyces cerevisiae. Genetics 1991, 129, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Chasovskikh, S.; Dimtchev, A.; Smulson, M.; Dritschilo, A. DNA transitions induced by binding of PARP-1 to cruciform structures in supercoiled plasmids. Cytom. Part A 2005, 68A, 21–27. [Google Scholar] [CrossRef]
- Ye, C.; Ji, G.; Li, L.; Liang, C. detectIR: A Novel Program for Detecting Perfect and Imperfect Inverted Repeats Using Complex Numbers and Vector Calculation. PLoS ONE 2014, 9, e113349. [Google Scholar] [CrossRef]
- Bowater, R.P.; Bohálová, N.; Brázda, V. Interaction of Proteins with Inverted Repeats and Cruciform Structures in Nucleic Acids. Int. J. Mol. Sci. 2022, 23, 6171. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, R.; Strehler, P.; Hennecke, H.; Fischer, H.-M. An imperfect inverted repeat is critical for DNA binding of the response regulator RegR of Bradyrhizobium japonicum. Nucleic Acids Res. 2000, 28, 4166–4171. [Google Scholar] [CrossRef]
- Svetec Miklenić, M.; Svetec, I.K. Palindromes in DNA—A Risk for Genome Stability and Implications in Cancer. Int. J. Mol. Sci. 2021, 22, 2840. [Google Scholar] [CrossRef]
- Gordenin, D.A.; Lobachev, K.S.; Degtyareva, N.P.; Malkova, A.L.; Perkins, E.; Resnick, M.A. Inverted DNA Repeats: A Source of Eukaryotic Genomic Instability. Mol. Cell. Biol. 1993, 13, 5315–5322. [Google Scholar]
- Voineagu, I.; Narayanan, V.; Lobachev, K.S.; Mirkin, S.M. Replication stalling at unstable inverted repeats: Interplay between DNA hairpins and fork stabilizing proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 9936–9941. [Google Scholar] [CrossRef]
- Murchie, A.I.H.; Bowater, R.; Aboul-Ela, F.; Lilley, D.M.J. Helix opening transitions in supercoiled DNA. Biochim. Biophys. Acta BBA Gene Struct. Expr. 1992, 1131, 1–15. [Google Scholar] [CrossRef]
- Lewis, S.M.; Coté, A.G. Palindromes and genomic stress fractures: Bracing and repairing the damage. DNA Repair 2006, 5, 1146–1160. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef]
- Lobachev, K.S.; Gordenin, D.A.; Resnick, M.A. The Mre11 Complex Is Required for Repair of Hairpin-Capped Double-Strand Breaks and Prevention of Chromosome Rearrangements. Cell 2002, 108, 183–193. [Google Scholar] [CrossRef]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef]
- Aparicio, T.; Baer, R.; Gautier, J. DNA double-strand break repair pathway choice and cancer. DNA Repair 2014, 19, 169–175. [Google Scholar] [CrossRef]
- Bacolla, A.; Tainer, J.A.; Vasquez, K.M.; Cooper, D.N. Translocation and deletion breakpoints in cancer genomes are associated with potential non-B DNA-forming sequences. Nucleic Acids Res. 2016, 44, 5673–5688. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Dai, W. Genomic Instability and Cancer. J. Carcinog. Mutagen. 2014, 5, 1000165. [Google Scholar]
- Yu, V.P.; Koehler, M.; Steinlein, C.; Schmid, M.; Hanakahi, L.A.; Gool, A.J.V.; West, S.C.; Venkitaraman, A.R. Gross chromosomal rearrangements and genetic exchange between nonhomologous chromosomes following BRCA2 inactivation. Genes Dev. 2000, 14, 1400–1406. [Google Scholar] [CrossRef]
- Mizuno, K.; Miyabe, I.; Schalbetter, S.A.; Carr, A.M.; Murray, J.M. Recombination-restarted replication makes inverted chromosome fusions at inverted repeats. Nature 2013, 493, 246–249. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Marotta, M.; Onodera, T.; Johnson, J.; Budd, G.T.; Watanabe, T.; Cui, X.; Giuliano, A.E.; Niida, A.; Tanake, H. Palindromic amplification of the ERBB2 oncogene in primary HER2-positive breast tumors. Sci. Rep. 2017, 7, 41921. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tapscott, S.J.; Trask, B.J.; Yao, M.-C. Short inverted repeats initiate gene amplification through the formation of a large DNA palindrome in mammalian cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8772–8777. [Google Scholar] [CrossRef]
- Albrecht, E.B.; Hunyady, A.B.; Stark, G.R.; Patterson, T.E. Mechanisms of sod2 Gene Amplification inSchizosaccharomyces pombe. Mol. Biol. Cell 2000, 11, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Franconi, C.P.; Sheridan, M.B.; Hacker, A.M.; Inagakai, H.; Glover, T.W.; Arlt, M.F.; Drabkin, H.A.; Gemmill, R.M.; Kurahashi, H.; et al. Analysis of the t(3;8) of Hereditary Renal Cell Carcinoma: A Palindrome-Mediated Translocation. Cancer Genet. 2014, 207, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, N.; Jacobsen, A.; Schultz, N.; Sander, C.; Lee, W. Genome-wide analysis of noncoding regulatory mutations in cancer. Nat. Genet. 2014, 46, 1160–1165. [Google Scholar] [CrossRef]
- Subramanian, S.; Chaparala, S.; Avali, V.; Ganapathiraju, M.K. A pilot study on the prevalence of DNA palindromes in breast cancer genomes. BMC Med. Genom. 2016, 9, 73. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer. Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2016, 4, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Randall, R.L.; Brothman, A.R.; Maxwell, T.; Coffin, C.M.; Goldsby, R.E. HER-2/neu Expression in Osteosarcoma Increases Risk of Lung Metastasis and Can Be Associated with Gene Amplification. J. Pediatr. Hematol. Oncol. 2003, 25, 27–32. [Google Scholar] [CrossRef]
- Ebb, D.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II Trial of Trastuzumab in Combination with Cytotoxic Chemotherapy for Treatment of Metastatic Osteosarcoma with Human Epidermal Growth Factor Receptor 2 Overexpression: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive Genomic Rearrangement Acquired in a Single Catastrophic Event during Cancer Development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef]
- Cortés-Ciriano, I.; Lee, J.J.; Xi, R.; Jain, D.; Jung, Y.L.; Yang, L.; Gordenin, D.; Klimczak, L.J.; Zhang, C.Z.; Pellman, D.S. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat. Genet. 2020, 52, 331–341. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Li, Z.; Dou, P.; Liu, T.; He, S. Application of Long Noncoding RNAs in Osteosarcoma: Biomarkers and Therapeutic Targets. Cell Physiol. Biochem. 2017, 42, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.; Fu, C.; Wang, X.; Zou, J.; Hua, H.; Bi, Z. Long noncoding RNA FGFR3-AS1 promotes osteosarcoma growth through regulating its natural antisense transcript FGFR3. Mol. Biol. Rep. 2016, 43, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, J.; Meng, H.; Yu, Z.; Wang, Z.; Yuan, X.; Chen, H.; Wang, A. A novel long non-coding RNA, hypoxia-inducible factor-2α promoter upstream transcript, functions as an inhibitor of osteosarcoma stem cells in vitro. Mol. Med. Rep. 2015, 11, 2534–2540. [Google Scholar] [CrossRef]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Ferreira, A.M.; Johnson, R.; Jungreis, I.; Loveland, J.; Mudge, J.M.; Sisu, C.; Wright, J.; Armstrong, J.; et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019, 47, D766–D773. [Google Scholar] [CrossRef]
- Karolchik, D.; Hinrichs, A.S.; Furey, T.S.; Roskin, K.M.; Sugnet, C.W.; Haussler, D.; Kent, W.J. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004, 32, D493–D496. [Google Scholar] [CrossRef]
- Moore, J.E.; Purcaro, M.J.; Pratt, H.E.; Epstein, C.B.; Shoresh, N.; Adrian, J.; Kawli, T.; Davis, C.A.; Dobin, A.; Kaul, R.; et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature 2020, 583, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Babraham Bioinformatics—FastQC a Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 10 March 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Benjamin, D.; Sato, T.; Cibulskis, K.; Getz, G.; Stewart, C.; Lichtenstein, L. Calling Somatic SNVs and Indels with Mutect2. bioRxiv 2019. bioRxiv:861054. [Google Scholar] [CrossRef]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A genomic mutational constraint map using variation in 76,156 human genomes. Nature 2024, 625, 92–100. [Google Scholar] [CrossRef]
- Perry, J.A.; Kiezun, A.; Tonzi, P.; Van Allen, E.M.; Carter, S.L.; Baca, S.C.; Cowley, G.S.; Bhatt, A.S.; Rheinbay, E.; Pedamallu, C.S.; et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc. Natl. Acad. Sci. USA 2014, 111, E5564–E5573. [Google Scholar] [CrossRef]
- Mayakonda, A.; Lin, D.-C.; Assenov, Y.; Plass, C.; Koeffler, H.P. Maftools: Efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018, 28, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.K.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Griffith, O.L.; Coffman, A.C.; Weible, J.V.; McMichael, J.F.; Spies, N.C.; Koval, J.; Das, I.; Callaway, M.B.; Eldred, J.M.; et al. DGIdb: Mining the druggable genome. Nat. Methods 2013, 10, 1209–1210. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in the Cancer Genome Atlas. Cell 2018, 173, 321–337.e10. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Birdwell, C.; Fiskus, W.; Kadia, T.M.; DiNardo, C.D.; Mill, C.P.; Bhalla, K.N. EVI1 dysregulation: Impact on biology and therapy of myeloid malignancies. Blood Cancer J. 2021, 11, 64. [Google Scholar] [CrossRef]
- Lozano Chinga, M.M.; Bertuch, A.A.; Afify, Z.; Dollerschell, K.; Hsu, J.I.; John, T.D.; Rao, E.S.; Rowe, R.G.; Sankaran, V.G.; Shimamura, A.; et al. Expanded phenotypic and hematologic abnormalities beyond bone marrow failure in MECOM-associated syndromes. Am. J. Med. Genet. Part A 2023, 191, 1826–1835. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, Z.; Zhao, W.; Wang, L.; Jiang, J.; Gu, L.; Li, M.; Jiang, L.; Wang, Y.; Bi, Y. PLCB1 Enhances Cell Migration and Invasion in Gastric Cancer Via Regulating Actin Cytoskeletal Remodeling and Epithelial–Mesenchymal Transition. Biochem. Genet. 2023, 61, 2618–2632. [Google Scholar] [CrossRef]
- Liang, S.; Guo, H.; Ma, K.; Li, X.; Wu, D.; Wang, Y.; Wang, W.; Zhang, S.; Cui, Y.; Liu, Y.; et al. A PLCB1–PI3K–AKT Signaling Axis Activates EMT to Promote Cholangiocarcinoma Progression. Cancer Res. 2021, 81, 5889–5903. [Google Scholar] [CrossRef] [PubMed]
- Soussi, T.; Wiman, K.G. TP53: An oncogene in disguise. Cell Death Differ. 2015, 22, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-J.; Liu, J.; Wang, S.Q.; Zhu, Y.; Gao, X.Y.; Tin, V.P.; Qin, J.; Wang, J.W.; Wong, M.P. NFATc2 enhances tumor-initiating phenotypes through the NFATc2/SOX2/ALDH axis in lung adenocarcinoma. Elife 2017, 6, e26733. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Yang, T.; Zhao, W.; Wang, N.; Li, P.; Zeng, X.; Zhang, W. Long non-coding RNA MALAT1 for promoting metastasis and proliferation by acting as a ceRNA of miR-144-3p in osteosarcoma cells. Oncotarget 2017, 8, 59417–59434. [Google Scholar] [CrossRef] [PubMed]
- Lux, S.; Milsom, M.D. EVI1-mediated Programming of Normal and Malignant Hematopoiesis. HemaSphere 2023, 7, e959. [Google Scholar] [CrossRef]
- Ade, C.; Roy-Engel, A.M.; Deininger, P.L. Alu elements: An intrinsic source of human genome instability. Curr. Opin. Virol. 2013, 3, 639–645. [Google Scholar] [CrossRef]
- Bourque, G.; Burns, K.H.; Gehring, M.; Gorbunova, V.; Seluanov, A.; Hammell, M.; Imbeault, M.; Izsvák, Z.; Levin, H.L.; Macfarlan, T.S.; et al. Ten things you should know about transposable elements. Genome Biol. 2018, 19, 199. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed]
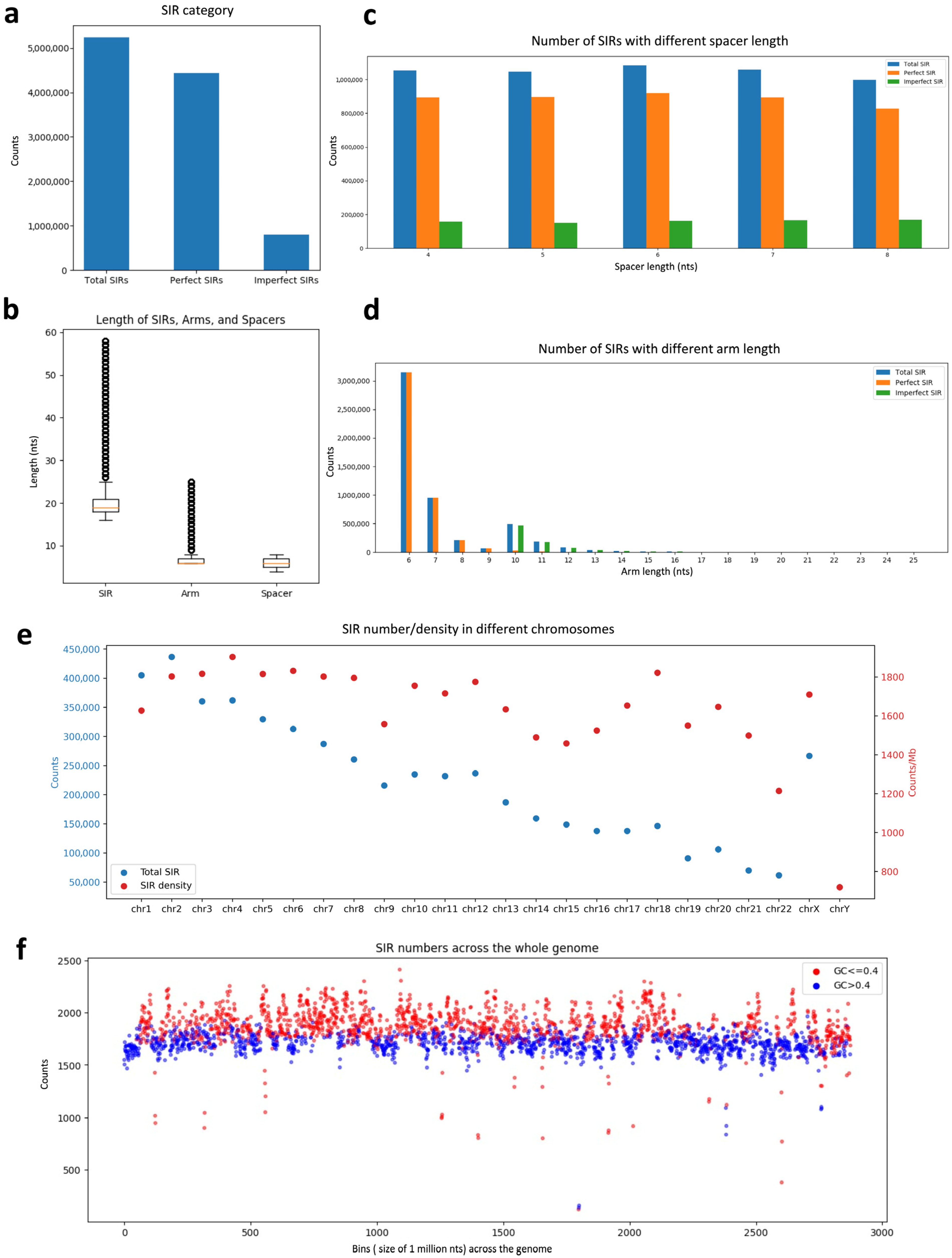
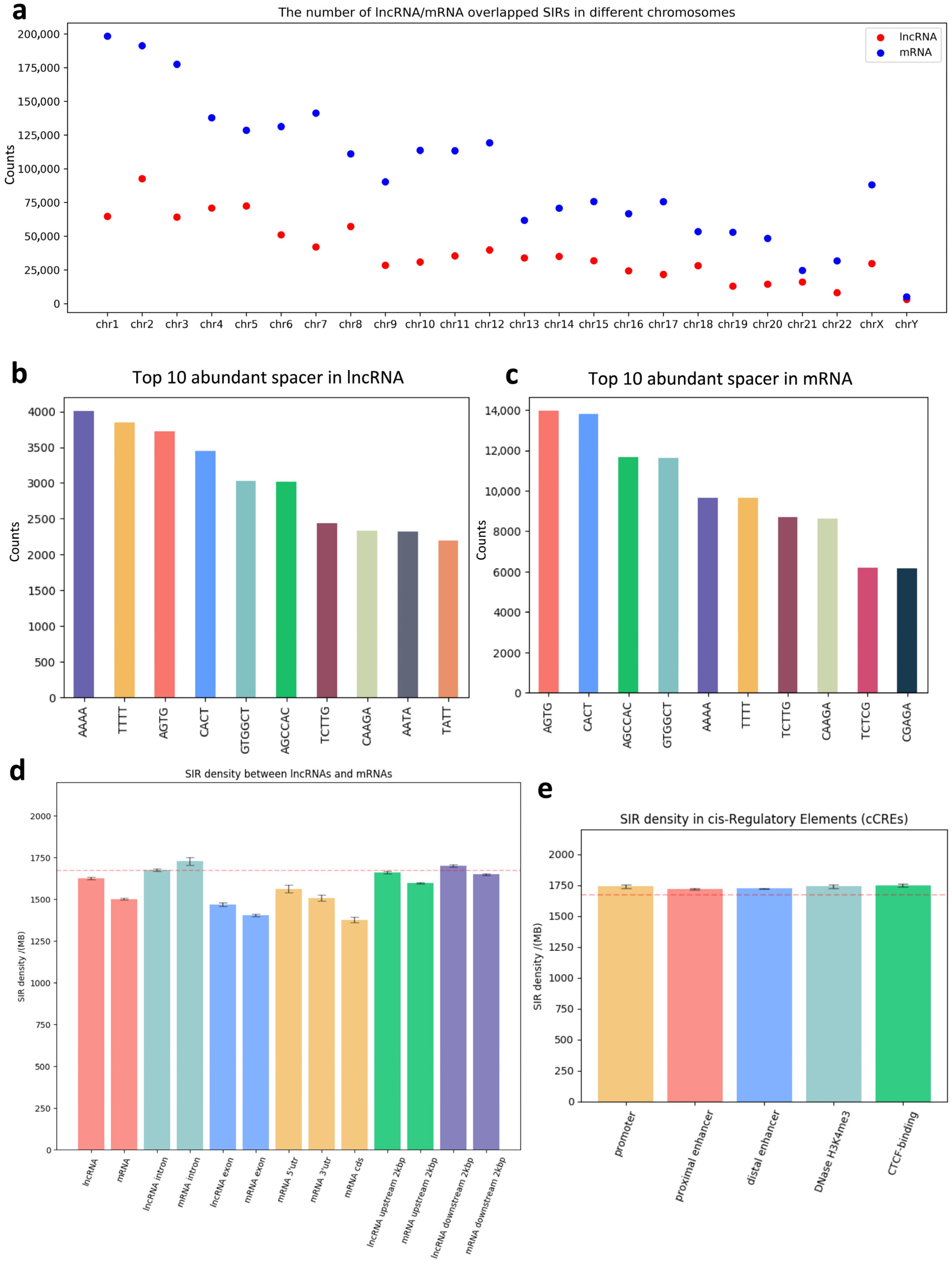
| Spacer (4 nt) | Total Counts | Spacer (5 nt) | Total Counts | Spacer (6 nt) | Total Counts | Spacer (7 nt) | Total Counts | Spacer (8 nt) | Total Counts |
|---|---|---|---|---|---|---|---|---|---|
| AGTG | 26,165 | TCTTG | 17,167 | AGCCAC | 22,180 | TATATCT | 9676 | AAGGAAAA | 3374 |
| CACT | 25,931 | CAAGA | 16,742 | GTGGCT | 22,152 | AGATATA | 9599 | TTTTCCTT | 3302 |
| AAAA | 22,020 | AACAG | 12,221 | GTGGCA | 8829 | TTTGTAG | 8677 | GCACTATT | 2378 |
| TTTT | 22,005 | TCTCG | 11,594 | TGCCAC | 8816 | CTACAAA | 8552 | AATAGTGC | 2330 |
| TATT | 12,522 | CGAGA | 11,582 | CACCAC | 8218 | TTTGCAG | 4247 | CGGGAATA | 1685 |
| AATA | 12,469 | TTTTT | 7657 | GTGGTG | 8129 | CTGCAAA | 4201 | GTGTGTGT | 1454 |
| CCTC | 11,573 | AAAAA | 7341 | TTCTTT | 4927 | CTCAGTA | 4180 | ACACACAC | 1437 |
| GAGG | 11,493 | TTTGT | 7219 | AAAGAA | 4917 | AGAAATA | 3742 | TGCAAGAG | 1400 |
| ATAA | 11,450 | TGTTC | 6891 | CGCCAC | 3952 | AATTAGG | 3714 | AAAAAAAA | 1169 |
| TTAT | 11,209 | GAACA | 6822 | GTGGCG | 3925 | CCTAATT | 3693 | TTTTTTTT | 1138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Liang, C. Short Inverted Repeats as Mutational Hotspots and Putative Drivers of Genome Instability in Osteosarcoma. Genes 2025, 16, 1202. https://doi.org/10.3390/genes16101202
Li M, Liang C. Short Inverted Repeats as Mutational Hotspots and Putative Drivers of Genome Instability in Osteosarcoma. Genes. 2025; 16(10):1202. https://doi.org/10.3390/genes16101202
Chicago/Turabian StyleLi, Minghua, and Chun Liang. 2025. "Short Inverted Repeats as Mutational Hotspots and Putative Drivers of Genome Instability in Osteosarcoma" Genes 16, no. 10: 1202. https://doi.org/10.3390/genes16101202
APA StyleLi, M., & Liang, C. (2025). Short Inverted Repeats as Mutational Hotspots and Putative Drivers of Genome Instability in Osteosarcoma. Genes, 16(10), 1202. https://doi.org/10.3390/genes16101202





