Genetics of Keratoconus: A Comprehensive Review
Abstract
1. Introduction
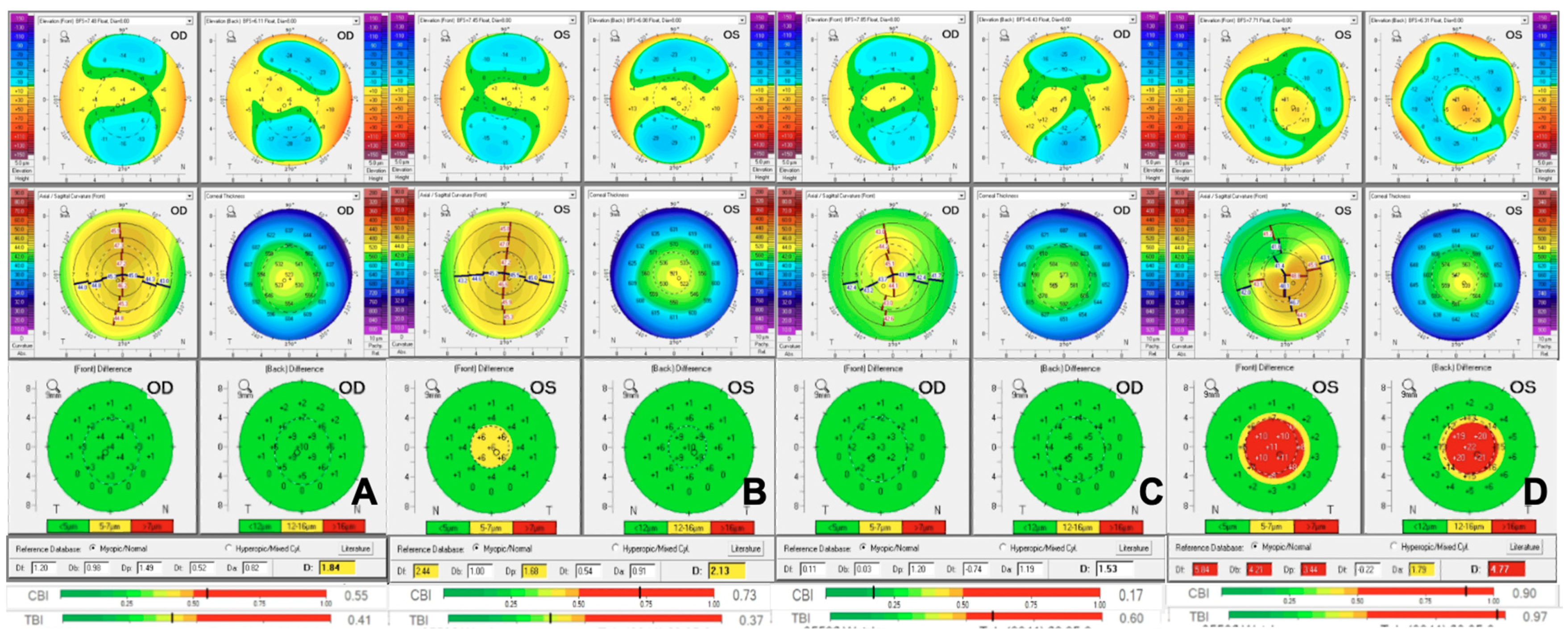
2. Understanding the Different Types of Genetic Studies
2.1. Candidate Genes
2.2. Linkage Studies
2.3. Genome Wide Association Studies (GWAS)
2.4. Genetic Expression Tests
3. Genetic Affections in KC
3.1. Familial KC
3.2. Syndromic KC
3.3. Genes Affected in KC
3.3.1. Visual System Homeobox 1 (VSX1)
3.3.2. Transforming Growth Factor Beta Induced (TGFBI)
3.3.3. Zinc-Finger E Homeobox-Binding (ZEB1)
3.3.4. microRNA 184 (MIR184)
3.3.5. Superoxide Dismutase 1 (SOD1)
3.3.6. Zinc Finger 469 (ZNF469)
3.3.7. Lysyl Oxidase (LOX)
3.3.8. Dedicator of Cytokinesis 9 (DOCK9)
3.3.9. Sodium Bicarbonate Trasporterlike Protein 11 (SLC4A11)
3.3.10. Tissue Inhibitor of Metalloproteinase 3 (TIMP3)
3.3.11. Interleukin 1 Alpha (IL1A)/Interleukin 1 Beta (IL1B)/: Interleukin 1 Receptor Antagonist (IL1RN)
3.3.12. Collagen Type IV Alpha 3 Chain (COL4A3)/Collagen Type IV Alpha 4 Chain (COL4A4)/Collagen Type V Alpha 1 Chain (COL5A1)
4. Future Directions in Genetics and KC
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAD | Belin Ambrosio Display |
| BCS | Brittle Cornea Syndrome |
| CBI | Corvis Biomechanical Index |
| CHED2 | Congenital Hereditary Endothelial Dystrophy |
| COL4A3 | Collagen Type IV Alpha 3 Chain |
| COL4A4 | Collagen Type IV Alpha 4 Chain |
| COL5A1 | Collagen Type V Alpha 1 Chain |
| DOCK9 | Dedicator of Cytokinesis 9 |
| FECD | Fuchs Endothelial Corneal Dystrophy |
| GWAS | Genome Wide Association Studies |
| IL1A | Interleukin 1 Alpha |
| IL1B | Interleukin 1 Beta |
| IL1RN | Interleukin 1 Receptor Antagonist |
| KC | Keratoconus |
| LOX | Lysyl Oxidase |
| MIR184 | microRNA 184 |
| PPCD | Posterior Polymorphous Corneal Dystrophy |
| SLC4A11 | Sodium Bicarbonate Transporter Like Protein 11 |
| SNP | Single Nucleotide Polymorphism |
| SOD1 | Superoxide Dismutase 1 |
| TBI | Tomography and Biomechanical Index |
| TGFBI | Transforming Growth Factor Beta Induced |
| VSX1 | Visual System Homeobox 1 |
| ZEB1 | Zinc-Finger E Homeobox-Binding |
| ZNF469 | Zinc Finger Protein 469 |
References
- Meek, K.M.; Knupp, C. Corneal Structure and Transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and Physiology of the Cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Lwigale, P.Y. Chapter Four Corneal Development Different Cells from a Common Progenitor. Prog. Mol. Biol. Transl. Sci. 2015, 134, 43–59. [Google Scholar] [CrossRef]
- Romero-Jiménez, M.; Santodomingo-Rubido, J.; Wolffsohn, J.S. Keratoconus: A Review. Contact Lens Anterior Eye 2010, 33, 157–166. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An Updated Review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Tan, D.; Rapuano, C.J.; Belin, M.W.; Ambrósio, R.; Guell, J.L.; Malecaze, F.; Nishida, K.; Sangwan, V.S.; Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global Consensus on Keratoconus and Ectatic Diseases. Cornea 2015, 34, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; Rodrigues, P.F.; Lamazales, L.L. Keratoconus Epidemiology: A Review. Saudi J. Ophthalmol. 2022, 36, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Netto, E.A.T.; Al-Otaibi, W.M.; Hafezi, N.L.; Kling, S.; Al-Farhan, H.M.; Randleman, J.B.; Hafezi, F. Prevalence of Keratoconus in Paediatric Patients in Riyadh, Saudi Arabia. Br. J. Ophthalmol. 2018, 102, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, H.; Khabazkhoob, M.; Fotouhi, A. Topographic Keratoconus Is Not Rare in an Iranian Population: The Tehran Eye Study. Ophthalmic Epidemiol. 2013, 20, 385–391. [Google Scholar] [CrossRef]
- Millodot, M.; Shneor, E.; Albou, S.; Atlani, E.; Gordon-Shaag, A. Prevalence and Associated Factors of Keratoconus in Jerusalem: A Cross-Sectional Study. Ophthalmic Epidemiol. 2011, 18, 91–97. [Google Scholar] [CrossRef]
- Bak-Nielsen, S.; Ramlau-Hansen, C.H.; Ivarsen, A.; Plana-Ripoll, O.; Hjortdal, J. Incidence and Prevalence of Keratoconus in Denmark—An Update. Acta Ophthalmol. 2019, 97, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Nangia, V.; Matin, A.; Kulkarni, M.; Bhojwani, K. Prevalence and Associations of Keratoconus in Rural Maharashtra in Central India: The Central India Eye and Medical Study. Am. J. Ophthalmol. 2009, 148, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Darwish, T.; Ghabra, M.; Kailani, O.; Haddeh, Y.; Askar, M.; Ali, A.; Ali, A.; Alhassan, S. Prevalence of Keratoconus in a Population-Based Study in Syria. J. Ophthalmol. 2022, 2022, 6064533. [Google Scholar] [CrossRef]
- Mohamed, Z.D. Keratoconus in Eastern Mediterranean Region: Prevalence and Risk Factors. Afr. Vis. Eye Heal. 2025, 84, a1044. [Google Scholar] [CrossRef]
- de Azevedo Magalhães, O.; Pagano, B.N.; Grellmann, L.V.; Zago, V.S.; Kronbauer, C.L. Prevalence of Keratoconus Among High School Students in Southern Brazil: A Community-Based Study. Eye Contact Lens Sci. Clin. Pr. 2024, 50, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Parmar, U.P.S.; Jhanji, V. Prevalence and Economic Burden of Keratoconus in the United States. Am. J. Ophthalmol. 2024, 259, 71–78. [Google Scholar] [CrossRef]
- Hashemi, H.; Heydarian, S.; Hooshmand, E.; Saatchi, M.; Yekta, A.; Aghamirsalim, M.; Valadkhan, M.; Mortazavi, M.; Hashemi, A.; Khabazkhoob, M. The Prevalence and Risk Factors for Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2019, 39, 263–270. [Google Scholar] [CrossRef]
- Vought, R.; Greenstein, S.A.; Gelles, J.; Hersh, P.S. The Pathophysiology of Keratoconus. Cornea 2025, 44, 137–143. [Google Scholar] [CrossRef]
- Tur, V.M.; MacGregor, C.; Jayaswal, R.; O’Brart, D.; Maycock, N. A Review of Keratoconus: Diagnosis, Pathophysiology, and Genetics. Surv. Ophthalmol. 2017, 62, 770–783. [Google Scholar] [CrossRef]
- Sharma, N.; Rao, K.; Maharana, P.K.; Vajpayee, R.B. Ocular Allergy and Keratoconus. Indian J. Ophthalmol. 2013, 61, 407–409. [Google Scholar] [CrossRef]
- Deshmukh, R.; Ong, Z.Z.; Rampat, R.; del Barrio, J.L.A.; Barua, A.; Ang, M.; Mehta, J.S.; Said, D.G.; Dua, H.S.; Ambrósio, R.; et al. Management of Keratoconus: An Updated Review. Front. Med. 2023, 10, 1212314. [Google Scholar] [CrossRef]
- Singh, R.B.; Koh, S.; Sharma, N.; Woreta, F.A.; Hafezi, F.; Dua, H.S.; Jhanji, V. Keratoconus. Nat. Rev. Dis. Prim. 2024, 10, 81. [Google Scholar] [CrossRef]
- Bykhovskaya, Y.; Rabinowitz, Y.S. Update on the Genetics of Keratoconus. Exp. Eye Res. 2021, 202, 108398. [Google Scholar] [CrossRef]
- Wheeler, J.; Hauser, M.A.; Afshari, N.A.; Allingham, R.R.; Liu, Y. The Genetics of Keratoconus: A Review. Reprod. Syst. Sex. Disord. 2012, (Suppl. 6) (Suppl. 6), 001. [Google Scholar] [CrossRef]
- Valgaeren, H.; Koppen, C.; Camp, G.V. A New Perspective on the Genetics of Keratoconus: Why Have We Not Been More Successful? Ophthalmic Genet. 2018, 39, 158–174. [Google Scholar] [CrossRef]
- Risch, N.; Merikangas, K. The Future of Genetic Studies of Complex Human Diseases. Science 1996, 273, 1516–1517. [Google Scholar] [CrossRef] [PubMed]
- Karolak, J.A.; Ginter-Matuszewska, B.; Tomela, K.; Kabza, M.; Nowak-Malczewska, D.M.; Rydzanicz, M.; Polakowski, P.; Szaflik, J.P.; Gajecka, M. Further Evaluation of Differential Expression of Keratoconus Candidate Genes in Human Corneas. PeerJ 2020, 8, e9793. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Botstein, D. Mapping Mendelian Factors Underlying Quantitative Traits Using RFLP Linkage Maps. Genetics 1989, 121, 185–199. [Google Scholar] [CrossRef]
- McCarthy, M.I.; Hirschhorn, J.N. Genome-Wide Association Studies: Potential next Steps on a Genetic Journey. Hum. Mol. Genet. 2008, 17, R156–R165. [Google Scholar] [CrossRef]
- Visscher, P.M.; Wray, N.R.; Zhang, Q.; Sklar, P.; McCarthy, M.I.; Brown, M.A.; Yang, J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet. 2017, 101, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bykhovskaya, Y.; Haritunians, T.; Siscovick, D.; Aldave, A.; Szczotka-Flynn, L.; Iyengar, S.K.; Rotter, J.I.; Taylor, K.D.; Rabinowitz, Y.S. A Genome-Wide Association Study Identifies a Potential Novel Gene Locus for Keratoconus, One of the Commonest Causes for Corneal Transplantation in Developed Countries. Hum. Mol. Genet. 2012, 21, 421–429. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Zadnik, K.; Barr, J.T.; Edrington, T.B.; Everett, D.F.; Jameson, M.; McMahon, T.T.; Shin, J.A.; Sterling, J.L.; Wagner, H.; Gordon, M.O. Baseline Findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2537–2546. [Google Scholar]
- Wang, Y.; Rabinowitz, Y.S.; Rotter, J.I.; Yang, H. Genetic Epidemiological Study of Keratoconus: Evidence for Major Gene Determination. Am. J. Méd. Genet. 2000, 93, 403–409. [Google Scholar] [CrossRef]
- Lapeyre, G.; Fournie, P.; Vernet, R.; Roseng, S.; Malecaze, F.; Bouzigon, E.; Touboul, D. Keratoconus Prevalence in Families: A French Study. Cornea 2020, 39, 1473–1479. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Wang, S.; Yang, K.; Gu, Y.; Fan, Q.; Wang, Q.; Zhu, M.; Guo, K.; Pang, C.; et al. A Hospital-Based Study on the Prevalence of Keratoconus in First-Degree Relatives of Patients with Keratoconus in Central China. J. Ophthalmol. 2022, 2022, 6609531. [Google Scholar] [CrossRef]
- Shneor, E.; Frucht-Pery, J.; Granit, E.; Gordon-Shaag, A. The Prevalence of Corneal Abnormalities in First-degree Relatives of Patients with Keratoconus: A Prospective Case-control Study. Ophthalmic Physiol. Opt. 2020, 40, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Naderan, M.; Rajabi, M.T.; Zarrinbakhsh, P.; Naderan, M.; Bakhshi, A. Association between Family History and Keratoconus Severity. Curr. Eye Res. 2016, 41, 1414–1418. [Google Scholar] [CrossRef]
- Szczotka-Flynn, L.; Slaughter, M.; McMahon, T.; Barr, J.; Edrington, T.; Fink, B.; Lass, J.; Belin, M.; Iyengar, S.K.; Group, C.S. Disease Severity and Family History in Keratoconus. Br. J. Ophthalmol. 2008, 92, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Tuft, S.J.; Hassan, H.; George, S.; Frazer, D.G.; Willoughby, C.E.; Liskova, P. Keratoconus in 18 Pairs of Twins. Acta Ophthalmol. 2012, 90, e482–e486. [Google Scholar] [CrossRef] [PubMed]
- Bitton, K.; Dubois, M.; Moran, S.; Gatinel, D. Discordant Keratoconus in Monozygotic Twins. Case Rep. Ophthalmol. 2022, 13, 313–317. [Google Scholar] [CrossRef]
- Gordon-Shaag, A.; Millodot, M.; Shneor, E. The Epidemiology and Etiology of Keratoconus. Int. J. Keratoconus Ectatic Corneal Dis. 2012, 1, 7–15. [Google Scholar] [CrossRef]
- Wagner, H.; Barr, J.T.; Zadnik, K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: Methods and Findings to Date. Contact Lens Anterior Eye 2007, 30, 223–232. [Google Scholar] [CrossRef]
- Kristianslund, O.; Drolsum, L. Prevalence of Keratoconus in Persons with Down Syndrome: A Review. BMJ Open Ophthalmol. 2021, 6, e000754. [Google Scholar] [CrossRef]
- Sun, E.; Kraus, C.L. The Ophthalmic Manifestations of Down Syndrome. Children 2023, 10, 341. [Google Scholar] [CrossRef]
- Alio, J.L.; Vega-Estrada, A.; Sanz, P.; Osman, A.A.; Kamal, A.M.; Mamoon, A.; Soliman, H. Corneal Morphologic Characteristics in Patients With Down Syndrome. JAMA Ophthalmol. 2018, 136, 971–978. [Google Scholar] [CrossRef]
- Woodward, M.A.; Blachley, T.S.; Stein, J.D. The Association Between Sociodemographic Factors, Common Systemic Diseases, and Keratoconus An Analysis of a Nationwide Heath Care Claims Database. Ophthalmology 2016, 123, 457–465.e2. [Google Scholar] [CrossRef]
- Bawazeer, A.M.; Hodge, W.G.; Lorimer, B. Atopy and Keratoconus: A Multivariate Analysis. Br. J. Ophthalmol. 2000, 84, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Unni, P.; Lee, H.J. Systemic Associations with Keratoconus. Life 2023, 13, 1363. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and Related Noninflammatory Corneal Thinning Disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Tanwar, M.; Kumar, M.; Nayak, B.; Pathak, D.; Sharma, N.; Titiyal, J.S.; Dada, R. VSX1 Gene Analysis in Keratoconus. Mol. Vis. 2010, 16, 2395–2401. [Google Scholar]
- Nejabat, M.; Naghash, P.; Dastsooz, H.; Mohammadi, S.; Alipour, M.; Fardaei, M. VSX1 and SOD1 Mutation Screening in Patients with Keratoconus in the South of Iran. J. Ophthalmic Vis. Res. 2017, 12, 135–140. [Google Scholar] [CrossRef]
- Héon, E.; Greenberg, A.; Kopp, K.K.; Rootman, D.; Vincent, A.L.; Billingsley, G.; Priston, M.; Dorval, K.M.; Chow, R.L.; McInnes, R.R.; et al. VSX1: A Gene for Posterior Polymorphous Dystrophy and Keratoconus. Hum. Mol. Genet. 2002, 11, 1029–1036. [Google Scholar] [CrossRef]
- Bykhovskaya, Y.; Margines, B.; Rabinowitz, Y.S. Genetics in Keratoconus: Where Are We? Eye Vis. 2016, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Wang, X.; Zheng, L.-B.; Wu, H.-J.; Yao, Y.-F. Analysis of the VSX1 Gene in Sporadic Keratoconus Patients from China. BMC Ophthalmol. 2017, 17, 173. [Google Scholar] [CrossRef]
- Tang, Y.G.; Picornell, Y.; Su, X.; Li, X.; Yang, H.; Rabinowitz, Y.S. Three VSX1 Gene Mutations, L159M, R166W, and H244R, Are Not Associated With Keratoconus. Cornea 2008, 27, 189–192. [Google Scholar] [CrossRef]
- Verma, A.; Das, M.; Srinivasan, M.; Prajna, N.V.; Sundaresan, P. Investigation of VSX1 Sequence Variants in South Indian Patients with Sporadic Cases of Keratoconus. BMC Res. Notes 2013, 6, 103. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kalantan, H.; Al-Muammar, A.M. Analysis of the VSX1 Gene in Keratoconus Patients from Saudi Arabia. Mol. Vis. 2011, 17, 667–672. [Google Scholar] [PubMed]
- Guan, T.; Wu, H.; Zhang, L.; Xu, D.; Zheng, L.; Yao, Y. A Novel VSX1 Gene Mutation Identified in a Sporadic Keratoconus Patient from China. Chin. J. Ophthalmol. 2018, 54, 212–217. [Google Scholar] [CrossRef]
- Bonis, P.D.; Laborante, A.; Pizzicoli, C.; Stallone, R.; Barbano, R.; Longo, C.; Mazzilli, E.; Zelante, L.; Bisceglia, L. Mutational Screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in Keratoconus. Mol. Vis. 2011, 17, 2482–2494. [Google Scholar]
- Vincent, A.L.; Jordan, C.; Sheck, L.; Niederer, R.; Patel, D.V.; McGhee, C.N.J. Screening the Visual System Homeobox 1 Gene in Keratoconus and Posterior Polymorphous Dystrophy Cohorts Identifies a Novel Variant. Mol. Vis. 2013, 19, 852–860. [Google Scholar] [PubMed]
- Jeoung, J.W.; Kim, M.K.; Park, S.S.; Kim, S.Y.; Ko, H.S.; Wee, W.R.; Lee, J.H. VSX1 Gene and Keratoconus. Cornea 2012, 31, 746–750. [Google Scholar] [CrossRef]
- Dash, D.P.; George, S.; O’Prey, D.; Burns, D.; Nabili, S.; Donnelly, U.; Hughes, A.E.; Silvestri, G.; Jackson, J.; Frazer, D.; et al. Mutational Screening of VSX1 in Keratoconus Patients from the European Population. Eye 2010, 24, 1085–1092. [Google Scholar] [CrossRef]
- Moussa, S.; Grabner, G.; Ruckhofer, J.; Dietrich, M.; Reitsamer, H. Genetics in Keratoconus—What Is New? Open Ophthalmol. J. 2017, 11, 201–210. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.-Y.; Jin, J.-J.; Shen, R.-J.; Mao, J.-Y.; Cheng, F.-F.; Chen, Z.-J.; Linardaki, E.; Voulgaraki, S.; Aslanides, I.M.; et al. Genetic Screening Revealed Latent Keratoconus in Asymptomatic Individuals. Front. Cell Dev. Biol. 2021, 9, 650344. [Google Scholar] [CrossRef] [PubMed]
- Zahir-Jouzdani, F.; Soleimani, M.; Mahbod, M.; Mottaghitalab, F.; Vakhshite, F.; Arefian, E.; Shahhoseini, S.; Dinarvand, R.; Atyabi, F. Corneal Chemical Burn Treatment through a Delivery System Consisting of TGF-Β1 siRNA: In Vitro and in Vivo. Drug Deliv. Transl. Res. 2018, 8, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Liu, C.; Ma, Z.; Ding, S. The Point Mutation and Polymorphism in Keratoconus Candidate Gene TGFBI in Chinese Population. Gene 2012, 503, 137–139. [Google Scholar] [CrossRef]
- Kabza, M.; Karolak, J.A.; Rydzanicz, M.; Szcześniak, M.W.; Nowak, D.M.; Ginter-Matuszewska, B.; Polakowski, P.; Ploski, R.; Szaflik, J.P.; Gajecka, M. Collagen Synthesis Disruption and Downregulation of Core Elements of TGF-β, Hippo, and Wnt Pathways in Keratoconus Corneas. Eur. J. Hum. Genet. 2017, 25, 582–590. [Google Scholar] [CrossRef]
- Lin, Q.; Zheng, L.; Shen, Z. A Novel Variant in TGFBI Causes Keratoconus in a Two-Generation Chinese Family. Ophthalmic Genet. 2022, 43, 159–163. [Google Scholar] [CrossRef]
- Stachon, T.; Ali, M.O.; Latta, L.; Huessein, G.H.; Mohamed, T.A.; Soliman, W.; Seitz, B.; Szentmáry, N. Effect of Thyroxine on Transforming Growth Factor Β1, Collagen I, and V Expression in Keratoconus Corneal Fibroblasts and Keratocytes, in Vitro. Curr. Eye Res. 2022, 47, 206–213. [Google Scholar] [CrossRef]
- Engler, C.; Chakravarti, S.; Doyle, J.; Eberhart, C.G.; Meng, H.; Stark, W.J.; Kelliher, C.; Jun, A.S. Transforming Growth Factor-β Signaling Pathway Activation in Keratoconus. Am. J. Ophthalmol. 2011, 151, 752–759.e2. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Liang, W.; Dean, D.C.; Zhang, L.; Liu, Y. Expression and Function of ZEB1 in the Cornea. Cells 2021, 10, 925. [Google Scholar] [CrossRef]
- Lechner, J.; Dash, D.P.; Muszynska, D.; Hosseini, M.; Segev, F.; George, S.; Frazer, D.G.; Moore, J.E.; Kaye, S.B.; Young, T.; et al. Mutational Spectrum of the ZEB1 Gene in Corneal Dystrophies Supports a Genotype–Phenotype Correlation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3215–3223. [Google Scholar] [CrossRef]
- González-Atienza, C.; Sánchez-Cazorla, E.; Villoldo-Fernández, N.; del Hierro, A.; Boto, A.; Guerrero-Carretero, M.; Nieves-Moreno, M.; Arruti, N.; Rodríguez-Solana, P.; Mena, R.; et al. Whole-Exome Sequencing of 24 Spanish Families: Candidate Genes for Non-Syndromic Pediatric Keratoconus. Genes 2023, 14, 1838. [Google Scholar] [CrossRef] [PubMed]
- Whigham, B.T.; Allingham, R.R. Developments in Ocular Genetics. Asia-Pac. J. Ophthalmol. 2013, 2, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Helwa, I.; Al-Muammar, A.; Strickland, S.; Hauser, M.A.; Allingham, R.R.; Liu, Y. Screening of the Seed Region of MIR184 in Keratoconus Patients from Saudi Arabia. BioMed Res. Int. 2015, 2015, 604508. [Google Scholar] [CrossRef]
- Ryan, D.G.; Oliveira-Fernandes, M.; Lavker, R.M. MicroRNAs of the Mammalian Eye Display Distinct and Overlapping Tissue Specificity. Mol. Vis. 2006, 12, 1175–1184. [Google Scholar] [PubMed]
- Hughes, A.E.; Bradley, D.T.; Campbell, M.; Lechner, J.; Dash, D.P.; Simpson, D.A.; Willoughby, C.E. Mutation Altering the miR-184 Seed Region Causes Familial Keratoconus with Cataract. Am. J. Hum. Genet. 2011, 89, 628–633. [Google Scholar] [CrossRef]
- Yu, J.; Ryan, D.G.; Getsios, S.; Oliveira-Fernandes, M.; Fatima, A.; Lavker, R.M. MicroRNA-184 Antagonizes microRNA-205 to Maintain SHIP2 Levels in Epithelia. Proc. Natl. Acad. Sci. USA 2008, 105, 19300–19305. [Google Scholar] [CrossRef]
- Pukl, S.S. Are miRNAs Dynamic Biomarkers in Keratoconus? A Review of the Literature. Genes 2022, 13, 588. [Google Scholar] [CrossRef]
- Lechner, J.; Bae, H.A.; Guduric-Fuchs, J.; Rice, A.; Govindarajan, G.; Siddiqui, S.; Farraj, L.A.; Yip, S.P.; Yap, M.; Das, M.; et al. Mutational Analysis of MIR184 in Sporadic Keratoconus and Myopia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5266–5272. [Google Scholar] [CrossRef]
- Udar, N.; Atilano, S.R.; Brown, D.J.; Holguin, B.; Small, K.; Nesburn, A.B.; Kenney, M.C. SOD1: A Candidate Gene for Keratoconus. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3345–3351. [Google Scholar] [CrossRef]
- Lopes, A.G.; de Almeida Júnior, G.C.; Teixeira, R.M.; de Mattos, L.C.; Mattos, C.C.B.d.; Castiglioni, L. Absence of the c.169+50delTAAACAG Mutation of SOD1 Gene in a Sample of Keratoconus Patients in Brazilian Population. BMC Res. Notes 2020, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.G.; de Almeida, G.C., Jr.; Miola, M.P.; Teixeira, R.M.; Pires, F.C.B.L.; Miani, R.A.; de Mattos, L.C.; Brandão, C.C.; Castiglioni, L. Absence of Significant Genetic Alterations in the VSX1, SOD1, TIMP3, and LOX Genes in Brazilian Patients with Keratoconus. Ophthalmic Genet. 2022, 43, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Muammar, A.M.; Kalantan, H.; Azad, T.A.; Sultan, T.; Abu-Amero, K.K. Analysis of the SOD1 Gene in Keratoconus Patients from Saudi Arabia. Ophthalmic Genet. 2015, 36, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Al-Raddadi, H.S.; Al-Barry, M.A.; Al-Harbi, E.; Samman, M.I.; Albalawi, A.M.; Basit, S. Sequence Analysis of the VSX1 and SOD1 Genes in Families with Keratoconus and a Review of the Literature. J. Taibah Univ. Med. Sci. 2016, 11, 115–120. [Google Scholar] [CrossRef]
- Lucas, S.E.M.; Zhou, T.; Blackburn, N.B.; Mills, R.A.; Ellis, J.; Leo, P.; Souzeau, E.; Ridge, B.; Charlesworth, J.C.; Lindsay, R.; et al. Rare, Potentially Pathogenic Variants in 21 Keratoconus Candidate Genes Are Not Enriched in Cases in a Large Australian Cohort of European Descent. PLoS ONE 2018, 13, e0199178. [Google Scholar] [CrossRef]
- Yu, X.; Chen, B.; Zhang, X.; Shentu, X. Identification of Seven Novel ZNF469 Mutations in Keratoconus Patients in a Han Chinese Population. Mol. Vis. 2017, 23, 296–305. [Google Scholar]
- Lechner, J.; Porter, L.F.; Rice, A.; Vitart, V.; Armstrong, D.J.; Schorderet, D.F.; Munier, F.L.; Wright, A.F.; Inglehearn, C.F.; Black, G.C.; et al. Enrichment of Pathogenic Alleles in the Brittle Cornea Gene, ZNF469, in Keratoconus. Hum. Mol. Genet. 2014, 23, 5527–5535. [Google Scholar] [CrossRef]
- Wright, E.M.M.B.; Spencer, H.L.; Daly, S.B.; Manson, F.D.C.; Zeef, L.A.H.; Urquhart, J.; Zoppi, N.; Bonshek, R.; Tosounidis, I.; Mohan, M.; et al. Mutations in PRDM5 in Brittle Cornea Syndrome Identify a Pathway Regulating Extracellular Matrix Development and Maintenance. Am. J. Hum. Genet. 2011, 88, 767–777. [Google Scholar] [CrossRef]
- Vincent, A.L.; Jordan, C.A.; Cadzow, M.J.; Merriman, T.R.; McGhee, C.N. Mutations in the Zinc Finger Protein Gene, ZNF469, Contribute to the Pathogenesis of Keratoconus. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5629–5635. [Google Scholar] [CrossRef]
- de Oteyza, G.G.; Engroba, J.F.; Charoenrook, V. New ZNF469 Mutations in Spanish Siblings with Brittle Cornea Syndrome. Cornea 2023, 42, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Wonneberger, W.; Sterner, B.; MacLean, U.; Claesson, M.; Johansson, L.H.; Skoog, I.; Zetterberg, M.; Zettergren, A. Genetic Variants in the FOXO1 and ZNF469 Genes Are Associated with Keratoconus in Sweden: A Case-Control Study. BMC Ophthalmol. 2024, 24, 36. [Google Scholar] [CrossRef]
- Karolak, J.A.; Gambin, T.; Rydzanicz, M.; Szaflik, J.P.; Polakowski, P.; Frajdenberg, A.; Mrugacz, M.; Podfigurna-Musielak, M.; Stankiewicz, P.; Gajecka, M. Evidence against ZNF469 Being Causative for Keratoconus in Polish Patients. Acta Ophthalmol. 2016, 94, 289–294. [Google Scholar] [CrossRef]
- Lucas, S.E.M.; Zhou, T.; Blackburn, N.B.; Mills, R.A.; Ellis, J.; Leo, P.; Souzeau, E.; Ridge, B.; Charlesworth, J.C.; Brown, M.A.; et al. Rare, Potentially Pathogenic Variants in ZNF469 Are Not Enriched in Keratoconus in a Large Australian Cohort of European Descent. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6248–6256. [Google Scholar] [CrossRef]
- Yildiz, E.; Bardak, H.; Gunay, M.; Bardak, Y.; Imamoglu, S.; Ozbas, H.; Bagci, O. Novel Zinc Finger Protein Gene 469 (ZNF469) Variants in Advanced Keratoconus. Curr. Eye Res. 2017, 42, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Bykhovskaya, Y.; Li, X.; Epifantseva, I.; Haritunians, T.; Siscovick, D.; Aldave, A.; Szczotka-Flynn, L.; Iyengar, S.K.; Taylor, K.D.; Rotter, J.I.; et al. Variation in the Lysyl Oxidase (LOX) Gene Is Associated with Keratoconus in Family-Based and Case-Control Studies. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Moshirfar, M.; Alizadeh, F.; Doroodgar, F.; Baradaran-Rafii, A.; Filutowski, O.; Niazi, F.; Ambrósio, R. Association of 2 Lysyl Oxidase Gene Single Nucleotide Polymorphisms with Keratoconus A Nationwide Registration Study. Ophthalmol. Sci. 2023, 3, 100247. [Google Scholar] [CrossRef]
- Shetty, R.; Sathyanarayanamoorthy, A.; Ramachandra, R.A.; Arora, V.; Ghosh, A.; Srivatsa, P.R.; Pahuja, N.; Nuijts, R.M.M.A.; Sinha-Roy, A.; Mohan, R.R.; et al. Attenuation of Lysyl Oxidase and Collagen Gene Expression in Keratoconus Patient Corneal Epithelium Corresponds to Disease Severity. Mol. Vis. 2014, 21, 12–25. [Google Scholar]
- Hasanian-Langroudi, F.; Saravani, R.; Validad, M.-H.; Bahari, G.; Yari, D. Association of Lysyl Oxidase (LOX) Polymorphisms with the Risk of Keratoconus in an Iranian Population. Ophthalmic Genet. 2015, 36, 309–314. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Hong, J.; Wu, D.; Xu, J. Association of Common Variants in LOX with Keratoconus: A Meta-Analysis. PLoS ONE 2015, 10, e0145815. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Cui, Y.; Yang, H.; Ping, X.; Wu, J.; Yu, X.; Jin, X.; Huang, X.; Shentu, X. Three Novel Variants Identified within ECM-Related Genes in Chinese Han Keratoconus Patients. Sci. Rep. 2020, 10, 5844. [Google Scholar] [CrossRef]
- Gajecka, M.; Radhakrishna, U.; Winters, D.; Nath, S.K.; Rydzanicz, M.; Ratnamala, U.; Ewing, K.; Molinari, A.; Pitarque, J.A.; Lee, K.; et al. Localization of a Gene for Keratoconus to a 5.6-Mb Interval on 13q32. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Karolak, J.A.; Rydzanicz, M.; Ginter-Matuszewska, B.; Pitarque, J.A.; Molinari, A.; Bejjani, B.A.; Gajecka, M. Variant c.2262A>C in DOCK9 Leads to Exon Skipping in Keratoconus Family. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7687–7690. [Google Scholar] [CrossRef] [PubMed]
- Czugala, M.; Karolak, J.A.; Nowak, D.M.; Polakowski, P.; Pitarque, J.; Molinari, A.; Rydzanicz, M.; Bejjani, B.A.; Yue, B.Y.J.T.; Szaflik, J.P.; et al. Novel Mutation and Three Other Sequence Variants Segregating with Phenotype at Keratoconus 13q32 Susceptibility Locus. Eur. J. Hum. Genet. 2012, 20, 389–397. [Google Scholar] [CrossRef]
- Reis, L.M.; Rodrigues, F.W.; da Silva, R.E.; Taleb, A.C.; de Ávila, M.P. Relation Analysis of the Occurrence of Single Nucleotide Polymorphism of the DOCK9 Gene in Keratoconus. Rev. Bras. Oftalmol. 2016, 75, 223–227. [Google Scholar] [CrossRef]
- Nowak, D.M.; Karolak, J.A.; Kubiak, J.; Gut, M.; Pitarque, J.A.; Molinari, A.; Bejjani, B.A.; Gajecka, M. Substitution at IL1RN and Deletion at SLC4A11 Segregating with Phenotype in Familial Keratoconus. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2207–2215. [Google Scholar] [CrossRef]
- Alvarez, B.V.; Piché, M.; Aizouki, C.; Rahman, F.; Derry, J.M.J.; Brunette, I.; Casey, J.R. Altered Gene Expression in Slc4a11−/− Mouse Cornea Highlights SLC4A11 Roles. Sci. Rep. 2021, 11, 20885. [Google Scholar] [CrossRef]
- Kao, L.; Azimov, R.; Abuladze, N.; Newman, D.; Kurtz, I. Human SLC4A11-C Functions as a DIDS-Stimulatable H+(OH−) Permeation Pathway: Partial Correction of R109H Mutant Transport. Am. J. Physiol.-Cell Physiol. 2015, 308, C176–C188. [Google Scholar] [CrossRef]
- Cheng, W.-Y.; Yang, S.-Y.; Huang, X.-Y.; Zi, F.-Y.; Li, H.-P.; Sheng, X.-L. Identification of Genetic Variants in Five Chinese Families with Keratoconus: Pathogenicity Analysis and Characteristics of Parental Corneal Topography. Front. Genet. 2022, 13, 978684. [Google Scholar] [CrossRef] [PubMed]
- Matthews, F.J.; Cook, S.D.; Majid, M.A.; Dick, A.D.; Smith, V.A. Changes in the Balance of the Tissue Inhibitor of Matrix Metalloproteinases (TIMPs)-1 and -3 May Promote Keratocyte Apoptosis in Keratoconus. Exp. Eye Res. 2007, 84, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Oum, B.S.; Choi, H.Y.; Lee, S.U.; Lee, J.S. Evaluation of Differentially Expressed Genes Identified in Keratoconus. Mol. Vis. 2009, 15, 2480–2487. [Google Scholar]
- Cao, K.; Sahebjada, S.; Richardson, A.J.; Baird, P.N. Do Age-Related Macular Degeneration Genes Show Association with Keratoconus? Eye Vis. 2019, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.-D.; Gao, H.; Xu, W.-H.; Shan, C.; Liu, Y.; Zhou, Z.-X.; Wang, K.; Li, P.-F. Systematically Displaying the Pathogenesis of Keratoconus via Multi-Level Related Gene Enrichment-Based Review. Front. Med. 2022, 8, 770138. [Google Scholar] [CrossRef]
- Nowak-Wąs, M.; Wąs, P.; Czuba, Z.; Wojnicz, R.; Wyględowska-Promieńska, D. Expression of Tissue Inhibitors of Metalloproteinases (TIMP-1, TIMP-2, TIMP-3, TIMP-4) in Blood Serum of Patients with Keratoconus. J. Clin. Med. 2024, 13, 1168. [Google Scholar] [CrossRef]
- Harati-Sadegh, M.; Sargazi, S.; Khorasani, M.; Ansari-Moghaddam, A.; Mirinejad, S.; Sheervalilou, R.; Saravani, R. IL1A and IL1B Gene Polymorphisms and Keratoconus Susceptibility: Evidence from an Updated Meta-Analysis. Ophthalmic Genet. 2021, 42, 503–513. [Google Scholar] [CrossRef]
- Kim, S.-H.; Mok, J.-W.; Kim, H.-S.; Joo, C.K. Association of –31T>C and –511 C>T Polymorphisms in the Interleukin 1 Beta (IL1B) Promoter in Korean Keratoconus Patients. Mol. Vis. 2008, 14, 2109–2116. [Google Scholar]
- Mikami, T.; Meguro, A.; Teshigawara, T.; Takeuchi, M.; Uemoto, R.; Kawagoe, T.; Nomura, E.; Asukata, Y.; Ishioka, M.; Iwasaki, M.; et al. Interleukin 1 Beta Promoter Polymorphism Is Associated with Keratoconus in a Japanese Population. Mol. Vis. 2013, 19, 845–851. [Google Scholar]
- West-Mays, J.A.; Sadow, P.M.; Tobin, T.W.; Strissel, K.J.; Cintron, C.; Fini, M.E. Repair Phenotype in Corneal Fibroblasts Is Controlled by an Interleukin-1 Alpha Autocrine Feedback Loop. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1367–1379. [Google Scholar]
- WILSON, S.E.; HE, Y.-G.; WENG, J.; LI, Q.; McDOWALL, A.W.; VITAL, M.; CHWANG, E.L. Epithelial Injury Induces Keratocyte Apoptosis: Hypothesized Role for the Interleukin-1 System in the Modulation of Corneal Tissue Organization and Wound Healing. Exp. Eye Res. 1996, 62, 325–338. [Google Scholar] [CrossRef]
- Palamar, M.; Onay, H.; Ozdemir, T.R.; Arslan, E.; Egrilmez, S.; Ozkinay, F.; Yagci, A. Relationship Between IL1β-511C>T and ILRN VNTR Polymorphisms and Keratoconus. Cornea 2014, 33, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bykhovskaya, Y.; Canedo, A.L.C.; Haritunians, T.; Siscovick, D.; Aldave, A.J.; Szczotka-Flynn, L.; Iyengar, S.K.; Rotter, J.I.; Taylor, K.D.; et al. Genetic Association of COL5A1 Variants in Keratoconus Patients Suggests a Complex Connection between Corneal Thinning and Keratoconus. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zheng, L.; Shen, Z.; Jie, L. A Novel Splice-Site Variation in COL5A1 Causes Keratoconus in an Indian Family. J. Ophthalmol. 2019, 2019, 2851380. [Google Scholar] [CrossRef] [PubMed]
- Stabuc-Silih, M.; Ravnik-Glavac, M.; Glavac, D.; Hawlina, M.; Strazisar, M. Polymorphisms in COL4A3 and COL4A4 Genes Associated with Keratoconus. Mol. Vis. 2009, 15, 2848–2860. [Google Scholar]
- Abdelghany, A.A.; Toraih, E.A.; Abdelaziz, E.Z.; El-Sherbeeny, N.A.; Fawzy, M.S. Association of Collagen Gene (COL4A3) Rs55703767 Variant with Response to Riboflavin/Ultraviolet A-Induced Collagen Cross-Linking in Female Patients with Keratoconus. Cornea 2020, 40, 88–98. [Google Scholar] [CrossRef]
- Rong, S.S.; Ma, S.T.U.; Yu, X.T.; Ma, L.; Chu, W.K.; Chan, T.C.Y.; Wang, Y.M.; Young, A.L.; Pang, C.P.; Jhanji, V.; et al. Genetic Associations for Keratoconus: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 4620. [Google Scholar] [CrossRef]
- Saravani, R.; Hasanian-Langroudi, F.; Validad, M.-H.; Yari, D.; Bahari, G.; Faramarzi, M.; Khateri, M.; Bahadoram, S. Evaluation of Possible Relationship Between COL4A4 Gene Polymorphisms and Risk of Keratoconus. Cornea 2015, 34, 318–322. [Google Scholar] [CrossRef]
- Skorodumova, L.O.; Belodedova, A.V.; Sharova, E.I.; Zakharova, E.S.; Iulmetova, L.N.; Bikbov, M.M.; Usubov, E.L.; Antonova, O.P.; Selezneva, O.V.; Levchenko, A.; et al. Rare Single Nucleotide Variants in COL5A1 Promoter Do Not Play a Major Role in Keratoconus Susceptibility Associated with Rs1536482. BMC Ophthalmol. 2021, 21, 357. [Google Scholar] [CrossRef]
- Bryant, G.; Moore, P.; Sathyamoorthy, M. The Association of a Single Nucleotide Variant in COL5A1 to Early Onset Keratoconus and Pectus Excavatum—Convergence of Extracellular Matrix Pathologies. Medicina 2024, 60, 974. [Google Scholar] [CrossRef]
- Kokolakis, N.S.; Gazouli, M.; Chatziralli, I.P.; Koutsandrea, C.; Gatzioufas, Z.; Peponis, V.G.; Droutsas, K.D.; Kalogeropoulos, C.; Anagnou, N.; Miltsakakis, D.; et al. Polymorphism Analysis of COL4A3 and COL4A4 Genes in Greek Patients with Keratoconus. Ophthalmic Genet. 2014, 35, 226–228. [Google Scholar] [CrossRef]
- Bisceglia, L.; Ciaschetti, M.; Bonis, P.D.; Campo, P.A.P.; Pizzicoli, C.; Scala, C.; Grifa, M.; Ciavarella, P.; Noci, N.D.; Vaira, F.; et al. VSX1 Mutational Analysis in a Series of Italian Patients Affected by Keratoconus: Detection of a Novel Mutation. Investig. Ophthalmol. Vis. Sci. 2005, 46, 39–45. [Google Scholar] [CrossRef]
- Moschos, M.M.; Kokolakis, N.; Gazouli, M.; Chatziralli, I.P.; Droutsas, D.; Anagnou, N.P.; Ladas, I.D. Polymorphism Analysis of VSX1 and SOD1 Genes in Greek Patients with Keratoconus. Ophthalmic Genet. 2015, 36, 213–217. [Google Scholar] [CrossRef]
- Karolak, J.A.; Polakowski, P.; Szaflik, J.; Szaflik, J.P.; Gajecka, M. Molecular Screening of Keratoconus Susceptibility Sequence Variants in VSX1, TGFBI, DOCK9, STK24, and IPO5 Genes in Polish Patients and Novel TGFBI Variant Identification. Ophthalmic Genet. 2016, 37, 37–43. [Google Scholar] [CrossRef]
- Loukovitis, E.; Sfakianakis, K.; Syrmakesi, P.; Tsotridou, E.; Orfanidou, M.; Bakaloudi, D.R.; Stoila, M.; Kozei, A.; Koronis, S.; Zachariadis, Z.; et al. Genetic Aspects of Keratoconus: A Literature Review Exploring Potential Genetic Contributions and Possible Genetic Relationships with Comorbidities. Ophthalmol. Ther. 2018, 7, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Saee-Rad, S.; Hashemi, H.; Miraftab, M.; Noori-Daloii, M.R.; Chaleshtori, M.H.; Raoofian, R.; Jafari, F.; Greene, W.; Fakhraie, G.; Rezvan, F.; et al. Mutation Analysis of VSX1 and SOD1 in Iranian Patients with Keratoconus. Mol. Vis. 2011, 17, 3128–3136. [Google Scholar] [PubMed]
- Kalantan, H.; Kondkar, A.A.; Sultan, T.; Azad, T.A.; Alsabaani, N.A.; AlQahtani, M.A.; Almummar, A.; Liu, Y.; Abu-Amero, K.K. Polymorphism Rs13334190 in Zinc Finger Protein 469 (ZNF469) Is Not a Risk Factor for Keratoconus in a Saudi Cohort. BMC Res. Notes 2017, 10, 652. [Google Scholar] [CrossRef]
- Alswailmi, F.K.; Bokhari, K.; Aladaileh, S.H.; Alanezi, A.A.; Azam, M.; Ahmad, A. Protective and Pathogenic Role of Collagen Subtypes Genes COL4A3 and COL4A4 Polymorphisms in the Onset of Keratoconus in South-Asian Pakistani Cohort. Saudi J. Biol. Sci. 2023, 30, 103503. [Google Scholar] [CrossRef]


| Gene Name | Loci | Variant Classification |
|---|---|---|
| VSX1 | 20p11-q11 | Variant of Uncertain Significance |
| TGFBI | 5q 31.1 | Variant of Uncertain Significance |
| ZEB1 | 10p11.22 | Variant of Uncertain Significance |
| MIR184 | 15q22-25 | Some Pathogenic (regarding syndromic KC) Some Variants of Uncertain Significance |
| SOD1 | 21q22.11 | Variant of Uncertain Significance |
| ZNF469 | 16q24 | Some Pathogenic |
| Some Variants of Uncertain Significance | ||
| LOX | 5q23.2 | Variant of Uncertain Significance |
| DOCK9 | 13q32 | Variant of Uncertain Significance |
| SLC4A11 | 20p13 | Variant of Uncertain Significance |
| TIMP3 | 22q12.3 | Variant of Uncertain Significance |
| IL1A/IL1B/IL1RN | 2q13-q14.3 | Likely Bening |
| COL4A3/COL4A4/COL5A1 | COL4A3: 2q36.3; COL4A4: 2q36.3; COL5A1: 9q34.2-q34. | Some Pathogenic Some Variants of Uncertain Significance |
| Location | Chromosome 20p11–q11 |
|---|---|
| Biological Role | Regulates cone opsin expression during early eye development, particularly in the corneal stroma and retina |
| Functional Impact | Disrupts protein interaction networks, particularly collagen, MMPs, and signaling pathways |
| Associated Conditions | BCS, Ehlers–Danlos, PPCD |
| Reported Variations | p.L17P, p.D144E, p.N151S, p.L159M, p.G160V, p.G160D, p.R166W, p.Q175H, p.H244R, p.P247R, p.R131P, p.G239R c.715G>C, p.Pro58Leu c.173C>T |
| Population-Specific Findings | Southern Indians: No mutations; 4 SNPs detected: rs12480307, rs6138482, rs56157240, IVS3-24C Saudi Arabians: 5 SNPs linked: g.8326G>A, g.10945G>T, g.11059A>C, g.5053G>T, g.8222A>G Chinese: Variants p.R131P and p.G160V in 3/50 patients, absent in controls Iranian and Italian: Mutations in ~5% of patients; SNPs: p.L17P, p.D144E, p.H244R, p.P247R, p.G239R New Zealanders: Novel c.173C>T p.Pro58Leu in KC + PPCD patient, not replicated England: No association found |
| Location | Chromosome 16q24 |
|---|---|
| Biological Role | Regulates ECM maintenance and corneal fiber homeostasis |
| Functional Impact | Suggested role in ECM remodeling and collagen remodeling |
| Associated Conditions | BCS, Ehlers–Danlos, PPCD |
| Reported Variations | 14 rare missense variants in Polynesian/Māori (46% of patients) 7 mutations in Han Chinese patients Allele mutations in 12.5% of KC patients p.Arg492Gln in Spanish family c.2972del, p.Pro991Hisfs62 in another Spanish family |
| Population-Specific Findings | Polynesian/Māori: High frequency of rare variants (46%) Han Chinese: 7 mutations detected Spanish: Two families with rare variants European: 12.5% of patients carried variants Polish: No enrichment, changes were polymorphisms Australian: No significant association Advanced KC patients: Variants linked in some studies |
| Gene | Location | Main Function | Variations Reported | Potential Phenotypical Impact | Populations Described |
|---|---|---|---|---|---|
| VSX1 [52,53,54,56,57,58,59,60,61,132] | 20p11-q11 | Cone opsin regulation, craniofacial regulation | p.L17P, p.D144E, p.N151S, p.L159M, p.G160V, p.G160D, p.R166W, p.Q175H, p.H244R, p.P247R, c.546A>G, p.R131P, p.L17P, p.D144E, p.H244R, p.P247R, g.8326 G>A, g.10945 G>T, g.11059 A>C, g.5053 G>T, g.8222 A>Gp.G239Rp.G160V rs12480307, c.627+23G>A rs6138482, c.627+84T>A rs56157240, c.504-24C>T IVS3-24C, c.173C>T p.Pro58Leu, p.G239R c.715G>C | Stromal thinning, PPCD overlap | Present in Saudi Arabian [59], Indian [58], Chinese [56,60], Iranian [53], Caucasians [61], Italian [132], New Zealanders [62] Absent in English [64], Korean [63], Greek [133], Saudi Arabian [59] (Inconclusive), American [57] |
| TGFBI [66,67,68,69,70,71,72] | 5q 31.1 | Modulates scar formation, fibrosis. Regulates cell adhesion, movement, and interaction in the ECM. | c.1406G>A | Decreased βig-h3 protein, fibrosis susceptibility, stromal ECM alterations | Present in Polish [69], Chinese [70], German [71] Absent in European populations [134], Chinese [135] |
| ZEB1 [73,74,75] | 10p11.22 | Regulates the expression of protein E-cadherin 1 (CDH1) | exon 7 c.1920G>T, missense ZEB1 mutation in p.Gln640His, p.Glu728Asp | PPCD overlap | Present in Spanish [75], Caucasian [74], Chinese [61] Absent in Chinese and Greek [66] |
| MIR184 [76,77,78,79,80,81,82] | 15q22-25 | miRNA regulation of INPPL1 and ITGB4 proteins | r.57C,T, +3A>G, +8C>A | Early onset cataracts overlap familial KC | Present in Irish [79], Saudi Arabians [77] Absent in Iranian [82] |
| SOD1 [53,61,83,86,87,133,136] | 21q22.11 | Manages oxidative stress regulation by dismutation of radicals | c.169+50delTAAACAG, g.12035C4A; g.13978T4A; g.12037G4A g.11931A4C | Oxidative stress imbalance | Present in Caucasian [83] Absent in Saudi Arabian [86], Brazilian [84], Australian [88] |
| ZNF469 [89,90,92,93,94,95,96,97] | 16q24 | ECM regulation, collagen maintenance, homeostasis of collagen fibers | p.Arg492Gln, rs2721051, rs9938149, c.2972del, p.Pro991Hisfs62 | BCS overlap | Present in Polynesian and Māori [92], Spanish [75,93], Caucasian [90] Absent in Polish [95], Australian [96], Saudi Arabian [137] |
| LOX [98,99,100,101,102] | 5q23.2 | Activation of collagen cross linking by catalyzing oxidative deamination | Rs10519694, rs2956540, rs1800449, rs2288393, | Potential stromal weakening | Present in Iranian [102], Chinese [103], Caucasian [32] Absent in Brazilian [61] |
| DOCK9 [104,105,106,107] | 13q32 | GTP/GDP exchange factor, CDC42 activation | c2262A>C, rs7995432 | Protein dysregulation | Present in Ecuadorians [105,106] Absent in Polish [105], Brazilian [107] |
| SLC4A11 [103,108,109,110,111] | 20p13 | Electrogenic Na+-coupled borate cotransporter | C.2558+149_2558+203del54, p.Gly769Arg | Induced apoptotic pathways, contributing to cellular degeneration, oxidative stress, mitochondrial dysfunction, and corneal edema | Present in Ecuadorians [108], Spanish [75], Chinese [103,111] |
| TIMP3 [112,113,115,116] | 22q12.3 | Endogenous MMP inhibitor | c.476C>T | ECM degradation imbalance, stromal apoptosis | Present in Chinese [103] Absent in Italian [114], Brazilian [85] |
| IL1A/IL1B/IL1RN [117,118,119,120,121] | 2q13-q14.3 | Inflammatory cytokines, immune modulation | Rs2071376, rs16944, rs1143627 | keratocyte apoptosis, ECM remodeling | Present in Korean [108,118], Japanese [119] Absent in Turkish [122] |
| COL4A3/COL4A4/COL5A1 [123,124,125,126,127,128,129,130] | COL4A3: 2q36.3; COL4A4: 2q36.3; COL5A1: 9q34.2-q34. | Collagen network formation and regulation in corneal stroma | P141L, D326Y, G895G, P482S, M1327V, V1516V, F1644F, rs2229813, rs2228557, c.1372C>T, rs1536482, rs2721051, rs1324183, rs1043208782, rs569248712 | Corneal collagen alterations | Present in Chinese [103], Caucasian [123,125,126,127], Indian [124], Iranian [128] Absent in Greek [131], Russian [129], Pakistani [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcelo-Canton, R.H.; Ting, D.S.J.; Mehta, J.S. Genetics of Keratoconus: A Comprehensive Review. Genes 2025, 16, 1147. https://doi.org/10.3390/genes16101147
Barcelo-Canton RH, Ting DSJ, Mehta JS. Genetics of Keratoconus: A Comprehensive Review. Genes. 2025; 16(10):1147. https://doi.org/10.3390/genes16101147
Chicago/Turabian StyleBarcelo-Canton, Raul Hernan, Darren S. J. Ting, and Jodhbir S. Mehta. 2025. "Genetics of Keratoconus: A Comprehensive Review" Genes 16, no. 10: 1147. https://doi.org/10.3390/genes16101147
APA StyleBarcelo-Canton, R. H., Ting, D. S. J., & Mehta, J. S. (2025). Genetics of Keratoconus: A Comprehensive Review. Genes, 16(10), 1147. https://doi.org/10.3390/genes16101147






