Plastid RNA Editing in Glycyrrhiza uralensis: Landscape Characterization and Comparative Assessment of RNA-Seq Library Strategies for Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Nucleic Acid Extraction, Library Preparation, and Sequencing
2.2. Plastid Genome Assembly, Annotation, Alignment, and Variant Calling
2.3. Identification of RNA Editing Sites
3. Results
3.1. Plastid Genome Assembly, Comparisons, and Annotation
3.2. RNA-Seq Alignment
4. Discussion
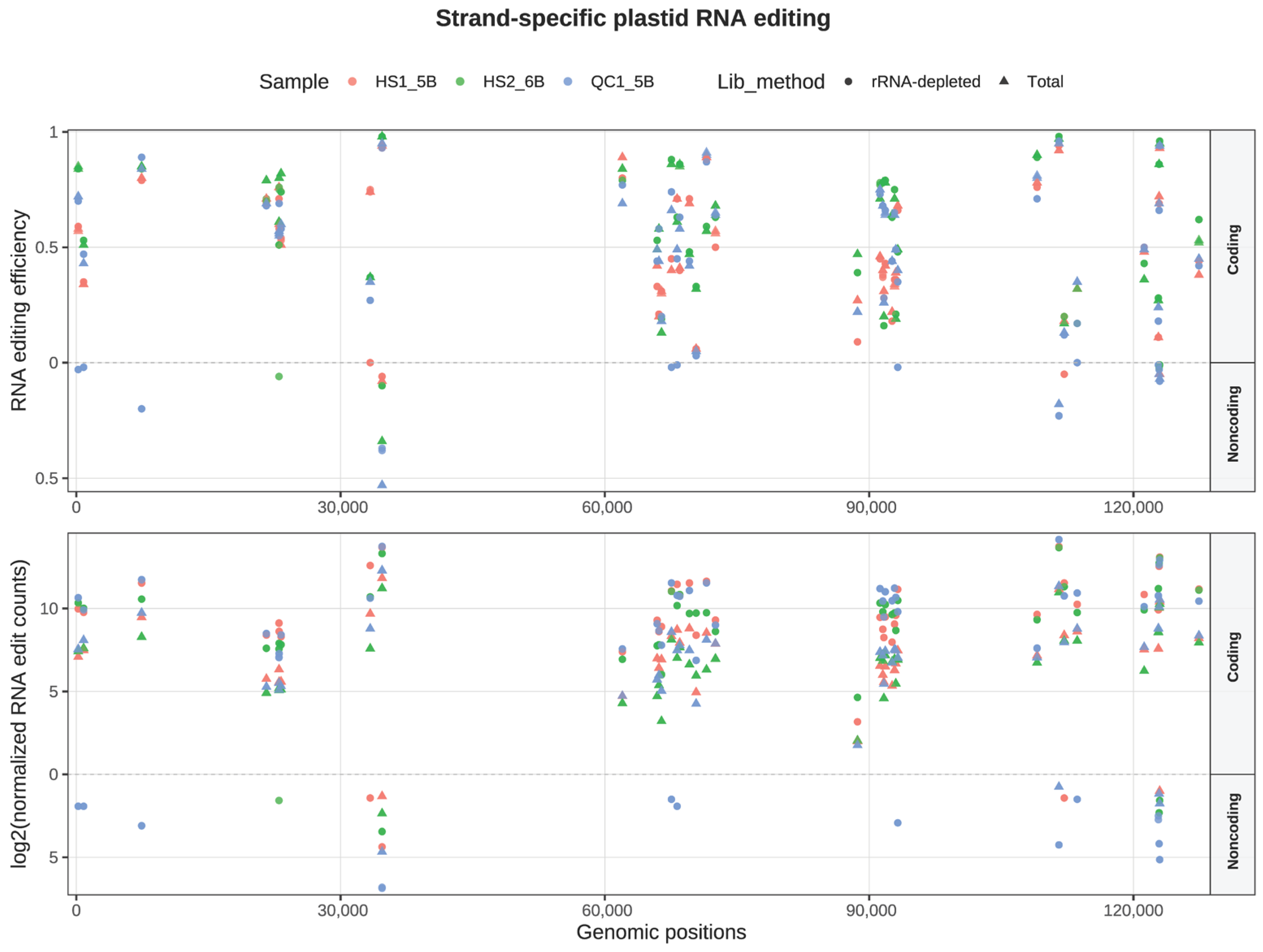
4.1. Divergent RNA Editing Landscapes Between G. uralensis and Vigna radiata
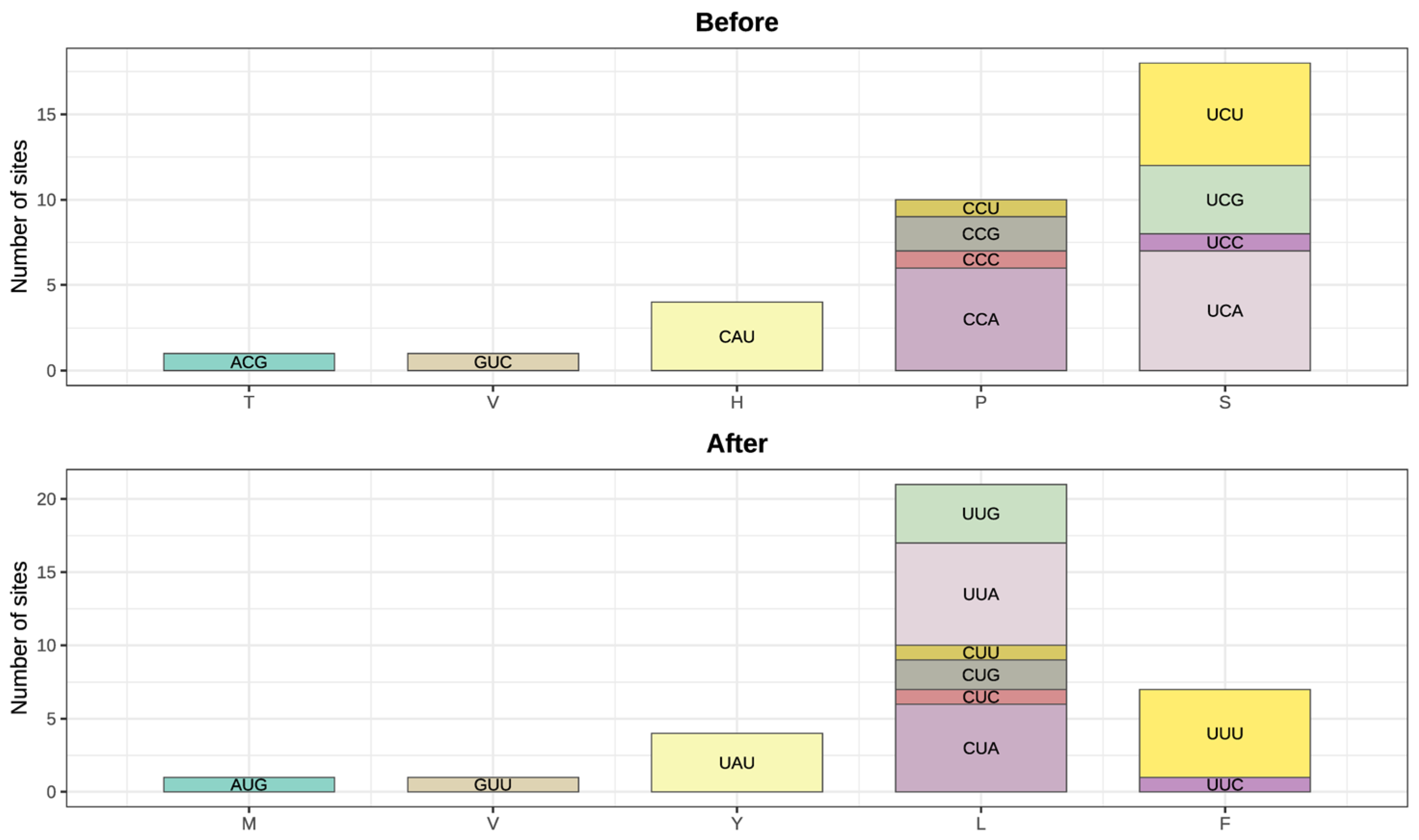
4.2. Distinguishing Authentic RNA Editing from Multiple Sources of Technical Artifacts
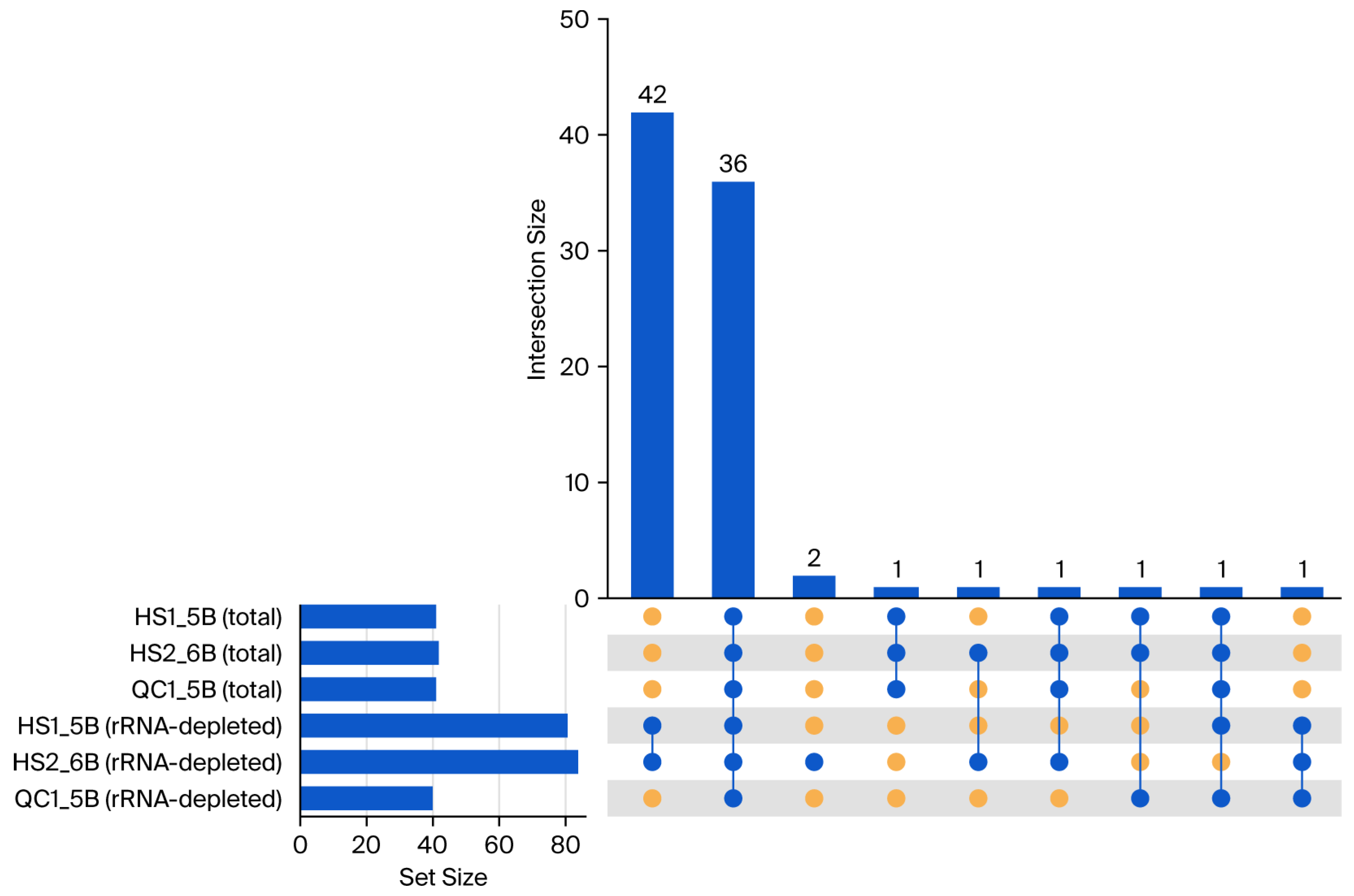
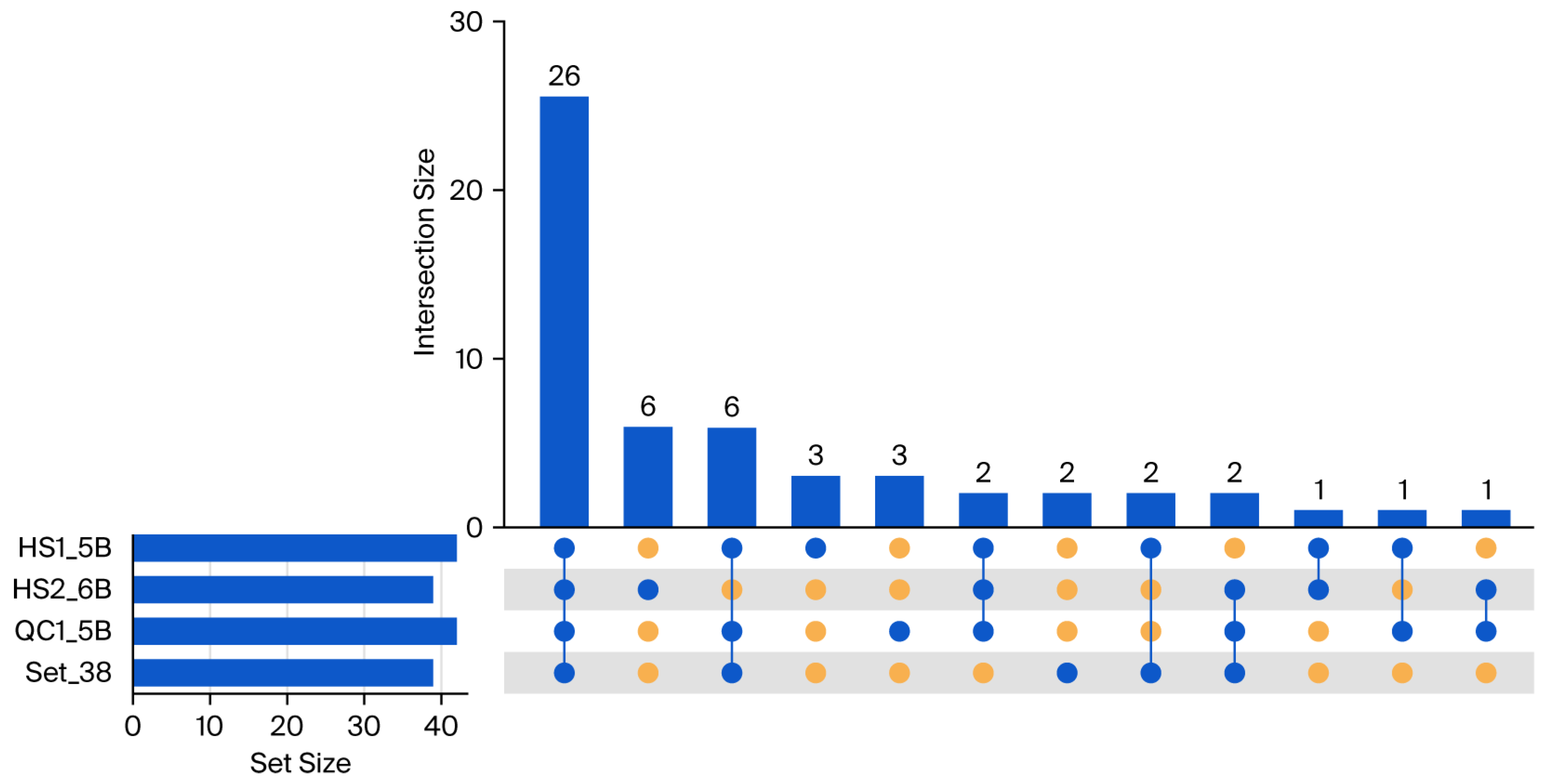
4.3. Evaluating Library Strategies: Superior Performance of Total RNA-Seq and Utility of mRNA-Seq in RNA Editing Identification
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chateigner-Boutin, A.-L.; Small, I. Plant RNA editing. RNA Biol. 2010, 7, 213–219. [Google Scholar] [CrossRef]
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Härtel, B.; Brennicke, A. RNA Editing in Plants and Its Evolution. Annu. Rev. Genet. 2013, 47, 335–352. [Google Scholar] [CrossRef]
- Knoop, V. C-to-U and U-to-C: RNA editing in plant organelles and beyond. J. Exp. Bot. 2022, 74, 2273–2294. [Google Scholar] [CrossRef]
- Mohammed, T.; Firoz, A.; Ramadan, A.M. RNA Editing in Chloroplast: Advancements and Opportunities. Curr. Issues Mol. Biol. 2022, 44, 5593–5604. [Google Scholar] [CrossRef]
- Chateigner-Boutin, A.-L.; Small, I. Organellar RNA editing. WIREs RNA 2011, 2, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Small, I.D.; Schallenberg-Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Dugardeyn, J.; Zhang, C.; Mühlenbock, P.; Eastmond, P.J.; Valcke, R.; De Coninck, B.; Öden, S.; Karampelias, M.; Cammue, B.P.A.; et al. The Arabidopsis thaliana RNA Editing Factor SLO2, which Affects the Mitochondrial Electron Transport Chain, Participates in Multiple Stress and Hormone Responses. Mol. Plant 2014, 7, 290–310. [Google Scholar] [CrossRef]
- Tang, F.; Barbacioru, C.; Wang, Y.; Nordman, E.; Lee, C.; Xu, N.; Wang, X.; Bodeau, J.; Tuch, B.B.; Siddiqui, A.; et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 2009, 6, 377–382. [Google Scholar] [CrossRef]
- Zhang, A.; Fang, J.; Zhang, X. Diversity of RNA editing in chloroplast transcripts across three main plant clades. Plant Syst. Evol. 2023, 309, 12. [Google Scholar] [CrossRef]
- Adiconis, X.; Borges-Rivera, D.; Satija, R.; DeLuca, D.S.; Busby, M.A.; Berlin, A.M.; Sivachenko, A.; Thompson, D.A.; Wysoker, A.; Fennell, T.; et al. Comparative analysis of RNA sequencing methods for degraded or low-input samples. Nat. Methods 2013, 10, 623–629. [Google Scholar] [CrossRef]
- Levin, J.Z.; Yassour, M.; Adiconis, X.; Nusbaum, C.; Thompson, D.A.; Friedman, N.; Gnirke, A.; Regev, A. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat. Methods 2010, 7, 709–715. [Google Scholar] [CrossRef]
- Ura, H.; Togi, S.; Niida, Y. A comparison of mRNA sequencing (RNA-Seq) library preparation methods for transcriptome analysis. BMC Genom. 2022, 23, 303. [Google Scholar] [CrossRef] [PubMed]
- Sarantopoulou, D.; Tang, S.Y.; Ricciotti, E.; Lahens, N.F.; Lekkas, D.; Schug, J.; Guo, X.S.; Paschos, G.K.; FitzGerald, G.A.; Pack, A.I.; et al. Comparative evaluation of RNA-Seq library preparation methods for strand-specificity and low input. Sci. Rep. 2019, 9, 13477. [Google Scholar] [CrossRef]
- Lin, C.P.; Ko, C.Y.; Kuo, C.I.; Liu, M.S.; Schafleitner, R.; Chen, L.F. Transcriptional Slippage and RNA Editing Increase the Diversity of Transcripts in Chloroplasts: Insight from Deep Sequencing of Vigna radiata Genome and Transcriptome. PLoS ONE 2015, 10, e0129396. [Google Scholar] [CrossRef]
- Nawae, W.; Yundaeng, C.; Naktang, C.; Kongkachana, W.; Yoocha, T.; Sonthirod, C.; Narong, N.; Somta, P.; Laosatit, K.; Tangphatsornruang, S.; et al. The Genome and Transcriptome Analysis of the Vigna mungo Chloroplast. Plants 2020, 9, 1247. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Report. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Chen, S. Ultrafast one-pass FASTQ data preprocessing, quality control, and deduplication using fastp. iMeta 2023, 2, e107. [Google Scholar] [CrossRef]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; dePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.-J.; Moore, M.J.; Li, D.-Z.; Yi, T.-S. PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 2019, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Lin, B.Y.; Mak, A.J.; Lowe, T.M. tRNAscan-SE 2.0: Improved detection and functional classification of transfer RNA genes. Nucleic Acids Res. 2021, 49, 9077–9096. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamada, K.D.; Tomii, K.; Katoh, K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 2018, 34, 2490–2492. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Lo Giudice, C.; Tangaro, M.A.; Pesole, G.; Picardi, E. Investigating RNA editing in deep transcriptome datasets with REDItools and REDIportal. Nat. Protoc. 2020, 15, 1098–1131. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant Review with the Integrative Genomics Viewer. Cancer Res. 2017, 77, e31–e34. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T. Cyclic Electron Transport Around Photosystem I: Genetic Approaches. Annu. Rev. Plant Biol. 2007, 58, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Peltier, G.; Cournac, L. Chlororespiration. Annu. Rev. Plant Biol. 2002, 53, 523–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Chen, H.; Xu, T.; Yang, W.; Fang, X. Solid-like condensation of MORF8 inhibits RNA editing under heat stress in Arabidopsis. Nat. Commun. 2025, 16, 2789. [Google Scholar] [CrossRef]
- Zhang, A.; Jiang, X.; Zhang, F.; Wang, T.; Zhang, X. Dynamic response of RNA editing to temperature in grape by RNA deep sequencing. Funct. Integr. Genom. 2020, 20, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.M.; Mohammed, T.; Firoz, A.; Alameldin, H.F.; Ali, H.M. RNA editing in chloroplast NADH dehydrogenase (ndhA) of salt stressed wild barley revealed novel type G to A. J. King Saud Univ.-Sci. 2023, 35, 102755. [Google Scholar] [CrossRef]
- Hao, W.; Liu, G.; Wang, W.; Shen, W.; Zhao, Y.; Sun, J.; Yang, Q.; Zhang, Y.; Fan, W.; Pei, S.; et al. RNA Editing and Its Roles in Plant Organelles. Front. Genet. 2021, 12, 757109. [Google Scholar] [CrossRef]
- Lu, Y. RNA editing of plastid-encoded genes. Photosynthetica 2018, 56, 48–61. [Google Scholar] [CrossRef]
- Hein, A.; Polsakiewicz, M.; Knoop, V. Frequent chloroplast RNA editing in early-branching flowering plants: Pilot studies on angiosperm-wide coexistence of editing sites and their nuclear specificity factors. BMC Evol. Biol. 2016, 16, 23. [Google Scholar] [CrossRef]
- Oldenkott, B.; Yang, Y.; Lesch, E.; Knoop, V.; Schallenberg-Rüdinger, M. Plant-type pentatricopeptide repeat proteins with a DYW domain drive C-to-U RNA editing in Escherichia coli. Commun. Biol. 2019, 2, 85. [Google Scholar] [CrossRef]
- Yamori, W.; Makino, A.; Shikanai, T. A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci. Rep. 2016, 6, 20147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shuai, J.; Ran, Z.; Zhao, J.; Wu, Z.; Liao, R.; Wu, J.; Ma, W.; Lei, M. Structural insights into NDH-1 mediated cyclic electron transfer. Nat. Commun. 2020, 11, 888. [Google Scholar] [CrossRef] [PubMed]
- RUMEAU, D.; PELTIER, G.; COURNAC, L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007, 30, 1041–1051. [Google Scholar] [CrossRef]
- Kleinman, C.L.; Majewski, J. Comment on “Widespread RNA and DNA Sequence Differences in the Human Transcriptome”. Science 2012, 335, 1302. [Google Scholar] [CrossRef]
- Parkhomchuk, D.; Borodina, T.; Amstislavskiy, V.; Banaru, M.; Hallen, L.; Krobitsch, S.; Lehrach, H.; Soldatov, A. Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res. 2009, 37, e123. [Google Scholar] [CrossRef] [PubMed]
- Incarnato, D.; Oliviero, S. The RNA Epistructurome: Uncovering RNA Function by Studying Structure and Post-Transcriptional Modifications. Trends Biotechnol. 2017, 35, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.; Fu, X.; Dai, N.; Corrêa, I.R., Jr.; Tanner, N.A.; Ong, J.L. Base modifications affecting RNA polymerase and reverse transcriptase fidelity. Nucleic Acids Res. 2018, 46, 5753–5763. [Google Scholar] [CrossRef]
- Schwartz, S.; Motorin, Y. Next-generation sequencing technologies for detection of modified nucleotides in RNAs. RNA Biol. 2017, 14, 1124–1137. [Google Scholar] [CrossRef]
- Motorin, Y.; Helm, M. RNA nucleotide methylation. WIREs RNA 2011, 2, 611–631. [Google Scholar] [CrossRef]
- Li, S.; Mason, C.E. The Pivotal Regulatory Landscape of RNA Modifications. Annu. Rev. Genom. Hum. Genet. 2014, 15, 127–150. [Google Scholar] [CrossRef]
- Debnath, T.K.; Xhemalçe, B. Deciphering RNA modifications at base resolution: From chemistry to biology. Brief. Funct. Genom. 2021, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Piskol, R.; Peng, Z.; Wang, J.; Li, J.B. Lack of evidence for existence of noncanonical RNA editing. Nat. Biotechnol. 2013, 31, 19–20. [Google Scholar] [CrossRef]
- Milenkovic, I.; Novoa, E.M. Dynamic rRNA modifications as a source of ribosome heterogeneity. Trends Cell Biol. 2025, 35, 604–614. [Google Scholar] [CrossRef]
- Genuth, N.R.; Barna, M. The Discovery of Ribosome Heterogeneity and Its Implications for Gene Regulation and Organismal Life. Mol. Cell 2018, 71, 364–374. [Google Scholar] [CrossRef]
- Schuster, G.; Lisitsky, I.; Klaff, P. Polyadenylation and Degradation of mRNA in the Chloroplast. Plant Physiol. 1999, 120, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Rorbach, J.; Bobrowicz, A.; Pearce, S.; Minczuk, M. Polyadenylation in Bacteria and Organelles. In Polyadenylation: Methods and Protocols; Rorbach, J., Bobrowicz, A.J., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 211–227. [Google Scholar]

| Sample | Raw Reads | Plastid Genome | Plastid-Mapped Reads | Plastid Mapping Ratio |
|---|---|---|---|---|
| HS1_5B | 292,305,370 | 127,702 bp | 16,070,696 | 5.49% |
| HS2_6B | 252,953,068 | 127,716 bp | 7,911,608 | 3.12% |
| QC1_5B | 327,037,602 | 127,670 bp | 19,483,022 | 5.95% |
| Sample | Library | Raw Reads | Plastid-Mapped Reads | Plastid Mapping Ratio |
|---|---|---|---|---|
| HS1_5B | Total RNA-seq | 127,089,854 | 32,020,931 | 25.19% |
| HS1_5B | rRNA-depleted RNA-seq | 70,544,244 | 23,316,279 | 33.05% |
| HS1_5B | mRNA-seq | 100,743,972 | 434,781 | 0.43% |
| HS2_6B | Total RNA-seq | 123,558,206 | 25,053,748 | 20.27% |
| HS2_6B | rRNA-depleted RNA-seq | 63,621,904 | 14,703,453 | 23.11% |
| HS2_6B | mRNA-seq | 103,140,076 | 147,072 | 0.14% |
| QC1_5B | Total RNA-seq | 112,646,262 | 33,606,579 | 29.83% |
| QC1_5B | rRNA-depleted RNA-seq | 66,603,282 | 26,073,981 | 39.14% |
| QC1_5B | mRNA-seq | 124,564,614 | 608,462 | 0.48% |
| Gene | Number of Editing Sites |
|---|---|
| accD | 2 |
| atpA | 1 |
| ndhA | 2 |
| ndhB | 8 |
| ndhD | 3 |
| ndhE | 1 |
| ndhF | 1 |
| ndhG | 2 |
| ndhH | 1 |
| petB | 2 |
| petL | 1 |
| psaI | 1 |
| psbF | 1 |
| psbL | 1 |
| psbZ | 1 |
| rpoA | 1 |
| rpoB | 4 |
| rps14 | 1 |
| Gene | Position on Gene | Codon Change | Amino Acid Change | Codon Position | Editing Efficiency | Supporting Reads |
|---|---|---|---|---|---|---|
| accD | 794 | TCG→TTG | S→L | 2 | 0.34~0.53 | 362~1036 |
| accD | 1403 | CCT→CTT | P→L | 2 | 0.57~0.84 | 273~1684 |
| atpA | 791 | CCC→CTC | P→L | 2 | 0.79~0.89 | 611~3605 |
| ndhA | 341 | TCA→TTA | S→L | 2 | 0.57~0.9 | 156~3556 |
| ndhA | 1073 | TCT→TTT | S→F | 2 | 0.42~0.71 | 193~3303 |
| ndhB | 95 | TCA→TTA | S→L | 2 | 0.45~0.77 | 188~2474 |
| ndhB | 413 | CCA→CTA | P→L | 2 | 0.38~0.78 | 129~1469 |
| ndhB | 532 | CAT→TAT | H→Y | 1 | 0.16~0.31 | 47~751 |
| ndhB | 692 | TCT→TTT | S→F | 2 | 0.42~0.79 | 182~2172 |
| ndhB | 782 | TCA→TTA | S→L | 2 | 0.18~0.64 | 81~1491 |
| ndhB | 1058 | TCA→TTA | S→L | 2 | 0.34~0.75 | 157~2515 |
| ndhB | 1201 | CAT→TAT | H→Y | 1 | 0.19~0.49 | 87~1711 |
| ndhB | 1427 | CCA→CTA | P→L | 2 | 0.35~0.68 | 234~2531 |
| ndhD | 383 | CCA→CTA | P→L | 2 | 0.13~0.31 | 18~538 |
| ndhD | 674 | TCG→TTG | S→L | 2 | 0.2~0.58 | 81~438 |
| ndhD | 878 | TCA→TTA | S→L | 2 | 0.33~0.53 | 51~696 |
| ndhE | 230 | CCG→CTG | P→L | 2 | 0.4~0.88 | 546~3135 |
| ndhF | 290 | TCA→TTA | S→L | 2 | 0.69~0.89 | 38~199 |
| ndhG | 50 | TCG→TTG | S→L | 2 | 0.4~0.86 | 393~1980 |
| ndhG | 347 | CCA→CTA | P→L | 2 | 0.45~0.71 | 253~3126 |
| ndhH | 505 | CAT→TAT | H→Y | 1 | 0.5~0.68 | 246~702 |
| petB | 12 | GTC→GTT | V→V | 3 | 0.12~0.2 | 446~3317 |
| petB | 611 | CCA→CTA | P→L | 2 | 0.92~0.98 | 3888~19,280 |
| petL | 5 | CCG→CTG | P→L | 2 | 0.36~0.5 | 147~2038 |
| psaI | 79 | CAT→TAT | H→Y | 1 | 0.38~0.62 | 484~2556 |
| psbF | 77 | TCT→TTT | S→F | 2 | 0.66~0.86 | 1960~6852 |
| psbL | 2 | ACG→ATG | T→M | 2 | 0.93~0.96 | 2429~9780 |
| psbZ | 50 | TCC→TTC | S→F | 2 | 0.27~0.74 | 376~6873 |
| rpoA | 200 | TCT→TTT | S→F | 2 | 0.71~0.9 | 206~889 |
| rpoB | 338 | TCT→TTT | S→F | 2 | 0.51~0.82 | 68~361 |
| rpoB | 551 | TCA→TTA | S→L | 2 | 0.55~0.8 | 62~442 |
| rpoB | 566 | TCG→TTG | S→L | 2 | 0.51~0.76 | 66~620 |
| rpoB | 2000 | TCT→TTT | S→F | 2 | 0.68~0.79 | 59~382 |
| rps14 | 80 | CCA→CTA | P→L | 2 | 0.93~0.98 | 4660~14,663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Rao, Y.; Lu, Y.; Fang, N.; Huang, Y.; Gong, L. Plastid RNA Editing in Glycyrrhiza uralensis: Landscape Characterization and Comparative Assessment of RNA-Seq Library Strategies for Detection. Genes 2025, 16, 1142. https://doi.org/10.3390/genes16101142
Ma H, Rao Y, Lu Y, Fang N, Huang Y, Gong L. Plastid RNA Editing in Glycyrrhiza uralensis: Landscape Characterization and Comparative Assessment of RNA-Seq Library Strategies for Detection. Genes. 2025; 16(10):1142. https://doi.org/10.3390/genes16101142
Chicago/Turabian StyleMa, Hui, Yixuan Rao, Yinxiao Lu, Na Fang, Yijia Huang, and Lei Gong. 2025. "Plastid RNA Editing in Glycyrrhiza uralensis: Landscape Characterization and Comparative Assessment of RNA-Seq Library Strategies for Detection" Genes 16, no. 10: 1142. https://doi.org/10.3390/genes16101142
APA StyleMa, H., Rao, Y., Lu, Y., Fang, N., Huang, Y., & Gong, L. (2025). Plastid RNA Editing in Glycyrrhiza uralensis: Landscape Characterization and Comparative Assessment of RNA-Seq Library Strategies for Detection. Genes, 16(10), 1142. https://doi.org/10.3390/genes16101142






