Dna2 Responds to Endogenous and Exogenous Replication Stress in Drosophila melanogaster
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Maintenance
2.2. Mutagen Sensitivity Assays
2.3. Mendelian Ratios
2.4. Fecundity
2.5. Egg Viability
2.6. Ovary Dissection and Fixation
2.7. Immunostaining
2.8. Imaging and Quantification of Germarium Size
2.9. Lifespan Assays
3. Results
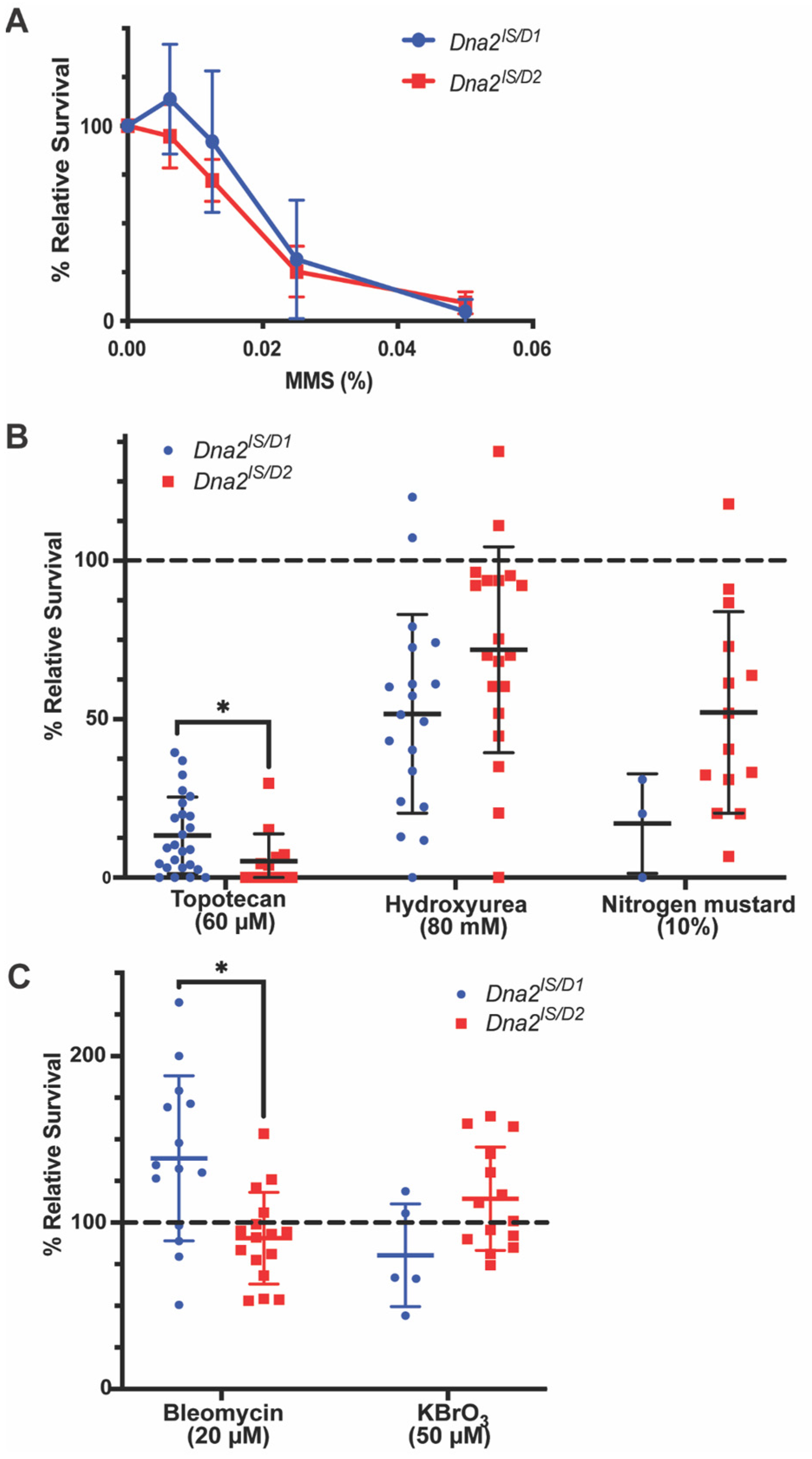
3.1. Dna2 Responds to Specific Types of DNA Damage
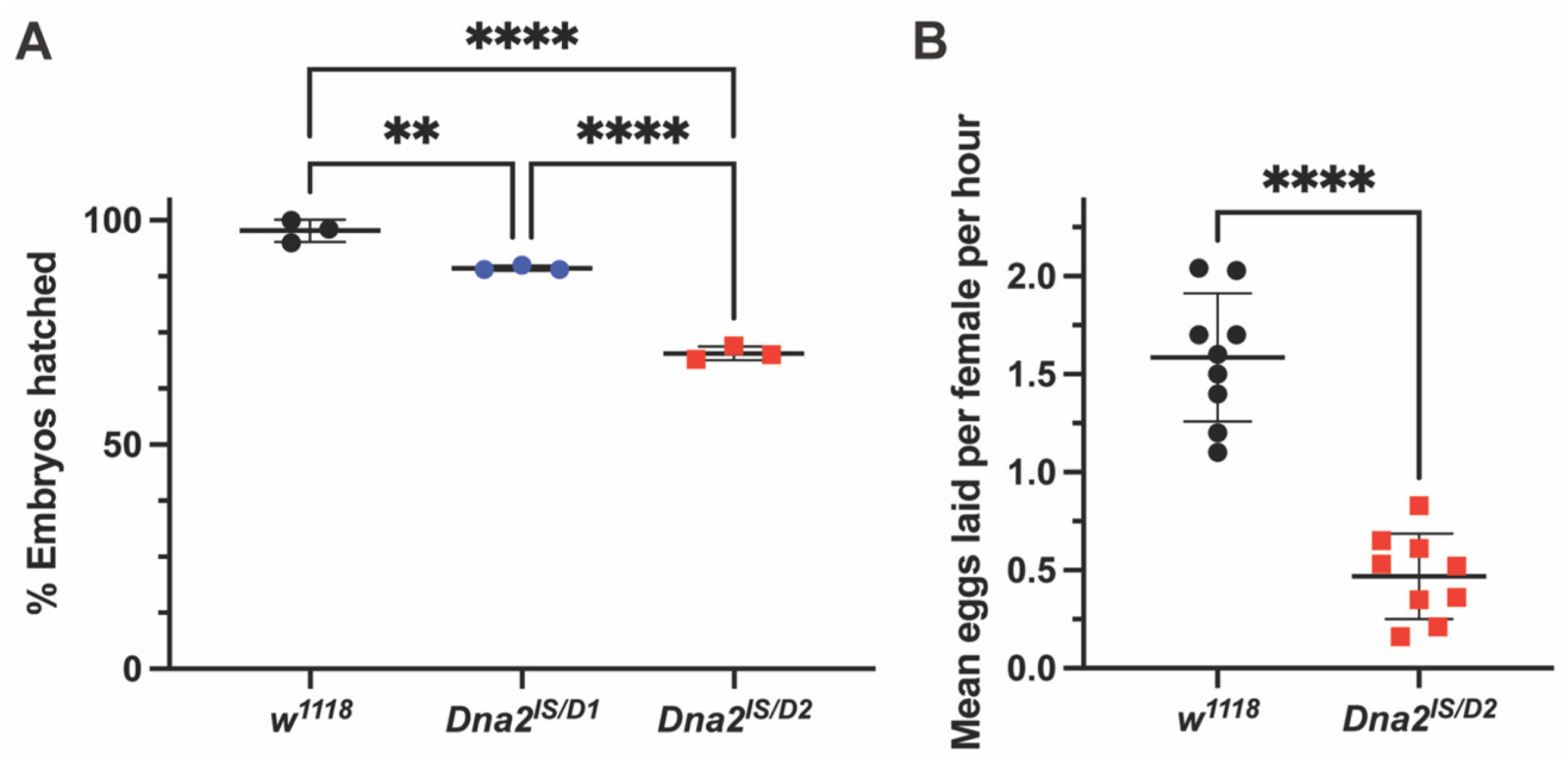
3.2. Dna2 Mutants Exhibit Reduced Viability and Reproductive Fitness
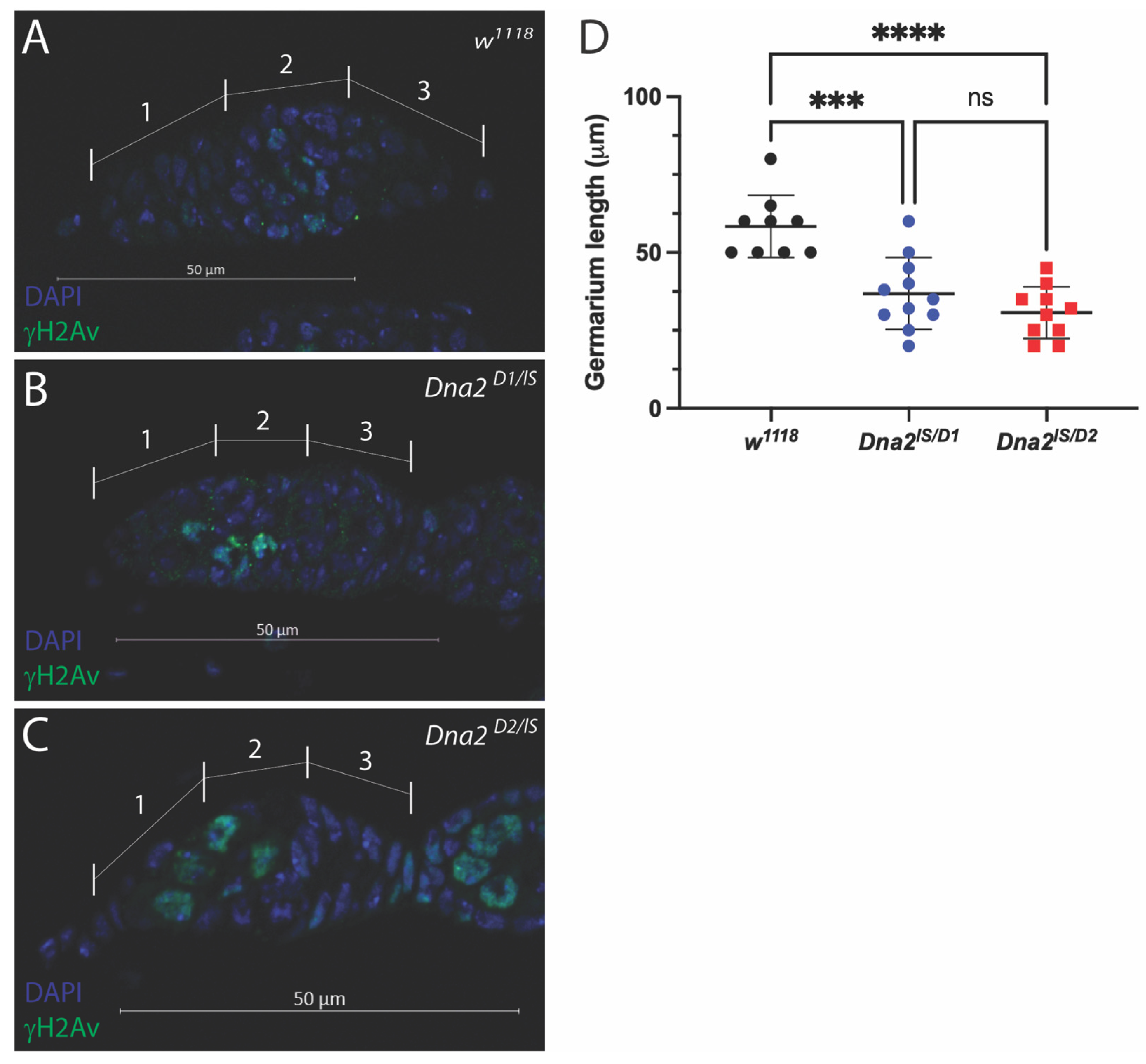
3.3. Dna2 Is Required to Prevent Replication Stress in the Female Germline, Independent of Meiosis
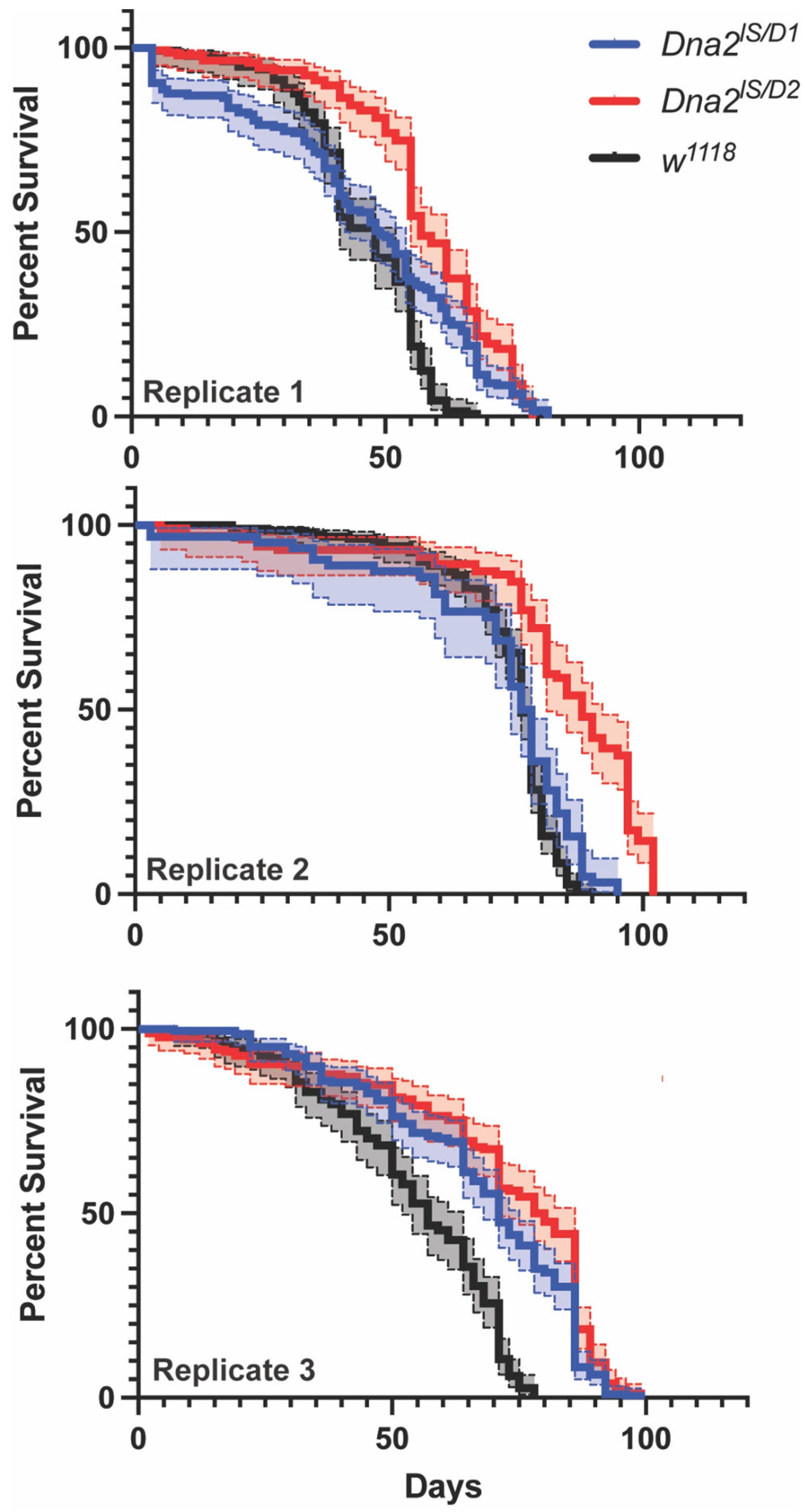
3.4. Dna2 Mutations Do Not Reduce Lifespan
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoeijmakers, J.H.J. DNA Damage, Aging, and Cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Meng, Y.; Campbell, J.L.; Shen, B. Multiple roles of DNA2 nuclease/helicase in DNA metabolism, genome stability and human diseases. Nucleic Acids Res. 2020, 48, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Appanah, R.; Jones, D.; Falquet, B.; Rass, U. Limiting homologous recombination at stalled replication forks is essential for cell viability: DNA2 to the rescue. Curr. Genet. 2020, 66, 1085–1092. [Google Scholar] [CrossRef]
- Falquet, B.; Ölmezer, G.; Enkner, F.; Klein, D.; Challa, K.; Appanah, R.; Gasser, S.M.; Rass, U. Disease-associated DNA2 nuclease–helicase protects cells from lethal chromosome under-replication. Nucleic Acids Res. 2020, 48, 7265–7278. [Google Scholar] [CrossRef]
- Hudson, J.J.R.; Rass, U. DNA2 in Chromosome Stability and Cell Survival—Is It All about Replication Forks? Int. J. Mol. Sci. 2021, 22, 3984. [Google Scholar] [CrossRef]
- Ölmezer, G.; Levikova, M.; Klein, D.; Falquet, B.; Fontana, G.A.; Cejka, P.; Rass, U. Replication intermediates that escape Dna2 activity are processed by Holliday junction resolvase Yen1. Nat. Commun. 2016, 7, 13157. [Google Scholar] [CrossRef]
- Levikova, M.; Pinto, C.; Cejka, P. The motor activity of DNA2 functions as an ssDNA translocase to promote DNA end resection. Genes Dev. 2017, 31, 493–502. [Google Scholar] [CrossRef]
- Miller, A.S.; Daley, J.M.; Pham, N.T.; Niu, H.; Xue, X.; Ira, G.; Sung, P. A novel role of the Dna2 translocase function in DNA break resection. Genes Dev. 2017, 31, 503–510. [Google Scholar] [CrossRef]
- Peng, G.; Dai, H.; Zhang, W.; Hsieh, H.J.; Pan, M.R.; Park, Y.Y.; Tsai, R.Y.-L.; Bedrosian, I.; Lee, J.-S.; Ira, G.; et al. Human Nuclease/Helicase DNA2 Alleviates Replication Stress by Promoting DNA End Resection. Cancer Res. 2012, 72, 2802–2813. [Google Scholar] [CrossRef]
- Budd, M.E.; Choe, W.-C.; Campbell, J.L. The Nuclease Activity of the Yeast Dna2 Protein, Which Is Related to the RecB-like Nucleases, Is Essential in Vivo. J. Biol. Chem. 2000, 275, 16518–16529. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, E.; Szczepanska, J.; Blasiak, J. DNA2—An Important Player in DNA Damage Response or Just Another DNA Maintenance Protein? Int. J. Mol. Sci. 2017, 18, 1562. [Google Scholar] [CrossRef]
- Zhou, C.; Pourmal, S.; Pavletich, N.P. Dna2 nuclease-helicase structure, mechanism and regulation by Rpa. eLife 2015, 4, e09832. [Google Scholar] [CrossRef]
- Mitchell, C.; Becker, V.; DeLoach, J.; Nestore, E.; Bolterstein, E.; Kohl, K.P. The Drosophila Mutagen-Sensitivity Gene mus109 Encodes DmDNA2. Genes 2022, 13, 312. [Google Scholar] [CrossRef]
- Boyd, J.B.; Golino, M.D.; Nguyen, T.D.; Green, M.M. Isolation and Characterization of X-of Drosophila Melanogaster Which Are Sensitive to Mutagens. Genetics 1976, 84, 485–506. [Google Scholar] [CrossRef]
- Mason, J.M.; Green, M.M.; Shaw, K.E.; Boyd, J.B. Genetic analysis of X-linked mutagen-sensitive mutants of Drosophila melanogaster. Mutat. Res. 1981, 81, 329–343. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Green, M.M.; Boyd, J.B. Isolation of two X-linked mutants in Drosophila melanogaster which are sensitive to γ-rays. Mutat. Res. Mol. Mech. Mutagen. 1978, 49, 139–143. [Google Scholar] [CrossRef]
- Beranek, D.T. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. Mol. Mech. Mutagen. 1990, 231, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Povirk, L.F.; Shuker, D.E. DNA damage and mutagenesis induced by nitrogen mustards. Mutat. Res. 1994, 318, 205–226. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Bolterstein, E.; Rivero, R.; Marquez, M.; McVey, M. The Drosophila Werner Exonuclease Participates in an Exonuclease-Independent Response to Replication Stress. Genetics 2014, 197, 643–652. [Google Scholar] [CrossRef]
- Epiney, D.G.; Salameh, C.; Cassidy, D.; Zhou, L.T.; Kruithof, J.; Milutinović, R.; Andreani, T.S.; Schirmer, A.E.; Bolterstein, E. Characterization of Stress Responses in a Drosophila Model of Werner Syndrome. Biomolecules 2021, 11, 1868. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 6 August 2025).
- Therneau, T.M.; Lumley, T.; Elizabeth, A.; Cynthia, C. Survival: Survival Analysis in R, R Package, version 3.8-3; The R foundation location: Vienna, Austria, 2024; Available online: https://cran.r-project.org/web/packages/survival/index.html (accessed on 6 August 2025).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Statistics for Biology and Health; Springer: New York, NY, USA, 2000; pp. xiii+350. [Google Scholar]
- Karanja, K.K.; Cox, S.W.; Duxin, J.P.; Stewart, S.A.; Campbell, J.L. DNA2 and EXO1 in replication-coupled, homology-directed repair and in the interplay between HDR and the FA/BRCA network. Cell Cycle 2012, 11, 3983–3996. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, M.; Li, Z.; Li, H.; Polaczek, P.; Dai, H.; Wu, Q.; Liu, C.; Karanja, K.K.; Popuri, V.; et al. A Selective Small Molecule DNA2 Inhibitor for Sensitization of Human Cancer Cells to Chemotherapy. eBioMedicine 2016, 6, 73–86. [Google Scholar] [CrossRef]
- Miller, D.E.; Cook, K.R.; Yeganeh Kazemi, N.; Smith, C.B.; Cockrell, A.J.; Hawley, R.S.; Bergman, C.M. Rare recombination events generate sequence diversity among balancer chromosomes in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2016, 113, E1352–E1361. [Google Scholar] [CrossRef]
- Miller, D.E.; Kahsai, L.; Buddika, K.; Dixon, M.J.; Kim, B.Y.; Calvi, B.R.; Sokol, N.S.; Hawley, R.S.; Cook, K.R. Identification and Characterization of Breakpoints and Mutations on Drosophila melanogaster Balancer Chromosomes. G3 Genes|Genomes|Genet. 2020, 10, 4271–4285. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.K.; Sherizen, D.E.; Bhagat, R.; Manheim, E.A.; McKim, K.S. Relationship of DNA double-strand breaks to synapsis in Drosophila. J. Cell Sci. 2003, 116, 3069–3077. [Google Scholar] [CrossRef]
- Lake, C.M.; Holsclaw, J.K.; Bellendir, S.P.; Sekelsky, J.; Hawley, R.S. The Development of a Monoclonal Antibody Recognizing the Drosophila melanogaster Phosphorylated Histone H2A Variant (-H2AV). G3 Genes|Genomes|Genet. 2013, 3, 1539–1543. [Google Scholar] [CrossRef]
- Lake, C.M.; Hawley, R.S. The Molecular Control of Meiotic Chromosomal Behavior: Events in Early Meiotic Prophase in Drosophila Oocytes. Annu. Rev. Physiol. 2012, 74, 425–451. [Google Scholar] [CrossRef]
- Madigan, J.P.; Chotkowski, H.L.; Glaser, R.L. DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis. Nucleic Acids Res. 2002, 30, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; McKim, K.S. Temporal Analysis of Meiotic DNA Double-Strand Break Formation and Repair in Drosophila Females. PLOS Genet. 2006, 2, e200. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, S.; Berti, M.; Levikova, M.; Pinto, C.; Gomathinayagam, S.; Vujanovic, M.; Zellweger, R.; Moore, H.; Lee, E.H.; Hendrickson, E.A.; et al. DNA2 drives processing and restart of reversed replication forks in human cells. J. Cell Biol. 2015, 208, 545–562. [Google Scholar] [CrossRef]
- Li, Z.; Liu, B.; Jin, W.; Wu, X.; Zhou, M.; Liu, V.Z.; Goel, A.; Shen, Z.; Zheng, L.; Shen, B. hDNA2 nuclease/helicase promotes centromeric DNA replication and genome stability. EMBO J. 2018, 37, e96729. [Google Scholar] [CrossRef]
- Budd, M.E.; Choe, W.C.; Campbell, J.L. DNA2 Encodes a DNA Helicase Essential for Replication of Eukaryotic Chromosomes. J. Biol. Chem. 1995, 270, 26766–26769. [Google Scholar] [CrossRef]
- Bonetti, D.; Martina, M.; Clerici, M.; Lucchini, G.; Longhese, M.P. Multiple Pathways Regulate 3′ Overhang Generation at S. cerevisiae Telomeres. Mol. Cell 2009, 35, 70–81. [Google Scholar] [CrossRef]
- Manfrini, N.; Guerini, I.; Citterio, A.; Lucchini, G.; Longhese, M.P. Processing of Meiotic DNA Double Strand Breaks Requires Cyclin-dependent Kinase and Multiple Nucleases. J. Biol. Chem. 2010, 285, 11628–11637. [Google Scholar] [CrossRef]
- Wawrousek, K.E.; Fortini, B.K.; Polaczek, P.; Chen, L.; Liu, Q.; Dunphy, W.G.; Campbell, J.L. Xenopus DNA2 is a helicase/nuclease that is found in complexes with replication proteins And-1/Ctf4 and Mcm10 and DSB response proteins Nbs1 and ATM. Cell Cycle 2010, 9, 1156–1166. [Google Scholar] [CrossRef]
- Zhai, B.; Zhang, S.; Li, B.; Zhang, J.; Yang, X.; Tan, Y.; Wang, Y.; Tan, T.; Yang, X.; Chen, B.; et al. Dna2 removes toxic ssDNA-RPA filaments generated from meiotic recombination-associated DNA synthesis. Nucleic Acids Res. 2023, 51, 7914–7935. [Google Scholar] [CrossRef] [PubMed]
- McVey, M.; Andersen, S.L.; Broze, Y.; Sekelsky, J. Multiple Functions of Drosophila BLM Helicase in Maintenance of Genome Stability. Genetics 2007, 176, 1979–1992. [Google Scholar] [CrossRef]
- Nakayama, M.; Yamaguchi, S.-I.; Sagisu, Y.; Sakurai, H.; Ito, F.; Kawasaki, K. Loss of RecQ5 leads to spontaneous mitotic defects and chromosomal aberrations in Drosophila melanogaster. DNA Repair 2009, 8, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ruchert, J.M.; Brady, M.M.; McMahan, S.; Lacey, K.J.; Latta, L.C.; Sekelsky, J.; Stoffregen, E.P. Blm helicase facilitates rapid replication of repetitive DNA sequences in early Drosophila development. Genetics 2022, 220, iyab169. [Google Scholar] [CrossRef]
- Sekelsky, J. DNA Repair in Drosophila: Mutagens, Models, and Missing Genes. Genetics 2017, 205, 471–490. [Google Scholar] [CrossRef]
- Thomas, A.M.; Hui, C.; South, A.; McVey, M. Common Variants of Drosophila melanogaster Cyp6d2 Cause Camptothecin Sensitivity and Synergize with Loss of Brca2. G3 Genes|Genomes|Genet. 2013, 3, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Karras, G.I.; Jentsch, S. The RAD6 DNA Damage Tolerance Pathway Operates Uncoupled from the Replication Fork and Is Functional Beyond S Phase. Cell 2010, 141, 255–267. [Google Scholar] [CrossRef]
- Keszenman, D.J.; Salvo, V.A.; Nunes, E. Effects of bleomycin on growth kinetics and survival of Saccharomyces cerevisiae: A model of repair pathways. J. Bacteriol. 1992, 174, 3125–3132. [Google Scholar] [CrossRef]
- Lisby, M.; Rothstein, R.; Mortensen, U.H. Rad52 forms DNA repair and recombination centers during S phase. Proc. Natl. Acad. Sci. USA 2001, 98, 8276–8282. [Google Scholar] [CrossRef]
- Thomas, M.S.; Pillai, G.S.; Butler, M.A.; Fernandez, J.; LaRocque, J.R. The epistatic relationship of Drosophila melanogaster CtIP and Rif1 in homology-directed repair of DNA double-strand breaks. G3 Genes|Genomes|Genet. 2024, 14, jkae210. [Google Scholar] [CrossRef]
- Yannuzzi, I.; Butler, M.A.; Fernandez, J.; LaRocque, J.R. The Role of Drosophila CtIP in Homology-Directed Repair of DNA Double-Strand Breaks. Genes 2021, 12, 1430. [Google Scholar] [CrossRef]
- Cypser, J.R.; Johnson, T.E. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B109–B114. [Google Scholar] [CrossRef] [PubMed]
- Le Bourg, E. A cold stress applied at various ages can increase resistance to heat and fungal infection in aged Drosophila melanogaster flies. Biogerontology 2011, 12, 185–193. [Google Scholar] [CrossRef]
- Wu, D.; Cypser, J.R.; Yashin, A.I.; Johnson, T.E. Multiple mild heat-shocks decrease the Gompertz component of mortality in Caenorhabditis elegans. Exp. Gerontol. 2009, 44, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Zada, D.; Sela, Y.; Matosevich, N.; Monsonego, A.; Lerer-Goldshtein, T.; Nir, Y.; Appelbaum, L. Parp1 promotes sleep, which enhances DNA repair in neurons. Mol. Cell 2021, 81, 4979–4993.e7. [Google Scholar] [CrossRef] [PubMed]
- Flatt, T. Survival costs of reproduction in Drosophila. Exp. Gerontol. 2011, 46, 369–375. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Observed Mutant Females | Observed Non-Mutant Females | Observed Non-Mutant Males | Expected Count (All Classes) | X2 | p-Value |
|---|---|---|---|---|---|---|
| Dna2lS/D1 | 4218 | 4341 | 3074 | 3877.667 | 251.797 | <0.0001 |
| Dna2lS/D2 | 5141 | 4918 | 3507 | 4522 | 349.236 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, I.; Shammari, S.; Sohail, H.; Villegas, C.; Wasim, Z.; Ip, S.H.; Becker, V.; Kohl, K.P.; Stoffregen, E.P.; Swanson, C.I.; et al. Dna2 Responds to Endogenous and Exogenous Replication Stress in Drosophila melanogaster. Genes 2025, 16, 1133. https://doi.org/10.3390/genes16101133
Rivera I, Shammari S, Sohail H, Villegas C, Wasim Z, Ip SH, Becker V, Kohl KP, Stoffregen EP, Swanson CI, et al. Dna2 Responds to Endogenous and Exogenous Replication Stress in Drosophila melanogaster. Genes. 2025; 16(10):1133. https://doi.org/10.3390/genes16101133
Chicago/Turabian StyleRivera, Ivan, Sabah Shammari, Hamiya Sohail, Christian Villegas, Zoha Wasim, Sze Hang Ip, Vada Becker, Kathryn P. Kohl, Eric P. Stoffregen, Christina I. Swanson, and et al. 2025. "Dna2 Responds to Endogenous and Exogenous Replication Stress in Drosophila melanogaster" Genes 16, no. 10: 1133. https://doi.org/10.3390/genes16101133
APA StyleRivera, I., Shammari, S., Sohail, H., Villegas, C., Wasim, Z., Ip, S. H., Becker, V., Kohl, K. P., Stoffregen, E. P., Swanson, C. I., & Bolterstein, E. (2025). Dna2 Responds to Endogenous and Exogenous Replication Stress in Drosophila melanogaster. Genes, 16(10), 1133. https://doi.org/10.3390/genes16101133





