Genetic Characteristics of Brazilian Patients with MH History
Abstract
1. Introduction
2. Materials and Methods
3. Molecular Analysis
4. Results
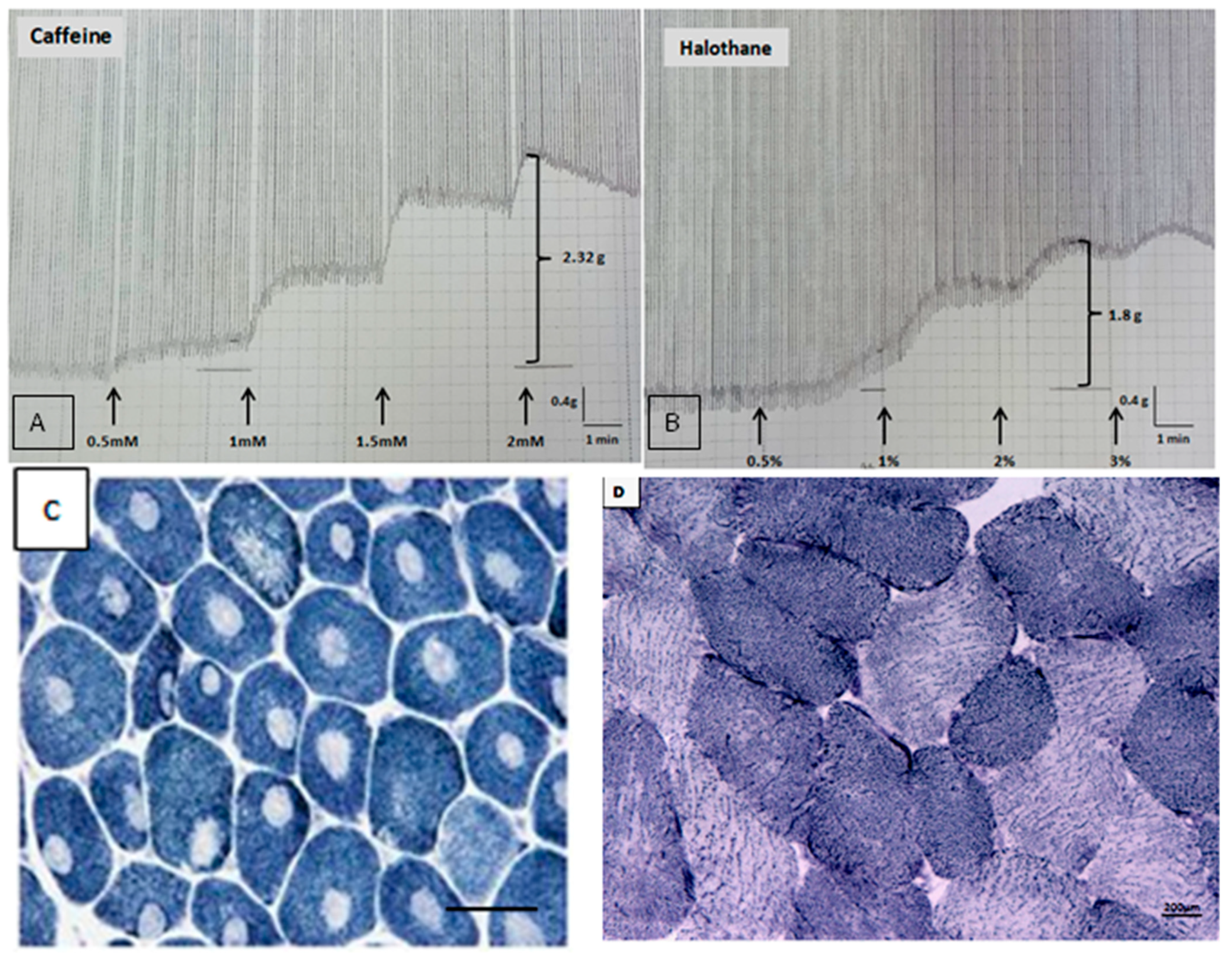
Clinical and Laboratory Findings in 61 Patients
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, H.; Sambuughin, N.; Riazi, S.; Dirksern, R. Malignant Hyperthermia Susceptibility. 2003 Dec 19 [Updated 2020 Jan 16]. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington, Seattle: Seattle, WA, USA, 1993–2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1146/ (accessed on 31 July 2025).
- Endo, Y.; Groom, L.; Celik, A.; Kraeva, N.; Lee, C.S.; Jung, S.Y.; Gardner, L.; Shaw, M.A.; Hamilton, S.L.; Hopkins, P.M.; et al. Variants in ASPH cause exertional heat illness and are associated with malignant hyperthermia susceptibility. Nat. Commun. 2022, 13, 3403. [Google Scholar] [CrossRef]
- Hopkins, P.M.; Rüffert, H.; Snoeck, M.M.; Girard, T.; Glahn, K.P.; Ellis, F.R.; Müller, C.R.; Urwyler, A. European Malignant Hyperthermia Group. European Malignant Hyperthermia Group guidelines for investigation of malignant hyperthermia susceptibility. Br. J. Anaesth. 2015, 115, 531–539. [Google Scholar] [CrossRef]
- Johnston, J.J.; Dirksen, R.T.; Girard, T.; Hopkins, P.M.; Kraeva, N.; Ognoon, M.; Radenbaugh, K.B.; Riazi, S.; Robinson, R.L.; Saddic, I.L.A.; et al. Updated variant curation expert panel criteria and pathogenicity classifications for 251 variants for RYR1-related malignant hyperthermia susceptibility. Hum. Mol. Genet. 2022, 31, 4087–4093. [Google Scholar] [CrossRef]
- Klingler, W.; Heiderich, S.; Girard, T.; Gravino, E.; Heffron, J.J.; Johannsen, S.; Jurkat-Rott, K.; Rüffert, H.; Schuster, F.; Snoeck, M.; et al. Functional and genetic characterization of clinical malignant hyperthermia crises: A multi-centre study. Orphanet J. Rare Dis. 2014, 9, 8. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Brandom, B.W.; Henker, R.A.; McAuliffe, J.J. Bayesian modeling to predict malignant hyperthermia susceptibility and pathogenicity of RYR1, CACNA1S and STAC3 variants. Pharmacogenomics 2019, 20, 989–1003. [Google Scholar] [CrossRef]
- Miller, D.M.; Daly, C.; Aboelsaod, E.M.; Gardner, L.; Hobson, S.J.; Riasat, K.; Shepherd, S.; Robinson, R.L.; Bilmen, J.G.; Gupta, P.K.; et al. Genetic epidemiology of malignant hyperthermia in the UK. Br. J. Anaesth. 2018, 121, 944–952. [Google Scholar] [CrossRef]
- Gillies, R.L.; Bjorksten, A.R.; Du Sart, D.; Hockey, B.M. Analysis of the entire ryanodine receptor type 1 and alpha 1 subunit of the dihydropyridine receptor (CACNA1S) coding regions for variants associated with malignant hyperthermia in Australian families. Anaesth. Intensive Care 2015, 43, 157–166. [Google Scholar] [CrossRef]
- Galli, L.; Orrico, A.; Cozzolino, S.; Pietrini, V.; Tegazzin, V.; Sorrentino, V. Mutations in the RYR1 gene in Italian patients at risk for malignant hyperthermia: Evidence for a cluster of novel mutations in the C-terminal region. Cell Calcium 2002, 32, 143–151. [Google Scholar] [CrossRef]
- Heytens, L.; Dos Santos Silva, M.; De Puydt, J.; Heytens, K.; De Ridder, W.; Baets, J.; Mortier, G. Malignant hyperthermia related DNA analysis (RYR1 gene) in Belgian families. Acta Anaesth. Belg. 2019, 70, 197–205. [Google Scholar]
- Kraeva, N.; Riazi, S.; Loke, J.; Frodis, W.; Crossan, M.L.; Nolan, K.; Kraev, A.; MacLennan, D.H. Ryanodine receptor type 1 gene mutations found in the Canadian malignant hyperthermia population. Can. J. Anesth. 2011, 58, 504–513. [Google Scholar] [CrossRef]
- Broman, M.; Islander, G.; Müller, C.R. Malignant hyperthermia, a Scandinavian update. Acta Anaesthesiol. Scand. 2015, 59, 951–961. [Google Scholar] [CrossRef]
- Yeh, H.M.; Liao, M.H.; Chu, C.L.; Lin, Y.H.; Sun, W.Z.; Lai, L.P.; Chen, P.L. Next-generation sequencing and bioinformatics to identify genetic causes of malignant hyperthermia. J. Formos. Med. Assoc. 2021, 120, 883–892. [Google Scholar] [CrossRef]
- Ibarra, M.C.A.; Wu, S.; Murayama, K.; Minami, N.; Ichihara, Y.; Kikuchi, H.; Noguchi, S.; Hayashi, Y.K.; Ochiai, R.; Nishino, I. Malignant hyperthermia in Japan: Mutation screening of the entire ryanodine receptor type 1 gene coding region by direct sequencing. Anesthesiology 2006, 104, 1146. [Google Scholar] [CrossRef]
- Galli, L.; Orrico, A.; Lorenzini, S.; Censini, S.; Falciani, M.; Covacci, A.; Tegazzin, V.; Sorrentino, V. Frequency and localization of mutations in the 106 exons of the RYR1 gene in 50 individuals with malignant hyperthermia. Hum. Mutat. 2006, 27, 830–839. [Google Scholar] [CrossRef]
- Sambuughin, N.; Holley, H.; Muldoon, S.; Brandom, B.W.; de Bantel, A.M.; Tobin, J.R.; Nelson, T.E.; Goldfarb, L.G. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the North American population. Anesthesiology 2005, 102, 515–521. [Google Scholar] [CrossRef]
- Bilmen, J.G.; Gupta, P.K. Keeping it in the family: Malignant Hyperthermia—How we predict, recognise and treat it. Braz. J. Anesthesiol. 2025, 75, 844645. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Larach, M.G.; Localio, A.R.; Allen, G.C.; Denborough, M.A.; Ellis, F.R.; Gronert, G.A.; Kaplan, R.F.; Muldoon, S.M.; Nelson, T.E.; Ording, H. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 1994, 80, 771–779. [Google Scholar] [CrossRef]
- Kraeva, N.; Heytens, L.; Jungbluth, H.; Treves, S.; Voermans, N.; Kamsteeg, E.; Ceuterick-de Groote, C.; Baets, J.; Riazi, S. Compound RYR1 heterozygosity resulting in a complex phenotype of malignant hyperthermia susceptibility and a core myopathy. Neuromuscul. Disord. 2015, 25, 567–576. [Google Scholar] [CrossRef]
- Silva, M.S.; Nakamura, R.; Arjona, M.R.; Monaco, T.P.C.D.; Malito, M.L.; Sampaio, T.O.; Oliveira, S.L.; Magalhães, J.S.A.; Machado-Costa, M.C.; Silva, H.C.A. STAC3 gene congenital myopathy and malignant hyperthermia: A crossroads between neurology and anesthesia. Arq. Neuropsiquiatr. 2025, 83, 1–6. [Google Scholar] [CrossRef]
- Foo, C.T.Y.; To, Y.H.; Irwanto, A.; Ng, A.Y.; Yan, B.; Chew, S.T.H.; Liu, J.; Ti, L.K. Variant landscape of the RYR1 gene based on whole genome sequencing of the Singaporean population. Sci. Rep. 2022, 12, 5429. [Google Scholar] [CrossRef]
- Natera-de Benito, D.; Ortez, C.; Jou, C.; Jimenez-Mallebrera, C.; Codina, A.; Carrera-García, L.; Expósito-Escudero, J.; Cesar, S.; Martorell, L.; Gallano, P.; et al. The Phenotype and Genotype of Congenital Myopathies Based on a Large Pediatric Cohort. Pediatr. Neurol. 2021, 115, 50–65. [Google Scholar] [CrossRef]
- Marti, P.; Pitarch-Castellano, I.; Muelas, N.; Azorín, I.; Fores, L.; Vilchez, R.; Sevilla, T.; Vilchez, J.J. Asymptomatic HyperCKemia in the Pediatric Population: A Prospective Study Utilizing Next-Generation Sequencing and Ancillary Tests. Neurology 2025, 104, e210116. [Google Scholar] [CrossRef]
- Wilmshurst, J.M.; Lillis, S.; Zhou, H.; Pillay, K.; Henderson, H.; Kress, W.; Müller, C.R.; Ndondo, A.; Cloke, V.; Cullup, T.; et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann. Neurol. 2010, 68, 717–726. [Google Scholar] [CrossRef]
- Monnier, N.; Marty, I.; Faure, J.; Castiglioni, C.; Desnuelle, C.; Sacconi, S.; Estournet, B.; Ferreiro, A.; Romero, N.; Laquerriere, A.; et al. Null mutations causing depletion of the type 1 ryanodine receptor (RYR1) are commonly associated with recessive structural congenital myopathies with cores. Hum. Mutat. 2008, 29, 670–678. [Google Scholar] [CrossRef]
- Manning, B.M.; Quane, K.A.; Ording, H.; Urwyler, A.; Tegazzin, V.; Lehane, M.; O’Halloran, J.; Hartung, E.; Giblin, L.M.; Lynch, P.J.; et al. Identification of novel mutations in the ryanodine-receptor gene (RYR1) in malignant hyperthermia: Genotype-phenotype correlation. Am. J. Hum. Genet. 1998, 62, 599–609. [Google Scholar] [CrossRef]
- Carpenter, D.; Robinson, R.L.; Quinnell, R.J.; Ringrose, C.; Hogg, M.; Casson, F.; Booms, P.; Iles, D.E.; Halsall, P.J.; Steele, D.S.; et al. Genetic variation in RYR1 and malignant hyperthermia phenotypes. Br. J. Anaesth. 2009, 103, 538–548. [Google Scholar] [CrossRef]
- Figueroa, L.; Kraeva, N.; Manno, C.; Toro, S.; Ríos, E.; Riazi, S. Abnormal calcium signalling and the caffeine-halothane contracture test. Br. J. Anaesth. 2019, 122, 32–41. [Google Scholar] [CrossRef]
- Kimura, L.; Ribeiro-Rodrigues, E.M.; De Mello Auricchio, M.T.; Vicente, J.P.; Batista Santos, S.E.; Mingroni-Netto, R.C. Genomic ancestry of rural African-derived populations from Southeastern Brazil. Am. J. Hum. Biol. 2013, 25, 35–41. [Google Scholar] [CrossRef]


| Patient | Variant | Criteria | Classification for AD-MH | Revel | ||
|---|---|---|---|---|---|---|
| EMHG > VCEP > ACMG | EMHG | VCEP | This Work | |||
| 54 | exon2:c.131G > A (p.Arg44His) | PS4_sup, PM1, PP3_mod | N/A | VUS | PP (PS4_mod, PM1, PP3_mod, PM5_sup) VECP | 0.93 |
| 48 | exon6:c:455C > A (p.Ala152Asp) | PS4_sup, PM1, PP3_mod | N/A | VUS | VUS (PS4_mod, PM1, PP3_mod) | 0.9 |
| 52 | exon6:c.487C > T (p.Arg163Cys) | PSb, PMb, PPa, PPb, PPc | P | P | - | 0.96 |
| 34 | exon17:c.1840C > T (p.Arg614Cys) | PSa, PMb, PPa, PPb, PPc | P | P | - | 0.93 |
| 59 | exon17:c.1840C > T (p.Arg614Cys) | PSa, PMb, PPa, PPb, PPc | P | P | - | 0.93 |
| 4 | exon29:c.4178A > G (p.Lys1393Arg) | BA1 | N/A | B | - | 0.56 |
| 11 | exon39:c.6544A > T (p.Ile2182Phe) | PS4_sup, PM1 | N/A | VUS | VUS (PS4_mod, PM1) | 0.76 |
| 6 | exon45:c.7292A > T (p.Asp2431Val) | PS4_sup, PM1_sup, PM5_sup, PP3_mod | N/A | VUS | PP (PS4_mod, PM1_sup, PM5_sup, PP3_mod) | 0.95 |
| 31 | exon 45:c.7300G > A (p.Gly2434Arg) | PSb, PMb, PPa, PPb, PPc | P | P | - | 0.97 |
| 56 | exon45:c.7304G > A (p.Arg2435His) | PSb, PMb, PPa, PPb, PPc | P | P | - | 0.94 |
| 50 | exon46:c.7358T > C (p.Ile2453Thr) | PS2_PM6_Mod, PS4_Sup, PM1, PP3_mod | N/A | PP | PP (PS2_mod, PS4_mod, PM1, PP3_mod) | 0.89 |
| 14 | exon46:c.7354C > T (p.Arg2452Trp) | PSb, PMb, PPa, PPc | P | PP | - | 0.83 |
| 28 | exon47:c.7523G > A (p.Arg2508His) | PSb, PMb, PPb, PPc | P | PP | - | 0.9 |
| 9 | exon73:c.10747G > C (p.Glu3583Gln) | BA1 | N/A | B | B (BP4_sup, BA1) | 0.32 |
| 20 | exon101:c.14545G > A (p.Val4849Ile) | PSb, PMb, PPa, PPc | P | P | - | 0.82 |
| 29 | exon101:c.14545G > A (p.Val4849Ile) | PSb, PMb, PPa, PPc | P | P | - | 0.82 |
| 40 | exon104:c.14918C > T (p.Pro4973Leu) | PMb, PPb, PPc | PP | PP | - | 0.9 |
| Patient | Variant | Criteria | Classification for AD-MH | Revel | ||
|---|---|---|---|---|---|---|
| EMHG/VCEP/ACMG | EMHG | VCEP | This Work | |||
| 1 | exon101:c.14524G > A (p.Val4842Met) # | PM1_sup, PP3_mod | N/A | VUS | - | 0.93 |
| intron:c.10348-6C > G (p.His3449ins33aafsX54) # | zero points; without applicable VCEP criteria | N/A | N/A | VUS for AD-MH (P for AR congenital myopathy) | 0.67 | |
| 12 | exon33:c.4711A > G (p.Ile1571Val) # | BS1 | N/A | PB | - | 0.56 |
| exon67:c.10097G > A (p.Arg3366His) # | BS1 | N/A | PB | - | 0.68 | |
| exon85:c.11798A > G (p.Tyr3933Cys) # | PP3_mod, BS1 | N/A | PB | - | 0.98 | |
| exon101:c.14545G > A (p.Val4849Ile) | PSb, PMb, PPa, PPc | P | P | - | 0.82 | |
| intron:c.9555-9G > A | BP4_sup, BA1 | N/A | N/A | PB | 0.01 | |
| 60 | exon28:c.4055C > G (p.Ala1352Gly) | BP4_sup, BS2_mod, BA1 | N/A | N/A | B | 0.27 |
| exon71:c.10485G > C (p.Lys3495Asn) | zero points; without applicable VCEP criteria | N/A | N/A | VUS | 0.58 | |
| 5 | exon73:c.10747G > C (p.Glu3583Gln) | BA1. BS2_SUP, BP4 | N/A | B | - | 0.32 |
| exon92:c.13459C > T (p.Leu4487Phe) | BP4 | N/A | N/A | VUS | 0.28 | |
| 10 | exon22:c.2767A > G (p.Met923Val) | zero points; without applicable VCEP criteria | N/A | N/A | VUS | 0.69 |
| exon90:c.12532G > A (p.Gly4178Ser) | PS4_sup, PP3_mod | N/A | VUS | - | 0.98 | |
| exon92:c.13502C > T (p.Pro4501Leu) | BP4_sup, BA1 | N/A | N/A | B | 0.42 | |
| 8 | exon79:c.11314C > T (p.Arg3772Trp)—Homozygous | PS4_sup, PM5_sup, PP3_mod | N/A | VUS | - | 0.94 |
| Patient | Variant | Criteria | Classification for AD-MH | Revel | ||
|---|---|---|---|---|---|---|
| EMHG > VCEP > ACMG | EMHG | VCEP | This Work | |||
| 17 | exon6:c.425-19A > G | 7BP4_mod, BS1_str | N/A | N/A | PB | 0.02 |
| 33 | exon6:c.452C > A (p.Pro151Gln) | PP3_sup, PM1_sup, PS4_sup | N/A | N/A | VUS | 0.96 |
| 25 | exon7:c.594A > G (p.Leu198Leu) | PM1, BP4_sup, BA1, BP7, BS2_str | N/A | N/A | B | 0.1 |
| 7 | exon20:c.2366G > A (p.(Arg789Gln) | zero points; without applicable VCEP criteria | N/A | N/A | VUS | 0.81 |
| 46 | Exon36:c.5999C > T (p.Ser2000Phe) | BP4 | N/A | N/A | VUS | 0.45 |
| 58 | exon 44: c.7123G > C (p.Gly2375Arg) | PS1_mod, PM1_sup, PP3_mod, PS4_sup | N/A | N/A | PP | 0.9 |
| 57 | exon46:c.7354C > G (p.Arg2452Gly) | PM1_sup, PM5_mod, PS4_sup | N/A | N/A | VUS | 0.79 |
| 45 | exon51:c.8197G > T (p.Gly2733Cys) | PP3_mod, PS4_mod | N/A | N/A | VUS | 0.87 |
| 37 | exon51:c.8197G > T (p.Gly2733Cys) | PP3_mod, PS4_mod | N/A | N/A | VUS | 0.87 |
| 47 | exon84:c.11716A > G (p.T3906A) | BP4_sup | N/A | N/A | VUS | 0.4 |
| 27 | exon91:c.12828_12829insGAGGGCGCGGCGGGGCTC (p.G4284_T4285insAAGLEG) | PS4_sup | N/A | N/A | VUS | N/A |
| 35 | exon91:c.12629A > G (p.Lys4210Arg) | BP4_sup | N/A | N/A | VUS | 0.34 |
| Patient | Variant | Gene | Criteria | Classification for AD-MH | Revel | ||
|---|---|---|---|---|---|---|---|
| EMHG > VCEP > ACMG | EMHG | VCEP | This Work | ||||
| 30 | 1. exon63: c.9472 + 30C > A | RYR1 | BP4_sup | N/A | N/A | VUS | 0.01 |
| 2.exon39:c.4718C > T (p.Thr1573Met) | CACNA1S | zero points | N/A | N/A | VUS | 0.42 | |
| 43 | 1.exon17:c.1840C > T (p.Arg614Cys) | RYR1 | PSa, PMb, PPa, PPb and PPc | P | P | - | 0.93 |
| 2.exon1:c.131G > A (p.Cys44Tyr) | CACNA1S | PM2_mod and PP3_sup | N/A | N/A | VUS | 0.67 | |
| 15 | 1.exon2:c.122T > C (p.Phe41Ser). | RYR1 | PM1_mod and PP3_mod | N/A | N/A | VUS | 0.93 |
| 2 exon26:c.3256C > T (p.Arg1086Cys) | CACNA1S | PP3, PM2_sup, PM5, PS4_sup | N/A | N/A | VUS | 0.96 | |
| 2 | c.851G > C (p.Trp284Ser) | STAC3 | PP3, PM2, PS3_sup, PM3, and PP1_str | N/A | N/A | P for Myopathy with risk for MH AR | 0.89 |
| 26 | c.851G > C (p.Trp284Ser) | STAC3 | PP3, PM2, PS3_sup, PM3, and PP1_str | N/A | N/A | P for Myopathy with risk for MH AR | 0.89 |
| 3 | c.851G > C (p.Trp284Ser) | STAC3 | PP3, PM2, PS3_sup, PM3, and PP1_str | N/A | N/A | P for Myopathy with risk for MH AR | 0.89 |
| Variant Absent (n = 20) | Variant Present (n = 41) | p | |
|---|---|---|---|
| Age | 29.5 ± 13.2 | 31.5 ± 17.9 | 0.61 * |
| Sex (female/male) | 6/14 | 19/22 | 0.148 † |
| Ethnic background Caucasian Afro Brazilian | 13 (65%) 7 (35%) | 30 (73.1%) 11 (26.8%) | 0.431 † |
| Personal antecedents of MH during anesthesia | 7 (35%) | 22 (53.6%) | 0.17 † |
| CK (IU/L; n = 54) | 87 (64–157.5) | 339 (162–563) | p < 0.0001 ‡ |
| Muscle weakness | 2 (10%) | 6 (14.6%) | 0.253 † |
| Ptosis/strabismus | 4 (20%) | 20 (48%) | 0.03 † |
| Muscle hypertrophy | 9 (45%) | 22 (53%) | 0.403 † |
| Cores (n = 51) | 0 (0%) | 8 (19.5%) | 0.013 † |
| IVCT result (n = 50) MHShc MHSh MHSc | 4 (20%) 13 (65%) 3 (15%) | 22 (73.3%) 4 (13.3%) 4 (13.3%) | 0.0002 † (MHShc vs. MHSh/MHSc, MHSh vs. MHShc/MHSc) |
| Contracture 2 mmol caffeine | 0 (0–0.3) | 1.6 (0.3–2.3) | p < 0.0001 ‡ |
| Contracture 2% halothane | 0.3 (0.2–0.4) | 2.2 (0.6–3.4) | p < 0.0001 ‡ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, H.C.A.; Mendonça, D.C.; Souza, B.W.; Santos, J.M.; Souza, L.S.; Junior, A.F.R.; Vasconcelos, F.T.G.R.; Andrade, P.V.; Oliveira, A.S.B.; Vainzof, M. Genetic Characteristics of Brazilian Patients with MH History. Genes 2025, 16, 1127. https://doi.org/10.3390/genes16101127
Silva HCA, Mendonça DC, Souza BW, Santos JM, Souza LS, Junior AFR, Vasconcelos FTGR, Andrade PV, Oliveira ASB, Vainzof M. Genetic Characteristics of Brazilian Patients with MH History. Genes. 2025; 16(10):1127. https://doi.org/10.3390/genes16101127
Chicago/Turabian StyleSilva, Helga C. A., Daniela C. Mendonça, Brandow W. Souza, Joilson M. Santos, Lucas S. Souza, Antonio F. R. Junior, Felipe T. G. R. Vasconcelos, Pamela V. Andrade, Acary S. B. Oliveira, and Mariz Vainzof. 2025. "Genetic Characteristics of Brazilian Patients with MH History" Genes 16, no. 10: 1127. https://doi.org/10.3390/genes16101127
APA StyleSilva, H. C. A., Mendonça, D. C., Souza, B. W., Santos, J. M., Souza, L. S., Junior, A. F. R., Vasconcelos, F. T. G. R., Andrade, P. V., Oliveira, A. S. B., & Vainzof, M. (2025). Genetic Characteristics of Brazilian Patients with MH History. Genes, 16(10), 1127. https://doi.org/10.3390/genes16101127







