The Ca2+-Regulated Protein Kinase CIPK1 Modulates Plant Response to Nitrate Deficiency in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Determination of Total N Content
2.3. Gene Expression Analysis
2.4. Yeast Two-Hybrid Assay
2.5. Statistical Analysis
3. Results
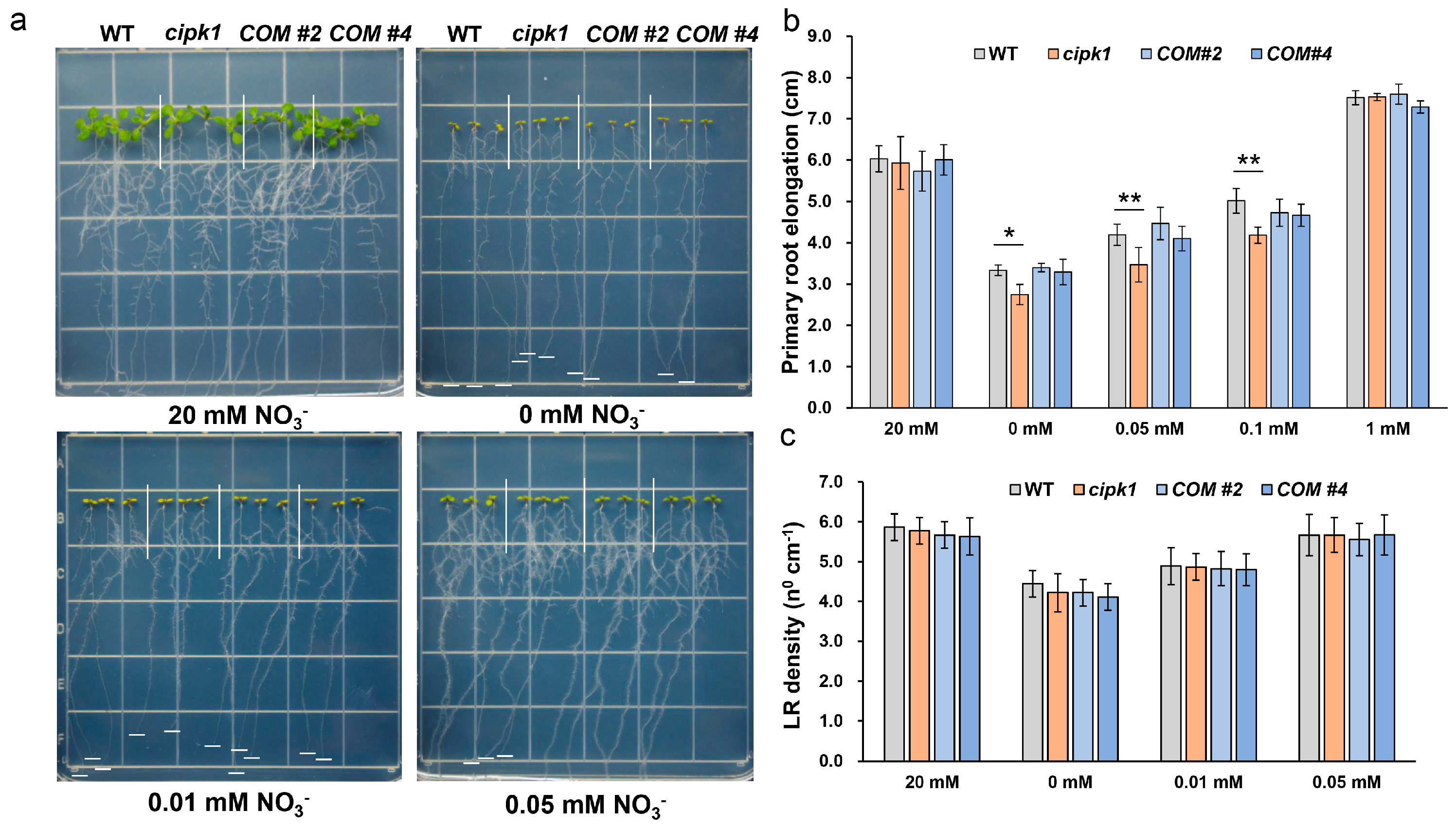
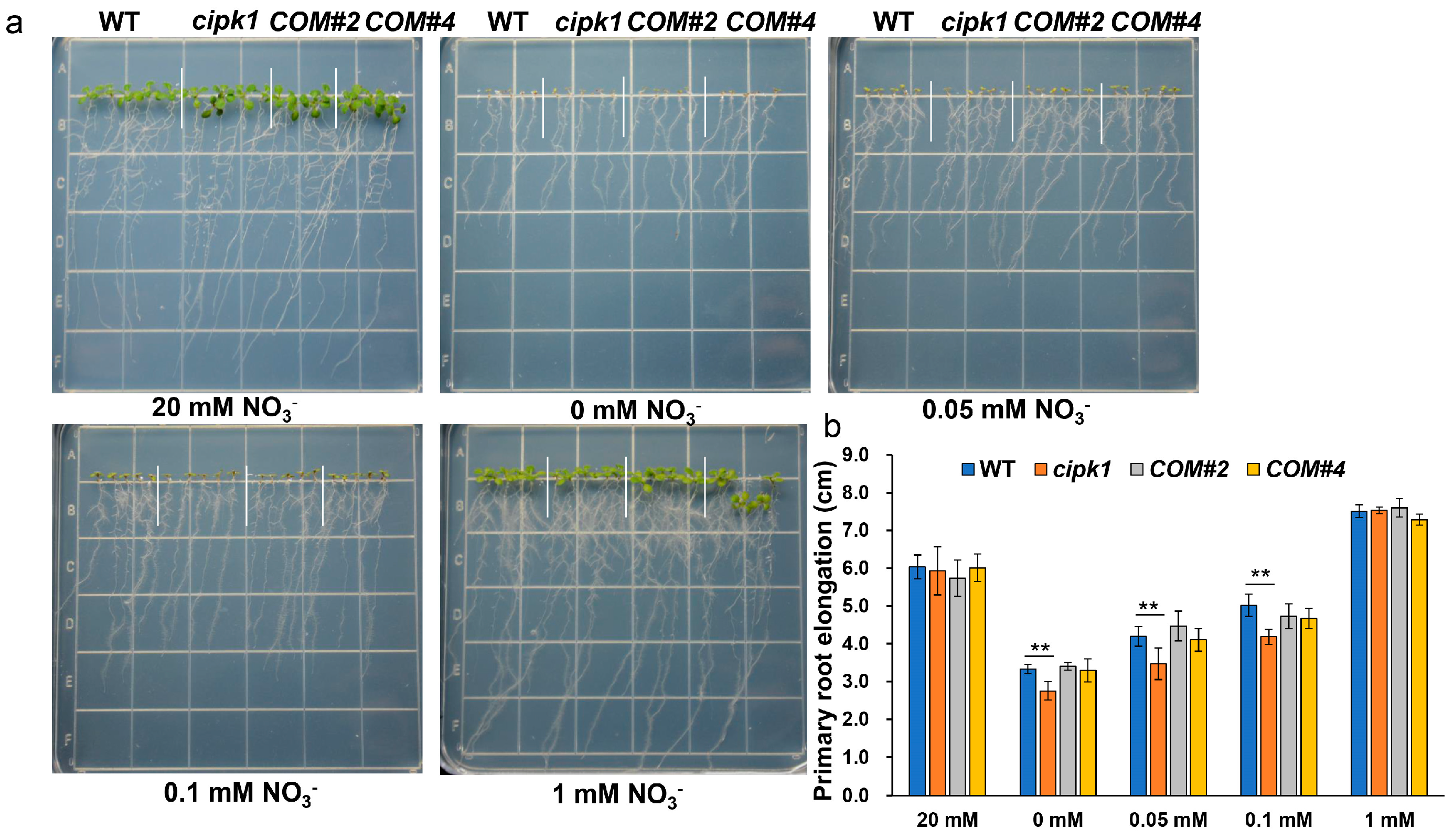
3.1. The cipk1 Mutant Shows Growth Inhibition Phenotype to Nitrate Deficiency
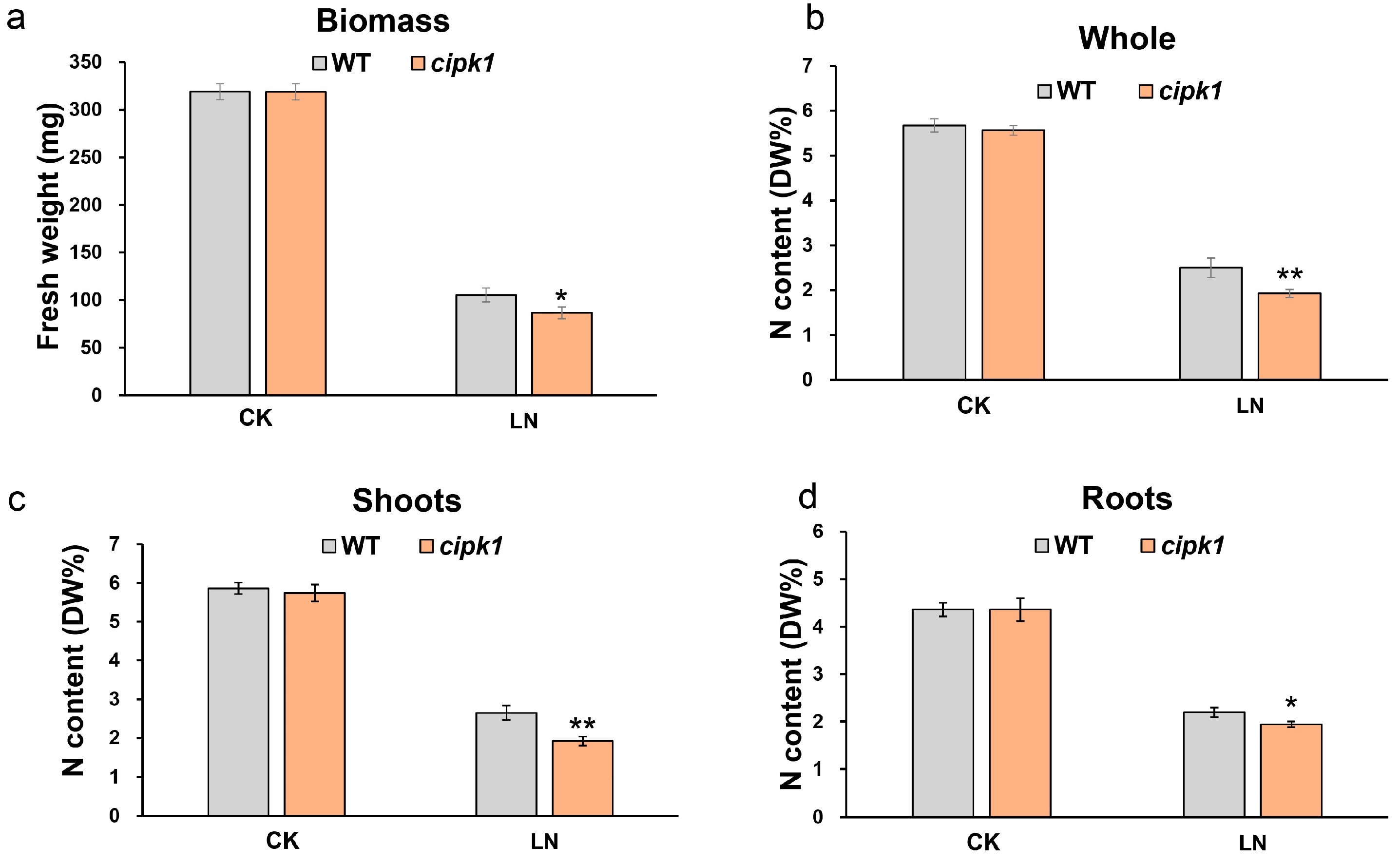
3.2. Mutation of CIPK1 Decreases Total N Accumulation
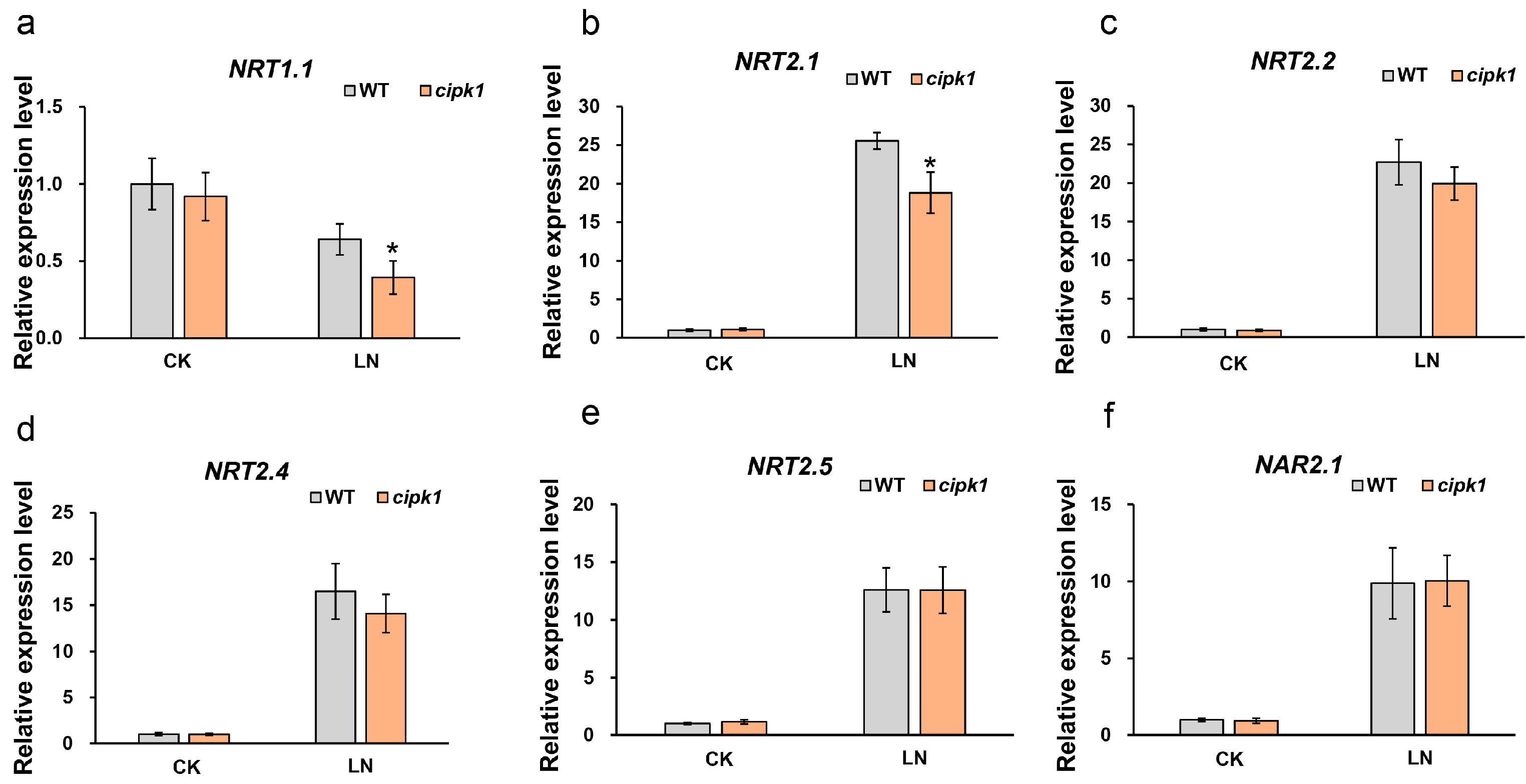
3.3. N-Starvation Marker Genes Expression Were Repressed in cipk1 Mutant under N Deficiency
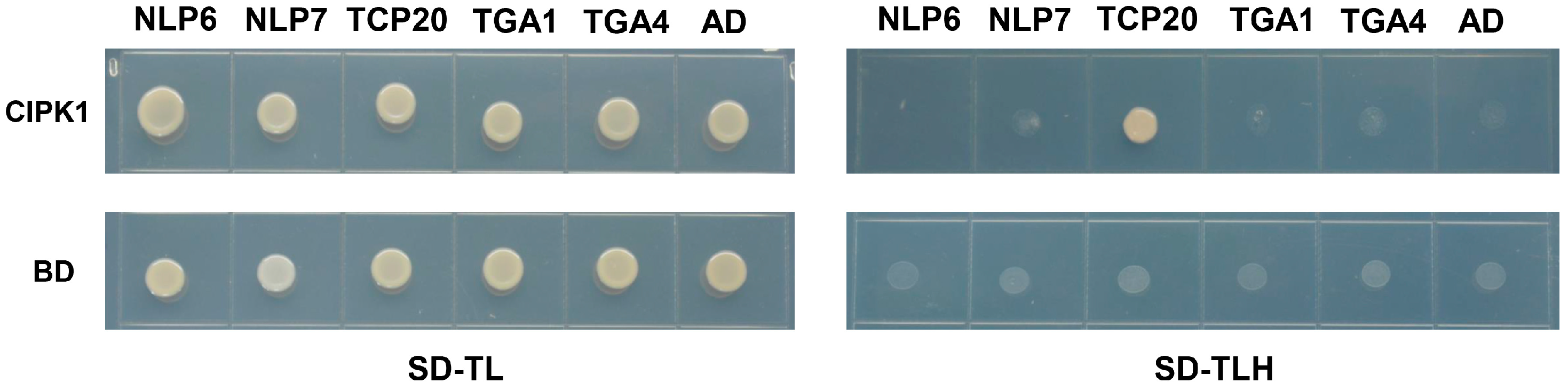
3.4. CIPK1 Interacts with TCP20 Transcription Factor
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, N.M.; Forde, B.G. Molecular and developmental biology of inorganic nitrogen nutrition. Arab. Book 2002, 1, e0011. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Yanagisawa, S. Emergence of a new step towards understanding the molecular mechanisms underlying nitrate-regulated gene expression. J. Exp. Bot. 2014, 65, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2004, 4, 1–36. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368. [Google Scholar] [CrossRef]
- Alboresi, A.; Gestin, C.; Leydecker, M.T.; Bedu, M.; Meyer, C.; Truong, H.N. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 2005, 28, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Bouguyon, E.; Perrine-Walker, F.; Pervent, M.; Rochette, J.; Cuesta, C.; Benkova, E.; Martiniere, A.; Bach, L.; Krouk, G.; Gojon, A.; et al. Nitrate controls root development through posttranscriptional regulation of the NRT1.1/NPF6.3 transporter/sensor. Plant Physiol. 2016, 172, 1237–1248. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutierrez, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef]
- Lin, Y.L.; Tsay, Y.F. Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis. J. Exp. Bot. 2017, 68, 2603–2609. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, Y.; Wang, Y.; Cheng, J.; Wang, J.; Chen, G.; Li, Q.; Wang, S.; Wang, Y.; Wang, S.S.; et al. Nitrate availability controls translocation of the transcription factor NAC075 for cell-type-specific reprogramming of root growth. Dev. Cell 2022, 57, 2638–2651.e2636. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cheng, Y.H.; Chen, K.E.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Tsay, Y.-F.; Schroeder, J.I.; Feldmann, K.A.; Crawford, N.M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 1993, 72, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Okamoto, M.; Crawford, N.M.; Siddiqi, M.Y.; Glass, A.D. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007, 143, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Lezhneva, L.; Boutet-Mercey, S.; Orsel, M.; Brehaut, V.; Miller, A.; Daniel-Vedele, F.; Sakakibara, H.; et al. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 2012, 24, 245–258. [Google Scholar] [CrossRef]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2014, 80, 230–241. [Google Scholar] [CrossRef]
- Liu, K.-H.; Huang, C.-Y.; Tsay, Y.-F. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 1999, 11, 865–874. [Google Scholar] [CrossRef]
- Deng, M.; Moureaux, T.; Caboche, M. Tungstate, a molybdate analog inactivating nitrate reductase, deregulates the expression of the nitrate reductase structural gene. Plant Physiol. 1989, 91, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Gowri, G.; Kenis, J.D.; Ingemarrson, B.; Redinbaugh, M.G.; Campbell, W.H. Nitrate reductase transcript is expressed in the primary response of maize to environmental nitrate. Plant Mol. Biol. 1992, 18, 55–64. [Google Scholar] [CrossRef]
- Marchive, C.; Roudier, F.; Castaings, L.; Brehaut, V.; Blondet, E.; Colot, V.; Meyer, C.; Krapp, A. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat. Commun. 2013, 4, 1713. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Riveras, E.; Vidal, E.A.; Gras, D.E.; Contreras-Lopez, O.; Tamayo, K.P.; Aceituno, F.; Gomez, I.; Ruffel, S.; Lejay, L.; et al. Systems approach identifies TGA1 and TGA4 transcription factors as important regulatory components of the nitrate response of Arabidopsis thaliana roots. Plant J. 2014, 80, 1–13. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, D.; Min, D.; Li, W.; Xu, Z.; Zhou, Y.; Li, L.; Chen, M.; Ma, Y. AtTGA4, a bZIP transcription factor, confers drought resistance by enhancing nitrate transport and assimilation in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2015, 457, 433–439. [Google Scholar] [CrossRef]
- Guan, P.; Ripoll, J.J.; Wang, R.; Vuong, L.; Bailey-Steinitz, L.J.; Ye, D.; Crawford, N.M. Interacting TCP and NLP transcription factors control plant responses to nitrate availability. Proc. Natl. Acad. Sci. USA 2017, 114, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.J.; Wang, C.; Li, K.; Luan, S. The CBL-CIPK calcium signaling network: Unified paradigm from 20 years of discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef]
- Xu, J.; Li, H.D.; Chen, L.Q.; Wang, Y.; Liu, L.L.; He, L.; Wu, W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Lin, S.H.; Hu, H.C.; Tsay, Y.F. CHL1 functions as a nitrate sensor in plants. Cell 2009, 138, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Leran, S.; Edel, K.H.; Pervent, M.; Hashimoto, K.; Corratge-Faillie, C.; Offenborn, J.N.; Tillard, P.; Gojon, A.; Kudla, J.; Lacombe, B. Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci. Signal. 2015, 8, ra43. [Google Scholar] [CrossRef]
- Ragel, P.; Rodenas, R.; Garcia-Martin, E.; Andres, Z.; Villalta, I.; Nieves-Cordones, M.; Rivero, R.M.; Martinez, V.; Pardo, J.M.; Quintero, F.J.; et al. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol. 2015, 169, 2863–2873. [Google Scholar] [CrossRef]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.S.; Zhu, J.K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar] [CrossRef]
- Qiu, Q.S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef]
- Tang, R.-j.; Zhao, F.-G.; Garcia, V.J.; Kleist, T.J.; Yang, L.; Zhang, H.-X.; Luan, S. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3134–3139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fu, D.; Sun, Z.; Ju, C.; Miao, C.; Wang, Z.; Xie, D.; Ma, L.; Gong, Z.; Wang, C. Tonoplast-associated calcium signaling regulates manganese homeostasis in Arabidopsis. Mol. Plant 2021, 14, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Zhang, Z.; Deng, J.; Miao, C.; Wang, Z.; Wallrad, L.; Javed, L.; Fu, D.; Zhang, T.; Kudla, J. Ca2+-dependent successive phosphorylation of vacuolar transporter MTP8 by CBL2/3-CIPK3/9/26 and CPK5 is critical for manganese homeostasis in Arabidopsis. Mol. Plant 2022, 15, 419–437. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Z.; Wallrad, L.; Wang, Z.; Höller, S.; Ju, C.; Schmitz-Thom, I.; Huang, P.; Wang, L.; Peiter, E. Ca2+-dependent phosphorylation of NRAMP1 by CPK21 and CPK23 facilitates manganese uptake and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2022, 119, e2204574119. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Liu, Y.; Zhang, T.; Liu, J.; You, Z.; Huang, P.; Zhang, Z.; Wang, C. Plasma membrane-associated calcium signaling modulates cadmium transport. New Phytol. 2023, 238, 313–331. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, Z.; Guo, S.; Fang, Y.; Zhang, Z.; Gao, H.; Ren, H.; Wang, C. Plasma membrane-associated calcium signaling regulates arsenate tolerance in Arabidopsis. Plant Physiol. 2023, 192, 910–926. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Liu, Y.; Fu, D.; You, Z.; Huang, P.; Gao, H.; Zhang, Z.; Wang, C. Calcium-dependent protein kinases CPK21 and CPK23 phosphorylate and activate the iron-regulated transporter IRT1 to regulate iron deficiency in Arabidopsis. Sci. China Life Sci. 2023, 66, 2646–2662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Fu, D.; Xie, D.; Wang, Z.; Zhao, Y.; Ma, X.; Huang, P.; Ju, C.; Wang, C. CBL1/9–CIPK23–NRAMP1 axis regulates manganese toxicity. New Phytol. 2023, 239, 660–672. [Google Scholar] [CrossRef]
- Hu, H.C.; Wang, Y.Y.; Tsay, Y.F. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009, 57, 264–278. [Google Scholar] [CrossRef]
- Ma, Q.; Tang, R.J.; Zheng, X.J.; Wang, S.M.; Luan, S. The calcium sensor CBL7 modulates plant responses to low nitrate in Arabidopsis. Biochem. Biophys. Res. Commun. 2015, 468, 59–65. [Google Scholar] [CrossRef]
- Chen, H.Y.; Chen, Y.N.; Wang, H.Y.; Liu, Z.T.; Frommer, W.B.; Ho, C.H. Feedback inhibition of AMT1 NH4(+)-transporters mediated by CIPK15 kinase. BMC Biol. 2020, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, C.; Hu, S.; Zuo, K. Arabidopsis calcium-dependent protein kinase CPK6 regulates drought tolerance under high nitrogen by the phosphorylation of NRT1.1. J. Exp. Bot. 2023, 74, 5682–5693. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Xie, D.; Jiang, C.; Wang, C.; Li, L.; Zhang, Y.; Tian, H.; Gao, H.; Wang, C. The Ca(2+) -regulated protein kinase CIPK1 integrates plant responses to phosphate deficiency in Arabidopsis thaliana. Plant Biol. 2020, 22, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, T.; Ju, C.; Deng, J.; Zhang, T.; Li, M.; Tian, H.; Wang, C. Abscisic acid signaling negatively regulates nitrate uptake via phosphorylation of NRT1.1 by SnRK2s in Arabidopsis. J. Integr. Plant Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; von Wiren, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.; von Wiren, N. Root nutrient foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef]
- Sakakibara, H.; Kobayashi, K.; Deji, A.; Sugiyama, T. Partial Characterization of the signaling pathway for the nitrate-dependent expression of genes for nitrogen-assimilatory enzymes using detached maize leaves. Plant Cell Physiol. 1997, 38, 837–843. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Mitsuyama, T.; Sugimoto, T.; Kleinhofs, A.; Warner, R.L.; Oji, Y. Effects of inhibitors for signaling components on the expression of the genes for nitrate reductase and nitrite reductase in excised barley leaves. Soil Sci. Plant Nutr. 1999, 45, 1015–1019. [Google Scholar] [CrossRef]
- Riveras, E.; Alvarez, J.M.; Vidal, E.A.; Oses, C.; Vega, A.; Gutierrez, R.A. The Calcium Ion Is a Second Messenger in the Nitrate Signaling Pathway of Arabidopsis. Plant Physiol. 2015, 169, 1397–1404. [Google Scholar] [CrossRef]
- Liu, K.H.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Sun Chung, H.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S.; et al. Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Filleur, S.; Diatloff, E.; Mounier, E.; Tillard, P.; Forde, B.G.; Gojon, A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc. Natl. Acad. Sci. USA 2006, 103, 19206–19211. [Google Scholar] [CrossRef] [PubMed]
- Mounier, E.; Pervent, M.; Ljung, K.; Gojon, A.; Nacry, P. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 2014, 37, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Remans, T.; Nacry, P.; Pervent, M.; Girin, T.; Tillard, P.; Lepetit, M.; Gojon, A. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006, 140, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Wang, R.; Nacry, P.; Breton, G.; Kay, S.A.; Pruneda-Paz, J.L.; Davani, A.; Crawford, N.M. Nitrate foraging by Arabidopsis roots is mediated by the transcription factor TCP20 through the systemic signaling pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 15267–15272. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Wang, Q.; Wang, L.; Cui, J. The Ca2+-Regulated Protein Kinase CIPK1 Modulates Plant Response to Nitrate Deficiency in Arabidopsis. Genes 2024, 15, 1235. https://doi.org/10.3390/genes15091235
Su H, Wang Q, Wang L, Cui J. The Ca2+-Regulated Protein Kinase CIPK1 Modulates Plant Response to Nitrate Deficiency in Arabidopsis. Genes. 2024; 15(9):1235. https://doi.org/10.3390/genes15091235
Chicago/Turabian StyleSu, Hang, Qian Wang, Lihu Wang, and Junjun Cui. 2024. "The Ca2+-Regulated Protein Kinase CIPK1 Modulates Plant Response to Nitrate Deficiency in Arabidopsis" Genes 15, no. 9: 1235. https://doi.org/10.3390/genes15091235
APA StyleSu, H., Wang, Q., Wang, L., & Cui, J. (2024). The Ca2+-Regulated Protein Kinase CIPK1 Modulates Plant Response to Nitrate Deficiency in Arabidopsis. Genes, 15(9), 1235. https://doi.org/10.3390/genes15091235






