Highlights
What are the main findings?
- We conducted a meta-analysis of all known genotype-by-environment interaction (GxE) studies in commercially farmed shrimp;
- The majority of studies were conducted in Pacific white shrimp (Litopenaeus vannamei);
- We detected low-to-moderate GxE in growth and body weight traits, but substantial GxE was evident for survival and disease resistance traits.
What is the implication of the main finding?
- Selection for growth in one environment should result in predictable improvement across a wide range of environments;
- Selection for survival is likely to be highly dependent on the specific environment in which shrimp are farmed, requiring tailored genetic improvement programmes;
- Further studies including opportunities for implementing GxE in genomic selection are required across different shrimp species.
Abstract
(1) Background: Genotype-by-environment interaction (G×E) can adversely impact genetic improvement programs. The presence of G×E is mainly measured as the genetic correlation between the same trait measured in different environments where departure from unity can be taken as presence of G×E. (2) Methods: To understand the extent of G×E in shrimp production, a review and meta-analysis was conducted using the results from 32 peer-reviewed studies. (3) Results: Of these, 22 G×E studies were conducted on Pacific white shrimp (Litopenaeus vannamei) with fewer studies reported in other shrimp species. The most frequently studied traits were growth and survival, with relatively few studies on traits of economic importance. The meta-analysis demonstrated a moderately high genetic correlation (rg = 0.72 ± 0.05) for growth, indicating low to moderate levels of G×E with some re-ranking of breeding values across environments. However, substantial G×E was evident for survival where only a moderate genetic correlation (rg = 0.58 ± 0.07) was observed for survival across different environments. A re-ranking of breeding values is likely for this trait and genetic improvement of shrimp for survival in one environment may not be effective in other environments. The results from ANOVA-based studies show that G×E accounted for 6.42 ± 1.05% and 7.13 ± 3.46% of the variation for growth and survival traits, respectively. (4) Conclusion: The significance of G×E necessitates tailored genetic improvement programs in commercial shrimp breeding. We discuss the scope and challenges of G×E for shrimp breeding programs, including opportunities of implementing G×E in genomic selection programs.
1. Introduction
Selective breeding for livestock and poultry has been conducted over many generations and often with spectacular results; for example, selective breeding has resulted in a threefold increase in efficiency in beef production in cattle, and in poultry it is responsible for an 85% improvement of production performance [1,2]. With the exception of a few major aquaculture species (e.g., salmon, tilapia and carp), relatively little development has taken place in the implementation of genetic improvement programs in aquaculture [3]. In shrimp, most of the genetic improvement and genotype-by-environment (G×E) studies have focused on Penaeus vannamei (Pacific white shrimp) and Penaeus monodon (black tiger prawn) which contribute 87% of the world production of cultured shrimp [4]. Although over the last three decades, global shrimp aquaculture has increased by approximately 400%, the ever increasing demand will require significantly higher production [5,6]. Sustainable increases in the production of shrimp require the development of genetically improved and high-quality seed stock. In spite of the development of various breeding programs in several countries, the rate of genetic gain of shrimp is lower than the potential gains because of several factors thought to inhibit selection response (e.g., challenges in domestication, inadequate supply of genetically improved seed stocks, poor survival in the production environments) [7]. Moreover, even if improved stock is available, the performance of such stocks is often highly variable in different environments. Therefore, improved productivity of the target trait along with minimizing its variability across environments is desirable [8], as high variation around an optimum trait value can have a significant negative impact on production outcomes. There are limitations in preventing such variability due to dynamic climatic and environmental features that influence phenotypes. The phenotypic expression of genetically similar individuals can alter as a direct response to environmental circumstances [9]. This leads to genotype-by-environment interaction (G×E), which is defined as a different phenotypic expression of the same trait in different environments by genetically identical organisms (Figure 1) [10]. G×E has been a central issue in animal and plant breeding and can affect selection response in genetic improvement programs. In some cases, G×E can cause re-ranking of breeding candidates in different environments [11]. This means that superior breeding candidates in one particular environment (e.g., tropical condition) may not be the best in another environment (e.g., temperate condition), thus significantly reducing selection efficiency [12].
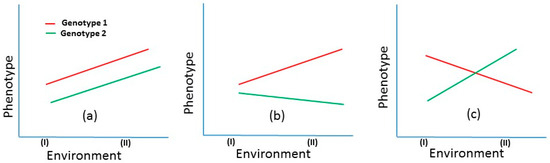
Figure 1.
Illustration of genotype-by-environment interaction (G×E) using two hypothetical genotypes across two environments. (a) Absence of G×E: Both genotypes show higher expression in environment II, with genotype 1 (red) consistently outperforming genotype 2 (green). (b) Heterogeneity of genetic variance: The performance gap between genotypes widens in environment II compared with environment I, indicating a mild G×E. (c) Re-ranking of genotypes: A strong G×E is demonstrated, where genotype 1 performs better in environment I, while genotype 2 excels in environment II. This crossover interaction shows how each genotype is better adapted to a specific environment.
Aquatic animals are farmed under a wide range of production environments, such as different geographical locations, water conditions, and management systems. These diverse conditions can lead to significant G×E effects in aquaculture production. G×E has been reported to exist in most of the economically important aquaculture species, including Atlantic salmon [13], rainbow trout [14], Atlantic cod [15], Arctic charr [16], sea bass [17], gilthead sea bream [18], turbot [19], common sole [20], rohu carp [21], and Nile tilapia [22]. Recently, G×E has also been broadly reported for shrimp species (Table 1). There are significant differences in G×E across studies as well as between estimates within a species. For example, a review on G×E interaction across different aquaculture species has indicated that moderate re-ranking is present for growth and survival, with mean genetic correlations across environments of 0.72 and 0.54, respectively [23].

Table 1.
Details of the G×E studies in commercially farmed shrimp species.
It is essential to understand the magnitude and pattern of G×E in shrimp aquaculture. Shrimp are typically raised in environments that experience extreme variation and where management practices vary within species and between farms; consequently, this can result in lack of uniformity in G×E estimates. Thus, while there is variation amongst published estimates, consensus estimates of G×E are not available to shrimp breeders and researchers.
The primary objective of this study was to review and synthesize the findings of the published studies on G×E and its impact on shrimp breeding programs. By employing a novel meta-analysis methodology, we improved the precision of the consensus G×E estimates derived from the published research. Finally, we discuss several related aspects, including the prospects of utilizing G×E information in genomic selection of shrimp species.
2. Meta-Analysis Methodology
We compiled the published studies which investigated G×E for economically important traits in all of the major farmed shrimp species. Additionally, we conducted a meta-analysis and provide summary weighted estimates of G×E, as described in the following sections.
2.1. Study Selection
Prospective G×E studies of aquaculture species were selected using Google Scholar search engine with the ‘topic’ search terms ‘genotype,’ ‘environment,’ ‘interaction,’ and ‘aquaculture.’ In addition, we conducted forward citation searches of key G×E studies. We collated a final list of 32 articles based on reported G×E results. Alternate search engines, such as PubMed and other databases, were also used but yielded no additional relevant papers beyond the 32 articles already identified. These articles either provided genetic correlation estimates of traits measured in different environments or estimated the effects of G×E using ANOVA-based analyses in shrimp. For each of the selected G×E studies, we recorded publication date, species name, breeding design, pedigree, number of individual/family sampled, type of study (e.g., marker/pedigree based), traits, environment type and presence of G×E. The list of studies included are presented in Table 1. Studies which were rejected from further analyses either failed to report appropriate G×E effects for inclusion in the meta-analysis [54], or referred to environments deemed in appropriate for this study, i.e., “sex” effect [55].
2.2. Basic Study Information and Weighted Estimation of Genetic Correlation
Several studies have reported G×E estimates for economically important traits such as body color, body composition, and feeding efficiency. For our meta-analysis, we compiled various traits and environmental conditions, categorizing them into three broad trait groups (growth, survival, and other traits) and two environmental groups (habitat and stress types), respectively. The “other traits” group encompasses body color, body composition, and feeding efficiency (Table 2). Due to the limited number of studies available for each individual shrimp species, we pooled data from all shrimp species in our meta-analysis to increase statistical power and derive broader insights across the genus Penaeus. Evidence for G×E was based on two main approaches. Firstly, we compiled genetic correlation estimates of the traits measured in different environments (rg) as a measure of G×E and used these estimates in the first meta-analysis. Growth and survival were the two traits with the highest number of reports.

Table 2.
Definition of groups of traits and environment.
Secondly, the effects of G×E were estimated by the partitioning of the sum of squares (SS) from ANOVA-based analyses. The percentage of variation attributable to G×E was estimated as Family×Environment SS / Total SS × 100, with ‘family’ representing the genotype, for the environment in the expression of the target trait. Thereafter, the overall G×E was calculated by averaging overall estimates.
Among the selected studies on G×E interaction, a total of 28 studies reported 136 genetic correlation estimates for traits recorded in different environments. A portion of estimates, approximately 25% of the total data, did not include standard errors of genetic correlation estimates. For these estimates, a conservative approximate standard error value of the upper quartile of known standard error estimates was used and attributed to the estimate. Weighted genetic correlation estimates were calculated for all traits where two or more estimates were available including estimates across species. Where there was only one estimate or trait mean in any class, the value was recorded as it appeared in the original study. Previous studies have identified the inverse of the sampling variance, i.e., inverse of the standard error squared, as the optimal weighting method for a weighted meta-analysis [56,57], and this weighting method was applied here as described by Hasan, Tulloch [58]. As expected, for genetic correlation estimates, standard errors tend to be smaller near the boundaries, and skewed away from the boundaries, namely from −1 to +1 for correlations. Consequently, for genetic correlations (rg), the estimates were transformed using Fisher’s z-transformation, namely resulting in an approximately normal scale, and the meta-analysis was performed on this scale. Summary values from the meta-analysis were then back-transformed into correlations for presentation [58].
We assumed that there are n studies with estimates ri and corresponding standard errors se(ri), i = 1, 2, …, n. A z-transform of each estimate, zi, i = 1, 2, …, n, was obtained. A delta method approximate standard error of each zi was obtained as se(zi) = se(ri)/(1−). The weighted mean of the zi was obtained, , where wi = [se(zi)]−2, with the corresponding overall standard error . Finally, these results were back-transformed to obtain the overall estimate of the correlation and corresponding delta method standard error,
2.3. Factors Affecting G×E Detection
To evaluate the relationship of trait and environmental groups with G×E estimates (e.g., rg values), a linear mixed effect model was fitted using the lme4 package in R. In the linear mixed model, the outcome variable was rg, the fixed effects were either trait categories (namely growth, survival and other, where other includes the traits of body color, body composition and feeding efficiency) or environmental categories (e.g., habitat and stress type), and the random effect was study identification number. We calculated estimated marginal means, also known as least squares means, with the emmeans package in R [59]. We performed pairwise comparison of estimated marginal means between traits and environments. For this pairwise comparison, we classified traits into three and environment into two major categories (Table 2). All data were analyzed in the R statistical environment (version: 3.6.3) [60].
3. Meta-Analysis Results
Growth and survival were the most frequently reported traits across all of the studies. This might be due to these traits being directly related to the economic return in shrimp farming. Moreover, these traits are easier to record as compared with other traits.
The most reported species was P. vannamei, consisting of 53% of all of the studies. The earliest G×E study on shrimp was conducted in 2002. There was no specific trend found in the number of studies published per year, although the highest number of studies was reported in 2020. By country, the highest number of the studies was found to have been conducted in China, followed by Australia (Table 1). All of the studies (n = 32) in which genotypic information is reported were derived from family-based breeding populations.
The weighted genetic correlation across different trait classes were 0.72 ± 0.05 for growth, 0.58 ± 0.07 for survival and 0.48 ± 0.27 for other traits (e.g., feeding efficiency, body color and body composition). The genetic correlation estimates were significantly different between trait groups (Table 3). The genetic correlation across environments were 0.65 ± 0.07 for habitat type (e.g., pond vs. tank) and 0.67 ± 0.08 for stress environment (e.g., pathogen presence vs. absence) (Figure 2). However, pairwise comparison revealed no significant difference between the two environment groups (Table 3).

Table 3.
Meta-estimates of genetic correlation across trait groups (a) and environments (b). “*” indicates that genetic correlation is significantly different between family groups (p < 0.05).
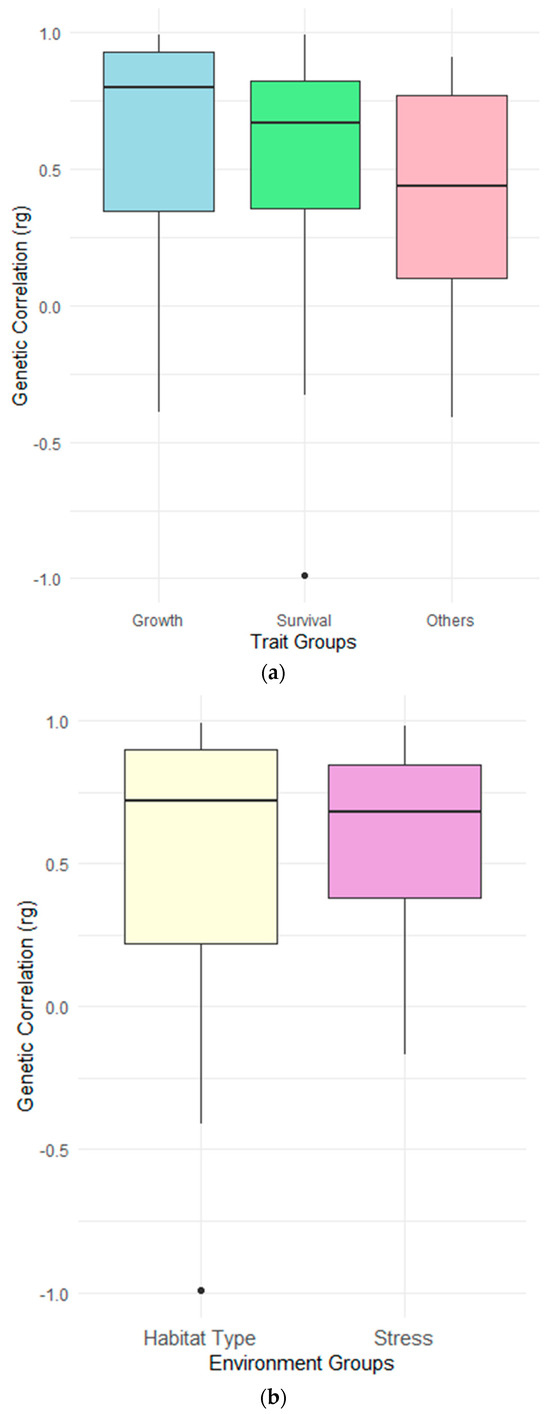
Figure 2.
Distribution of rg across studies of G×E in shrimp species shown as boxplots based on (a) three trait groups (growth, survival and other) and (b) across two different environmental classification (habitat type and stress). Boxplots show median (central line), interquartile range (box), and range (whiskers, extending to 1.5 times the interquartile range) of rg values. Points beyond whiskers represent potential outliers. rg values closer to 1 suggest weaker G×E effects, while values closer to 0 or less indicate stronger G×E effects.
The results from the meta-analysis of the studies (n = 4) on ANOVA SS reveal that habitat type and stress type had significant interactions with family (genotype) for the shrimp growth and survival traits (Table 4). The G×E explained 6.42 ± 1.05% of the variation in growth, and 7.13 ± 3.46% of the variation for the survival traits. A higher level of variability in G×E interaction was observed for survival traits (Figure 3).

Table 4.
Variance explained by G×E from ANOVA-based studies.
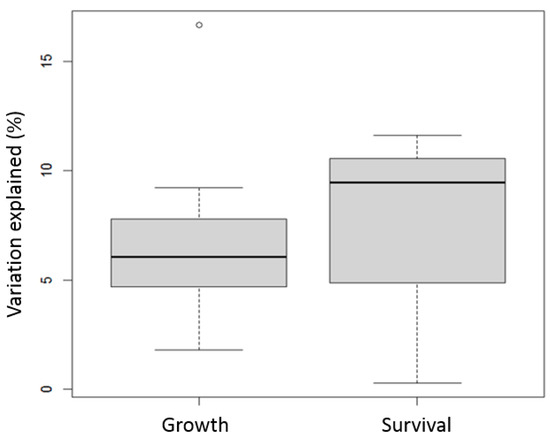
Figure 3.
Boxplots of percent of phenotypic variation explained by G×E in ANOVA-based studies for growth and survival traits. Boxplots show median (central line), interquartile range (box), and range (whiskers, extending to 1.5 times the interquartile range) of % variation explained. Points beyond whiskers represent potential outliers.
4. Discussion and Implications for the Shrimp Industry
G×E has been reported for many traits of economic importance for a wide range of shrimp and aquaculture species. An overview of the data available so far suggests that the outcomes from these studies are highly variable, nevertheless, these meta-analyses have provided important baseline information for breeders to gain an overall understanding of G×E in shrimp aquaculture. G×E typically manifests in one of two ways: either as a re-ranking of genotypes (breeding values) across different environments or as a heterogeneity of genetic variances for the same trait measured in different environments. The former is more important than the latter, because re-ranking indicates that a single genotype is not optimal across all environments. Alternatively, when there is no re-ranking (rg = 1), selection in one particular environment leads to the same genetic gain across all environments. The aim of our meta-analysis was to assess the current state of G×E in shrimp breeding programs. We focused on G×E in shrimp as no such analysis has previously been conducted.
4.1. G×E in Growth Traits
Our meta-analysis revealed that the mean rg value for growth traits in shrimp is 0.72 ± 0.05. This high positive rg value implies that selection for improved growth performance in one environment is likely to lead to similar genetic responses in the same direction in other environments. The results indicate low to moderate overall G×E for growth traits in shrimp, implying that the extent of re-ranking might be minimal for these traits within shrimp species [34,41]. Similar to our findings of G×E for growth traits in shrimp, studies on other aquaculture species have shown high rg value and low G×E for growth traits (e.g., sea bass [61], sole [20], gilthead seabream [18], and Nile tilapia [62]) and is consistent with those presented in the review by Sae-Lim, Gjerde [23] for diverse aquaculture species. Despite the high average rg value for growth traits in shrimp species, a high degree of variability in rg values was observed (Figure 2). Variation in rearing environments on a farm showed a lower impact of G×E effect on growth traits in shrimp, whereas significantly greater variability in G×E was shown across diverse environments, from rearing to grow out. For example, Caballero-Zamora, Montaldo [38] reported negligible G×E for growth traits reared in different ponds (rg = 0.99 ± 0.03) for P. vannamei. Similarly, the presence of low G×E for growth trait was presented by Krishna, Gopikrishna [47] for P. monodon in ponds (rg = 0.78). Sui, Luan [39] reported a high genetic correlation (0.94 ± 0.06) for body weight traits in two farms for P. vannamei. Gitterle, Rye [29] reported low G×E for growth traits for P. vannamei, as the genetic correlation was close to unity, and it was consistent across the test environments. Argue, Arce [24] reported a weak G×E for growth traits between round and raceway tanks in P. vannamei. In contrast with these findings, Nguyen, Ninh [44] reported substantial G×E for growth traits (rg = −0.15 and 0.39) for P. vannamei reared in pond and tank environments. In this study, the authors have reported that ponds are a better environment in which to measure performance when compared with tanks. Jerry, Preston [53] reported sex-specific G×E for body weight trait across different ponds in P. japonicas. Argue, Arce [24] reported significant G×E for body weight traits between pond and a bio-secure aquaculture system. The interaction accounted for 1.81% of the variation. Pérez-Rostro, Racotta [27] reported the extent of G×E in P. vannamei reared in different locations and rearing conditions and found that there is an absence of G×E for harvest size traits and therefore a re-ranking of individual breeding values was not evident. Overall, findings from these studies suggest that, in most cases, G×E might be insignificant for growth traits when shrimp are farmed in different rearing environments.
Wide variability was observed in G×E for growth traits in environments with different rearing density. Rearing density, which is considered a source of resource competition, is regarded as an important environmental factor leading to G×E interaction. Luan, Wang [37] reported low G×E for body weight traits for P. vannamei reared in two densities (rg = 0.71 ± 0.11). Similarly, Campos-Montes, Montaldo [34] have reported that there is no evidence of G×E for bodyweight in P. vannamei produced in different farming methods (e.g., semi-intensive, intensive and super-intensive). Tan, Luan [41] examined the impact of G×E on the growth of P. vannamei in two different densities and found high genetic correlations (0.94 to 0.98). These authors found slightly better production in this species in the low-density condition. In contrast, Ibarra and Famula [33] reported the presence of a substantial G×E effect on the body weight traits in P. vannamei reared in two different densities (rg = 0.56 ± 0.12). These authors have recommended that this species should be grown in higher densities as overall production was higher under such conditions. Castillo-Juárez, Casares [32] have reported the presence of G×E for body weight in P. vannamei reared in low and high density, although rg = 0.80 ± 0.08 to 0.86 ± 0.04 suggests the effects may have been small. Overall, these findings suggest G×E for density could impact the efficiency of selective breeding programs for the economically important traits of shrimp, if stocks produced from the breeding programs are cultivated across a wide range of densities.
Environmental stress is another significant environmental impact factors studied in G×E investigations. Here environmental stress is usually defined by water temperature, salinity, and hypoxia. Growth traits showed stronger G×E effects when the rearing environment was stressful. Li, Luan [36] found significant G×E for growth traits for P. vannamei at different rearing temperatures. Coman, Crocos [51] have reported the presence of significant genotype-by-temperature interactions for growth and survival traits across a wide range of rearing temperature conditions.
Growth traits are generally considered non-fitness traits and are less influenced by environmental variation [63,64]. For example, growth traits tend to have significantly higher additive genetic variation compared with survival traits [65]. However, G×E for growth traits is evident in shrimp when the rearing environment is stressful (e.g., temperature, salinity, ammonia tolerance etc.). This implies that re-ranking can be evident even for non-fitness traits (e.g., growth) when the environment is stressful and adversely affects survival. This finding in shrimp is relatively consistent with those of several other aquaculture species, including tilapia, salmon and rainbow trout, where significant G×E was observed for growth traits under highly stressful environments [23]. Therefore, it is likely that G×E effects may have a significant impact on the selection of growth traits in shrimp breeding programs when the environment is stressful and affects survival as described in the following section.
4.2. G×E in Survival Traits
Survival traits are the second most reported traits for G×E interaction studies in shrimp. In fact, it is the most important set of economic traits in shrimp aquaculture, with a direct impact on profitable shrimp farming, and thus warrants inclusion into the overall breeding objective. Our meta-analysis revealed a genetic correlation of rg = 0.58 ± 0.07 for survival traits, suggesting a strong G×E and noticeable re-ranking of breeding values for this trait across the different environments which impact differential survival. Moreover, we observed extreme variability in G×E for this trait across the range of −0.99 to 0.99 for rg values (Figure 2). This indicates that re-ranking for survival is of significant importance and it should be noted that a full re-ranking is possible for survival traits under some environments. In general, this suggests that this trait is strongly influenced by numerous environmental factors. Similar to our findings regarding shrimp species, relatively strong G×E effects for survival traits have been reported for other farmed aquaculture species here including Atlantic salmon and rainbow trout [66,67]. Considering individual studies, Lu, Luan [40] reported a medium strength rg of survival traits in P. vannamei, e.g., 0.39 for survival time and 0.39 for survival status, between two different saline environments with high ammonia concentration. This suggests a strong G×E for ammonia tolerance across different salinity levels. Li, Luan [36] found that G×E for survival in different water temperatures was 0.29 ± 0.22 (rg) and suggested this trait could be controlled by different sets of loci in different temperatures for P. vannamei. Luan, Wang [37] reported variable estimates (low to high) of genotype-by-pond interaction (rg = 0.001 ± 0.17 to 0.80 ± 0.06) for survival across different generations of M. rosenbergii. Gitterle, Salte [28] reported a low genotype-by-farm interaction for survival traits. A similar finding of high genetic correlation between replicated tanks for survival (WSSV resistance) was also reported by Gitterle, Salte [28]. Caballero-Zamora, Montaldo [38] reported moderate to high genetic correlation (ranging 0.49 to 0.93) for survival traits across different ponds, suggesting the general pond environment has a less pronounced impact on survival than other environmental conditions. Moss, Moss [35] examined the genetic correlation for survival of P. vannamei infected with different TSV isolates, which ranged from 0.35 to 0.99. This suggests that virulence can vary significantly among various strains of the same pathogenic species and can affect the G×E of survival of P. vannamei. Tan, Luan [41] examined the effect of density on the survival of P. vannamei and found low G×E (rg = 0.77 ± 0.09), suggesting the genetic component of variation in survival might be less affected by stocking density. Gitterle, Gjerde [31] found that survival traits displayed significant interaction of genotype-by-infection based on the infection protocol used (e.g., IO = individual oral vs. WB = water-borne infection) to infect the experimental P. vannamei with white spot syndrome virus (WSSV). These authors observed significant re-ranking of families when family breeding values were predicted based on the two infection protocols. There are several approaches for the inoculation of pathogens, including feeding of infected tissues, oral delivery, and intramuscular injection. This study provided insight about how these methods can be an important source of non-genetic variability for evaluating the genetic merit of animals [31]. Hayes, Gitterle [46] found higher G×E for survival between tanks (rg = 0.09) in P. vannamei and speculated that this phenomena occurred due to lack of genetic variation. Noble, Coman [48] found moderate G×E (rg = 0.35 to 0.58) for GAV-induced mortality across different groups of tanks challenged on different days. Moss, Moss [35] found a positive phenotypic correlation of 0.55 and 0.88 for mean family survival for TSV challenge test and growth in pond. Despite re-ranking, this positive correlation suggests that pathogen tolerance can be improved under commercial farm conditions by means of selective breeding. Coman, Crocos [51] examined the effect of temperature on growth and survival traits in P. japonicus, where the G×E accounted for 2.5 to 10.9% of the variability of the growth and survival traits, respectively. Coman, Crocos [52] examined the effect of density on the growth and survival of P. japonicus and found a small but significant G×E for growth but not for survival traits. The interaction accounted for 2.3% of the variation of growth.
In shrimp breeding programs, the aim is to improve overall survival and resilience against multiple environmental disturbances. Survival is a complex trait which is characterized by its high variability in expression and is difficult to measure. We observed a substantial level of G×E and re-ranking of shrimp species for survival traits across studies (Figure 2). This level of re-ranking for survival traits can result from the presence of various mortality factors expressed in different environments, and there might be limited additive genetic variation for resilience against these factors. Hoffmann and Merilä [9] have proposed that changes in the genetic response of a trait due to unfavorable conditions can be caused by increases in environmental and phenotypic variance, and that this can cause a decrease in the relative importance of additive effects and variance because of limited expression of additive gene effects (i.e., lowered heritability).
4.3. G×E in Other Economically-Important Traits
Aside from growth and survival, only a handful of studies have been conducted to examine the impact of G×E on other economically important traits such as feed efficiency, body composition and body color. The meta-correlation value (rg) of these traits was 0.48 ± 0.27. However, this is an overall composite estimate across traits and it is thus important to look into results from individual studies for specific details. Pérez-Rostro, Racotta [27] studied the impact of acute hypoxia on the expression of the body composition traits of P. vannamei. The authors reported that biochemical traits (e.g., body protein percent) showed variation depending on oxygen condition. However, the authors reported lack of significant G×E for lactate concentration in hypoxic or normoxic situations. Glencross, Tabrett [68] reported the existence of significant G×E on the impact of diet on growth in P. monodon and demonstrated that genetically selected shrimp can perform better if they are fed with an improved diet. A low-quality diet regime might have restricted the animal in expressing its full genetic potential. Dai, Kong [42] investigated the extent of G×E for feed efficiency traits in P. vannamei in different stocking density and conditions (e.g., individual vs. group reared). They found that moderate G×E was evident for the FER trait but was insignificant for growth expressed as ADG. Giang, Knibb [43] examined the G×E for body color in two environments (viz. two locations) and found it to be significant (rg = −0.40 to 0.16). This study signifies that rearing in different environments can improve the body color in shrimp.
Overall, results from this meta-analysis identified the substantial presence of G×E in shrimp, which signifies the importance of implementing environment-specific breeding programs. For this purpose, “break-even correlation” or BEC can be used to decide whether running several environment-specific breeding programs will be cost effective or not. BEC is the point at which genetic gain is similar across different breeding plans. Under this scheme distinctive breeding strategies are recommended when rg across environments is lower than the BEC. For aquaculture species this BEC is assumed to be higher (e.g., ≥0.7) compared with livestock (e.g., 0.61 to 0.7) [69,70]. This is because, in aquaculture species, selection is generally carried out based on SIB testing combined with higher selection intensity [71]. Selection intensity in particular has a very large and proportional effect on BEC [69]. In our meta-analysis, rg values for growth, survival and other (feeding, body coloration and body composition) traits were 0.73 ± 0.06, 0.51 ± 0.09 and 0.62 ± 0.10, respectively. Therefore, higher genetic gain can be obtained for growth traits in different environments within a single breeding program in a common environment. However, environment-specific breeding programs will lead to higher genetic gain in shrimp species for survival and other traits. However, the breeder needs to consider the cost–benefit analysis in this kind of scenario [72].
4.4. Prospects to Overcome G×E in Breeding Programs
G×E interaction has always been viewed as problematic in breeding programs under traditional selection breeding programs, which rely on pedigree information and performance testing; this becomes more complex when several environments need to be considered. However, genomic selection with reference populations reared in several environments can be far more effective [73]. Such multi-environment reference populations could be combined with high-throughput genomics and phenomics [74]. The use of large training datasets on genotypes and phenotypes can be utilized to select animals for improved resilience across environments. The multi-environment reference population can facilitate the prediction of accurate genomic enabled breeding values (GEBV) for the genotyped selection candidates. This is because genotyping and phenotyping of large number of selection candidates increases the possibility of creating specialized lines by genomic selection methods, coupled with the BEC usually being higher with genomic selection than with a conventional selection breeding approach [75]. By utilizing genetic variance among populations, G×E can play a significant positive role for increasing resilience. Mulder [75] compared the accuracy of EBVs in an extreme environment by using a reaction norm (RN) model, which considers G×E interactions, and a conventional model, which ignores them. The study found that the RN model yielded higher EBV accuracy. Furthermore, the author investigated the effectiveness of incorporating G×E information in genomic selection using large reference populations. The results demonstrate that the RN model was capable of reducing environmental sensitivity by selecting resilient animals. This method of GS outperformed traditional breeding methods by 9 to 140% across environments [75]. The author evaluated the advantage of genomic selection in fish in the presence of G×E, where the production performance of the reference population was measured in commercial farms, and found that the selection accuracy greatly improved under such a scheme. Chu, Alemu [76] evaluated the impact of level of G×E on genetic gain in the genomic breeding schemes for broiler chicken and found that when G×E is strong (rg = 0.5 to 0.7), genomic selection of birds reared in a commercial production environment yielded higher genetic gain than when production data from the bio-secure environment was used. From these studies it is becoming clear that G×E can be managed successfully by using genomic information whilst increasing the accuracy of EBV estimation, provided that the reference population is large. Once the models are trained using data from diverse environments, GS in aquaculture shrimp species can be performed in the centralized nucleus facilities. This approach eliminates the need for progeny testing across different environments.
In the face of changing global climatic conditions, the incorporation of G×E information in future breeding programs will be crucial [77]. Aquaculture seed stocks adapted to current environmental conditions may not perform optimally in future changing conditions and climate change may cause a sudden outbreak of existing and novel pathogens. This becomes even more important when popular seed stocks are being used across the globe. To overcome these challenges, the application of selective breeding to develop resilient animals will be of great importance [78].
Disease in shrimp farming is a major constraint to obtain profitable yields. Most shrimp pathogens are transmitted vertically, and disease is often the result of a massive viral amplification generally triggered by various environmental and physiological stresses. Based on detailed environmental data, the likelihood of pathogen attack can be predicted both in the short and long term [79]. Such predictions can potentially be carried out for the coming year or season based on currently available weather forecast data in combination with historical data on diseases, pathogens and climatic factors [80]. The ability to predict phenotypes in a specific environment is important to manage disease and pests in a sustainable and dynamic way. Although no such studies have been conducted in shrimp, studies on wheat have shown that specific disease outbreaks can be successfully predicted for different environments. For example, wheat rust Ug99 disease dynamics/epidemics have been predicted in east Africa by integrating the current status and distribution of the rust, prevailing winds, climatic factors for rust survival and sporulation, wheat production zones, historical migration patterns of rust races, and responses to the existing cultivars to the rusts [81]. Similar approaches can be taken to develop disease-resistant shrimp. To attain this, detailed environmental data, along with genotype and phenotype data, need to be considered in the breeding programs [82]. This detailed environmental data should include multiple environmental trials, geographic and historical weather information, measurement of habitat characteristics (e.g., pond soil and water properties) and the evaluation of companion organisms (e.g., susceptible pathogens and microbes) at an ecosystem level. So far, variation in environmental factors has not been properly integrated into shrimp breeding programs. This is largely due to the fact that environmental factors have been considered at a gross level, e.g., only major environmental parameters are considered such as location and station and is generally treated as a ‘black box’ effect that interacts with the genotype to affect phenotype without considering individual components. Moreover, environmental parameters are dynamic throughout the animal grow-out periods. To circumvent these limitations, precise dissection of complex environmental factors for both target environments and for specific genotypes needs to be carried out for sustainable management, control and optimization of environmental factors for enhanced genetic improvement program [82,83,84]. Therefore, genomic selection and use of ‘big data’ can be successfully utilized to exploit G×E as a ‘game changer’ tool of genetic improvement across environments.
Throughout our review it is apparent that there is a lack of strategies for assessing a large number of individual family pedigrees. In many cases, a mass selection method is applied in shrimp breeding, where animals with superior phenotypic values for target traits are chosen as the breeders for the next generation. However, if individuals are chosen in the absence of particular environmental factors (e.g., disease factors) that are highly correlated, then, over generations, the performance of the animal for the selected traits might decrease. Alternatively, a family-based selection scheme considers multiple-trait expression in different environments, which provides a powerful tool with which to improve multiple traits simultaneously. Therefore, to better manage the G×E issue in shrimp breeding, family-based selection or a genomic selection scheme should be preferred.
5. Conclusions and Recommendation
Most of the studies have focused on only a single major shrimp species used in commercial aquaculture (e.g., P. vannamei), with the main traits of interest being growth and survival. However, many other economically important species and traits (e.g., feed efficiency, nutrition, body composition and body color) also need to be considered for G×E studies. In addition, our review revealed that there is a lack of standard documentation for G×E effects across studies. For proper utilization of G×E information in breeding programs and to ensure reproducibility, minimum standard information should be provided from each study (e.g., family information, population size, and a standardized definition of environmental parameters etc.).
This review underscores the potential of genomic selection in addressing G×E interactions for shrimp breeding programs. Conventional practices of rearing shrimp families in individual tanks or ponds can lead to confounding of genetic and environmental effects, whereas communal family rearing with marker-based pedigree estimation mitigates these effects and improves the accuracy of genomic breeding values. To optimize breeding programs, it is crucial to ensure that disease challenge test conditions closely resemble grow-out environments, and that environmental information is incorporated into the selection index. Controlling G×E effects can be achieved by maintaining separate genetic lines or assessing sibling performance in different environments, combining evaluation data to maintain genetic diversity.
Traditionally, separate tanks/ponds are used for producing shrimp families for maintaining genetic lines and the evaluation of traits. This facilitates easy tracing of families and is convenient for stocking equal numbers of animals/individuals per family. This separate rearing environment can introduce a significant confounding of family traits with tank-specific environment effects. In contrast, as demonstrated by Jerry, Preston [53], communal family rearing has been proposed to eliminate such effects by means of marker-based pedigree estimation. This can ultimately eliminate the potential non-genetic effects (e.g., common environment effect due to rearing system) and increase the reliability of measures of genetic value [85].
The measurement of disease resistance through a disease challenge test is only effective for selection breeding programs where the challenge conditions are very similar to the grow-out conditions. Therefore, it is critically important to optimize the grow-out environment to be as similar as possible to the selection environment. It can be done by incorporating production environment information in the selection index. To limit re-ranking, maintaining separate genetic lines for different environments is essential to secure long-term response to the selection and maintenance of genetic diversity. Another approach to control G×E effects would be to test sibling production performance in different production environments and to use combined genetic evaluation of the production data from both environments.
Author Contributions
Conceptualization, M.M.H., M.S.K. and H.W.R.; methodology, M.M.H., M.S.K. and P.C.T.; software, M.M.H., M.S.K. and P.C.T.; formal analysis, M.M.H., M.S.K. and P.C.T.; data curation, M.M.H.; writing—original draft preparation, M.M.H.; writing—review and editing, M.M.H., M.S.K., H.W.R. and P.C.T.; visualization, M.M.H.; supervision, M.S.K., H.W.R. and P.C.T. All authors have read and agreed to the published version of the manuscript.
Funding
M.M.H. was supported by a scholarship from the Australian Government Research Training Pro-gram.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Eenennaam, A.L. Application of genome editing in farm animals: Cattle. Transgenic Res. 2019, 28 (Suppl. 2), 93–100. [Google Scholar] [CrossRef] [PubMed]
- Athrey, G. Poultry genetics and breeding. In Animal Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 317–330. [Google Scholar]
- Gjedrem, T.; Robinson, N.; Rye, M. The importance of selective breeding in aquaculture to meet future demands for animal protein: A review. Aquaculture 2012, 350, 117–129. [Google Scholar] [CrossRef]
- Andriantahina, F.; Liu, X.; Feng, T.; Xiang, J. Current status of genetics and genomics of reared penaeid shrimp: Information relevant to access and benefit sharing. Mar. Biotechnol. 2013, 15, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Gjedrem, T. Genetic improvement for the development of efficient global aquaculture: A personal opinion review. Aquaculture 2012, 344, 12–22. [Google Scholar] [CrossRef]
- Anderson, J.; Valderrama, D.; Darryl, J. Shrimp Production Review; Global Outlook for Aquaculture Leadership (GOAL): Dublin, Ireland, 2017. [Google Scholar]
- Van Sang, N.; Luan, N.T.; Van Hao, N.; Van Nhien, T.; Vu, N.T.; Nguyen, N.H. Genotype by environment interaction for survival and harvest body weight between recirculating tank system and pond culture in Penaeus monodon. Aquaculture 2020, 525, 735278. [Google Scholar] [CrossRef]
- Mulder, H.A.; Bijma, P.; Hill, W.G. Selection for uniformity in livestock by exploiting genetic heterogeneity of residual variance. Genet. Sel. Evol. 2008, 40, 37. [Google Scholar]
- Hoffmann, A.A.; Merilä, J. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 1999, 14, 96–101. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Addison Wesley Longman: Harlow, UK, 1996. [Google Scholar]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer: Sunderland, MA, USA, 1998. [Google Scholar]
- Mulder, H.; Bijma, P. Effects of genotype × environment interaction on genetic gain in breeding programs. J. Anim. Sci. 2005, 83, 49–61. [Google Scholar] [CrossRef]
- Drangsholt, T.; Gjerde, B.; Ødegård, J.; Finne-Fridell, F.; Evensen, Ø.; Bentsen, H. Quantitative genetics of disease resistance in vaccinated and unvaccinated Atlantic salmon (Salmo salar L.). Heredity 2011, 107, 471–477. [Google Scholar] [CrossRef]
- Kause, A.; Ritola, O.; Paananen, T.; Mäntysaari, E.; Eskelinen, U. Selection against early maturity in large rainbow trout Oncorhynchus mykiss: The quantitative genetics of sexual dimorphism and genotype-by-environment interactions. Aquaculture 2003, 228, 53–68. [Google Scholar] [CrossRef]
- Kolstad, K.; Thorland, I.; Refstie, T.; Gjerde, B. Genetic variation and genotype by location interaction in body weight, spinal deformity and sexual maturity in Atlantic cod (Gadus morhua) reared at different locations off Norway. Aquaculture 2006, 259, 66–73. [Google Scholar] [CrossRef]
- Nilsson, J.; Brännäs, E.; Eriksson, L.-O. The Swedish Arctic charr breeding programme. Hydrobiologia 2010, 650, 275–282. [Google Scholar] [CrossRef]
- Saillant, E.; Dupont-Nivet, M.; Haffray, P.; Chatain, B. Estimates of heritability and genotype–environment interactions for body weight in sea bass (Dicentrarchus labrax L.) raised under communal rearing conditions. Aquaculture 2006, 254, 139–147. [Google Scholar] [CrossRef]
- Navarro, A.; Zamorano, M.J.; Hildebrandt, S.; Ginés, R.; Aguilera, C.; Afonso, J.M. Estimates of heritabilities and genetic correlations for growth and carcass traits in gilthead seabream (Sparus auratus L.), under industrial conditions. Aquaculture 2009, 289, 225–230. [Google Scholar] [CrossRef]
- Guan, J.; Hu, Y.; Wang, M.; Wang, W.; Kong, J.; Luan, S. Estimating genetic parameters and genotype-by-environment interactions in body traits of turbot in two different rearing environments. Aquaculture 2016, 450, 321–327. [Google Scholar] [CrossRef]
- Mas-Muñoz, J.; Blonk, R.; Schrama, J.W.; van Arendonk, J.; Komen, H. Genotype by environment interaction for growth of sole (Solea solea) reared in an intensive aquaculture system and in a semi-natural environment. Aquaculture 2013, 410, 230–235. [Google Scholar] [CrossRef]
- Gjerde, B.; Mahapatra, K.D.; Reddy, P.V.; Saha, J.N.; Jana, R.K.; Meher, P.K.; Sahoo, M.; Khaw, H.L.; Gjedrem, T.; Rye, M. Genetic parameters for growth and survival in rohu carp (Labeo rohita). Aquaculture 2019, 503, 381–388. [Google Scholar] [CrossRef]
- Khaw, H.L.; Ponzoni, R.W.; Hamzah, A.; Abu-Bakar, K.R.; Bijma, P. Genotype by production environment interaction in the GIFT strain of Nile tilapia (Oreochromis niloticus). Aquaculture 2012, 326, 53–60. [Google Scholar] [CrossRef]
- Sae-Lim, P.; Gjerde, B.; Nielsen, H.M.; Mulder, H.; Kause, A. A review of genotype-by-environment interaction and micro-environmental sensitivity in aquaculture species. Rev. Aquac. 2016, 8, 369–393. [Google Scholar] [CrossRef]
- Argue, B.J.; Arce, S.M.; Lotz, J.M.; Moss, S.M. Selective breeding of Pacific white shrimp (Litopenaeus vannamei) for growth and resistance to Taura Syndrome Virus. Aquaculture 2002, 204, 447–460. [Google Scholar] [CrossRef]
- Pérez-Rostro, C.I.; Ibarra, A.M. Quantitative genetic parameter estimates for size and growth rate traits in Pacific white shrimp, Penaeus vannamei (Boone 1931) when reared indoors. Aquac. Res. 2003, 34, 543–553. [Google Scholar] [CrossRef]
- Pérez-Rostro, C.I.; Ibarra, A.M. Heritabilities and genetic correlations of size traits at harvest size in sexually dimorphic Pacific white shrimp (Litopenaeus vannamei) grown in two environments. Aquac. Res. 2003, 34, 1079–1085. [Google Scholar] [CrossRef]
- Pérez-Rostro, C.I.; Racotta, I.S.; Ibarra, A.M. Decreased genetic variation in metabolic variables of Litopenaeus vannamei shrimp after exposure to acute hypoxia. J. Exp. Mar. Biol. Ecol. 2004, 302, 189–200. [Google Scholar] [CrossRef]
- Gitterle, T.; Salte, R.; Gjerde, B.; Cock, J.; Johansen, H.; Salazar, M.; Lozano, C.; Rye, M. Genetic (co)variation in resistance to White Spot Syndrome Virus (WSSV) and harvest weight in Penaeus (Litopenaeus) vannamei. Aquaculture 2005, 246, 139–149. [Google Scholar] [CrossRef]
- Gitterle, T.; Rye, M.; Salte, R.; Cock, J.; Johansen, H.; Lozano, C.; Suárez, J.A.; Gjerde, B. Genetic (co)variation in harvest body weight and survival in Penaeus (Litopenaeus) vannamei under standard commercial conditions. Aquaculture 2005, 243, 83–92. [Google Scholar] [CrossRef]
- Gitterle, T.; Ødegård, J.; Gjerde, B.; Rye, M.; Salte, R. Genetic parameters and accuracy of selection for resistance to White Spot Syndrome Virus (WSSV) in Penaeus (Litopenaeus) vannamei using different statistical models. Aquaculture 2006, 251, 210–218. [Google Scholar] [CrossRef]
- Gitterle, T.; Gjerde, B.; Cock, J.; Salazar, M.; Rye, M.; Vidal, O.; Lozano, C.; Erazo, C.; Salte, R. Optimization of experimental infection protocols for the estimation of genetic parameters of resistance to White Spot Syndrome Virus (WSSV) in Penaeus (Litopenaeus) vannamei. Aquaculture 2006, 261, 501–509. [Google Scholar] [CrossRef]
- Castillo-Juárez, H.; Casares, J.C.Q.; Campos-Montes, G.; Villela, C.C.; Ortega, A.M.; Montaldo, H.H. Heritability for body weight at harvest size in the Pacific white shrimp, Penaeus (Litopenaeus) vannamei, from a multi-environment experiment using univariate and multivariate animal models. Aquaculture 2007, 273, 42–49. [Google Scholar] [CrossRef]
- Ibarra, A.M.; Famula, T.R. Genotype by environment interaction for adult body weights of shrimp Penaeus vannamei when grown at low and high densitie. Genet. Sel. Evol. 2008, 40, 541. [Google Scholar] [CrossRef]
- Campos-Montes, G.R.; Montaldo, H.H.; Martínez-Ortega, A.; Castillo-Juárez, H. Genotype by environment interaction effects for body weight at 130 days of age in the Pacific white shrimp [Penaeus (Litopenaeus) vannamei]. Vet. México 2009, 40, 255–268. [Google Scholar]
- Moss, D.R.; Moss, S.M.; Lotz, J.M. Estimation of genetic parameters for survival to multiple isolates of Taura syndrome virus in a selected population of Pacific white shrimp Penaeus (Litopenaeus) vannamei. Aquaculture 2013, 416, 78–84. [Google Scholar] [CrossRef]
- Li, W.; Luan, S.; Luo, K.; Sui, J.; Xu, X.; Tan, J.; Kong, J. Genetic parameters and genotype by environment interaction for cold tolerance, body weight and survival of the Pacific white shrimp Penaeus vannamei at different temperatures. Aquaculture 2015, 441, 8–15. [Google Scholar] [CrossRef]
- Luan, S.; Wang, J.; Yang, G.; Luo, K.; Chen, X.; Gao, Q.; Hu, H.; Kong, J. Genetic parameters of survival for six generations in the giant freshwater prawn Macrobrachium rosenbergii. Aquac. Res. 2015, 46, 1345–1355. [Google Scholar] [CrossRef]
- Caballero-Zamora, A.; Montaldo, H.H.; Campos-Montes, G.R.; Cienfuegos-Rivas, E.G.; Martínez-Ortega, A.; Castillo-Juárez, H. Genetic parameters for body weight and survival in the Pacific White Shrimp Penaeus (Litopenaeus) vannamei affected by a White Spot Syndrome Virus (WSSV) natural outbreak. Aquaculture 2015, 447, 102–107. [Google Scholar] [CrossRef]
- Sui, J.; Luan, S.; Luo, K.; Meng, X.; Lu, X.; Cao, B.; Li, W.; Chai, Z.; Liu, N.; Xu, S. Genetic parameters and response to selection for harvest body weight of Pacific white shrimp, Litopenaeus vannamei. Aquac. Res. 2016, 47, 2795–2803. [Google Scholar] [CrossRef]
- Lu, X.; Luan, S.; Cao, B.; Meng, X.; Sui, J.; Dai, P.; Luo, K.; Shi, X.; Hao, D.; Han, G. Estimation of genetic parameters and genotype-by-environment interactions related to acute ammonia stress in Pacific white shrimp (Litopenaeus vannamei) juveniles at two different salinity levels. PLoS ONE 2017, 12, e0173835. [Google Scholar] [CrossRef]
- Tan, J.; Luan, S.; Luo, K.; Guan, J.; Li, W.; Sui, J.; Guo, Z.; Xu, S.; Kong, J. Heritability and genotype by environment interactions for growth and survival in Litopenaeus vannamei at low and high densities. Aquac. Res. 2017, 48, 1430–1438. [Google Scholar] [CrossRef]
- Dai, P.; Kong, J.; Meng, X.; Luo, K.; Lu, X.; Chen, B.; Cao, B.; Luan, S. Genotype by environment interaction for feed efficiency trait of the juvenile Pacific white shrimp Litopenaeus vannamei held in individuals vs. in groups. Aquaculture 2019, 500, 506–513. [Google Scholar] [CrossRef]
- Giang, C.T.; Knibb, W.; Ninh, N.H.; Nguyen, N.H. Prospects for Genetic Improvement in Objective Measurements of Body Colour in Pacific Whiteleg Shrimp (Litopenaeus vannamei). J. Mar. Sci. Eng. 2019, 7, 460. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Ninh, N.H.; Hung, N.H. Evaluation of two genetic lines of Pacific White leg shrimp Liptopenaeus vannamei selected in tank and pond environments. Aquaculture 2020, 516, 734522. [Google Scholar] [CrossRef]
- Cala-Moreno, N.; Campos-Montes, G.; Caballero-Zamora, A.; Berruecos-Villalobos, J.; Castillo-Juárez, H. Genotype-by-environment interaction in white shrimp associated with White Spot Disease. Abanico Vet. 2021, 11, e2020-95. [Google Scholar]
- Hayes, B.J.; Gitterle, T.; Gopikrishna, G.; Gopal, C.; Krishna, G.; Jahageerdar, S.; Lozano, C.; Alavandi, S.; Paulpandi, S.; Ravichandran, P. Limited evidence for genetic variation for resistance to the white spot syndrome virus in Indian populations of Penaeus monodon. Aquac. Res. 2010, 41, e872–e877. [Google Scholar] [CrossRef]
- Krishna, G.; Gopikrishna, G.; Gopal, C.; Jahageerdar, S.; Ravichandran, P.; Kannappan, S.; Pillai, S.M.; Paulpandi, S.; Kiran, R.P.; Saraswati, R. Genetic parameters for growth and survival in Penaeus monodon cultured in India. Aquaculture 2011, 318, 74–78. [Google Scholar] [CrossRef]
- Noble, T.H.; Coman, G.J.; Wade, N.M.; Thomson, P.C.; Raadsma, H.W.; Khatkar, M.S.; Guppy, J.L.; Jerry, D.R. Genetic parameters for tolerance to gill-associated virus under challenge-test conditions in the black tiger shrimp (Penaeus monodon). Aquaculture 2020, 516, 734428. [Google Scholar] [CrossRef]
- Jiang, S.; Mo, X.; Zhou, F.; Huang, J.; Yang, Q.; Yang, L.; Jiang, S. Genetic Evaluation of Body Weight and Survival of Black Tiger Shrimp (Penaeus monodon) fed on Different Dietary Levels of Fish Meal Protein. Pak. J. Zool. 2021, 53, 1–6. [Google Scholar] [CrossRef]
- Hasan, M.M.; Thomson, P.C.; Raadsma, H.W.; Khatkar, M.S. Genetic analysis of digital image derived morphometric traits of black tiger shrimp (Penaeus monodon) by incorporating G × E investigations. Front. Genet. 2022, 13, 1007123. [Google Scholar] [CrossRef]
- Coman, G.J.; Crocos, P.J.; Preston, N.P.; Fielder, D. The effects of temperature on the growth, survival and biomass of different families of juvenile Penaeus japonicus Bate. Aquaculture 2002, 214, 185–199. [Google Scholar] [CrossRef]
- Coman, G.J.; Crocos, P.J.; Preston, N.P.; Fielder, D. The effects of density on the growth and survival of different families of juvenile Penaeus japonicus Bate. Aquaculture 2004, 229, 215–223. [Google Scholar] [CrossRef]
- Jerry, D.R.; Preston, N.P.; Crocos, P.J.; Keys, S.; Meadows, J.R.; Li, Y. Application of DNA parentage analyses for determining relative growth rates of Penaeus japonicus families reared in commercial ponds. Aquaculture 2006, 254, 171–181. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Wang, L.; Guan, T.; Chang, G.; Wu, N.; Li, J. Heritability Estimates of Growth-Related Traits in Oriental River Prawns, Macrobrachium nipponense. Aquac. Res. 2023, 2023, 8315364. [Google Scholar] [CrossRef]
- Sui, J.; Luan, S.; Yang, G.; Xia, Z.; Tang, Q.; Luo, K.; Meng, X.; Kong, J. Effects of the individual rearing stage on the growth traits of candidate giant freshwater prawns (Macrobrachium rosenbergii). Aquac. Int. 2022, 30, 1659–1673. [Google Scholar] [CrossRef]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Marín-Martínez, F.; Sánchez-Meca, J. Weighting by Inverse Variance or by Sample Size in Random-Effects Meta-Analysis. Educ. Psychol. Meas. 2010, 70, 56–73. [Google Scholar] [CrossRef]
- Hasan, M.M.; Tulloch, R.L.; Thomson, P.C.; Raadsma, H.W.; Khatkar, M.S. Meta-analysis of genetic parameters of production traits in cultured shrimp species. Fish Fish. 2020, 21, 1150–1174. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated marginal means, aka least-squares means. (R package version 1.3), 2018.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Dupont-Nivet, M.; Karahan-Nomm, B.; Vergnet, A.; Merdy, O.; Haffray, P.; Chavanne, H.; Chatain, B.; Vandeputte, M. Genotype by environment interactions for growth in European seabass (Dicentrarchus labrax) are large when growth rate rather than weight is considered. Aquaculture 2010, 306, 365–368. [Google Scholar] [CrossRef]
- Trọng, T.Q.; Mulder, H.A.; van Arendonk, J.A.; Komen, H. Heritability and genotype by environment interaction estimates for harvest weight, growth rate, and shape of Nile tilapia (Oreochromis niloticus) grown in river cage and VAC in Vietnam. Aquaculture 2013, 384, 119–127. [Google Scholar] [CrossRef]
- Visscher, P.M.; Hill, W.G.; Wray, N.R. Heritability in the genomics era—Concepts and misconceptions. Nat. Rev. Genet. 2008, 9, 255–266. [Google Scholar] [CrossRef]
- Mousseau, T.A.; Roff, D.A. Natural selection and the heritability of fitness components. Heredity 1987, 59, 181–197. [Google Scholar] [CrossRef]
- Kenkel, C.; Setta, S.; Matz, M.V. Heritable differences in fitness-related traits among populations of the mustard hill coral, Porites astreoides. Heredity 2015, 115, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Rye, M.; Lillevik, K.M.; Gjerde, B. Survival in early life of Atlantic salmon and rainbow trout: Estimates of heritabilities and genetic correlations. Aquaculture 1990, 89, 209–216. [Google Scholar] [CrossRef]
- Vehviläinen, H.; Kause, A.; Quinton, C.; Koskinen, H.; Paananen, T. Survival of the currently fittest: Genetics of rainbow trout survival across time and space. Genetics 2008, 180, 507–516. [Google Scholar] [CrossRef]
- Glencross, B.; Tabrett, S.; Irvin, S.; Wade, N.; Anderson, M.; Blyth, D.; Smith, D.; Coman, G.; Preston, N. An analysis of the effect of diet and genotype on protein and energy utilization by the black tiger shrimp, Penaeus monodon—Why do genetically selected shrimp grow faster? Aquac. Nutr. 2013, 19, 128–138. [Google Scholar] [CrossRef]
- Mulder, H.; Veerkamp, R.; Ducro, B.; Van Arendonk, J.; Bijma, P. Optimization of dairy cattle breeding programs for different environments with genotype by environment interaction. J. Dairy Sci. 2006, 89, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- James, J. Selection in two environments. Heredity 1961, 16, 145–152. [Google Scholar] [CrossRef]
- Sonesson, A.K.; Meuwissen, T.H. Testing strategies for genomic selection in aquaculture breeding programs. Genet. Sel. Evol. 2009, 41, 37. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L.; Ninh, N.H. Accounting for genotype by environment interaction in economic appraisal of genetic improvement programs in common carp Cyprinus carpio. Aquaculture 2008, 285, 47–55. [Google Scholar] [CrossRef]
- Nirea, K.; Meuwissen, T. Improving production efficiency in the presence of genotype by environment interactions in pig genomic selection breeding programmes. J. Anim. Breed. Genet. 2017, 134, 119–128. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating high-throughput phenotyping into genetic gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]
- Mulder, H.A. Genomic selection improves response to selection in resilience by exploiting genotype by environment interactions. Front. Genet. 2016, 7, 178. [Google Scholar] [CrossRef]
- Chu, T.T.; Alemu, S.W.; Norberg, E.; Sørensen, A.C.; Henshall, J.; Hawken, R.; Jensen, J. Benefits of testing in both bio-secure and production environments in genomic selection breeding programs for commercial broiler chicken. Genet. Sel. Evol. 2018, 50, 52. [Google Scholar] [CrossRef]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Iung, L.H.d.S.; Carvalheiro, R.; Neves, H.H.d.R.; Mulder, H.A. Genetics and genomics of uniformity and resilience in livestock and aquaculture species: A review. J. Anim. Breed. Genet. 2020, 137, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Khiem, N.M.; Takahashi, Y.; Oanh, D.T.H.; Hai, T.N.; Yasuma, H.; Kimura, N. The use of machine learning to predict acute hepatopancreatic necrosis disease (AHPND) in shrimp farmed on the east coast of the Mekong Delta of Vietnam. Fish. Sci. 2020, 86, 673–683. [Google Scholar] [CrossRef]
- Gillberg, J.; Marttinen, P.; Mamitsuka, H.; Kaski, S. Modelling G × E with historical weather information improves genomic prediction in new environments. Bioinformatics 2019, 35, 4045–4052. [Google Scholar] [CrossRef]
- Singh, R.P.; Hodson, D.P.; Jin, Y.; Huerta-Espino, J.; Kinyua, M.G.; Wanyera, R.; Njau, P.; Ward, R.W. Current status, likely migration and strategies to mitigate the threat to wheat production from rzace Ug99 (TTKS) of stem rust pathogen. CABI Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2007, 1, 054. [Google Scholar] [CrossRef]
- Rana, M.; Rahman, A.; Hugo, D.; McCulloch, J.; Hellicar, A. Investigating data-driven approaches to understand the interaction between water quality and physiological response of sentinel oysters in natural environment. Comput. Electron. Agric. 2020, 175, 105545. [Google Scholar] [CrossRef]
- Xu, Y. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Dabrowski, J.; McCulloch, J. Dissolved oxygen prediction in prawn ponds from a group of one step predictors. Inf. Process. Agric. 2020, 7, 307–317. [Google Scholar] [CrossRef]
- Kong, Z.; Kong, J.; Hao, D.; Lu, X.; Jian, T.; Meng, X.; Luo, K.; Cao, B.; Sui, J.; Li, X. Reducing the Common Environmental Effect on Litopenaeus vannamei Body Weight by Rearing Communally at Early Developmental Stages and Using a Reconstructed Pedigree. J. Ocean Univ. China 2020, 19, 923–930. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).