The Clinical and Mutational Spectrum of Bardet–Biedl Syndrome in Saudi Arabia
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Characteristics and Clinical Features
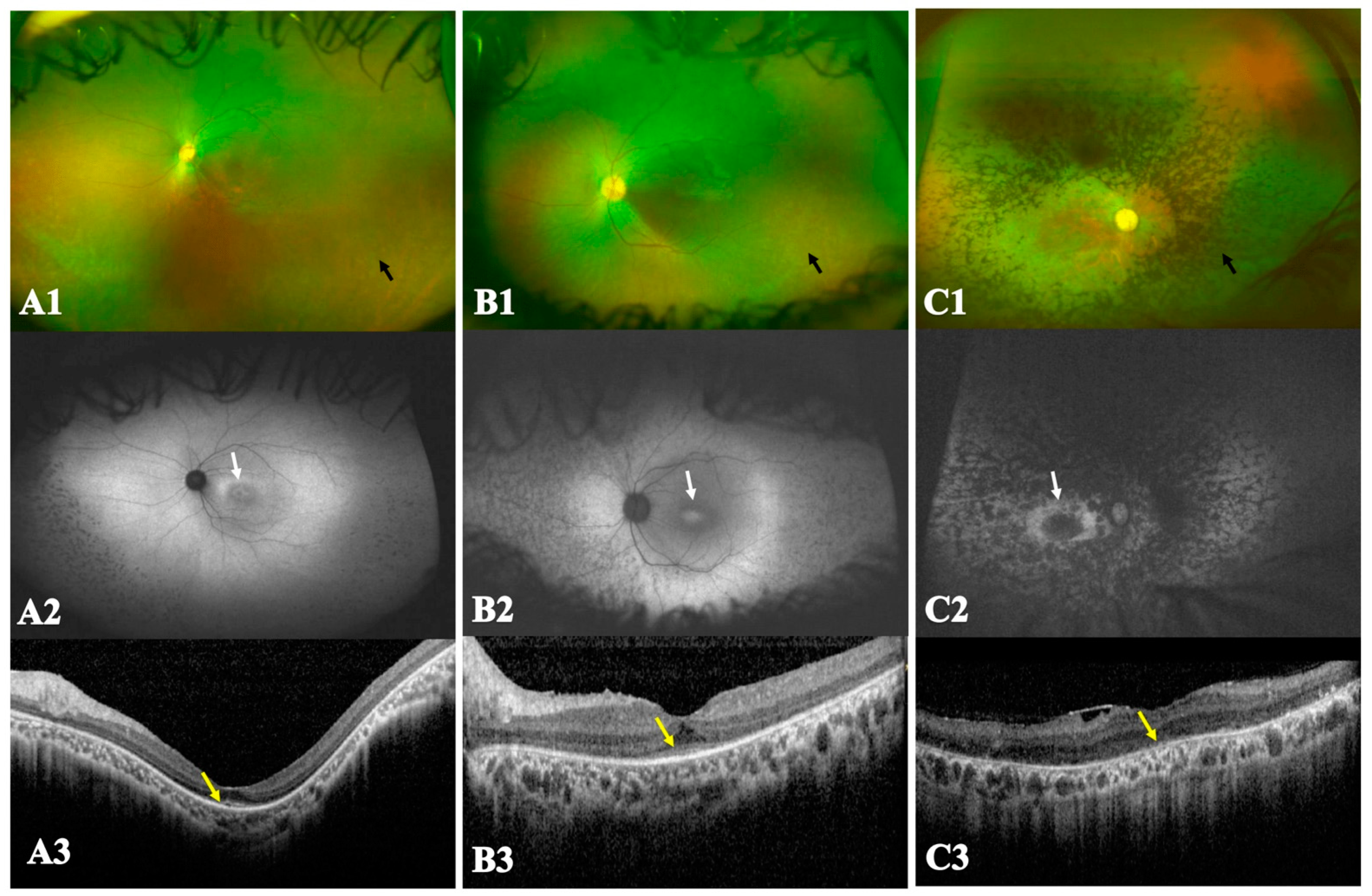
3.2. Multimodal Imaging and Electroretinographic Features
3.3. Molecular Genetics
| Proband | Gene Group | Gene | Allele 1 | Allele 2 | Pathogenicity |
|---|---|---|---|---|---|
| 1 | Others | ARL6 (NM_001278293) | c.431C>T p.(Ser144Phe) [9,22,23] | c.431C>T p.(Ser144Phe) | Likely pathogenic |
| 2 | BBSome | BBS1 (NM_024649) | c.124+1G>A [24,25] | c.951+58C>T p. (Gly318Valfs*61) [26] | Pathogenic |
| 3 | Chaperonin complex | BBS10 (NM_024685) | c.924G>T p.(Leu308Phe) [27] | c.924G>T (p.Leu308Phe) | Pathogenic |
| 4 | BBSome complex | BBS4 (NM_033028) | c.157-2A>G [9,28] | c.157-2A>G | Pathogenic |
| 5 | BBSome complex | BBS4 (NM_033028) | c.157-2A>G [9,28] | c.157-2A>G | Pathogenic |
| 6 | BBSome complex | BBS4 (NM_033028) | c.157-2A>G [9,28] | c.157-2A>G | Pathogenic |
| 7 | BBSome complex | BBS5 (NM_152384) | c.966dupT: p.(Ala323Cysfs*57) [29] | c.966dupT: p.(Ala323Cysfs*57) | Pathogenic |
| 8 | BBSome complex | BBS9 (NM_198428) | c.617+3A>C [*] | c.617+3A>C | VUS |
| 9 | BBSome complex | BBS5 (NM_152384) | c.900+1G>A [*] | c.900+1G>A | Pathogenic |
| 10 | Chaperonin complex | MKKS (NM_170784) | c.116C>T p.(Pro39Leu) [24,25] | c.116C>T p.(Pro39Leu) | Likely pathogenic |
| 11 | Others | ARL6 (NM_001278293) | c.362G>A p.(Arg121His) [30,31] | c.362G>A p.(Arg121His) | Likely pathogenic |
| 12 | Chaperonin complex | MKKS (NM_170784) | c.295T>C p.(Cys99Arg) [32,33] | c.295T>C p.(Cys99Arg) | Likely pathogenic |
| 13 | Chaperonin complex | MKKS (NM_170784) | c.116C>T p.(Pro39Leu) [24,25] | c.116C>T p.(Pro39Leu) | Likely pathogenic |
| 14 | BBSome complex | BBS4 (NM_033028) | c.157-2A>G [9,28] | c.157-2A>G | Pathogenic |
| 15 | BBSome complex | BBS4 (NM_033028) | c.1106+2T>A [34,35] | c.1106+2T>A | Pathogenic |
| 16 | BBSome complex | BBS4 (NM_033028) | c.157-2A>G [9,28] | c.157-2A>G | Pathogenic |
| 17 | Chaperonin complex | BBS10 (NM_024685) | c.1195_1197delCTT (p. Leu399del) [*] | c.1195_1197delCTT (p. Leu399del) | Likely pathogenic |
| 18 | BBSome complex | BBS2 (NM_031885) | c.471G>A p.(Thr157=) [36] | c.944G>A p.(Arg315Gln) [37] | Likely pathogenic |
| 19 | BBSome complex | BBS1 (NM_024649) | c.951+58C>T p.(Gly318Valfs*61) [26] | c.951+58C>T p.(Gly318Valfs*61) | Pathogenic |
| 20 | BBSome complex | BBS4 (NM_033028) | c.1159G>T p.(Glu387*) [*] | c.1159G>T p.(Glu387*) | Pathogenic |
| 21 | BBSome complex | BBS1 (NM_024649) | c.951+58C>T p.(Gly318Valfs*61) [26] | c.951+58C>T p.(Gly318Valfs*61) | Pathogenic |
| 22 | BBSome complex | BBS4 (NM_033028) | c.157-2A>G [9,28] | c.157-2A>G | Pathogenic |
| 23 | BBSome complex | BBS4 (NM_033028) | c.262delG p.(Glu88Asnfs*54) [*] | c.1311_1312insT p.(Lys438*) [*] | Pathogenic |
| 24 | BBSome complex | BBS9 (NM_198428) | c.832C>T p.(Arg278*) [38] | c.832C>T p.(Arg278*) | Pathogenic |
| 25 | Chaperonin complex | MKKS (NM_170784) | c.116C>T p.(Pro39Leu) [24,25] | c.116C>T p.(Pro39Leu) | Pathogenic |
| 26 | Chaperonin complex | BBS12 (NM_001178007) | c.787dupT p.(Tyr263Leufs*4) [25] | c.787dupT p.(Tyr263Leufs*4) | Pathogenic |
| 27 | BBSome complex | BBS1 (NM_024649) | c.951+58C>T p.(Gly318Valfs*61) [26] | c.951+58C>T p.(Gly318Valfs*61) | Pathogenic |
| 28 | Chaperonin complex | BBS12 (NM_001178007) | c.787dupT p.(Tyr263Leufs*4) [25] | c.787dupT p.(Tyr263Leufs*4) | Pathogenic |
| 29 | Chaperonin complex | MKKS (NM_170784) | c.116C>T p.(Pro39Leu) [24,25] | c.116C>T p.(Pro39Leu) | Pathogenic |
| 30 | Chaperonin complex | MKKS (NM_170784) | c.116C>T p.(Pro39Leu) [24,25] | c.116C>T p.(Pro39Leu) | Pathogenic |
| 31 | BBSome complex | BBS5 (NM_152384) | c.966dupT: p.(Ala323Cysfs*57) [29] | c.966dupT: p.(Ala323Cysfs*57) | Pathogenic |
3.4. Genotype–Phenotype Correlation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharon, D.; Ben-Yosef, T.; Goldenberg-Cohen, N.; Pras, E.; Gradstein, L.; Soudry, S.; Mezer, E.; Zur, D.; Abbasi, A.H.; Zeitz, C.; et al. A nationwide genetic analysis of inherited retinal diseases in Israel as assessed by the Israeli inherited retinal disease consortium (IIRDC). Hum. Mutat. 2020, 41, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Beales, P.L.; Elcioglu, N.; Woolf, A.S.; Parker, D.; Flinter, F.A. New criteria for improved diagnosis of Bardet-Biedl syndrome: Results of a population survey. J. Med. Genet. 1999, 36, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Grudzinska Pechhacker, M.K.; Jacobson, S.G.; Drack, A.V.; Scipio, M.D.; Strubbe, I.; Pfeifer, W.; Duncan, J.L.; Dollfus, H.; Goetz, N.; Muller, J.; et al. Comparative Natural History of Visual Function From Patients with Biallelic Variants in BBS1 and BBS10. Investig. Ophthalmol. Vis. Sci. 2021, 62, 26. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, E.; Beales, P.L. Bardet-Biedl syndrome. Eur. J. Hum. Genet. 2013, 21, 8–13. [Google Scholar] [CrossRef]
- M’Hamdi, O.; Ouertani, I.; Chaabouni-Bouhamed, H. Update on the genetics of bardet-biedl syndrome. Mol. Syndromol. 2014, 5, 51–56. [Google Scholar] [CrossRef]
- Khan, S.A.; Muhammad, N.; Khan, M.A.; Kamal, A.; Rehman, Z.U.; Khan, S. Genetics of human Bardet-Biedl syndrome, an updates. Clin. Genet. 2016, 90, 3–15. [Google Scholar] [CrossRef]
- Kleinendorst, L.; Alsters, S.I.M.; Abawi, O.; Waisfisz, Q.; Boon, E.M.J.; van den Akker, E.L.T.; van Haelst, M.M. Second case of Bardet-Biedl syndrome caused by biallelic variants in IFT74. Eur. J. Hum. Genet. 2020, 28, 943–946. [Google Scholar] [CrossRef]
- Khan, A.O.; Decker, E.; Bachmann, N.; Bolz, H.J.; Bergmann, C. C8orf37 is mutated in Bardet-Biedl syndrome and constitutes a locus allelic to non-syndromic retinal dystrophies. Ophthalmic Genet. 2016, 37, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Abu Safieh, L.; Aldahmesh, M.A.; Shamseldin, H.; Hashem, M.; Shaheen, R.; Alkuraya, H.; Al Hazzaa, S.A.; Al-Rajhi, A.; Alkuraya, F.S. Clinical and molecular characterisation of Bardet-Biedl syndrome in consanguineous populations: The power of homozygosity mapping. J. Med. Genet. 2010, 47, 236–241. [Google Scholar] [CrossRef]
- Farag, T.I.; Teebi, A.S. High incidence of Bardet Biedl syndrome among the Bedouin. Clin. Genet. 1989, 36, 463–464. [Google Scholar] [CrossRef]
- Magliyah, M.S.; AlSulaiman, S.M.; Schatz, P.; Nowilaty, S.R. Evolution of macular hole in enhanced S-cone syndrome. Doc. Ophthalmol. 2021, 142, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.G.; Michaelides, M.; Luong, V.A.; Holder, G.E.; Bird, A.C.; Webster, A.R.; Moore, A.T.; Fitzke, F.W. Functional correlates of fundus autofluorescence abnormalities in patients with RPGR or RIMS1 mutations causing cone or cone rod dystrophy. Br. J. Ophthalmol. 2008, 92, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.G.; Michaelides, M.; Saihan, Z.; Bird, A.C.; Webster, A.R.; Moore, A.T.; Fitzke, F.W.; Holder, G.E. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc. Ophthalmol. 2008, 116, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Magliyah, M.S.; Geuer, S.; Alsalamah, A.K.; Lenzner, S.; Drasdo, M.; Schatz, P. Association of the Recurrent Rare Variant c.415T>C p.Phe139Leu in CLN5 with a Recessively Inherited Macular Dystrophy. JAMA Ophthalmol. 2021, 139, 339–343. [Google Scholar] [CrossRef] [PubMed]
- VarSome. The Human Genomics Community. Available online: https://varsome.com/ (accessed on 14 August 2021).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- gnomAD. Available online: https://gnomad.broadinstitute.org/ (accessed on 14 August 2021).
- ClinVar. Available online: https://www.ncbi.nlm.nih.gov/clinvar/ (accessed on 14 August 2021).
- An Open Source DNA Variation Database System. Available online: https://www.lovd.nl/ (accessed on 14 August 2021).
- Human Splicing Finder—Version 3.1. Available online: http://umd.be/Redirect.html (accessed on 14 August 2021).
- Desmet, F.O.; Hamroun, D.; Lalande, M.; Collod-Béroud, G.; Claustres, M.; Béroud, C. Human Splicing Finder: An online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, H.E.; Shaheen, R.; Ewida, N.; Bubshait, D.K.; Alkuraya, H.; Almardawi, E.; Howaidi, A.; Sabr, Y.; Abdalla, E.M.; Alfaifi, A.Y.; et al. The morbid genome of ciliopathies: An update. Genet. Med. 2020, 22, 1051–1060. [Google Scholar] [CrossRef]
- Abu-Safieh, L.; Al-Anazi, S.; Al-Abdi, L.; Hashem, M.; Alkuraya, H.; Alamr, M.; Sirelkhatim, M.O.; Al-Hassnan, Z.; Alkuraya, B.; Mohamed, J.Y.; et al. In search of triallelism in Bardet-Biedl syndrome. Eur. J. Hum. Genet. 2012, 20, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Abouelhoda, M.; Sobahy, T.; El-Kalioby, M.; Patel, N.; Shamseldin, H.; Monies, D.; Al-Tassan, N.; Ramzan, K.; Imtiaz, F.; Shaheen, R.; et al. Clinical genomics can facilitate countrywide estimation of autosomal recessive disease burden. Genet. Med. 2016, 18, 1244–1249. [Google Scholar] [CrossRef]
- Abualsaud, D.; Hashem, M.; AlHashem, A.; Alkuraya, F.S. Survey of disorders of sex development in a large cohort of patients with diverse Mendelian phenotypes. Am. J. Med. Genet. A 2021, 185, 2789–2800. [Google Scholar] [CrossRef]
- Scheidecker, S.; Hull, S.; Perdomo, Y.; Studer, F.; Pelletier, V.; Muller, J.; Stoetzel, C.; Schaefer, E.; Defoort-Dhellemmes, S.; Drumare, I.; et al. Predominantly Cone-System Dysfunction as Rare Form of Retinal Degeneration in Patients with Molecularly Confirmed Bardet-Biedl Syndrome. Am. J. Ophthalmol. 2015, 160, 364–372.e1. [Google Scholar] [CrossRef] [PubMed]
- Stoetzel, C.; Laurier, V.; Davis, E.E.; Muller, J.; Rix, S.; Badano, J.L.; Leitch, C.C.; Salem, N.; Chouery, E.; Corbani, S.; et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat. Genet. 2006, 38, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Katsanis, N.; Eichers, E.R.; Ansley, S.J.; Lewis, R.A.; Kayserili, H.; Hoskins, B.E.; Scambler, P.J.; Beales, P.L.; Lupski, J.R. BBS4 is a minor contributor to Bardet-Biedl syndrome and may also participate in triallelic inheritance. Am. J. Hum. Genet. 2002, 71, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamed, M.H.; van Lennep, C.; Hynes, A.M.; Chrystal, P.; Eley, L.; Al-Fadhly, F.; El Sayed, R.; Simms, R.J.; Meyer, B.; Sayer, J.A. Functional modelling of a novel mutation in BBS5. Cilia 2014, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Duncan, J.L.; Maranhao, B.; Kozak, I.; Branham, K.; Gabriel, L.; Lin, J.H.; Barteselli, G.; Navani, M.; Suk, J.; et al. Genetic analysis of 10 pedigrees with inherited retinal degeneration by exome sequencing and phenotype-genotype association. Physiol. Genom. 2017, 49, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, H.L.; Gudiseva, H.V.; Kishaba, K.T.; Suk, J.J.; Verma, R.; Tadimeti, K.; Thorson, J.A.; Ayyagari, R. A Report on Molecular Diagnostic Testing for Inherited Retinal Dystrophies by Targeted Genetic Analyses. Genet. Test. Mol. Biomark. 2017, 21, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Méjécase, C.; Kozak, I.; Moosajee, M. The genetic landscape of inherited eye disorders in 74 consecutive families from the United Arab Emirates. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Kerr, E.N.; Bhan, A.; Héon, E. Exploration of the cognitive, adaptive and behavioral functioning of patients affected with Bardet-Biedl syndrome. Clin. Genet. 2016, 89, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Jespersgaard, C.; Fang, M.; Bertelsen, M.; Dang, X.; Jensen, H.; Chen, Y.; Bech, N.; Dai, L.; Rosenberg, T.; Zhang, J.; et al. Molecular genetic analysis using targeted NGS analysis of 677 individuals with retinal dystrophy. Sci. Rep. 2019, 9, 1219. [Google Scholar] [CrossRef]
- Yates, C.L.; Monaghan, K.G.; Copenheaver, D.; Retterer, K.; Scuffins, J.; Kucera, C.R.; Friedman, B.; Richard, G.; Juusola, J. Whole-exome sequencing on deceased fetuses with ultrasound anomalies: Expanding our knowledge of genetic disease during fetal development. Genet. Med. 2017, 19, 1171–1178. [Google Scholar] [CrossRef]
- Shevach, E.; Ali, M.; Mizrahi-Meissonnier, L.; McKibbin, M.; El-Asrag, M.; Watson, C.M.; Inglehearn, C.F.; Ben-Yosef, T.; Blumenfeld, A.; Jalas, C.; et al. Association between missense mutations in the BBS2 gene and nonsyndromic retinitis pigmentosa. JAMA Ophthalmol. 2015, 133, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Deveault, C.; Billingsley, G.; Duncan, J.L.; Bin, J.; Theal, R.; Vincent, A.; Fieggen, K.J.; Gerth, C.; Noordeh, N.; Traboulsi, E.I.; et al. BBS genotype-phenotype assessment of a multiethnic patient cohort calls for a revision of the disease definition. Hum. Mutat. 2011, 32, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Stoetzel, C.; Vincent, M.C.; Leitch, C.C.; Laurier, V.; Danse, J.M.; Hellé, S.; Marion, V.; Bennouna-Greene, V.; Vicaire, S.; et al. Identification of 28 novel mutations in the Bardet-Biedl syndrome genes: The burden of private mutations in an extensively heterogeneous disease. Hum. Genet. 2010, 127, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Cuzcano, A.; Koenekoop, R.K.; Senechal, A.; De Baere, E.B.; de Ravel, T.; Banfi, S.; Kohl, S.; Ayuso, C.; Sharon, D.; Hoyng, C.B.; et al. BBS1 mutations in a wide spectrum of phenotypes ranging from nonsyndromic retinitis pigmentosa to Bardet-Biedl syndrome. Arch. Ophthalmol. 2012, 130, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Oke, S.; MacDonald, I.M. Validating Ellipsoid Zone Area Measurement with Multimodal Imaging in Choroideremia. Transl. Vis. Sci. Technol. 2021, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, P.R.; Baye, L.M.; Nishimura, D.Y.; Searby, C.C.; Bugge, K.; Yang, B.; Mullins, R.F.; Stone, E.M.; Sheffield, V.C.; Slusarski, D.C. Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet. 2010, 6, e1000884. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, P.R.; Aldahmesh, M.A.; Alkuraya, F.S.; Sheffield, V.C.; Slusarski, D.C. Functional analysis of BBS3 A89V that results in non-syndromic retinal degeneration. Hum. Mol. Genet. 2011, 20, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Riazuddin, S.A.; Iqbal, M.; Wang, Y.; Masuda, T.; Chen, Y.; Bowne, S.; Sullivan, L.S.; Waseem, N.H.; Bhattacharya, S.; Daiger, S.P.; et al. A splice-site mutation in a retina-specific exon of BBS8 causes nonsyndromic retinitis pigmentosa. Am. J. Hum. Genet. 2010, 86, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Castro-Sánchez, S.; Álvarez-Satta, M.; Cortón, M.; Guillén, E.; Ayuso, C.; Valverde, D. Exploring genotype-phenotype relationships in Bardet-Biedl syndrome families. J. Med. Genet. 2015, 52, 503–513. [Google Scholar] [CrossRef]
- Riise, R.; Andréasson, S.; Borgaström, M.K.; Wright, A.F.; Tommerup, N.; Rosenberg, T.; Tornqvist, K. Intrafamilial variation of the phenotype in Bardet-Biedl syndrome. Br. J. Ophthalmol. 1997, 81, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.P.; Carrigan, M.; Collins, K.; Dempsey, H.; Dockery, A.; Farrar, G.J.; Kenna, P.F. Intrafamilial Phenotype Variation Associated with BBS1 Met390Arg. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3257. [Google Scholar]
- Daniels, A.B.; Sandberg, M.A.; Chen, J.; Weigel-DiFranco, C.; Fielding Hejtmancic, J.; Berson, E.L. Genotype-phenotype correlations in Bardet-Biedl syndrome. Arch. Ophthalmol. 2012, 130, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Niederlova, V.; Modrak, M.; Tsyklauri, O.; Huranova, M.; Stepanek, O. Meta-analysis of genotype-phenotype associations in Bardet-Biedl syndrome uncovers differences among causative genes. Hum. Mutat. 2019, 40, 2068–2087. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, G.; Bin, J.; Fieggen, K.J.; Duncan, J.L.; Gerth, C.; Ogata, K.; Wodak, S.S.; Traboulsi, E.I.; Fishman, G.A.; Paterson, A.; et al. Mutations in chaperonin-like BBS genes are a major contributor to disease development in a multiethnic Bardet-Biedl syndrome patient population. J. Med. Genet. 2010, 47, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Mockel, A.; Perdomo, Y.; Stutzmann, F.; Letsch, J.; Marion, V.; Dollfus, H. Retinal dystrophy in Bardet-Biedl syndrome and related syndromic ciliopathies. Prog. Retin. Eye Res. 2011, 30, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Liew, G.; Michaelides, M.; Bunce, C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009-2010. BMJ Open 2014, 4, e004015. [Google Scholar] [CrossRef] [PubMed]
- Chandra, B.; Tung, M.L.; Hsu, Y.; Scheetz, T.; Sheffield, V.C. Retinal ciliopathies through the lens of Bardet-Biedl Syndrome: Past, present and future. Prog. Retin. Eye Res. 2022, 89, 101035. [Google Scholar] [CrossRef]
- May-Simera, H.L.; Wan, Q.; Jha, B.S.; Hartford, J.; Khristov, V.; Dejene, R.; Chang, J.; Patnaik, S.; Lu, Q.; Banerjee, P.; et al. Primary Cilium-Mediated Retinal Pigment Epithelium Maturation Is Disrupted in Ciliopathy Patient Cells. Cell Rep. 2018, 22, 189–205. [Google Scholar] [CrossRef]

| Patient | Age | Sex | Age of Onset of Visual Symptoms | Earliest Visual Symptoms | Systemic Features | Consanguinity |
|---|---|---|---|---|---|---|
| 1 | 23 | M | Early childhood | Nystagmus and poor night vision | Obesity and polydactyly | 1st cousins |
| 2A | 12 | F | Early childhood | Poor night vision | Obesity | No |
| 2B | 22 | F | Early childhood | Nystagmus and poor night vision | Obesity | No |
| 3A | 26 | M | Early childhood | Nystagmus and poor night vision | Obesity, polydactyly and cardiac disease | No |
| 3B | 28 | F | Early infancy | Nystagmus and poor night vision | Obesity and polydactyly | No |
| 4A | 19 | M | Early childhood | Poor day and night vision | Obesity and polydactyly | same tribe |
| 4B | 24 | F | Early childhood | Poor day and night vision | Obesity and polydactyly | same tribe |
| 5A | 13 | F | Early infancy | Nystagmus, poor night vision and poor navigation | Obesity and cognitive disability | 1st cousins |
| 5B | 4 | F | Early infancy | Nystagmus, poor night vision and poor navigation | Obesity and polydactyly | 1st cousins |
| 5C | 11 | M | Early infancy | Nystagmus and poor night vision | Obesity, polydactyly and hypothyroidism | 1st cousins |
| 6 | 33 | M | Early childhood | Poor night vision | None | 1st cousins |
| 7 | 15 | M | Early infancy | Nystagmus | Obesity, polydactyly renal impairment and cognitive disability | 1st cousins |
| 8 A | 13 | M | Early infancy | Poor night vision | Obesity, polydactyly and hypothyroidism | 1st cousins |
| 8B | 9 | F | Early childhood | Poor night vision | Obesity and brachydactyly | 1st cousins |
| 9 | 15 | M | Early childhood | Poor night vision | Obesity, polydactyly, renal impairment and hypogonadism | 1st cousins |
| 10 | 33 | M | Early childhood | Poor night vision and day vision | Obesity and polydactyly | 1st cousins |
| 11 | 28 | F | Adulthood | Poor day vision | None | 1st cousins |
| 12 | 22 | M | Early childhood | Poor night vision | None | 1st cousins |
| 13A | 15 | M | Early infancy | Poor navigation | Obesity and polydactyly | No |
| 13B | 16 | F | Early infancy | Poor navigation | Obesity and polydactyly | No |
| 13C | 11 | M | Early infancy | Nystagmus | Obesity and polydactyly | No |
| 14 | 10 | M | Early infancy | Nystagmus and poor night vision | Obesity and cognitive disability | 1st cousins |
| 15 | 33 | M | Early childhood | Poor day vision | Obesity and cognitive disability | 1st cousins |
| 16 | 21 | F | Early childhood | Poor day and night vision | Obesity, polydactyly and cognitive disability | 1st cousins |
| 17 | 28 | M | Early childhood | Poor day and night vision | Obesity and polydactyly | No |
| 18A | 35 | M | Early childhood | Poor day and night vision | Obesity and polydactyly | No |
| 18B | 30 | M | Early childhood | Poor day and night vision | Obesity | No |
| 19 | 20 | M | Early childhood | Poor navigation Poor day and night vision | Obesity and polydactyly | Same tribe |
| 20A | 31 | F | Early childhood | Poor night vision | Obesity, polydactyly, renal impairment, cognitive disability and splenomegaly | 1st cousins |
| 20B | 22 | F | Early infancy | Nystagmus, poor day and night vision | Obesity and renal impairment | 1st cousins |
| 21 | 34 | F | Early childhood | Poor day vision | Obesity and cognitive disability | No |
| 22 | 6 | M | Early infancy | Nystagmus | Obesity | 1st cousins |
| 23 | 34 | F | Adulthood | Poor night vision | Obesity | 2nd cousins |
| 24 | 17 | M | Early childhood | Nystagmus, poor day and night vision | Obesity, polydactyly, cognitive disability and hypogonadism | 1st cousins |
| 25 | 15 | F | Early childhood | Nystagmus, poor day and night vision | None | 1st cousins |
| 26 | 19 | F | Early childhood | Poor night vision | Obesity | 1st cousins |
| 27A | 30 | F | Early childhood | Poor day and night vision | Obesity, polydactyly and benign lung tumor | 1st cousins |
| 27B | 24 | M | Early childhood | Poor day and night vision | Obesity, short stature | 1st cousins |
| 28A | 28 | F | Early childhood | Nystagmus, poor day and night vision | Obesity, polydactyly and cognitive disability | Same tribe |
| 28B | 19 | M | Early childhood | Nystagmus, poor day and night vision | Obesity, cognitive disability and hypothyroidism | Same tribe |
| 28C | 34 | M | Early childhood | Poor day and night vision | Obesity, polydactyly and syndactyly cognitive disability | Same tribe |
| 28D | 30 | F | Early childhood | Poor day and night vision | Obesity, diabetes, cognitive disability, renal impairment and hypothyroidism | Same tribe |
| 29 | 17 | M | Early childhood | Poor day and night vision | Obesity and polydactyly | 2nd cousins |
| 30A | 13 | M | Early childhood | Nystagmus, poor night vision | Obesity, polydactyly, syndactyly, cognitive disability and delay speech | 1st cousins |
| 30B | 8 | M | Early childhood | Esotropia and poor night vision | Obesity, polydactyly, and cognitive disability | 1st cousins |
| 31 | 10 | M | Early childhood | Exotropia, nystagmus and poor day and night vision | Obesity, cognitive disability and hypogonadism | 1st cousins |
| Patient | Age | VA at Presentation (Age) | VA at Last Visit (Age) | Fundus Images | FAF Finding | Phenotype | OCT Finding | ERG |
|---|---|---|---|---|---|---|---|---|
| 1 | 23 | 20/300 (14Y) | HM OU (21Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and macular atrophy | Patches of decreased AF around the arcades with patchy loss of AF at the macula | CRD | Partial disorganization of retinal lamination with loss of EZ. | NA |
| 2A | 12 | 20/100 and 20/400 (5 Y) | 20/300 and 20/400 (13 Y) | Retinal atrophy, vascular attenuation and macular pigment alteration. | Patches of decreased AF at the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 2B | 23 | LP OU (23Y) | LP OU (23Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and macular atrophy. | Patches of decreased AF of the retina with patchy loss of AF at the macula. | CRD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 3A | 26 | 1/200 OU (19 Y) | 1/200 OU (25Y) | Vascular attenuation midperipheral bone spicules and macular atrophy. | Patches of decreased AF at the midperipheral retina with patchy loss of AF at the macula. | Generalized photoreceptor involvement | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 3B | 28 | CF OU (14 Y) | HM OU (25) | Vascular attenuation, midperipheral bone spicules and macular pigment alteration. | Patches of decreased AF at the midperiphery with a central patch of increased AF at the macular surrounded by an annulus of decreased AF. | Generalized photoreceptor involvement | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 4A | 19 | CF OU (12 Y) | CF OU (12Y) | Retinal atrophy and vascular attenuation. | NA | Not classified | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 4B | 24 | HM OD and 2/200 (18 Y) | HM OD and 2/200 (18 Y) | Retinal atrophy, vascular attenuation and macular atrophy. | NA | Not classified | NA | UD |
| 5A | 13 | 20/100 OU (9 Y) | 20/100 and 20/60 (13 Y) | Retinal atrophy and vascular attenuation. | Patches of decreased AF with perifoveal annulus of increased AF. | RCD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 5B | 4 | F and F (1Y) | F and F (4 Y) | NA | NA | Not classified | NA | Reduced photonic and scotopic responses (Figure S5) |
| 5C | 11 | 20/100 and 20/200 (9Y) | 20/100 and 20/200 (11 Y) | Retinal atrophy and vascular attenuation. | Patches of decreased AF at the midperiphery with perifoveal annulus of increased AF. | RCD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 6 | 33 | LP OU (27 Y) | LP OU (34 Y) | Vascular attenuation, Midperipheral bone spicules, and macular atrophy. | Patches of decreased AF in the midperiphery with patchy loss of AF at the macula. | Generalized photoreceptor involvement. | Partial disorganization of retinal lamination, with loss of EZ. | UD |
| 7 | 15 | HM/LP (12Y) | HM/LP (16 Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and macular atrophy. | Patches of decreased AF in the midperiphery with patchy loss of AF at the macula. | Generalized photoreceptor involvement. | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 8A | 13 | F and F (8 Y) | 20/100 and 20/80 (14Y) | Retinal atrophy, vascular attenuation and macular pigment alteration. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Unremarkable lamination with disrupted EZ. | NA |
| 8B | 9 | F and F (4 Y) | 20/80 and 20/100 (9 Y) | Retinal atrophy, vascular attenuation and bull’s eye maculopathy. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Unremarkable lamination with disrupted EZ. | UD |
| 9 | 15 | 20/100 OU (10Y) | 20/100 OU (16Y) | Retinal atrophy, vascular attenuation, and bull’s eye maculopathy. | Patches of decreased AF at the midperiphery and around the arcades with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 10 | 33 | HM OU (30 Y) | HM OU (34Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and macular atrophy. | Patches of decreased AF at the midperiphery and around the arcades with patchy loss of AF at the macula. | Generalized photoreceptor involvement. | Severe disorganization of retinal lamination with loss of EZ. | NA |
| 11 | 28 | 2/200 (23Y) | 2/200 (29Y) | Retinal atrophy, vascular attenuation, Scanty midperipheral bone spicules, scanty nummular pigmentations and bull’s eye maculopathy. | Patches and nummular dots of decreased AF at the midperiphery and around the arcades with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 12 | 21 FU 6 Y | 20/100 and 20/125 (16 Y) | 20/400 OU (22 Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and bull’s eye maculopathy. | Patches of decreased AF at the midperiphery and around the arcades with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 13A | 15 FU 3 Y | 20/70 OU (12 Y) | 20/200 and 20/100 (15 Y) | Retinal atrophy | Perifoveal annulus of increased AF | RCD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 13B | 16 | 2/200 (13 Y) | 3/200 and 20/400 (16 Y) | Retinal atrophy, vascular attenuation, and bull’s eye maculopathy. | A central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with disrupted EZ. | Reduced scotopic response and unrecordable photopic response. (Figure S5) |
| 13C | 10 | CF OU (6 Y) | CF OU (10 Y) | Retinal atrophy, vascular attenuation, and macular pigment alteration. | A central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 14 | 10 | F and F (4 Y) | 20/300 and 5/200 (11 Y) | Retinal atrophy, vascular and macular pigment alteration. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 15 | 33 | HM and LP (28 Y) | HM and LP (34 Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and bull’s eye maculopathy. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Severe disorganization of retinal lamination, with loss of EZ. | NA |
| 16 | 21 | LP OU (17 Y) | LP OU (22Y) | Retinal atrophy, vascular attenuation, Scanty midperipheral bone spicules and bull’s eye maculopathy. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 17 | 28 | 1/200 and 20/300 (23Y) | 1/200 and 20/300 (29 Y) | Retinal atrophy, vascular attenuation, Scanty midperipheral bone spicules and bull’s eye maculopathy. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 18A | 35 | HM OU (29 Y) | LP OU (35 Y) | Retinal atrophy, vascular attenuation, Scanty midperipheral bone spicule and macular pigment alteration. | Patches of decreased AF at the midperiphery and around the arcades with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 18B | 30 | 20/400 and 20/300 (23 Y) | HM and 20/300 (30 Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules, scanty nummular pigmentations and macular pigment alteration. | Patches and nummular dots of decreased AF at the midperiphery and around the arcades with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 19 | 20 | 20/300 and 20/100 (18 Y) | 20/100 and 20/100 (20 Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and macular pigment alteration. | Midperipheral hypo autofluorescence patches, a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 20A | 31 | HM OU (27 Y) | HM OU (30 Y) | Retinal atrophy, vascular attenuation, and scanty midperipheral bone spicules. | Patches of decreased AF in the midperiphery with we could not assess the macular AF features due cataract. | CRD | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 20B | 22 | LP OU (19 Y) | LP OU (22 Y) | Retinal atrophy, vascular attenuation and macular pigment alteration. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 21 | 34 | LP OU (32 Y) | LP OU (34 Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and bull’s eye maculopathy. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Severe disorganization of retinal lamination with disrupted EZ. | NA |
| 22 | 6 | F andF (4 Y) | F andF (6Y) | Retinal atrophy and vascular attenuation and macular pigment alteration. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 23 | 34 | 20/60 and 4/200 (23 Y) | 20/300 OU (33 y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and macular atrophy. | Patches of decreased AF in the midperiphery with a patchy loss of AF at the macula. | CRD | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 24 | 17 | CF OU (14 Y) | CF OU (18 Y) | Retinal atrophy and bull’s eye maculopathy. | A central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with disrupted EZ. | UD |
| 25 | 15 | 6/200 and 20/100 (7 Y) | 20/300 AND 20/400 (14 Y) | Retinal atrophy, vascular attenuation, and bull’s eye maculopathy. | A central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Severe disorganization of retinal lamination, with loss of EZ. | UD |
| 26 | 20 | 20/70 and 20/60 (12 Y) | 20/80 and 30/100 (20 Y) | Retinal atrophy, vascular attenuation, and macular pigment alteration. | Patch of increased AF at the macula. | CRD | Severe disorganization of retinal lamination with disrupted EZ. | NA |
| 27A | 30 | HM OU (24 Y) | LP OU (28 Y) | Retinal atrophy, vascular attenuation, midperipheral bone spicules and macular atrophy. | Patches of decreased AF in the midperiphery with a patchy loss of AF at the macula. | Not classified | NA | NA |
| 27B | 18 | LP OU (18 Y) | LP OU (18 Y) | Vascular attenuation, midperipheral bone spicules and macular atrophy. | Patches of decreased AF in the midperiphery with a patchy loss of AF at the macula. | Not classified | NA | NA |
| 28A | 28 | 20/300 OU (20 Y) | HM OU (28 Y) | vascular attenuation, midperipheral bone spicules and macular pigment alteration. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 28B | 19 | HM (6 Y) | HM OU (18 Y) | Coats’-like picture, retinal atrophy, vascular attenuation, scanty midperipheral bone spicules, macular atrophy and superior temporal peripheral cryotherapy scars. | Patches of decreased AF in the midperiphery with a patchy loss of AF at the macula. | CRD | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 28C | 34 | HM OU (29 Y) | LP OU (31 Y) | Vascular attenuation, midperipheral bone spicules, scanty nummular pigmentations, laser scars around the arcades and bull’s eye maculopathy. | Patches and nummular dots of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | CRD | Partial disorganization of retinal lamination with loss of EZ. | NA |
| 28D | 30 | LP OU (22 Y) | LP OU (30 Y) | Vascular attenuation, midperipheral bone spicules, scanty nummular pigmentations and macular atrophy, ARGUS II Implant OD. | Patches and nummular dots of decreased AF in the midperiphery and around the arcades with a patchy loss of AF at the macula. | CRD | Severe disorganization of retinal lamination with loss of EZ. | UD |
| 29 | 17 | 20/160 OU (14 Y) | 20/300 OU (15 Y) | Retinal atrophy, vascular attenuation, and macular atrophy. | Patches of decreased AF in the midperiphery with a central patch of increased AF at the macula surrounded by an annulus of decreased AF. | Not classified | NA | NA |
| 30A | 13 | 20/100 And 20/160 (8 Y) | 20/100 20/160 (13 Y) | Retinal atrophy, vascular attenuation, scanty midperipheral bone spicules, and macular atrophy, left round pigmented lesion surrounded by lacunae (CHRPE). | Patches of decreased AF at the midperiphery and around the arcades with a central patch of increased AF at the macula surrounded by an annulus of decreased AF, left peripheral round lesion with decreased AF. | CRD. | Partial disorganization of retinal lamination with loss of EZ. | UD |
| 30B | 8 | F and F (3 Y) | 20/200 20/300 (8 Y) | Retinal atrophy, vascular attenuation, hypopigmented and macular pigment alteration. | A patch of decreased AF at the macula. | RCD | Unremarkable lamination with disrupted EZ. | UD |
| 31 | 10 | F and F (8 Y) | 20/400 OU (10 Y) | Retinal atrophy, vascular attenuation, and macular pigment alteration. | A patch of decreased AF at the macula. | Generalized photoreceptor involvement. | Unremarkable lamination with disrupted EZ. | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milibari, D.; Nowilaty, S.R.; Ba-Abbad, R. The Clinical and Mutational Spectrum of Bardet–Biedl Syndrome in Saudi Arabia. Genes 2024, 15, 762. https://doi.org/10.3390/genes15060762
Milibari D, Nowilaty SR, Ba-Abbad R. The Clinical and Mutational Spectrum of Bardet–Biedl Syndrome in Saudi Arabia. Genes. 2024; 15(6):762. https://doi.org/10.3390/genes15060762
Chicago/Turabian StyleMilibari, Doaa, Sawsan R. Nowilaty, and Rola Ba-Abbad. 2024. "The Clinical and Mutational Spectrum of Bardet–Biedl Syndrome in Saudi Arabia" Genes 15, no. 6: 762. https://doi.org/10.3390/genes15060762
APA StyleMilibari, D., Nowilaty, S. R., & Ba-Abbad, R. (2024). The Clinical and Mutational Spectrum of Bardet–Biedl Syndrome in Saudi Arabia. Genes, 15(6), 762. https://doi.org/10.3390/genes15060762









