Enhanced Learning and Memory in Patients with CRB1 Retinopathy
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Participants
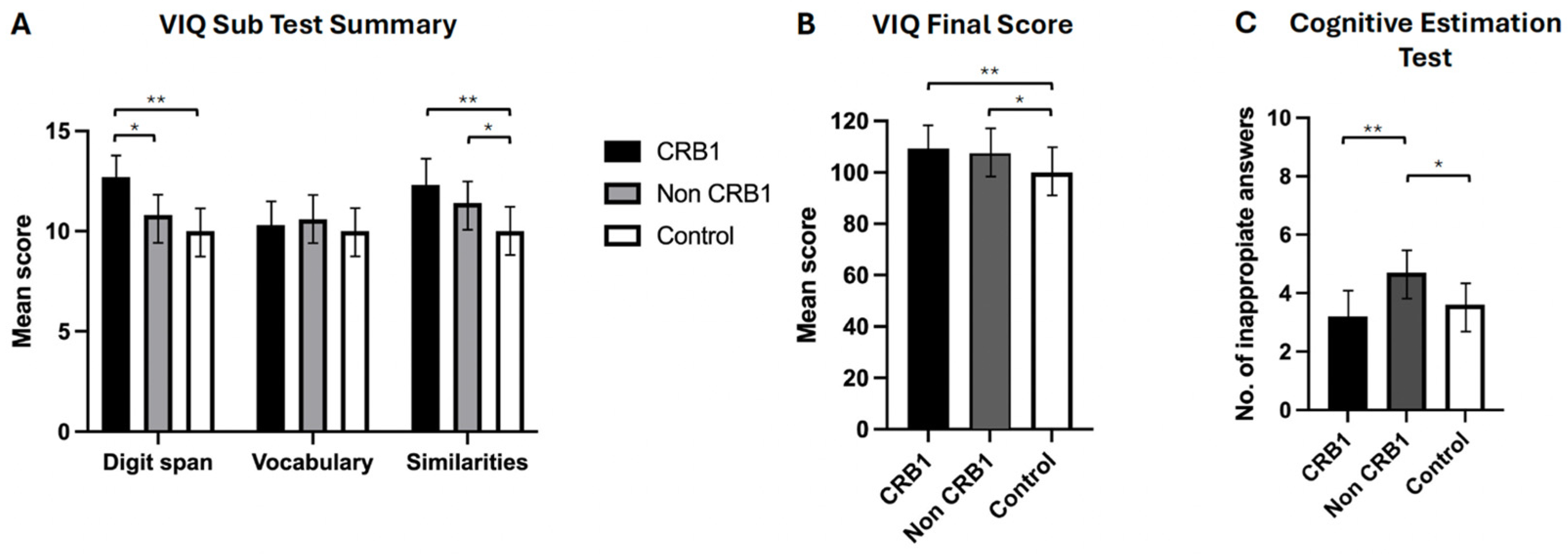
Subjects with CRB1 Retinopathy Have Enhanced Cognitive Function
- No significant differences were observed between the two groups of retinopathy subjects in the story recall immediate (p = 0.111) and delayed (p = 0.057) memory tests [Figure 2A] or in the verbal fluency phonemic subtest (p = 0.363) [Figure 2B]. CRB1 retinopathy subjects scored significantly higher than non-CRB1 retinopathy subjects in the list learning tasks of immediate (p = 0.001) and delayed memory (p = 0.007) [Figure 2C], in the verbal fluency semantic subtest (p = 0.017) [Figure 2B], and in the Hayling test of mental processing speed (p = 0.068) [Figure 2D]. Additionally, CRB1 retinopathy subjects scored higher in the cognitive estimation test of higher executive function (p = 0.020) [Figure 3C] and in the verbal IQ digit span subtest compared to the non-CRB1 group (p = 0.037) [Figure 3A]. No significant differences were found in overall verbal IQ (p = 0.142) [Figure 3B] or in the verbal IQ vocabulary (p = 0.436) and similarities (p = 0.208) subtests [Figure 3A].
- CRB1 retinopathy subjects scored significantly higher than the normal population in both story recall (p = 0.0001) memory tests [Figure 2A], in immediate (p = 0.0001) and delayed (p = 0.0004) list learning tests [Figure 2C], both verbal fluency tests (p = 0.0001) [Figure 3B], in the digit span (p = 0.0003) verbal IQ subtest which assesses immediate short term memory recall, verbal IQ similarities subtest (p = 0.002), and overall verbal IQ tests (p = 0.001) [Figure 3A,B]. There were no significant differences in the Hayling test of mental processing speed (p = 0.349) [Figure 2D], in the vocabulary verbal IQ subtest (p = 0.648) [Figure 3A], and in cognitive estimation tests of higher executive function (p = 0.403) [Figure 3C].
- Non-CRB1 retinopathy subjects scored significantly higher than the normal population in story recall (p = 0.0001) [Figure 2A]. No significant differences were seen in the list learning immediate (p = 0.6603) and delayed (p = 0.800) memory tests [Figure 2C] and in the verbal IQ digit span (p = 0.060) and vocabulary (p = 0.366) subtests [Figure 3A]. Additionally, non-CRB1 retinopathy subjects scored significantly higher than the normal population in both verbal fluency tests (p = 0.0001) [Figure 3B], in the similarities verbal IQ subtest (p = 0.001) [Figure 3A], and in the overall verbal IQ tests (p = 0.001) [Figure 3B]. They, however, scored significantly worse than the normal population in the Hayling test of mental processing speed (p = 0.0001) [Figure 2D] and cognitive estimation tests of higher executive function (p = 0.004) [Figure 3C].
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehrenberg, M.; Pierce, E.A.; Cox, G.F.; Fulton, A.B. CRB1: One gene, many phenotypes. Semin. Ophthalmol. 2013, 28, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, N.; Moore, A.T.; Weleber, R.G.; Michaelides, M. Leber congenital amaurosis/early-onset severe retinal dystrophy: Clinical features, molecular genetics and therapeutic interventions. Br. J. Ophthalmol. 2017, 101, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.D.; Georgiou, M.; Alswaiti, Y.; Kabbani, J.; Fujinami, K.; Fujinami-Yokokawa, Y.; Khoda, S.; Mahroo, O.A.; Robson, A.G.; Webster, A.R.; et al. CRB1-Associated Retinal Dystrophies: Genetics, Clinical Characteristics, and Natural History. Am. J. Ophthalmol. 2023, 246, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.T.A.; Talib, M.; van Schooneveld, M.J.; Wijnhold, J.; van Genderen, M.M.; Florijn, R.J.; ten Brink, J.B.; Cremers, F.P.M.; Meester-Smoor, M.A.; Klaver, C.C.W.; et al. A two-year prospective natural history study in patients with CRB1-associated retinal dystrophies: Establishing clinical endpoints for future gene therapy trials. In Acta Ophthalmologica; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Owen, N.; Toms, M.; Tian, Y.; Toualbi, L.; Richardson, R.; Young, R.; Tracey-White, D.; Dhami, P.; Beck, S.; Moosajee, M. Loss of the crumbs cell polarity complex disrupts epigenetic transcriptional control and cell cycle progression in the developing retina. J. Pathol. 2023, 259, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.-T.; Talib, M.; van Schooneveld, M.J.; Wijnholds, J.; van Genderen, M.M.; Schalij-Delfos, N.E.; Klaver, C.C.; Talsma, H.E.; Fiocco, M.; Florijn, R.J.; et al. CRB1-Associated Retinal Dystrophies: A Prospective Natural History Study in Anticipation of Future Clinical Trials. Arch. Ophthalmol. 2022, 234, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Talib, M.; Van Cauwenbergh, C.; De Zaeytijd, J.; Van Wynsberghe, D.; De Baere, E.; Boon, C.J.F.; Leroy, B.P. CRB1-associated retinal dystrophies in a Belgian cohort: Genetic characteristics and long-term clinical follow-up. Br. J. Ophthalmol. 2022, 106, 696–704. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, A.I.; Ghiani, M.; de Kok, Y.J.; Wijnholds, J.; Ballabio, A.; Cremers, F.P.; Broccoli, V. Isolation of Crb1, a mouse homolo Drosophila crumbs, and analysis of its expression pattern in eye and Brain. Mech. Dev. 2002, 110, 203–207. [Google Scholar] [CrossRef]
- Weissman, I.L. Stem Cells: Units of development, units of regeneration, and units in evolution. Cell 2000, 100, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. The neuropsychology of human memory. Annu. Rev. Neurosci. 1982, 5, 241–273. [Google Scholar] [CrossRef]
- Riedel, G.; Micheau, J.; Lam, A.G.M.; Roloff, E.V.L.; Martin, S.J.; Bridge, H.; Hoz, L.D.; Poeschel, B.; McCulloch, J.; Morris, R.G. Reversible neural activation reveals hippocampal participation in several memory processes. Nat. Neurosc. 1999, 2, 898–906. [Google Scholar] [CrossRef]
- Dong, H.-W.; Swanson, L.W.; Chen, L.; Fanselow, M.S.; Toga, A.W. Genomic–anatomic evidence for distinct functional domains in hippocampal field CA1. Proc. Natl. Acad. Sci. USA 2009, 106, 11794–11799. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.A. Highly Expressed Genes within Hippocampal Sector CA1: Implications for the Physiology of Memory. Neurol. Int. 2014, 6, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.S.; Karagiannis, G.S.; Constantinidou, A. Midkine (MDK) growth factor: A key player in cancer progression and a promising therapeutic target. Oncogene 2020, 39, 2040–2054. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, E.; Kadomatsu, K.; Yuasa, S.; Muramatsu, H.; Mamiya, T.; Nabeshima, T.; Fan, Q.; Ishiguro, K.; Igakura, T.; Matsubara, S.; et al. Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells 1998, 3, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, E.; Beales, P.L. Bardet-Biedl Syndrome. Eur. J. Hum. Genet. 2013, 21, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xie, Y.A.; Abouzeid, H.; Gordon, C.T.; Fiorentino, A.; Sun, Z.; Lehman, A.; Osman, I.S.; Dharmat, R.; Riveiro-Alvarez, R.; et al. Mutations in the Spliceosome Component CWC27 Cause Retinal Degeneration with or without Additional Developmental Anomalies. Am. J. Hum. Genet. 2017, 100, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Fasham, J.; Arno, G.; Lin, S.; Xu, M.; Carss, K.J.; Hull, S.; Lane, A.; Robson, A.G.; Wenger, O.; Self, J.E.; et al. Delineating the Expanding Phenotype Associated with SCAPER Gene Mutation. Am. J. Med. Genet. Part A 2019, 179, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Sisodiya, S.M.; Thompson, P.J.; Need, A.; Harris, S.E.; Weale, M.E.; Wilkie, S.E.; Michaelides, M.; Free, S.L.; Walley, N.; Gumbs, C.; et al. Genetic enhancement of cognition in a kindred with cone-rod dystrophy due to RIMS1 mutation. J. Med. Genet. 2007, 44, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gutierrez, M.P.; Schiff, E.R.; Wright, G.; Waseem, N.; Mahroo, O.A.; Michaelides, M.; Moore, A.T.; Webster, A.R.; Arno, G.; Genomics England Research Consortium. Dominant Cone Rod Dystrophy, Previously Assigned to a Missense Variant in RIMS1, Is Fully Explained by Co- Inheritance of a Dominant Allele of PROM1. Invest. Ophthalmol. Vis. Sci. 2022, 63, 14. [Google Scholar] [CrossRef]
- Kempermann, G.; Chesler, E.J.; Lu, L.; Williams, R.W.; Gage, F.H. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 780–785. [Google Scholar] [CrossRef]
- Arrigoni, F.I.; Matarin, M.; Thompson, P.J.; Michaelides, M.; McClements, M.E.; Redmond, E.; Clarke, L.; Ellins, E.; Mohamed, S.; Pavord, I.; et al. Extended extraocular phenotype of PROM1 mutation in kindreds with known autosomal dominant macular dystrophy. Eur. J. Hum. Genet. 2011, 19, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.J.; Mitchell, T.N.; Free, S.L.; Williamson, K.A.; Hanson, I.M.; van Heyningen, V.; Moore, A.T.; Sisodiya, S.M. Cognitive functioning in humans with mutations of the PAX6 gene. Neurology 2004, 62, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medarde, A.; Barhoum, R.; Riquelme, R.; Porteros, A.; Núñez, A.; De Luis, A.; Rivas, J.D.L.; De La Villa, P.; Varela-Nieto, I.; Santos, E. RasGRF1 disruption causes retinal photoreception defects and associated transcriptomic alterations. J. Neurochem. 2009, 110, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. WAIS-R Administration and Scoring Manual; New York Psychological corporation: New York, NY, USA, 1981. [Google Scholar]
- Wechsler, D. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale, 3rd ed.; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W. Neuropsychological Assessment, 4th ed.; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Hull, T.; Mason, H. Performance of blind children on digit-span tests. J. Vis. Impair. Blind. 1995, 89, 166–169. [Google Scholar] [CrossRef]
- Pigeon, C.; Marin-Lamellet, C. Evaluation of the attentional capacities and working memory of early and late blind persons. Acta Psychol. 2015, 155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bliss, I.; Kujala, T.; Hämäläinen, H. Comparison of blind and sighted participants’ performance in a letter recognition working memory task. Cogn. Brain Res. 2004, 18, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, A.; Lam, J.S.; Proulx, M.J. Congenital blindness improves semantic and episodic memory. Behav. Brain Res. 2013, 244, 162–165. [Google Scholar] [CrossRef]
- Röder, B.; Rösler, F. Memory for environmental sounds in sighted, congenitally blind and late blind adults: Evidence for cross-modal compensation. Int. J. Psychophysiol. 2003, 50, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Withagen, A.; Kappers, A.M.; Vervloed, M.P.; Knoors, H.; Verhoeven, L. Short term memory and working memory in blind versus sighted children. Res. Dev. Disabil. 2013, 34, 2161–2172. [Google Scholar] [CrossRef]
- Keller, J.N. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res. Rev. 2006, 5, 1–13. [Google Scholar] [CrossRef]
- Wang, H.; Ferguson, G.D.; Pineda, V.V.; Cundiff, P.E.; Storm, D.R. Overexpression of type-1 adenylyl cyclase in mouse Forebrain enhances recognition memory and LTP. Nat. Neurosci. 2004, 7, 635–642. [Google Scholar] [CrossRef]
- Zeng, H.; Chattarji, S.; Barbarosie, M.; Rondi-Reig, L.; Philpot, B.D.; Miyakawa, T.; Bear, M.F.; Tonegawa, S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell 2001, 107, 617–629. [Google Scholar] [CrossRef]
- Balschun, D.; Wolfer, D.P.; Bertocchini, F.; Barone, V.; Conti, A.; Zuschratter, W.; Missiaen, L.; Lipp, H.; Frey, J.U.; Sorrentino, V. Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J. 1999, 18, 5264–5273. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Silva, A.J. The molecular and cellular biology of enhanced cognition. Nat. Rev. Neurosci. 2009, 10, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Malleret, G.; Hen, R.; Guillou, J.-L.; Segu, L.; Buhot, M.-C. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the morris water maze. J. Neurosci. 1999, 19, 6157–6168. [Google Scholar] [CrossRef]
- Tang, Y.-P.; Shimizu, E.; Dube, G.R.; Rampon, C.; Kerchner, G.A.; Zhuo, M.; Liu, G.; Tsien, J.Z. Genetic enhancement of learning and memory in mice. Nature 1999, 401, 63–69. [Google Scholar] [CrossRef]
- Cui, Y.; Jin, J.; Zhang, X.; Xu, H.; Yang, L.; Du, D.; Zeng, Q.; Tsien, J.Z.; Yu, H.; Cao, X. Forebrain NR2B overexpression facilitating the prefrontal cortex long-term potentiation and enhancing working memory function in mice. PLoS ONE 2011, 6, e20312. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cui, Z.; Feng, R.; Tang, Y.; Qin, Z.; Mei, B.; Tsien, J.Z. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur. J. Neurosci. 2007, 25, 1815–1822. [Google Scholar] [CrossRef]
- Tan, D.P.; Liu, Q.-Y.; Koshiya, N.; Gu, H.; Alkon, D. Enhancement of long-term memory retention and short-term synaptic plasticity in cbl-b null mice. Proc. Natl. Acad. Sci. USA 2006, 103, 5125–5130. [Google Scholar] [CrossRef]
- Nakamura, K.; Manabe, T.; Watanabe, M.; Mamiya, T.; Ichikawa, R.; Kiyama, Y.; Sanbo, M.; Yagi, T.; Inoue, Y.; Nabeshima, T.; et al. Enhancement of hippocampal LTP, reference memory and sensorimotor gating in mutant mice lacking a telencephalon-specific cell adhesion molecule. Eur. J. Neurosci. 2001, 13, 179–189. [Google Scholar] [CrossRef]
- Suzuki, A.; Fukushima, H.; Mukawa, T.; Toyoda, H.; Wu, L.-J.; Zhao, M.-G.; Xu, H.; Shang, Y.; Endoh, K.; Iwamoto, T.; et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J. Neurosci. 2011, 31, 8786–8802. [Google Scholar] [CrossRef] [PubMed]
- Scoville, W.B.; Milner, B. Loss of recent memory after Bilateral Hippocampal lesions. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Dolón, J.F.; Paniagua, A.E.; Valle, V.; Segurado, A.; Arévalo, R.; Velasco, A.; Lillo, C. Expression and localization of the polarity protein CRB2 in adult mouse brain: A comparison with the CRB1rd8 mutant mouse model. Sci. Rep. 2018, 8, 11652. [Google Scholar] [CrossRef] [PubMed]
- Collignon, O.; Charbonneau, G.; Lassonde, M.; Lepore, F. Early visual deprivation alters multisensory processing in peripersonal space. Neuropsychologia 2009, 47, 3236–3243. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.A.; Lomber, S.G. Somatosensory and visual crossmodal plasticity in the anterior auditory field of early-deaf cats. Hear. Res. 2011, 280, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Renier, L.; De Volder, A.G.; Rauschecker, J.P. Cortical plasticity and preserved function in early blindness. Neurosci. Biobehav. Rev. 2014, 41, 53–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lura, Y.; Udo, H. Behavioral analyses of visually impaired Crx knockout mice revealed sensory compensation in exploratory activities on elevated platforms. Behav. Brain Res. 2014, 258, 1–7. [Google Scholar]
- Tillman, M.H.; Bashaw, W.L. Multivariate analysis of the WISC scales for blind and sighted children. Psychol. Rep. 1968, 23, 523–526. [Google Scholar] [CrossRef]
- Pozar, L. Effects of long-term sensory deprivation on recall of verbal material. Stud. Psychol. 1982, 24, 311. [Google Scholar]
- Rauschecker, J.P. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995, 18, 36–43. [Google Scholar] [CrossRef]
- Lazzouni, L.; Lepore, F. Compensatory plasticity: Time matters. Front. Hum. Neurosci. 2014, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Gougoux, F.; Zatorre, R.J.; Lassonde, M.; Voss, P.; Lepore, F. A functional neuroimaging study of sound localization: Visual cortex activity predicts performance in early-blind individuals. PLOS Biol. 2005, 3, e27. [Google Scholar] [CrossRef] [PubMed]
- Burton, H.; Sinclair, R.J.; Agato, A. Recognition memory for Braille or spoken words: An fMRI study in early blind. Brain Res. 2012, 1438, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, G.V.; Bauer, C.M.; Merabet, L.B. Using structural and functional brain imaging to uncover how the brain adapts to blindness. Ann. Neurosci. Psychol. 2015, 2, 7. [Google Scholar] [CrossRef]
- Amedi, A.; Raz, N.; Pianka, P.; Malach, R.; Zohary, E. Early ‘visual’ cortex activation correlates with superior verbal memory performance in the blind. Nat. Neurosci. 2003, 6, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Davey, A.; Elias, M.F.; Siegler, I.C.; Lele, U.; Martin, P.; Johnson, M.A.; Hausman, D.B.; Poon, L.W. Cognitive function, physical performance, health, and disease: Norms from the Georgia Centenarian Study. Exp. Aging Res. 2010, 36, 394–425. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.K.; Bobilev, A.M.; Branch, A.; Lauderdale, J.D. Structural and functional consequences of PAX6 mutations in the brain: Implications for aniridia. Brain Res. 2021, 1756, 147283. [Google Scholar] [CrossRef]
- Price, J.R.; Mount, G.G.; Coles, E.A. Evaluating the visually impaired: Neuropsychological technique. J. Vis. Impair. Blind. 1987, 48, 20–30. [Google Scholar] [CrossRef]
- Coughlan, A.K.; Hollows, S.E. The Adult Memory and Information Processing Battery (AMIPB) Test Manual; Publication A. K. Coughlan, St James University Hospital: Leeds, UK, 1986. [Google Scholar]
- Spreen, O.; Strauss, E. A compendium of Neuropsychological Tests; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Burgess, P.W.; Shallice, T. The Hayling Island and Brixton Test Manual; Thames Valley Test Co.: Bury St Edmunds, UK, 1997. [Google Scholar]
- Shallice, T.; Evans, M.E. The involvement of the frontal lobes in cognitive estimation. Cortex 1978, 14, 294–303. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, G.A.; Rodriguez-Martinez, A.C.; Conn, H.; Matarin, M.; Thompson, P.; Moore, A.T.; Ba-Abbad, R.; Webster, A.R.; Moosajee, M. Enhanced Learning and Memory in Patients with CRB1 Retinopathy. Genes 2024, 15, 660. https://doi.org/10.3390/genes15060660
Wright GA, Rodriguez-Martinez AC, Conn H, Matarin M, Thompson P, Moore AT, Ba-Abbad R, Webster AR, Moosajee M. Enhanced Learning and Memory in Patients with CRB1 Retinopathy. Genes. 2024; 15(6):660. https://doi.org/10.3390/genes15060660
Chicago/Turabian StyleWright, Genevieve A., Ana Catalina Rodriguez-Martinez, Hanne Conn, Mar Matarin, Pamela Thompson, Anthony T. Moore, Rola Ba-Abbad, Andrew R. Webster, and Mariya Moosajee. 2024. "Enhanced Learning and Memory in Patients with CRB1 Retinopathy" Genes 15, no. 6: 660. https://doi.org/10.3390/genes15060660
APA StyleWright, G. A., Rodriguez-Martinez, A. C., Conn, H., Matarin, M., Thompson, P., Moore, A. T., Ba-Abbad, R., Webster, A. R., & Moosajee, M. (2024). Enhanced Learning and Memory in Patients with CRB1 Retinopathy. Genes, 15(6), 660. https://doi.org/10.3390/genes15060660






