Are the Head and Tail Domains of Intermediate Filaments Really Unstructured Regions?
Abstract
1. Introduction

1.1. The Role of Head and Tail Domains in IF Assembly
1.2. Structural Data
1.3. Interactions
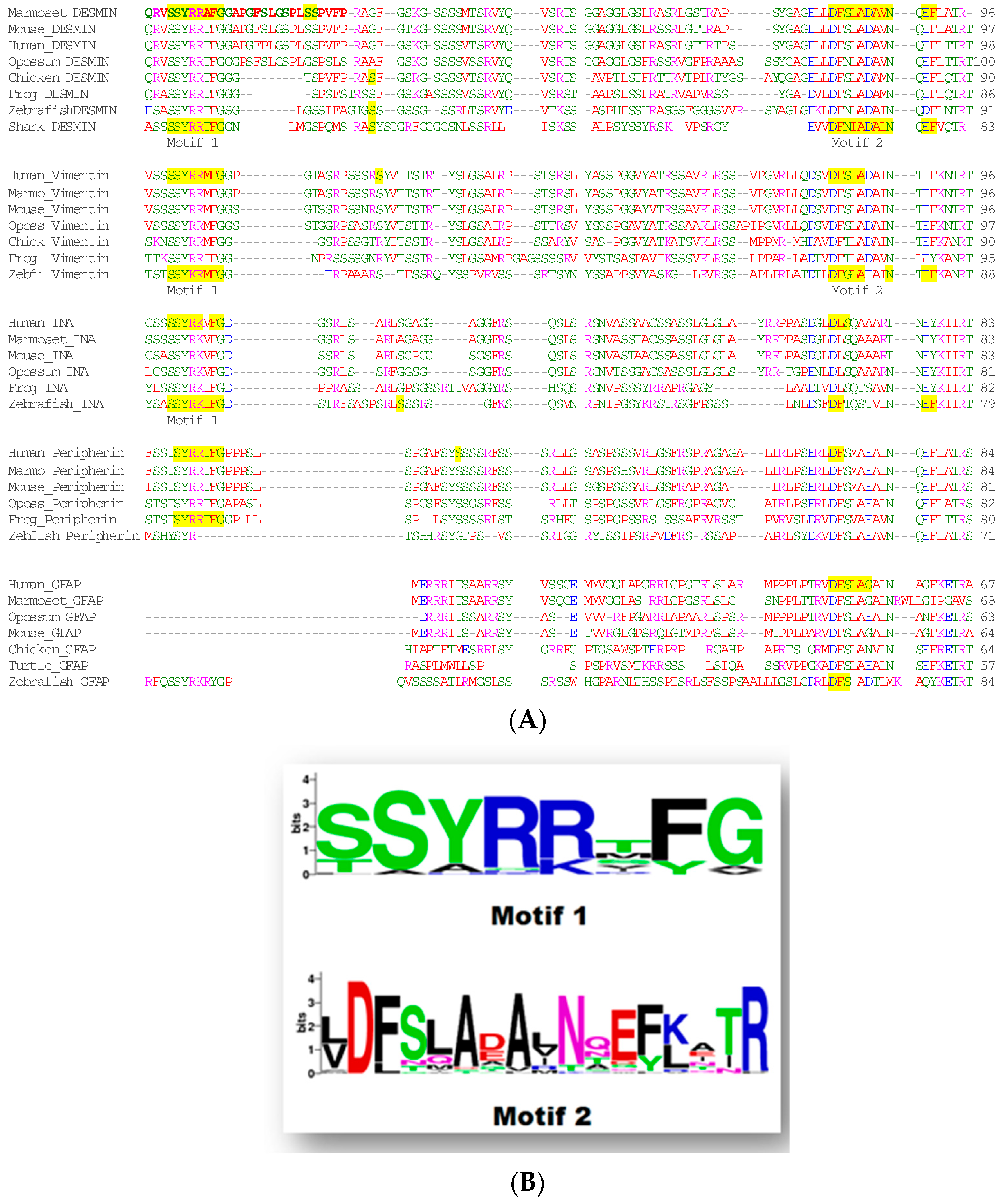
1.4. Low-Complexity Regions
2. Discussion—Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrmann, H.; Aebi, U. Intermediate Filaments: Molecular Structure, Assembly Mechanism, and Integration Into Functionally Distinct Intracellular Scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.F.; Parry, D.A.D. Intermediate Filament Structure: 3. Analysis of Sequence Homologies. Int. J. Biol. Macromol. 1988, 10, 79–98. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filament Assembly: Fibrillogenesis Is Driven by Decisive Dimer-Dimer Interactions. Curr. Opin. Struct. Biol. 1998, 8, 177–185. [Google Scholar] [CrossRef]
- Hesse, M.; Magin, T.M.; Weber, K. Genes for Intermediate Filament Proteins and the Draft Sequence of the Human Genome: Novel Keratin Genes and a Surprisingly High Number of Pseudogenes Related to Keratin Genes 8 and 18. J. Cell Sci. 2001, 114, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Aebi, U. Intermediate Filaments: Structure and Assembly. Cold Spring Harb. Perspect. Biol. 2016, 8, a018242. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Strelkov, S.V. History and Phylogeny of Intermediate Filaments: Now in Insects. BMC Biol. 2011, 9, 16. [Google Scholar] [CrossRef]
- Dodemont, H.; Riemer, D.; Weber, K. Structure of an Invertebrate Gene Encoding Cytoplasmic Intermediate Filament (IF) Proteins: Implications for the Origin and the Diversification of IF Proteins. EMBO J. 1990, 9, 4083–4094. [Google Scholar] [CrossRef]
- Kollmar, M. Polyphyly of Nuclear Lamin Genes Indicates an Early Eukaryotic Origin of the Metazoan-Type Intermediate Filament Proteins. Sci. Rep. 2015, 5, 10652. [Google Scholar] [CrossRef]
- Kreplak, L.; Aebi, U.; Herrmann, H. Molecular Mechanisms Underlying the Assembly of Intermediate Filaments. Exp. Cell Res. 2004, 301, 77–83. [Google Scholar] [CrossRef]
- Strelkov, S.V.; Herrmann, H.; Aebi, U. Molecular Architecture of Intermediate Filaments. Bioessays 2003, 25, 243–251. [Google Scholar] [CrossRef]
- Herrmann, H.; Bär, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate Filaments: From Cell Architecture to Nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Kreplak, L.; Buehler, M.J. Nanomechanical Properties of Vimentin Intermediate Filament Dimers. Nanotechnology 2009, 20, 425101. [Google Scholar] [CrossRef] [PubMed]
- Kreplak, L.; Fudge, D. Biomechanical Properties of Intermediate Filaments: From Tissues to Single Filaments and Back. BioEssays 2007, 29, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Chernyatina, A.A.; Nicolet, S.; Aebi, U.; Herrmann, H.; Strelkov, S.V. Atomic Structure of the Vimentin Central α-Helical Domain and Its Implications for Intermediate Filament Assembly. Proc. Natl. Acad. Sci. USA 2012, 109, 13620–13625. [Google Scholar] [CrossRef]
- Eldirany, S.A.; Ho, M.; Hinbest, A.J.; Lomakin, I.B.; Bunick, C.G. Human Keratin 1/10-1B Tetramer Structures Reveal a Knob-Pocket Mechanism in Intermediate Filament Assembly. EMBO J. 2019, 38, e100741. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Jo, I.; Kang, S.; Hong, S.; Kim, S.; Jeong, S.; Kim, Y.-H.; Park, B.-J.; Ha, N.-C. Structural Basis for Lamin Assembly at the Molecular Level. Nat. Commun. 2019, 10, 3757. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, S.; Herrmann, H.; Aebi, U.; Strelkov, S.V. Atomic Structure of Vimentin Coil 2. J. Struct. Biol. 2010, 170, 369–376. [Google Scholar] [CrossRef]
- Chang, L.; Goldman, R.D. Intermediate Filaments Mediate Cytoskeletal Crosstalk. Nat. Rev. Mol. Cell Biol. 2004, 5, 601–613. [Google Scholar] [CrossRef]
- Tsikitis, M.; Galata, Z.; Mavroidis, M.; Psarras, S.; Capetanaki, Y. Intermediate Filaments in Cardiomyopathy. Biophys. Rev. 2018, 10, 1007–1031. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D.G. CNS Stem Cells Express a New Class of Intermediate Filament Protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Merdes, A.; Gounari, F.; Georgatos, S.D. The 47-kD Lens-Specific Protein Phakinin Is a Tailless Intermediate Filament Protein and an Assembly Partner of Filensin. J. Cell Biol. 1993, 123, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Bader, B.L.; Magin, T.M.; Hatzfeld, M.; Franke, W.W. Amino Acid Sequence and Gene Organization of Cytokeratin No. 19, an Exceptional Tail-less Intermediate Filament Protein. EMBO J. 1986, 5, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Goulielmos, G.; Gounari, F.; Remington, S.; Müller, S.; Häner, M.; Aebi, U.; Georgatos, S.D. Filensin and Phakinin form a Novel Type of Beaded Intermediate Filaments and Coassemble de Novo in Cultured Cells. J. Cell Biol. 1996, 132, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Wedig, T.; Porter, R.M.; Lane, E.B.; Aebi, U. Characterization of Early Assembly Intermediates of Recombinant Human Keratins. J. Struct. Biol. 2002, 137, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Häner, M.; Brettel, M.; Müller, S.A.; Goldie, K.N.; Fedtke, B.; Lustig, A.; Franke, W.W.; Aebi, U. Structure and Assembly Properties of the Intermediate Filament Protein Vimentin: The Role of Its Head, Rod and Tail Domains. J. Mol. Biol. 1996, 264, 933–953. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mücke, N.; Katus, H.A.; Herrmann, H.; Bär, H. Disease Mutations in the “Head” Domain of the Extra-Sarcomeric Protein Desmin Distinctly Alter Its Assembly and Network-Forming Properties. J. Mol. Med. 2009, 87, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Hess, J.F.; Budamagunta, M.S.; Voss, J.C.; FitzGerald, P.G. Site-Directed Spin Labeling and Electron Paramagnetic Resonance Determination of Vimentin Head Domain Structure*. J. Biol. Chem. 2010, 285, 15278–15285. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Hess, J.F.; Budamagunta, M.S.; FitzGerald, P.G.; Voss, J.C. Head and Rod 1 Interactions in Vimentin*. J. Biol. Chem. 2009, 284, 7330–7338. [Google Scholar] [CrossRef] [PubMed]
- Usman, S.; Aldehlawi, H.; Nguyen, T.K.N.; Teh, M.-T.; Waseem, A. Impact of N-Terminal Tags on De Novo Vimentin Intermediate Filament Assembly. Int. J. Mol. Sci. 2022, 23, 6349. [Google Scholar] [CrossRef]
- Beuttenmüller, M.; Chen, M.; Janetzko, A.; Kühn, S.; Traub, P. Structural Elements of the Amino-Terminal Head Domain of Vimentin Essential for Intermediate Filament Formation in Vivo and in Vitro. Exp. Cell Res. 1994, 213, 128–142. [Google Scholar] [CrossRef]
- Herrmann, H.; Hofmann, I.; Franke, W.W. Identification of a Nonapeptide Motif in the Vimentin Head Domain Involved in Intermediate Filament Assembly. J. Mol. Biol. 1992, 223, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, P.-J.; Lilina, A.V.; Hashim, H.M.; Dlabolová, L.; Fiala, J.; Beelen, S.; Kukačka, Z.; Harvey, J.N.; Novák, P.; Strelkov, S.V. Molecular Structure of Soluble Vimentin Tetramers. Sci. Rep. 2023, 13, 8841. [Google Scholar] [CrossRef]
- Georgakopoulou, S.; Möller, D.; Sachs, N.; Herrmann, H.; Aebi, U. Near-UV Circular Dichroism Reveals Structural Transitions of Vimentin Subunits during Intermediate Filament Assembly. J. Mol. Biol. 2009, 386, 544–553. [Google Scholar] [CrossRef]
- Stuurman, N.; Heins, S.; Aebi, U. Nuclear Lamins: Their Structure, Assembly, and Interactions. J. Struct. Biol. 1998, 122, 42–66. [Google Scholar] [CrossRef]
- Ralton, J.E.; Lu, X.; Hutcheson, A.M.; Quinlan, R.A. Identification of Two N-Terminal Non-Alpha-Helical Domain Motifs Important in the Assembly of Glial Fibrillary Acidic Protein. J. Cell Sci. 1994, 107, 1935–1948. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Interactive Analysis of Phylogeny and Character Evolution Using the Computer Program MacClade. Folia Primatol. 1989, 53, 190–202. [Google Scholar] [CrossRef]
- Eibauer, M.; Weber, M.S.; Kronenberg-Tenga, R.; Beales, C.T.; Boujemaa-Paterski, R.; Turgay, Y.; Sivagurunathan, S.; Kraxner, J.; Köster, S.; Goldman, R.D.; et al. Vimentin Filaments Integrate Low Complexity Domains in a Highly Complex Helical Structure. bioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Eibauer, M.; Weber, M.S.; Turgay, Y.; Sivagurunathan, S.; Goldman, R.D.; Medalia, O. The Molecular Architecture of Vimentin Filaments. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Bray, D.J.; Walsh, T.R.; Noro, M.G.; Notman, R. Complete Structure of an Epithelial Keratin Dimer: Implications for Intermediate Filament Assembly. PLoS ONE 2015, 10, e0132706. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Chou, Y.H.; Prahlad, V.; Parry, D.A.; Marekov, L.N.; Wu, K.C.; Jang, S.I.; Goldman, R.D. A High Molecular Weight Intermediate Filament-Associated Protein in BHK-21 Cells Is Nestin, a Type VI Intermediate Filament Protein. Limited Co-Assembly in Vitro to Form Heteropolymers with Type III Vimentin and Type IV Alpha-Internexin. J. Biol. Chem. 1999, 274, 9881–9890. [Google Scholar] [CrossRef] [PubMed]
- Ching, G.Y.; Liem, R.K. Roles of Head and Tail Domains in Alpha-Internexin’s Self-Assembly and Coassembly with the Neurofilament Triplet Proteins. J. Cell Sci. 1998, 111 Pt 3, 321–333. [Google Scholar] [CrossRef]
- Heitlinger, E.; Peter, M.; Lustig, A.; Villiger, W.; Nigg, E.A.; Aebi, U. The Role of the Head and Tail Domain in Lamin Structure and Assembly: Analysis of Bacterially Expressed Chicken Lamin A and Truncated B2 Lamins. J. Struct. Biol. 1992, 108, 74–89. [Google Scholar] [CrossRef]
- Isobe, K.; Gohara, R.; Ueda, T.; Takasaki, Y.; Ando, S. The Last Twenty Residues in the Head Domain of Mouse Lamin A Contain Important Structural Elements for Formation of Head-to-Tail Polymers in Vitro. Biosci. Biotechnol. Biochem. 2007, 71, 1252–1259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izumi, M.; Vaughan, O.A.; Hutchison, C.J.; Gilbert, D.M. Head and/or CaaX Domain Deletions of Lamin Proteins Disrupt Preformed Lamin A and C But Not Lamin B Structure in Mammalian Cells. Mol. Biol. Cell 2000, 11, 4323–4337. [Google Scholar] [CrossRef] [PubMed]
- Strelkov, S.V.; Schumacher, J.; Burkhard, P.; Aebi, U.; Herrmann, H. Crystal Structure of the Human Lamin A Coil 2B Dimer: Implications for the Head-to-Tail Association of Nuclear Lamins. J. Mol. Biol. 2004, 343, 1067–1080. [Google Scholar] [CrossRef]
- Goulielmos, G.; Remington, S.; Schwesinger, F.; Georgatos, S.D.; Gounari, F. Contributions of the Structural Domains of Filensin in Polymer Formation and Filament Distribution. J. Cell Sci. 1996, 109, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, M.; Nakamura, A.; Kuratani, Y.; Takada, M.; Iwamoto, S.; Oka, M.; Ando, S. Effects of Truncations in the N- and C-Terminal Domains of Filensin on Filament Formation with Phakinin in Cell-Free Conditions and Cultured Cells. FEBS Open Bio 2023, 13, 1990–2004. [Google Scholar] [CrossRef]
- Geisler, N. Proteinchemical Characterization of Three Structurally Distinct Domains along the Protofilament Unit of Desmin 10 Nm Filaments. Cell 1982, 30, 277–286. [Google Scholar] [CrossRef]
- Premchandar, A.; Mücke, N.; Poznański, J.; Wedig, T.; Kaus-Drobek, M.; Herrmann, H.; Dadlez, M. Structural Dynamics of the Vimentin Coiled-Coil Contact Regions Involved in Filament Assembly as Revealed by Hydrogen-Deuterium Exchange. J. Biol. Chem. 2016, 291, 24931–24950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lin, Y.; Kato, M.; Mori, E.; Liszczak, G.; Sutherland, L.; Sysoev, V.O.; Murray, D.T.; Tycko, R.; McKnight, S.L. Transiently Structured Head Domains Control Intermediate Filament Assembly. Proc. Natl. Acad. Sci. USA 2021, 118, e2022121118. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kato, M.; McKnight, S.L. How Do Disordered Head Domains Assist in the Assembly of Intermediate Filaments? Curr. Opin. Cell Biol. 2023, 85, 102262. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Mack, J.W.; Korge, B.P.; Gan, S.Q.; Haynes, S.R.; Steven, A.C. Glycine Loops in Proteins: Their Occurrence in Certain Intermediate Filament Chains, Loricrins and Single-Stranded RNA Binding Proteins. Int. J. Biol. Macromol. 1991, 13, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Badowski, C.; Sim, A.Y.L.; Verma, C.; Szeverényi, I.; Natesavelalar, C.; Terron-Kwiatkowski, A.; Harper, J.; O’Toole, E.A.; Lane, E.B. Modeling the Structure of Keratin 1 and 10 Terminal Domains and Their Misassembly in Keratoderma. J. Investig. Dermatol. 2017, 137, 1914–1923. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Parry, D.A. The Conserved H1 Domain of the Type II Keratin 1 Chain Plays an Essential Role in the Alignment of Nearest Neighbor Molecules in Mouse and Human Keratin 1/Keratin 10 Intermediate Filaments at the Two- to Four-Molecule Level of Structure. J. Biol. Chem. 1993, 268, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, J.; Jeong, S.; Kang, S.; Park, B.-J.; Ha, N.-C. Beta-Strand-Mediated Dimeric Formation of the Ig-like Domains of Human Lamin A/C and B1. Biochem. Biophys. Res. Commun. 2021, 550, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, M.; Malka-Gibor, E.; Laser-Azogui, A.; Doron, O.; Herrmann, H.; Beck, R. Composite Bottlebrush Mechanics: α-Internexin Fine-Tunes Neurofilament Network Properties. Soft Matter 2015, 11, 5839–5849. [Google Scholar] [CrossRef]
- Hess, J.F.; Budamagunta, M.S.; Aziz, A.; FitzGerald, P.G.; Voss, J.C. Electron Paramagnetic Resonance Analysis of the Vimentin Tail Domain Reveals Points of Order in a Largely Disordered Region and Conformational Adaptation upon Filament Assembly. Protein Sci. 2013, 22, 47–55. [Google Scholar] [CrossRef]
- Vlachakis, D.; Tsilafakis, K.; Kostavasili, I.; Kossida, S.; Mavroidis, M. Unraveling Desmin’s Head Domain Structure and Function. Cells 2024, 13, 603. [Google Scholar] [CrossRef]
- Heimburg, T.; Schuenemann, J.; Weber, K.; Geisler, N. Specific Recognition of Coiled Coils by Infrared Spectroscopy: Analysis of the Three Structural Domains of Type III Intermediate Filament Proteins. Biochemistry 1996, 35, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Kouloumenta, A.; Mavroidis, M.; Capetanaki, Y. Proper Perinuclear Localization of the TRIM-like Protein Myospryn Requires Its Binding Partner Desmin. J. Biol. Chem. 2007, 282, 35211–35221. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Q.; Oiso, N.; Novak, E.K.; Gautam, R.; O’Brien, E.P.; Tinsley, C.L.; Blake, D.J.; Spritz, R.A.; Copeland, N.G.; et al. Hermansky-Pudlak Syndrome Type 7 (HPS-7) Results from Mutant Dysbindin, a Member of the Biogenesis of Lysosome-Related Organelles Complex 1 (BLOC-1). Nat. Genet. 2003, 35, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.A.; Tinsley, C.L.; Blake, D.J. Myospryn Is a Novel Binding Partner for Dysbindin in Muscle. J. Biol. Chem. 2004, 279, 10450–10458. [Google Scholar] [CrossRef] [PubMed]
- Dayal, A.A.; Medvedeva, N.V.; Nekrasova, T.M.; Duhalin, S.D.; Surin, A.K.; Minin, A.A. Desmin Interacts Directly with Mitochondria. Int. J. Mol. Sci. 2020, 21, 8122. [Google Scholar] [CrossRef] [PubMed]
- Chernoivanenko, I.S.; Matveeva, E.A.; Gelfand, V.I.; Goldman, R.D.; Minin, A.A. Mitochondrial Membrane Potential Is Regulated by Vimentin Intermediate Filaments. FASEB J. 2015, 29, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Kouklis, P.D.; Hutton, E.; Fuchs, E. Making a Connection: Direct Binding between Keratin Intermediate Filaments and Desmosomal Proteins. J. Cell Biol. 1994, 127, 1049–1060. [Google Scholar] [CrossRef]
- Meng, J.-J.; Bornslaeger, E.A.; Green, K.J.; Steinert, P.M.; Ip, W. Two-Hybrid Analysis Reveals Fundamental Differences in Direct Interactions between Desmoplakin and Cell Type-Specific Intermediate Filaments *. J. Biol. Chem. 1997, 272, 21495–21503. [Google Scholar] [CrossRef]
- Favre, B.; Begré, N.; Bouameur, J.-E.; Lingasamy, P.; Conover, G.M.; Fontao, L.; Borradori, L. Desmoplakin Interacts with the Coil 1 of Different Types of Intermediate Filament Proteins and Displays High Affinity for Assembled Intermediate Filaments. PLoS ONE 2018, 13, e0205038. [Google Scholar] [CrossRef]
- Favre, B.; Schneider, Y.; Lingasamy, P.; Bouameur, J.-E.; Begré, N.; Gontier, Y.; Steiner-Champliaud, M.-F.; Frias, M.A.; Borradori, L.; Fontao, L. Plectin Interacts with the Rod Domain of Type III Intermediate Filament Proteins Desmin and Vimentin. Eur. J. Cell Biol. 2011, 90, 390–400. [Google Scholar] [CrossRef]
- Bouameur, J.-E.; Favre, B.; Fontao, L.; Lingasamy, P.; Begré, N.; Borradori, L. Interaction of Plectin with Keratins 5 and 14: Dependence on Several Plectin Domains and Keratin Quaternary Structure. J. Investig. Dermatol. 2014, 134, 2776–2783. [Google Scholar] [CrossRef]
- Snider, N.T.; Omary, M.B. Post-Translational Modifications of Intermediate Filament Proteins: Mechanisms and Functions. Nat. Rev. Mol. Cell Biol. 2014, 15, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Gomi, H.; Colucci-Guyon, E.; Itohara, S. Protective Role of Phosphorylation in Turnover of Glial Fibrillary Acidic Protein in Mice. J. Neurosci. 2002, 22, 6972–6979. [Google Scholar] [CrossRef]
- Omary, M.B.; Ku, N.-O.; Tao, G.-Z.; Toivola, D.M.; Liao, J. ‘Heads and Tails’ of Intermediate Filament Phosphorylation: Multiple Sites and Functional Insights. Trends Biochem. Sci. 2006, 31, 383–394. [Google Scholar] [CrossRef]
- Jeong, S.; Ahn, J.; Jo, I.; Kang, S.-M.; Park, B.-J.; Cho, H.-S.; Kim, Y.-H.; Ha, N.-C. Cyclin-Dependent Kinase 1 Depolymerizes Nuclear Lamin Filaments by Disrupting the Head-to-Tail Interaction of the Lamin Central Rod Domain. J. Biol. Chem. 2022, 298, 102256. [Google Scholar] [CrossRef]
- Omary, M.B. “IF-Pathies”: A Broad Spectrum of Intermediate Filament-Associated Diseases. J. Clin. Investig. 2009, 119, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Betz, R.C.; Planko, L.; Eigelshoven, S.; Hanneken, S.; Pasternack, S.M.; Büssow, H.; Van Den Bogaert, K.; Wenzel, J.; Braun-Falco, M.; Rütten, A.; et al. Loss-of-Function Mutations in the Keratin 5 Gene Lead to Dowling-Degos Disease. Am. J. Hum. Genet. 2006, 78, 510–519. [Google Scholar] [CrossRef]
- Brenner, M.; Johnson, A.B.; Boespflug-Tanguy, O.; Rodriguez, D.; Goldman, J.E.; Messing, A. Mutations in GFAP, Encoding Glial Fibrillary Acidic Protein, Are Associated with Alexander Disease. Nat. Genet. 2001, 27, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Finsterer, J.; Steger, C.; Wexberg, P.; Gatterer, E.; Khazen, C.; Stix, G.; Gerull, B.; Höftberger, R.; Weidinger, F. Novel c.367_369del LMNA Mutation Manifesting as Severe Arrhythmias, Dilated Cardiomyopathy, and Myopathy. Heart Lung 2012, 41, 382–386. [Google Scholar] [CrossRef]
- van Spaendonck-Zwarts, K.Y.; van Hessem, L.; Jongbloed, J.D.H.; de Walle, H.E.K.; Capetanaki, Y.; van der Kooi, A.J.; van Langen, I.M.; van den Berg, M.P.; van Tintelen, J.P. Desmin-Related Myopathy. Clin. Genet. 2011, 80, 354–366. [Google Scholar] [CrossRef]
- Pallari, H.-M.; Eriksson, J.E. Intermediate Filaments as Signaling Platforms. Sci. STKE 2006, 2006, pe53. [Google Scholar] [CrossRef]
- Herrmann, H.; Aebi, U. Intermediate Filaments and Their Associates: Multi-Talented Structural Elements Specifying Cytoarchitecture and Cytodynamics. Curr. Opin. Cell Biol. 2000, 12, 79–90. [Google Scholar] [CrossRef]
- Bott, C.J.; Winckler, B. Intermediate Filaments in Developing Neurons: Beyond Structure. Cytoskeleton 2020, 77, 110–128. [Google Scholar] [CrossRef]
- Wang, Q.; Tolstonog, G.V.; Shoeman, R.; Traub, P. Sites of Nucleic Acid Binding in Type I−IV Intermediate Filament Subunit Proteins. Biochemistry 2001, 40, 10342–10349. [Google Scholar] [CrossRef] [PubMed]
- Shoeman, R.L.; Hüttermann, C.; Hartig, R.; Traub, P. Amino-Terminal Polypeptides of Vimentin Are Responsible for the Changes in Nuclear Architecture Associated with Human Immunodeficiency Virus Type 1 Protease Activity in Tissue Culture Cells. MBoC 2001, 12, 143–154. [Google Scholar] [CrossRef]
- Samson, C.; Petitalot, A.; Celli, F.; Herrada, I.; Ropars, V.; Le Du, M.-H.; Nhiri, N.; Jacquet, E.; Arteni, A.-A.; Buendia, B.; et al. Structural Analysis of the Ternary Complex between Lamin A/C, BAF and Emerin Identifies an Interface Disrupted in Autosomal Recessive Progeroid Diseases. Nucleic Acids Res. 2018, 46, 10460–10473. [Google Scholar] [CrossRef] [PubMed]
- Krimm, I.; Ostlund, C.; Gilquin, B.; Couprie, J.; Hossenlopp, P.; Mornon, J.-P.; Bonne, G.; Courvalin, J.-C.; Worman, H.J.; Zinn-Justin, S. The Ig-like Structure of the C-Terminal Domain of Lamin A/C, Mutated in Muscular Dystrophies, Cardiomyopathy, and Partial Lipodystrophy. Structure 2002, 10, 811–823. [Google Scholar] [CrossRef]
- Etourneaud, L.; Moussa, A.; Rass, E.; Genet, D.; Willaume, S.; Chabance-Okumura, C.; Wanschoor, P.; Picotto, J.; Thézé, B.; Dépagne, J.; et al. Lamin B1 Sequesters 53BP1 to Control Its Recruitment to DNA Damage. Sci. Adv. 2021, 7, eabb3799. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed]
- King, O.D.; Gitler, A.D.; Shorter, J. The Tip of the Iceberg: RNA-Binding Proteins with Prion-like Domains in Neurodegenerative Disease. Brain Res. 2012, 1462, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Ntountoumi, C.; Vlastaridis, P.; Mossialos, D.; Stathopoulos, C.; Iliopoulos, I.; Promponas, V.; Oliver, S.; Amoutzias, G. Low Complexity Regions in the Proteins of Prokaryotes Perform Important Functional Roles and Are Highly Conserved. Nucleic Acids Res. 2019, 47, 9998–10009. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, H.; Kwon, I. Phase Separation of Low-Complexity Domains in Cellular Function and Disease. Exp. Mol. Med. 2022, 54, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, A. On the Role of Phase Separation in the Biogenesis of Membraneless Compartments. EMBO J. 2022, 41, e109952. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Kato, M.; Whitwam, T.; Kim, J.H.; Montell, D.J. An Atypical Tropomyosin in Drosophila with Intermediate Filament-like Properties. Cell Rep. 2016, 16, 928–938. [Google Scholar] [CrossRef]
- Sysoev, V.O.; Kato, M.; Sutherland, L.; Hu, R.; McKnight, S.L.; Murray, D.T. Dynamic Structural Order of a Low-Complexity Domain Facilitates Assembly of Intermediate Filaments. Proc. Natl. Acad. Sci. USA 2020, 117, 23510–23518. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Kumar, R.; Kumar, M. Low Complexity and Disordered Regions of Proteins Have Different Structural and Amino Acid Preferences. Mol. BioSyst. 2015, 11, 585–594. [Google Scholar] [CrossRef]
- Kumari, B.; Kumar, R.; Chauhan, V.; Kumar, M. Comparative Functional Analysis of Proteins Containing Low-Complexity Predicted Amyloid Regions. PeerJ 2018, 6, e5823. [Google Scholar] [CrossRef]
- Agnetti, G.; Halperin, V.L.; Kirk, J.A.; Chakir, K.; Guo, Y.; Lund, L.; Nicolini, F.; Gherli, T.; Guarnieri, C.; Caldarera, C.M.; et al. Desmin Modifications Associate with Amyloid-like Oligomers Deposition in Heart Failure. Cardiovasc. Res. 2014, 102, 24–34. [Google Scholar] [CrossRef]
- Rainer, P.P.; Dong, P.; Sorge, M.; Fert-Bober, J.; Holewinski, R.J.; Wang, Y.; Foss, C.A.; An, S.S.; Baracca, A.; Solaini, G.; et al. Desmin Phosphorylation Triggers Preamyloid Oligomers Formation and Myocyte Dysfunction in Acquired Heart Failure. Circ. Res. 2018, 122, e75–e83. [Google Scholar] [CrossRef] [PubMed]
- Kedia, N.; Arhzaouy, K.; Pittman, S.K.; Sun, Y.; Batchelor, M.; Weihl, C.C.; Bieschke, J. Desmin Forms Toxic, Seeding-Competent Amyloid Aggregates That Persist in Muscle Fibers. Proc. Natl. Acad. Sci. USA 2019, 116, 16835–16840. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A.; Hughes, M.P.; Hu, C.J.; Sawaya, M.R.; Salwinski, L.; Pan, H.; French, S.W.; Seidler, P.M.; Eisenberg, D.S. Identifying Amyloid-Related Diseases by Mapping Mutations in Low-Complexity Protein Domains to Pathologies. Nat. Struct. Mol. Biol. 2022, 29, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.P.; Sawaya, M.R.; Boyer, D.R.; Goldschmidt, L.; Rodriguez, J.A.; Cascio, D.; Chong, L.; Gonen, T.; Eisenberg, D.S. Atomic Structures of Low-Complexity Protein Segments Reveal Kinked β Sheets That Assemble Networks. Science 2018, 359, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompson, M.J.; Balbirnie, M.; Wiltzius, J.J.W.; McFarlane, H.T.; et al. Atomic Structures of Amyloid Cross-Beta Spines Reveal Varied Steric Zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Kouklis, P.D.; Papamarcaki, T.; Merdes, A.; Georgatos, S.D. A Potential Role for the COOH-Terminal Domain in the Lateral Packing of Type III Intermediate Filaments. J. Cell Biol. 1991, 114, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Pasotti, M.; Pilotto, A.; Pellegrini, C.; Grasso, M.; Previtali, S.; Repetto, A.; Bellini, O.; Azan, G.; Scaffino, M.; et al. Desmin Accumulation Restrictive Cardiomyopathy and Atrioventricular Block Associated with Desmin Gene Defects. Eur. J. Heart Fail. 2006, 8, 477–483. [Google Scholar] [CrossRef] [PubMed]
- van Tintelen, J.P.; Van Gelder, I.C.; Asimaki, A.; Suurmeijer, A.J.H.; Wiesfeld, A.C.P.; Jongbloed, J.D.H.; van den Wijngaard, A.; Kuks, J.B.M.; van Spaendonck-Zwarts, K.Y.; Notermans, N.; et al. Severe Cardiac Phenotype with Right Ventricular Predominance in a Large Cohort of Patients with a Single Missense Mutation in the DES Gene. Heart Rhythm. 2009, 6, 1574–1583. [Google Scholar] [CrossRef]
- Lapouge, K.; Fontao, L.; Champliaud, M.-F.; Jaunin, F.; Frias, M.A.; Favre, B.; Paulin, D.; Green, K.J.; Borradori, L. New Insights into the Molecular Basis of Desmoplakinand Desmin-Related Cardiomyopathies. J. Cell Sci. 2006, 119, 4974–4985. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsilafakis, K.; Mavroidis, M. Are the Head and Tail Domains of Intermediate Filaments Really Unstructured Regions? Genes 2024, 15, 633. https://doi.org/10.3390/genes15050633
Tsilafakis K, Mavroidis M. Are the Head and Tail Domains of Intermediate Filaments Really Unstructured Regions? Genes. 2024; 15(5):633. https://doi.org/10.3390/genes15050633
Chicago/Turabian StyleTsilafakis, Konstantinos, and Manolis Mavroidis. 2024. "Are the Head and Tail Domains of Intermediate Filaments Really Unstructured Regions?" Genes 15, no. 5: 633. https://doi.org/10.3390/genes15050633
APA StyleTsilafakis, K., & Mavroidis, M. (2024). Are the Head and Tail Domains of Intermediate Filaments Really Unstructured Regions? Genes, 15(5), 633. https://doi.org/10.3390/genes15050633





