Molecular Mechanisms Associated with the Development of the Metritis Complex in Dairy Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Phenotypic Data
2.2. DNA Extraction and Genotypic Data
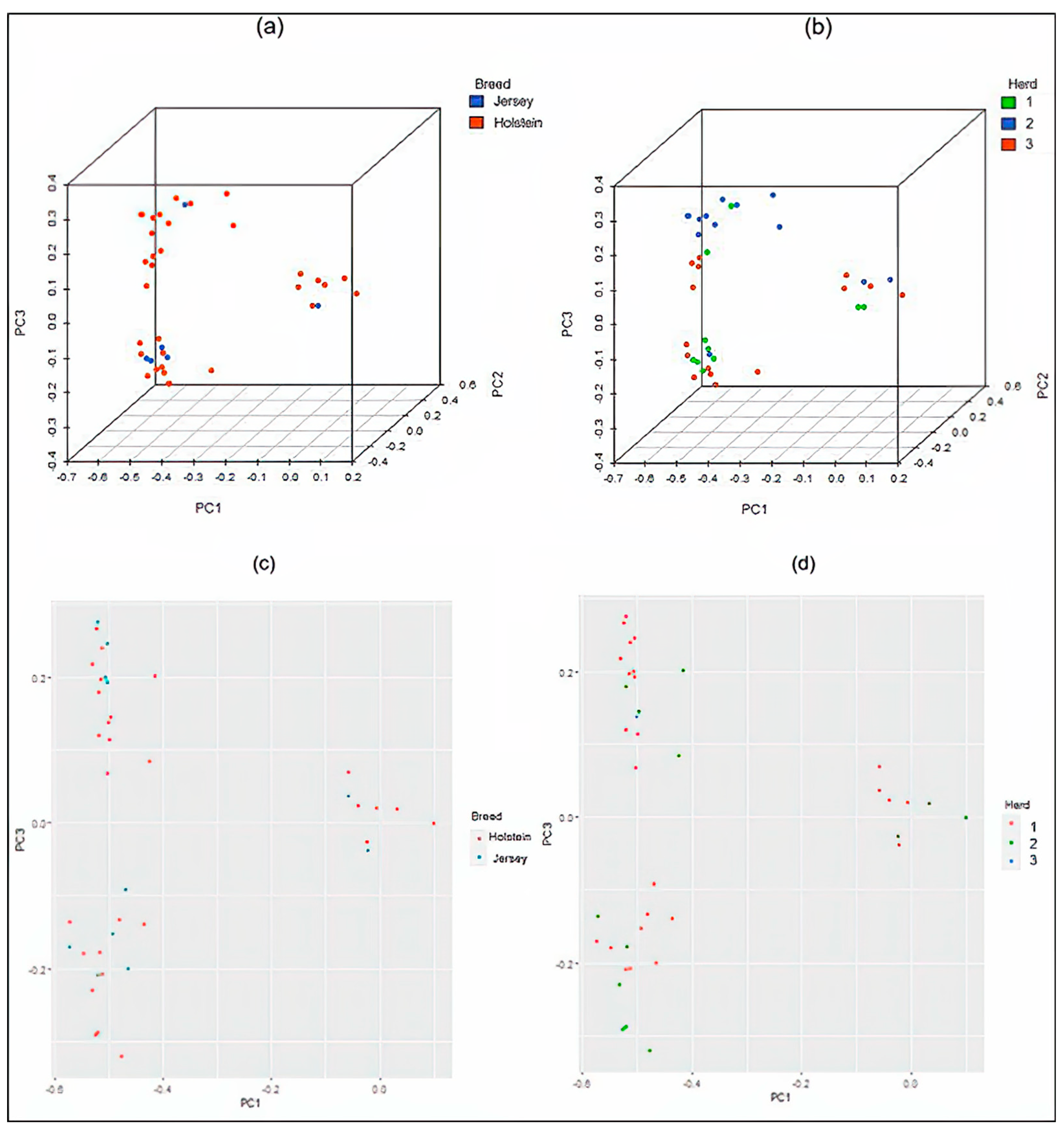
2.3. Population Structure
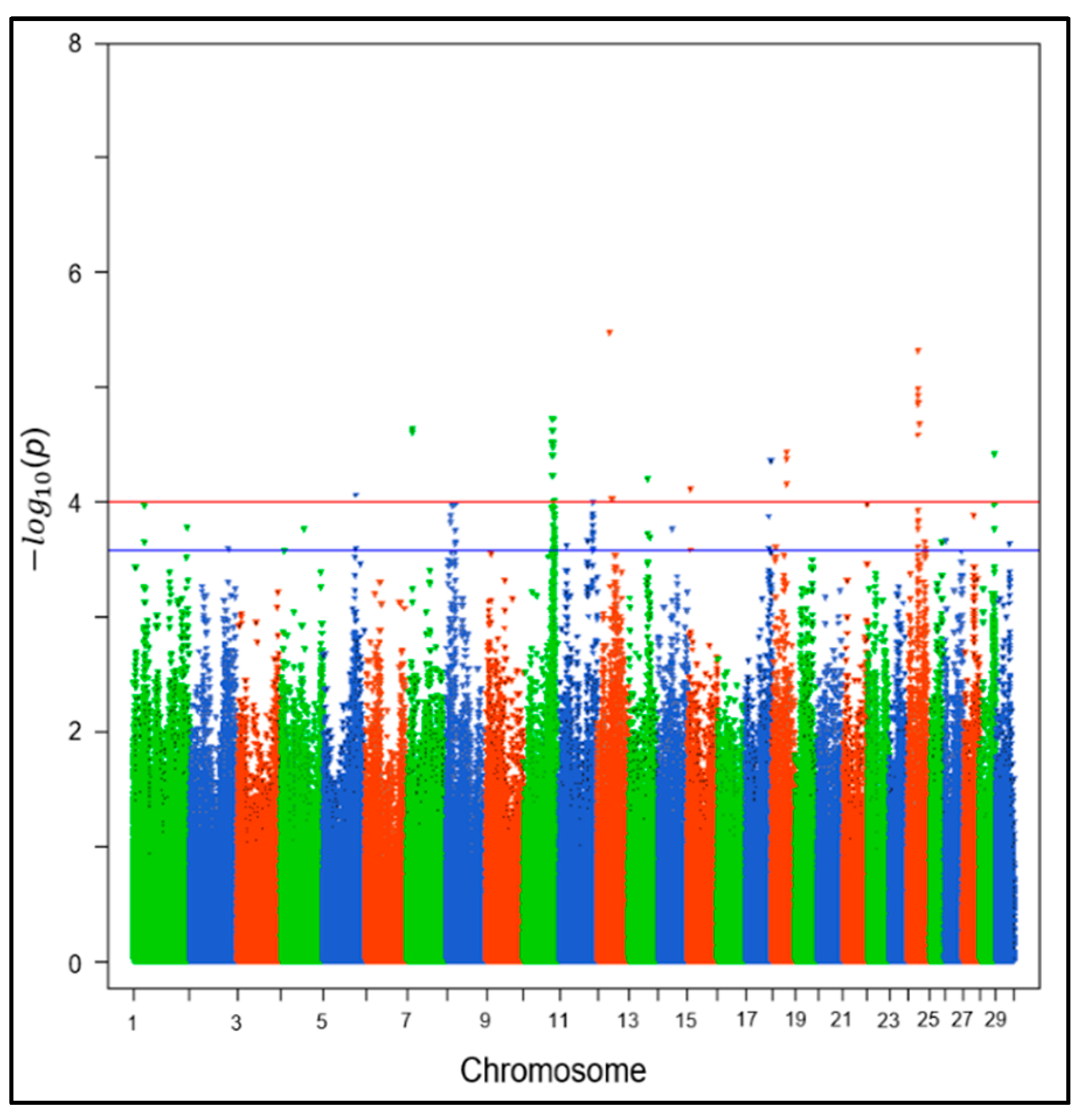
2.4. Genome-Wide Association Study (GWAS) and Genomic Heritability
2.5. In-Silico Functional Analyses
3. Results
3.1. Population Structure
3.2. Genome-Wide Association Study (GWAS) and Genomic Heritability
3.3. In-Silico Functional Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.A.; Nash, D.M.; Herath, S. Uterine Diseases in Cattle after Parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef]
- Basbas, C.; Garzon, A.; Silva-del-Rio, N.; Byrne, B.A.; Karle, B.; Aly, S.S.; Champagne, J.D.; Williams, D.R.; Lima, F.S.; Machado, V.S.; et al. Evaluation of Antimicrobial Resistance and Risk Factors for Recovery of Intrauterine Escherichia Coli from Cows with Metritis on California Commercial Dairy Farms. Sci. Rep. 2022, 12, 13937. [Google Scholar] [CrossRef]
- Pérez-Báez, J.; Silva, T.V.; Risco, C.A.; Chebel, R.C.; Cunha, F.; De Vries, A.; Santos, J.E.P.; Lima, F.S.; Pinedo, P.; Schuenemann, G.M.; et al. The Economic Cost of Metritis in Dairy Herds. J. Dairy Sci. 2021, 104, 3158–3168. [Google Scholar] [CrossRef]
- Figueiredo, C.C.; Merenda, V.R.; de Oliveira, E.B.; Lima, F.S.; Chebel, R.C.; Galvão, K.N.; Santos, J.E.P.; Bisinotto, R.S. Failure of Clinical Cure in Dairy Cows Treated for Metritis Is Associated with Reduced Productive and Reproductive Performance. J. Dairy Sci. 2021, 104, 7056–7070. [Google Scholar] [CrossRef]
- Giuliodori, M.J.; Magnasco, R.P.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Risco, C.A.; de la Sota, R.L. Metritis in Dairy Cows: Risk Factors and Reproductive Performance. J. Dairy Sci. 2013, 96, 3621–3631. [Google Scholar] [CrossRef]
- Overton, M.; Fetrow, J. Economics of Postpartum Uterine Health. In Proceedings of the Dairy Cattle Reproduction Council Convention Annual Meet, Omaha, NE, USA, 7 November 2008; pp. 39–44. [Google Scholar]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle1. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Sandals, W.C.D.; Curtis, R.A.; Cote, J.F.; Martin, S.W. The Effect of Retained Placenta and Metritis Complex on Reproductive Performance in Dairy Cattle A Case Control Study. Can. Vet. J. 1979, 20, 131–135. [Google Scholar]
- Egger-Danner, C.; Cole, J.B.; Pryce, J.E.; Gengler, N.; Heringstad, B.; Bradley, A.; Stock, K.F. Invited Review: Overview of New Traits and Phenotyping Strategies in Dairy Cattle with a Focus on Functional Traits. Animal 2015, 9, 191–207. [Google Scholar] [CrossRef]
- Freebern, E.; Santos, D.J.A.; Fang, L.; Jiang, J.; Parker Gaddis, K.L.; Liu, G.E.; VanRaden, P.M.; Maltecca, C.; Cole, J.B.; Ma, L. GWAS and Fine-Mapping of Livability and Six Disease Traits in Holstein Cattle. BMC Genom. 2020, 21, 41. [Google Scholar] [CrossRef]
- Schaid, D.J.; Chen, W.; Larson, N.B. From Genome-Wide Associations to Candidate Causal Variants by Statistical Fine-Mapping. Nat. Rev. Genet. 2018, 19, 491–504. [Google Scholar] [CrossRef]
- Hirschhorn, J.N.; Daly, M.J. Genome-Wide Association Studies for Common Diseases and Complex Traits. Nat. Rev. Genet. 2005, 6, 95–108. [Google Scholar] [CrossRef]
- May, K.; Sames, L.; Scheper, C.; König, S. Genomic Loci and Genetic Parameters for Uterine Diseases in First-Parity Holstein Cows and Associations with Milk Production and Fertility. J. Dairy Sci. 2022, 105, 509–524. [Google Scholar] [CrossRef]
- Parker Gaddis, K.L.; Cole, J.B.; Clay, J.S.; Maltecca, C. Genomic Selection for Producer-Recorded Health Event Data in US Dairy Cattle. J. Dairy Sci. 2014, 97, 3190–3199. [Google Scholar] [CrossRef]
- Guarini, A.R.; Lourenco, D.A.L.; Brito, L.F.; Sargolzaei, M.; Baes, C.F.; Miglior, F.; Misztal, I.; Schenkel, F.S. Genetics and Genomics of Reproductive Disorders in Canadian Holstein Cattle. J. Dairy Sci. 2019, 102, 1341–1353. [Google Scholar] [CrossRef]
- Weller, J.I.; Ezra, E.; van Straten, M. Genetic and Environmental Analysis of Diseases with Major Economic Impact in Israeli Holsteins. J. Dairy Sci. 2019, 102, 10030–10038. [Google Scholar] [CrossRef]
- Naderi, S.; Bohlouli, M.; Yin, T.; König, S. Genomic Breeding Values, SNP Effects and Gene Identification for Disease Traits in Cow Training Sets. Anim. Genet. 2018, 49, 178–192. [Google Scholar] [CrossRef]
- Zaitlen, N.; Kraft, P. Heritability in the Genome-Wide Association Era. Hum. Genet. 2012, 131, 1655–1664. [Google Scholar] [CrossRef]
- Sheldon, I.M. The Metritis Complex in Cattle. In Veterinary Reproduction and Obstetrics; Noakes, D.E., Parkinson, T.J., Eds.; England GCW: St. Louis, MO, USA, 2019; pp. 408–433. ISBN 978-0-7020-7233-8. [Google Scholar]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining Postpartum Uterine Disease in Cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Snelling, W.M.; Hoff, J.L.; Li, J.H.; Kuehn, L.A.; Keel, B.N.; Lindholm-Perry, A.K.; Pickrell, J.K. Assessment of Imputation from Low-Pass Sequencing to Predict Merit of Beef Steers. Genes 2020, 11, 1312. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. GigaScience 2015, 4, 7. [Google Scholar] [CrossRef]
- Ligges, U.; Mächler, M. Scatterplot3d—An R Package for Visualizing Multivariate Data. J. Stat. Softw. 2003, 8, 1–20. [Google Scholar] [CrossRef]
- R Core Team. 2021 R: A Language and Environment for Statistical Computing 2021; R Core Team: Vienna, Austria,, 2021. [Google Scholar]
- Wickham, H.; Chang, W. Package ‘Ggplot2’. Create Elegant Data Visualisations Using the Grammar of Graphics. 2016, Version 2.1. pp. 1–189. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pd (accessed on 18 April 2023).
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A Tool for Genome-Wide Complex Trait Analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef]
- Kurz, J.P.; Yang, Z.; Weiss, R.B.; Wilson, D.J.; Rood, K.A.; Liu, G.E.; Wang, Z. A Genome-Wide Association Study for Mastitis Resistance in Phenotypically Well-Characterized Holstein Dairy Cattle Using a Selective Genotyping Approach. Immunogenetics 2019, 71, 35–47. [Google Scholar] [CrossRef]
- Klein, S.-L.; Scheper, C.; May, K.; König, S. Genetic and Nongenetic Profiling of Milk β-Hydroxybutyrate and Acetone and Their Associations with Ketosis in Holstein Cows. J. Dairy Sci. 2020, 103, 10332–10346. [Google Scholar] [CrossRef]
- Parker Gaddis, K.L.; Null, D.J.; Cole, J.B. Explorations in Genome-Wide Association Studies and Network Analyses with Dairy Cattle Fertility Traits. J. Dairy Sci. 2016, 99, 6420–6435. [Google Scholar] [CrossRef]
- Welderufael, B.G.; Løvendahl, P.; De Koning, D.-J.; Janss, L.L.G.; Fikse, W.F. Genome-Wide Association Study for Susceptibility to and Recoverability From Mastitis in Danish Holstein Cows. Front. Genet. 2018, 9, 141. [Google Scholar] [CrossRef]
- Turner, S.D. qqman: An R package for visualizing GWAS results using QQ and manhattan plots. Biorxiv 2014, 005165. [Google Scholar] [CrossRef]
- Da, Y.; Wang, C.; Wang, S.; Hu, G. Mixed Model Methods for Genomic Prediction and Variance Component Estimation of Additive and Dominance Effects Using SNP Markers. PLoS ONE 2014, 9, e87666. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded Annotation Database and Novel Algorithms to Better Extract Biology from Large Gene Lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Park, C.A.; Wu, X.-L.; Reecy, J.M. Animal QTLdb: An Improved Database Tool for Livestock Animal QTL/Association Data Dissemination in the Post-Genome Era. Nucleic Acids Res. 2013, 41, D871–D879. [Google Scholar] [CrossRef]
- Galvão, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium Review: The Uterine Microbiome Associated with the Development of Uterine Disease in Dairy Cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef]
- Horlock, A.D.; Piersanti, R.L.; Ramirez-Hernandez, R.; Yu, F.; Ma, Z.; Jeong, K.C.; Clift, M.J.D.; Block, J.; Santos, J.E.P.; Bromfield, J.J.; et al. Uterine Infection Alters the Transcriptome of the Bovine Reproductive Tract Three Months Later. Reproduction 2020, 160, 93–107. [Google Scholar] [CrossRef]
- de Lima, F.S. de Recent Advances and Future Directions for Uterine Diseases Diagnosis, Pathogenesis, and Management in Dairy Cows. Anim. Reprod. 2020, 17, e20200063. [Google Scholar] [CrossRef]
- Kim, D.-U.; Lee, S.-C.; Jeong, J.-K.; Choi, I.-S.; Moon, S.-H.; Kang, H.-G.; Kim, I.-H. Effects of Dystocia on the Postpartum Complications, Milk Production and Reproductive Performance in Dairy Cows. J. Vet. Clin. 2016, 33, 87–92. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Postpartum Uterine Disease and Dairy Herd Reproductive Performance: A Review. Vet. J. 2008, 176, 102–114. [Google Scholar] [CrossRef]
- Lombard, J.E.; Garry, F.B.; Tomlinson, S.M.; Garber, L.P. Impacts of Dystocia on Health and Survival of Dairy Calves. J. Dairy Sci. 2007, 90, 1751–1760. [Google Scholar] [CrossRef]
- Syrjälä, P.; Anttila, M.; Dillard, K.; Fossi, M.; Collin, K.; Nylund, M.; Autio, T. Causes of Bovine Abortion, Stillbirth and Neonatal Death in Finland 1999–2006. Acta Vet. Scand. 2007, 49, S3. [Google Scholar] [CrossRef]
- Fouz, R.; Gandoy, F.; Sanjuán, M.L.; Yus, E.; Diéguez, F.J. Factors Associated with 56-Day Non-Return Rate in Dairy Cattle. Pesqui. Agropecuária Bras. 2011, 46, 648–654. [Google Scholar] [CrossRef][Green Version]
- Opsomer, G.; Gröhn, Y.T.; Hertl, J.; Coryn, M.; Deluyker, H.; De Kruif, A. Risk Factors for Post Partum Ovarian Dysfunction in High Producing Dairy Cows in Belgium: A Field Study. Theriogenology 2000, 53, 841–857. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Gomes, G.; Greco, L.F.; Cerri, R.L.A.; Vieira-Neto, A.; Monteiro, P.L.J.; Lima, F.S.; Bisinotto, R.S.; Thatcher, W.W.; Santos, J.E.P. Carryover Effect of Postpartum Inflammatory Diseases on Developmental Biology and Fertility in Lactating Dairy Cows. J. Dairy Sci. 2016, 99, 2201–2220. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk Factors for Postpartum Uterine Diseases in Dairy Cows. J. Dairy Sci. 2010, 93, 5764–5771. [Google Scholar] [CrossRef]
- Galvão, K.N. Uterine Diseases in Dairy Cows: Understanding the Causes and Seeking Solutions. Anim. Reprod. 2013, 10, 228–238. [Google Scholar]
- Mu, T.; Hu, H.; Ma, Y.; Wen, H.; Yang, C.; Feng, X.; Wen, W.; Zhang, J.; Gu, Y. Identifying Key Genes in Milk Fat Metabolism by Weighted Gene Co-Expression Network Analysis. Sci. Rep. 2022, 12, 6836. [Google Scholar] [CrossRef]
- Xing, Y.; Peng, K.; Yi, Q.; Yu, D.; Shi, H.; Yang, G.; Yin, S. TMEM30A Is Essential for Hair Cell Polarity Maintenance in Postnatal Mouse Cochlea. Cell. Mol. Biol. Lett. 2023, 28, 23. [Google Scholar] [CrossRef]
- Griffin, S.; Healey, G.D.; Sheldon, I.M. Isoprenoids Increase Bovine Endometrial Stromal Cell Tolerance to the Cholesterol-Dependent Cytolysin from Trueperella Pyogenes. Biol. Reprod. 2018, 99, 749–760. [Google Scholar] [CrossRef]
- He, M.; Zhang, W.; Dong, Y.; Wang, L.; Fang, T.; Tang, W.; Lv, B.; Chen, G.; Yang, B.; Huang, P.; et al. Pro-Inflammation NF-κB Signaling Triggers a Positive Feedback via Enhancing Cholesterol Accumulation in Liver Cancer Cells. J. Exp. Clin. Cancer Res. 2017, 36, 15. [Google Scholar] [CrossRef]
- Song, N.; Wang, X.; Gui, L.; Raza, S.H.A.; Luoreng, Z.; Zan, L. MicroRNA-214 Regulates Immunity-Related Genes in Bovine Mammary Epithelial Cells by Targeting NFATc3 and TRAF3. Mol. Cell. Probes 2017, 35, 27–33. [Google Scholar] [CrossRef]
- Aylon, Y.; Oren, M. The Hippo Pathway, P53 and Cholesterol. Cell Cycle 2016, 15, 2248–2255. [Google Scholar] [CrossRef]
- Prusinkiewicz, M.A.; Gameiro, S.F.; Ghasemi, F.; Dodge, M.J.; Zeng, P.Y.F.; Maekebay, H.; Barrett, J.W.; Nichols, A.C.; Mymryk, J.S. Survival-Associated Metabolic Genes in Human Papillomavirus-Positive Head and Neck Cancers. Cancers 2020, 12, 253. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. The SREBP Pathway: Regulation of Cholesterol Metabolism by Proteolysis of a Membrane-Bound Transcription Factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.; Sordillo, L.M. Role of Lipid Mediators in the Regulation of Oxidative Stress and Inflammatory Responses in Dairy Cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Strüve, K.; Herzog, K.; Magata, F.; Piechotta, M.; Shirasuna, K.; Miyamoto, A.; Bollwein, H. The Effect of Metritis on Luteal Function in Dairy Cows. BMC Vet. Res. 2013, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.; Kang, H.; Choi, H.; Choi, W.; Jun, H. Reactive Oxygen Species-induced Changes in Glucose and Lipid Metabolism Contribute to the Accumulation of Cholesterol in the Liver during Aging. Aging Cell 2019, 18, e12895. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Oguejiofor, C.; Swangchan-Uthai, T.; Carr, S.; Wathes, D. Relationships between Circulating Urea Concentrations and Endometrial Function in Postpartum Dairy Cows. Animals 2015, 5, 748–773. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.; Wilke, T.; Petzinger, E. The Solute Carrier Family SLC10: More than a Family of Bile Acid Transporters Regarding Function and Phylogenetic Relationships. Naunyn. Schmiedebergs Arch. Pharmacol. 2006, 372, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Argraves, W.S.; Morales, C.R. Immunolocalization of Cubilin, Megalin, Apolipoprotein J, and Apolipoprotein A-I in the Uterus and Oviduct. Mol. Reprod. Dev. 2004, 69, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M.; Stefansson, S.; Twal, W.O.; Drake, C.J.; Fleming, P.; Remaley, A.; Brewer, H.B.; Argraves, W.S. Cubilin, the Endocytic Receptor for Intrinsic Factor-Vitamin B 12 Complex, Mediates High-Density Lipoprotein Holoparticle Endocytosis. Proc. Natl. Acad. Sci. USA 1999, 96, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Pleckaityte, M. Cholesterol-Dependent Cytolysins Produced by Vaginal Bacteria: Certainties and Controversies. Front. Cell. Infect. Microbiol. 2020, 9, 452. [Google Scholar] [CrossRef]
- Liu, J.; Liang, Q.; Wang, T.; Ma, B.; Wang, X.; Li, P.; Shaukat, A.; Guo, X.; Deng, G. IFN-τ Mediated miR-26a Targeting PTEN to Activate PI3K/AKT Signalling to Alleviate the Inflammatory Damage of bEECs. Sci. Rep. 2022, 12, 9410. [Google Scholar] [CrossRef]
- Huang, X.; Tan, J.; Chen, X.; Liu, M.; Zhu, H.; Li, W.; He, Z.; Han, J.; Ma, C. Akt Phosphorylation Influences Persistent Chlamydial Infection and Chlamydia-Induced Golgi Fragmentation Without Involving Rab14. Front. Cell. Infect. Microbiol. 2021, 11, 675890. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, I.; Papafragkos, I.; Kolliniati, O.; Lapi, I.; Tsatsanis, C.; Vergadi, E. Akt Inhibition Promotes Autophagy and Clearance of Group B Streptococcus from the Alveolar Epithelium. Pathogens 2022, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tang, Y.-D.; Zhai, J.; Zheng, C. The RING Finger Protein Family in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Srinivasan, S.; Boyer, S.N.; Wazer, D.E.; Band, V. The E6 Oncoproteins of High-Risk Papillomaviruses Bind to a Novel Putative GAP Protein, E6TP1, and Target It for Degradation. Mol. Cell. Biol. 1999, 19, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR Signaling Pathway and mTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Dossou, A.S.; Basu, A. The Emerging Roles of mTORC1 in Macromanaging Autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.-C.; Hsu, S.-C.; Hsieh, Y.-H.; Tsou, W.-L.; Chuang, T.-C.; Liu, J.-Y.; Kao, M.-C. Downregulation of HER2 by RIG1 Involves the PI3K/Akt Pathway in Ovarian Cancer Cells. Carcinogenesis 2008, 29, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, D.; Zhu, L.; Jia, H.; Cai, J.; Li, P.; Han, B.; Wang, D.; Li, H.; Fan, J.; et al. SGSM2 Inhibits Thyroid Cancer Progression by Activating RAP1 and Enhancing Competitive RAS Inhibition. Cell Death Dis. 2022, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.M.; Da Silveira, J.C.; Perrini, C.; Del Collado, M.; Gebremedhn, S.; Tesfaye, D.; Meirelles, F.V.; Perecin, F. The Role of the PI3K-Akt Signaling Pathway in the Developmental Competence of Bovine Oocytes. PLoS ONE 2017, 12, e0185045. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. Role of Akt Signaling in Vascular Homeostasis and Angiogenesis. Circ. Res. 2002, 90, 1243–1250. [Google Scholar] [CrossRef]
- Sipka, A.S.; Chandler, T.L.; Behling-Kelly, E.L.; Overton, T.R.; Mann, S. The Effect of Ex Vivo Lipopolysaccharide Stimulation and Nutrient Availability on Transition Cow Innate Immune Cell AKT/mTOR Pathway Responsiveness. J. Dairy Sci. 2020, 103, 1956–1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Wang, M.; Zhang, L.; Zan, L.; Yang, W. Bta-miR-34b Controls Milk Fat Biosynthesis via the Akt/mTOR Signaling Pathway by Targeting RAI14 in Bovine Mammary Epithelial Cells. J. Anim. Sci. Biotechnol. 2021, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Wientjes, Y.C.; Calus, M.P.; Goddard, M.E.; Hayes, B.J. Impact of QTL Properties on the Accuracy of Multi-Breed Genomic Prediction. Genet. Sel. Evol. 2015, 47, 42. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, E.C.G.; Bauersachs, S.; Tietze, M.; Simianer, H.; Tetens, J.; Thaller, G.; Reinhardt, F.; Wolf, E.; König, S. Exploration of Relationships between Production and Fertility Traits in Dairy Cattle via Association Studies of SNPs within Candidate Genes Derived by Expression Profiling: Relationships between Production and Fertility at the Genomic Level. Anim. Genet. 2011, 42, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.; Cormican, P.; Lim, J.J.; Chapwanya, A.; O’Farrelly, C.; Meade, K.G. Qualitative and Quantitative Differences in Endometrial Inflammatory Gene Expression Precede the Development of Bovine Uterine Disease. Sci. Rep. 2020, 10, 18275. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and Innate Immunity Shape the Development of Postpartum Uterine Disease and the Impact of Endometritis in Dairy Cattle. Annu. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef]
| Herd | Sample | Breed | Phenotype 1 | ||
|---|---|---|---|---|---|
| n | Jersey | Holstein | Case | Control | |
| 1 | 525 | 262 | 263 | 171 | 354 |
| 2 | 980 | 4 | 976 | 158 | 822 |
| 3 | 462 | 0 | 462 | 113 | 349 |
| Total | 1967 | 266 | 1701 | 442 | 1525 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, L.; Campos-Chillon, F.; Sargolzaei, M.; Peterson, D.G.; Sprayberry, K.A.; McArthur, G.; Anderson, P.; Golden, B.; Pokharel, S.; Abo-Ismail, M.K. Molecular Mechanisms Associated with the Development of the Metritis Complex in Dairy Cattle. Genes 2024, 15, 439. https://doi.org/10.3390/genes15040439
Sanchez L, Campos-Chillon F, Sargolzaei M, Peterson DG, Sprayberry KA, McArthur G, Anderson P, Golden B, Pokharel S, Abo-Ismail MK. Molecular Mechanisms Associated with the Development of the Metritis Complex in Dairy Cattle. Genes. 2024; 15(4):439. https://doi.org/10.3390/genes15040439
Chicago/Turabian StyleSanchez, Leanna, Fernando Campos-Chillon, Mehdi Sargolzaei, Daniel G. Peterson, Kim A. Sprayberry, Garry McArthur, Paul Anderson, Bruce Golden, Siroj Pokharel, and Mohammed K. Abo-Ismail. 2024. "Molecular Mechanisms Associated with the Development of the Metritis Complex in Dairy Cattle" Genes 15, no. 4: 439. https://doi.org/10.3390/genes15040439
APA StyleSanchez, L., Campos-Chillon, F., Sargolzaei, M., Peterson, D. G., Sprayberry, K. A., McArthur, G., Anderson, P., Golden, B., Pokharel, S., & Abo-Ismail, M. K. (2024). Molecular Mechanisms Associated with the Development of the Metritis Complex in Dairy Cattle. Genes, 15(4), 439. https://doi.org/10.3390/genes15040439





