Causal Associations of Glaucoma and Age-Related Macular Degeneration with Cataract: A Bidirectional Two-Sample Mendelian Randomisation Study
Abstract
1. Introduction
2. Materials and Methods
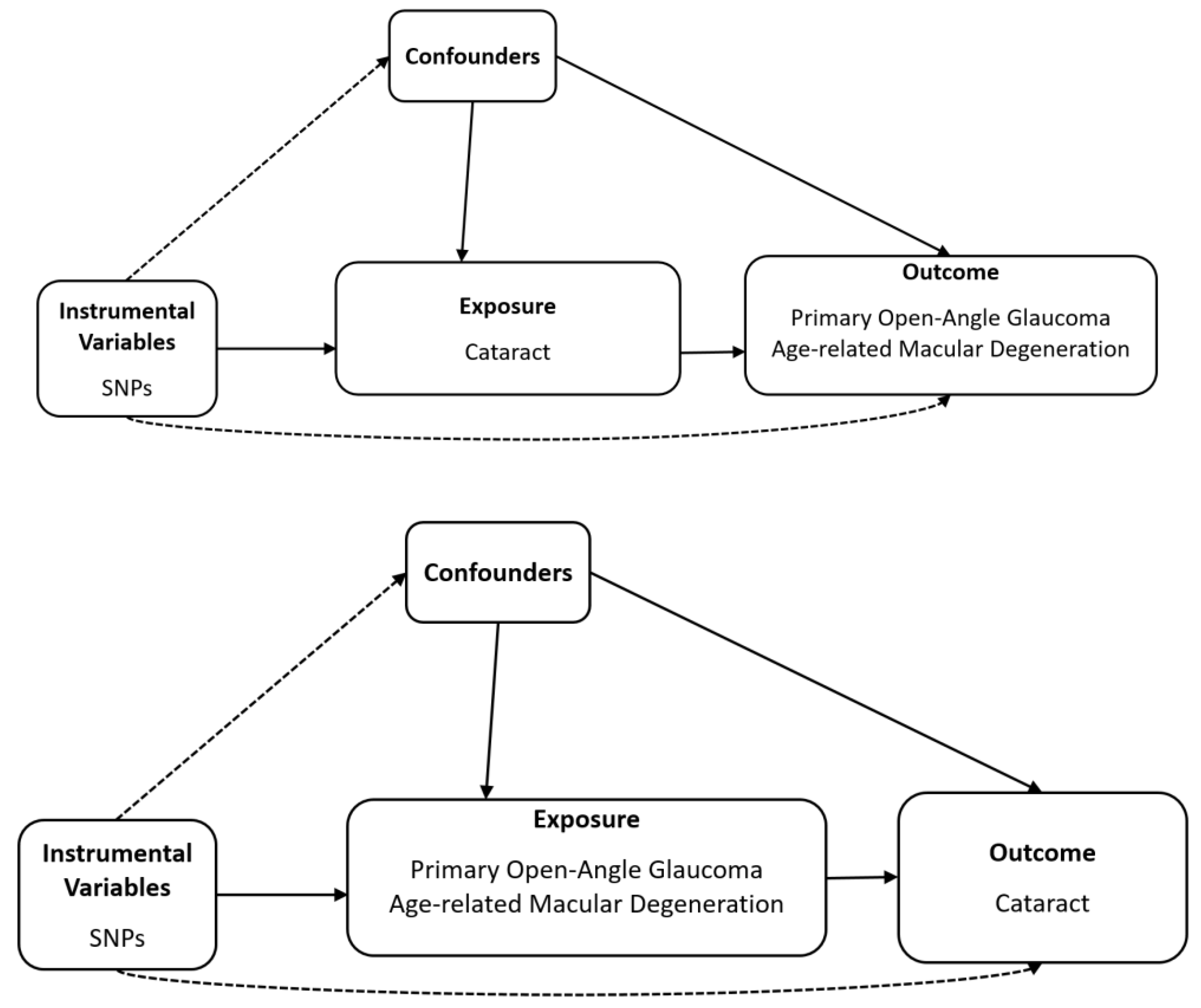
2.1. Study Design
2.2. Data Source
2.3. Selection of the Genetic Instrumental Variables
2.4. Mendelian Randomisation
3. Results
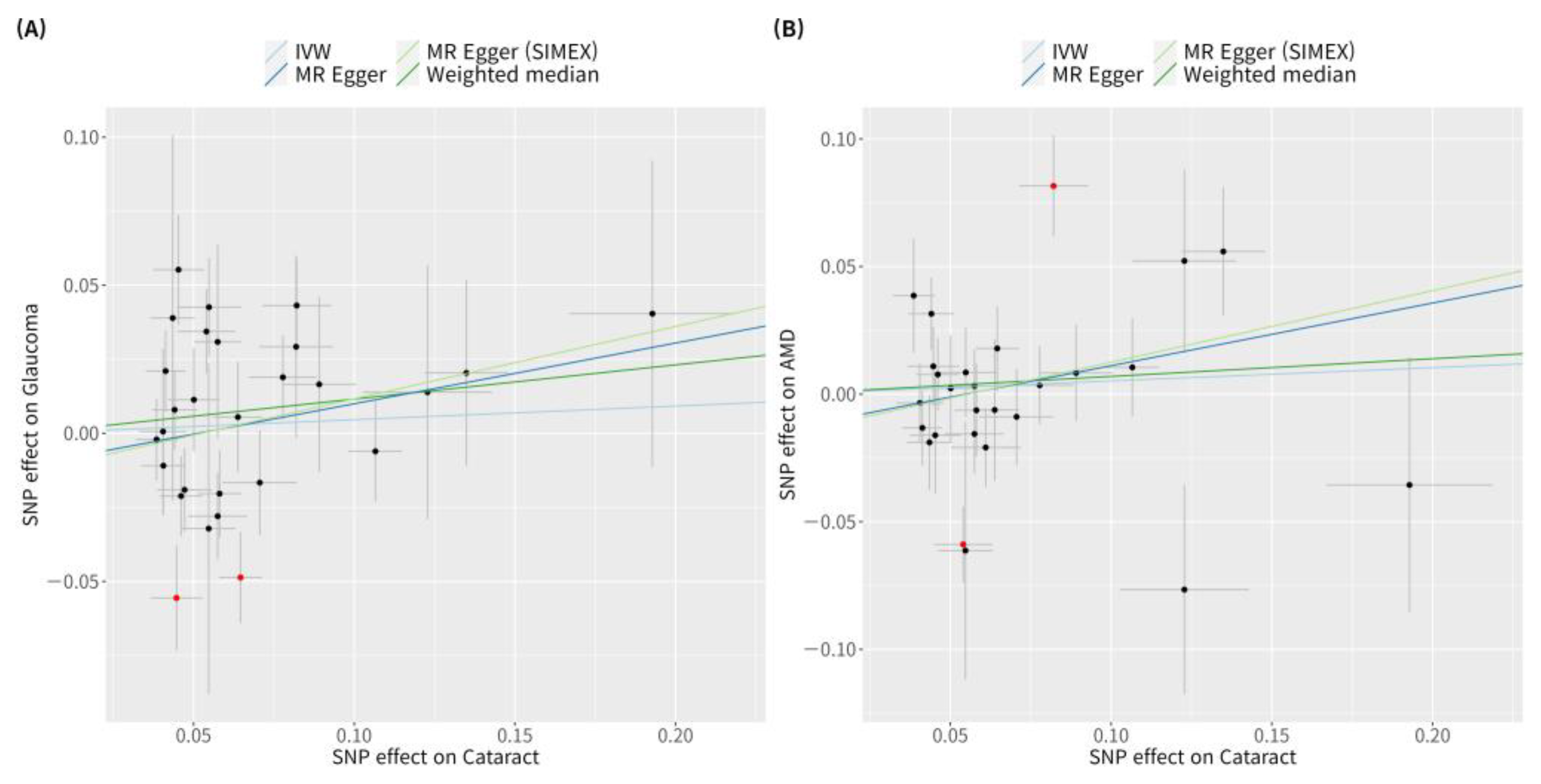
3.1. Genetic Instrumental Variables for the Causal Association of Cataract with Glaucoma or AMD
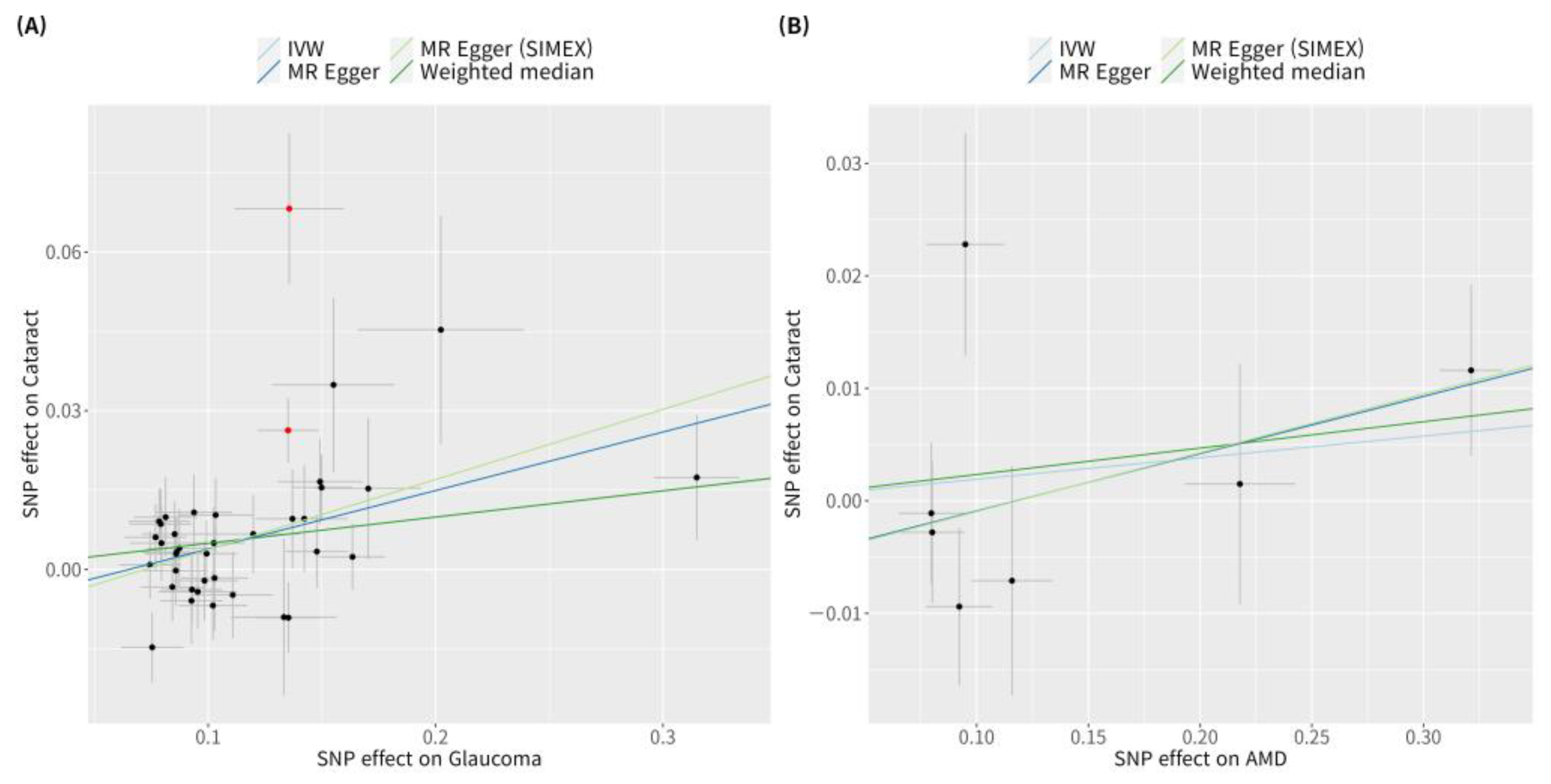
3.2. Genetic Instrumental Variables for the Causal Association of Glaucoma or AMD with Cataract
3.3. Heterogeneity and Horizontal Pleiotropy of Instrumental Variables
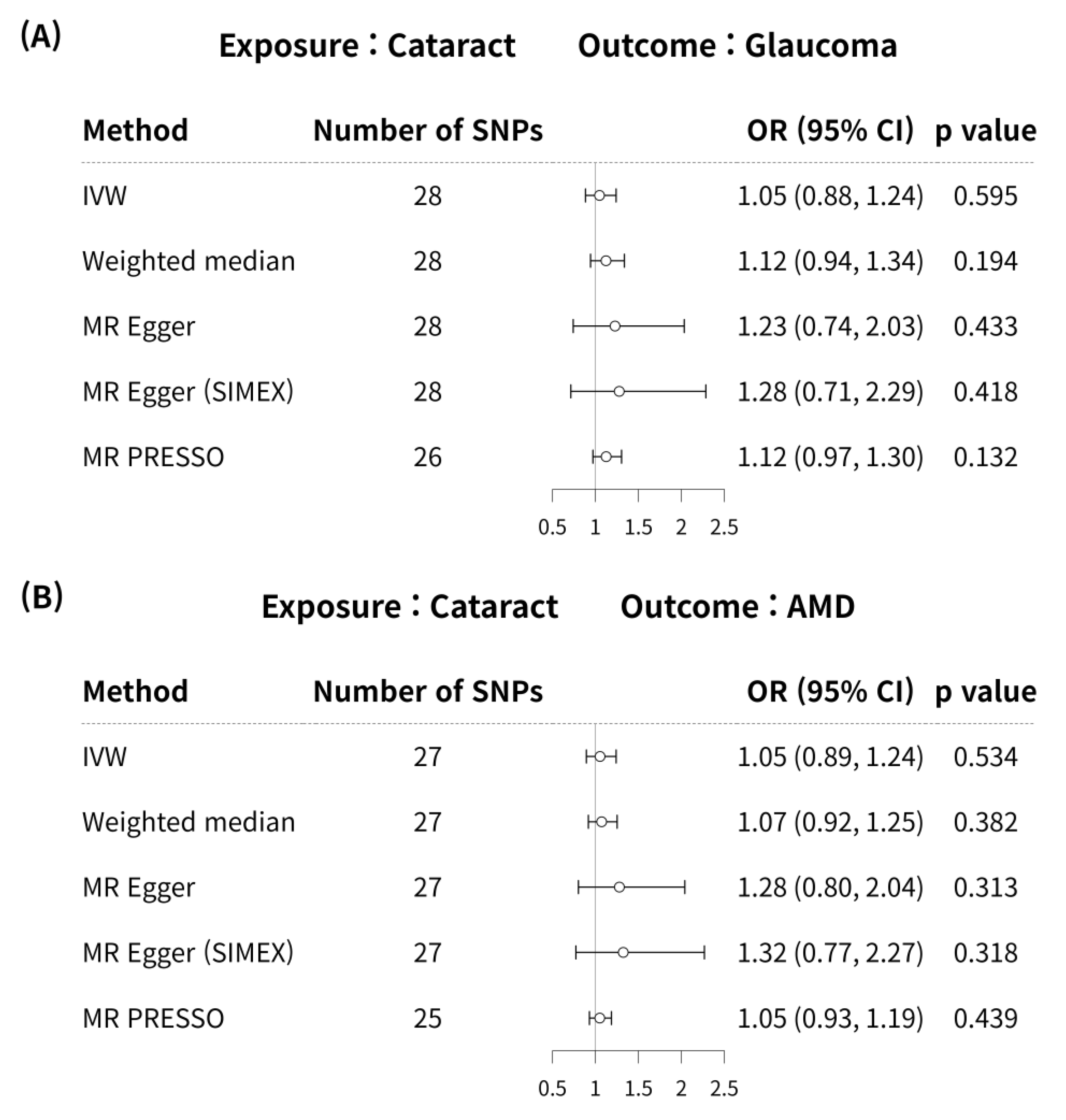
3.4. Mendelian Randomisation for Causal Association of Cataract with Glaucoma and AMD
3.5. Mendelian Randomisation (Reverse-Wise) for Causal Association of Glaucoma or AMD with Cataract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.M.; Afshari, N.A. The global state of cataract blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Layana, A.; Cabrera-Lopez, F.; Garcia-Arumi, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef]
- Christenbury, J.G.; Folgar, F.A.; O’Connell, R.V.; Chiu, S.J.; Farsiu, S.; Toth, C.A.; Age-related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 2013, 120, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dai, Y.; Chen, Y.; Yu, D.Y.; Cringle, S.J.; Chen, J.; Kong, X.; Wang, X.; Jiang, C. Primary angle closure glaucoma: What we know and what we don’t know. Prog. Retin. Eye Res. 2017, 57, 26–45. [Google Scholar] [CrossRef]
- Jefferis, J.M.; Taylor, J.P.; Collerton, J.; Jagger, C.; Kingston, A.; Davies, K.; Kirkwood, T.; Clarke, M.P. The association between diagnosed glaucoma and cataract and cognitive performance in very old people: Cross-sectional findings from the newcastle 85+ study. Ophthalmic Epidemiol. 2013, 20, 82–88. [Google Scholar] [CrossRef]
- Lee, C.S.; Gibbons, L.E.; Lee, A.Y.; Yanagihara, R.T.; Blazes, M.S.; Lee, M.L.; McCurry, S.M.; Bowen, J.D.; McCormick, W.C.; Crane, P.K.; et al. Association Between Cataract Extraction and Development of Dementia. JAMA Intern. Med. 2022, 182, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Rajavi, Z.; Moezzi-Ghadim, H.; Kamrava, K. The effect of trabeculectomy on cataract formation or progression. J. Ophthalmic Vis. Res. 2009, 4, 84–89. [Google Scholar] [PubMed]
- Dada, T.; Bhartiya, S.; Begum Baig, N. Cataract Surgery in Eyes with Previous Glaucoma Surgery: Pearls and Pitfalls. J. Curr. Glaucoma Pract. 2013, 7, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.E.; Munoz, B.; West, S.K.; Tielsch, J.M.; Schein, O.D. Is there an association between cataract surgery and age-related macular degeneration? Data from three population-based studies. Am. J. Ophthalmol. 2003, 135, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Chew, E.Y. Cataract surgery and the risk of progression of macular degeneration. Curr. Opin. Ophthalmol. 2023, 34, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Rim, T.H.; Lee, C.S.; Lee, S.C.; Kim, S.; Kim, S.S.; Epidemiologic Survey Committee of the Korean Ophthalmologica. Association between Previous Cataract Surgery and Age-Related Macular Degeneration. Semin. Ophthalmol. 2017, 32, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Howard, K.P.; Lee, K.E.; Iyengar, S.K.; Sivakumaran, T.A.; Klein, R. The relationship of cataract and cataract extraction to age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology 2012, 119, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Arepalli, S.; Kaiser, P.K. Pipeline therapies for neovascular age related macular degeneration. Int. J. Retin. Vitr. 2021, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Kaiserman, I.; Kaiserman, N.; Elhayany, A.; Vinker, S. Cataract surgery is associated with a higher rate of photodynamic therapy for age-related macular degeneration. Ophthalmology 2007, 114, 278–282. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 2015, 181, 251–260. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Huang, Y.; Guan, P.; Huang, D.; Yu, H.; Yang, X.; Liu, L. Mendelian randomization analyses in ocular disease: A powerful approach to causal inference with human genetic data. J. Transl. Med. 2022, 20, 621. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Lee, Y. Causal Association between Iritis or Uveitis and Glaucoma: A Two-Sample Mendelian Randomisation Study. Genes 2023, 14, 642. [Google Scholar] [CrossRef]
- Choquet, H.; Khawaja, A.P.; Jiang, C.; Yin, J.; Melles, R.B.; Glymour, M.M.; Hysi, P.G.; Jorgenson, E. Association Between Myopic Refractive Error and Primary Open-Angle Glaucoma: A 2-Sample Mendelian Randomization Study. JAMA Ophthalmol. 2022, 140, 864–871. [Google Scholar] [CrossRef]
- Chong, R.S.; Li, H.; Cheong, A.J.Y.; Fan, Q.; Koh, V.; Raghavan, L.; Nongpiur, M.E.; Cheng, C.Y. Mendelian Randomization Implicates Bidirectional Association between Myopia and Primary Open-Angle Glaucoma or Intraocular Pressure. Ophthalmology 2023, 130, 394–403. [Google Scholar] [CrossRef]
- Han, X.; Ong, J.S.; An, J.; Craig, J.E.; Gharahkhani, P.; Hewitt, A.W.; MacGregor, S. Association of Myopia and Intraocular Pressure with Retinal Detachment in European Descent Participants of the UK Biobank Cohort: A Mendelian Randomization Study. JAMA Ophthalmol. 2020, 138, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Melles, R.B.; Sangani, P.; Hoffmann, T.J.; Hysi, P.G.; Glymour, M.M.; Jorgenson, E.; Lachke, S.A.; Choquet, H. Association of Behavioral and Clinical Risk Factors with Cataract: A Two-Sample Mendelian Randomization Study. Invest. Ophthalmol. Vis. Sci. 2023, 64, 19. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Choquet, H.; Paylakhi, S.; Kneeland, S.C.; Thai, K.K.; Hoffmann, T.J.; Yin, J.; Kvale, M.N.; Banda, Y.; Tolman, N.G.; Williams, P.A.; et al. A multiethnic genome-wide association study of primary open-angle glaucoma identifies novel risk loci. Nat. Commun. 2018, 9, 2278. [Google Scholar] [CrossRef]
- Winkler, T.W.; Grassmann, F.; Brandl, C.; Kiel, C.; Gunther, F.; Strunz, T.; Weidner, L.; Zimmermann, M.E.; Korb, C.A.; Poplawski, A.; et al. Genome-wide association meta-analysis for early age-related macular degeneration highlights novel loci and insights for advanced disease. BMC Med. Genom. 2020, 13, 120. [Google Scholar] [CrossRef]
- Loh, M.; Zhang, W.; Ng, H.K.; Schmid, K.; Lamri, A.; Tong, L.; Ahmad, M.; Lee, J.J.; Ng, M.C.Y.; Petty, L.E.; et al. Identification of genetic effects underlying type 2 diabetes in South Asian and European populations. Commun. Biol. 2022, 5, 329. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G.; Collaboration, C.C.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.A.; Seo, J.H. Causal Association of Obesity and Dyslipidemia with Type 2 Diabetes: A Two-Sample Mendelian Randomization Study. Genes 2022, 13, 2407. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.A.; Thompson, J.R. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 2016, 45, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Publisher Correction: Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 1196. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Greco, M.F.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lee, S.; Won, S. Causal Evaluation of Laboratory Markers in Type 2 Diabetes on Cancer and Vascular Diseases Using Various Mendelian Randomization Tools. Front. Genet. 2020, 11, 597420. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.T.; Yoon, B.W.; Seo, J.H. Analysis of risk allele frequencies of single nucleotide polymorphisms related to open-angle glaucoma in different ethnic groups. BMC Med. Genom. 2021, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.T.; Yoon, B.W.; Seo, J.H. Comparison of risk allele frequencies of single nucleotide polymorphisms associated with age-related macular degeneration in different ethnic groups. BMC Ophthalmol. 2021, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.W.; Shin, H.T.; Seo, J.H. Risk Allele Frequency Analysis and Risk Prediction of Single-Nucleotide Polymorphisms for Prostate Cancer. Genes 2022, 13, 2039. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.W.; Shin, H.T.; Seo, J. Risk Allele Frequency Analysis of Single-Nucleotide Polymorphisms for Vitamin D Concentrations in Different Ethnic Group. Genes 2021, 12, 1530. [Google Scholar] [CrossRef] [PubMed]
- Katan, M.B. Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet 1986, 1, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, S.; Zhu, J.; Luo, D.; Song, W.; Zhou, M. Plasma lipid levels and risk of primary open angle glaucoma: A genetic study using Mendelian randomization. BMC Ophthalmol. 2020, 20, 390. [Google Scholar] [CrossRef]
- Choquet, H.; Melles, R.B.; Yin, J.; Hoffmann, T.J.; Thai, K.K.; Kvale, M.N.; Banda, Y.; Hardcastle, A.J.; Tuft, S.J.; Glymour, M.M.; et al. A multiethnic genome-wide analysis of 44,039 individuals identifies 41 new loci associated with central corneal thickness. Commun. Biol. 2020, 3, 301. [Google Scholar] [CrossRef]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Tai, E.S.; Aung, T.; Tay, W.T.; Saw, S.M.; Seielstad, M.; Wong, T.Y. Relation of age-related cataract with obesity and obesity genes in an Asian population. Am. J. Epidemiol. 2009, 169, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, H.; Zhao, X.; Pan, Y. Association between Cataract Surgery and Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2022, 2022, 6780901. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Sadun, A.A.; Bassi, C.J. Optic nerve damage in Alzheimer’s disease. Ophthalmology 1990, 97, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, S.; Ding, Y.; Li, L.; Huang, C.; Bao, M.; Li, S.; Wang, Q. Dissecting causal associations of type 2 diabetes with 111 types of ocular conditions: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1307468. [Google Scholar] [CrossRef] [PubMed]
- Lachke, S.A.; Alkuraya, F.S.; Kneeland, S.C.; Ohn, T.; Aboukhalil, A.; Howell, G.R.; Saadi, I.; Cavallesco, R.; Yue, Y.; Tsai, A.C.; et al. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 2011, 331, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hauser, M.A.; Akafo, S.K.; Qin, X.; Miura, S.; Gibson, J.R.; Wheeler, J.; Gaasterland, D.E.; Challa, P.; Herndon, L.W.; et al. Investigation of known genetic risk factors for primary open angle glaucoma in two populations of African ancestry. Invest. Ophthalmol. Vis. Sci. 2013, 54, 6248–6254. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef]
- Donato, L.; Alibrandi, S.; Scimone, C.; Rinaldi, C.; Dascola, A.; Calamuneri, A.; D’Angelo, R.; Sidoti, A. The impact of modifier genes on cone-rod dystrophy heterogeneity: An explorative familial pilot study and a hypothesis on neurotransmission impairment. PLoS ONE 2022, 17, e0278857. [Google Scholar] [CrossRef]
- Hirbo, J.B.; Pasutto, F.; Gamazon, E.R.; Evans, P.; Pawar, P.; Berner, D.; Sealock, J.; Tao, R.; Straub, P.S.; Konkashbaev, A.I.; et al. Analysis of genetically determined gene expression suggests role of inflammatory processes in exfoliation syndrome. BMC Genom. 2023, 24, 75. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Zhu, Y.; Yuan, G.; Yang, J.; Yu, W. Mendelian Randomization and Transcriptome-Wide Association Analysis Identified Genes That Were Pleiotropically Associated with Intraocular Pressure. Genes 2023, 14, 1027. [Google Scholar] [CrossRef] [PubMed]
- Bontzos, G.; Agiorgiotakis, M.; Detorakis, E.T. Long-term Follow-up of Patients receiving Intraocular Pressure-lowering Medications as Cataract Surgery Candidates: A Case-control Study. J. Curr. Glaucoma Pract. 2017, 11, 107–112. [Google Scholar] [CrossRef]
- Kuppens, E.V.; van Best, J.A.; Sterk, C.C. Is glaucoma associated with an increased risk of cataract? Br. J. Ophthalmol. 1995, 79, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Fisher, S.A.; Fritsche, L.G.; Keilhauer, C.N.; Lichtner, P.; Meitinger, T.; Weber, B.H. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum. Mol. Genet. 2005, 14, 3227–3236. [Google Scholar] [CrossRef]
- De Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, G.R.; Curcio, C.A.; Swaroop, A. Age-related macular degeneration: Genetics and biology coming together. Annu. Rev. Genom. Hum. Genet. 2014, 15, 151–171. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef]
- DeAngelis, M.M.; Owen, L.A.; Morrison, M.A.; Morgan, D.J.; Li, M.; Shakoor, A.; Vitale, A.; Iyengar, S.; Stambolian, D.; Kim, I.K.; et al. Genetics of age-related macular degeneration (AMD). Hum. Mol. Genet. 2017, 26, R45–R50. [Google Scholar] [CrossRef]
- Kuan, V.; Warwick, A.; Hingorani, A.; Tufail, A.; Cipriani, V.; Burgess, S.; Sofat, R.; International AMD Genomics Consortium (IAMDGC). Association of Smoking, Alcohol Consumption, Blood Pressure, Body Mass Index, and Glycemic Risk Factors with Age-Related Macular Degeneration: A Mendelian Randomization Study. JAMA Ophthalmol. 2021, 139, 1299–1306. [Google Scholar] [CrossRef]
- Grover, S.; Sharma, M.; International Age-Related Macular Degeneration Genomics Consortium (IAMDGC). Sleep, Pain, and Neurodegeneration: A Mendelian Randomization Study. Front. Neurol. 2022, 13, 765321. [Google Scholar] [CrossRef]
- Jiang, L.; Li, J.C.; Tang, B.S.; Guo, J.F.; Shen, L. Lack of bidirectional association between age-related macular degeneration and Alzheimer’s disease: A Mendelian randomization study. Alzheimer’s Dement. 2022, 18, 2725–2729. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Cheng, J.; Wang, M.; Zhong, Y.; Shi, G.; Yu, A.Y. Causal Associations of Thyroid Function and Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study. Am. J. Ophthalmol. 2022, 239, 108–114. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G. Mendelian Randomization Implicates High-Density Lipoprotein Cholesterol-Associated Mechanisms in Etiology of Age-Related Macular Degeneration. Ophthalmology 2017, 124, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ong, J.S.; An, J.; Hewitt, A.W.; Gharahkhani, P.; MacGregor, S. Using Mendelian randomization to evaluate the causal relationship between serum C-reactive protein levels and age-related macular degeneration. Eur. J. Epidemiol. 2020, 35, 139–146. [Google Scholar] [CrossRef]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A.; Findl, O.; Dick, H.B.; Desmettre, T.; Ledesma-Gil, G.; Curcio, C.A.; Turner, P.L. The Blue Light Hazard Versus Blue Light Hype. Am. J. Ophthalmol. 2022, 240, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kessel, L.; Erngaard, D.; Flesner, P.; Andresen, J.; Tendal, B.; Hjortdal, J. Cataract surgery and age-related macular degeneration. An evidence-based update. Acta Ophthalmol. 2015, 93, 593–600. [Google Scholar] [CrossRef]
- Casparis, H.; Lindsley, K.; Kuo, I.C.; Sikder, S.; Bressler, N.B. Surgery for cataracts in people with age-related macular degeneration. Cochrane Database Syst. Rev. 2012, 6, CD006757. [Google Scholar] [CrossRef]
- Minelli, C.; Del Greco, M.F.; van der Plaat, D.A.; Bowden, J.; Sheehan, N.A.; Thompson, J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int. J. Epidemiol. 2021, 50, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zheng, Z.; Fang, H.; Yang, J. A generalized linear mixed model association tool for biobank-scale data. Nat. Genet. 2021, 53, 1616–1621. [Google Scholar] [CrossRef] [PubMed]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segre, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef] [PubMed]

| Trait | Data Source | No. of Participants | Population | No. of Variants | Reference |
|---|---|---|---|---|---|
| Cataract | BBJ + UKB | 670,603 77,713 cases + 592,890 controls | East Asian + European | 25,845,331 | [28] |
| Glaucoma | GERA cohort + UKB | 240,302 12,315 cases + 227,987 controls | Multi-ethnic: 214,102 European; 5103 African unspecified; 3571 Other admixed ancestry; 1847 African American or Afro-Caribbean; 5189 Hispanic or Latin American; 5370 East Asian; 5120 South Asian. | 7,760,820 | [29] |
| AMD | 11 sources of data, including the IAMDGC and UKB | 105,248 14,034 cases + 91,214 controls | European | 11,703,383 | [30] |
| Exposure | Outcome | Heterogeneity | Horizontal Pleiotropy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MR-Egger | MR-Egger (SIMEX) | ||||||||||
| N | F | I2 (%) | p * | p † | p ‡ | Intercept, β (SE) | p | Intercept, β (SE) | p | ||
| Cataract | Glaucoma | 28 | 132.84 | 94.31 | <0.001 | <0.001 | <0.001 | −0.011 (0.016) | 0.517 | −0.013 (0.018) | 0.489 |
| Cataract | AMD | 27 | 147.63 | 89.53 | <0.001 | <0.001 | <0.001 | −0.013 (0.015) | 0.393 | −0.016 (0.018) | 0.384 |
| Glaucoma | Cataract | 39 | 182.81 | 81.86 | 0.001 | 0.002 | 0.001 | −0.007 (0.005) | 0.173 | −0.010 (0.006) | 0.121 |
| AMD | Cataract | 7 | 169.33 | 97.34 | 0.168 | 0.165 | 0.308 | −0.006 (0.007) | 0.413 | −0.006 (0.007) | 0.409 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.H.; Lee, Y. Causal Associations of Glaucoma and Age-Related Macular Degeneration with Cataract: A Bidirectional Two-Sample Mendelian Randomisation Study. Genes 2024, 15, 413. https://doi.org/10.3390/genes15040413
Seo JH, Lee Y. Causal Associations of Glaucoma and Age-Related Macular Degeneration with Cataract: A Bidirectional Two-Sample Mendelian Randomisation Study. Genes. 2024; 15(4):413. https://doi.org/10.3390/genes15040413
Chicago/Turabian StyleSeo, Je Hyun, and Young Lee. 2024. "Causal Associations of Glaucoma and Age-Related Macular Degeneration with Cataract: A Bidirectional Two-Sample Mendelian Randomisation Study" Genes 15, no. 4: 413. https://doi.org/10.3390/genes15040413
APA StyleSeo, J. H., & Lee, Y. (2024). Causal Associations of Glaucoma and Age-Related Macular Degeneration with Cataract: A Bidirectional Two-Sample Mendelian Randomisation Study. Genes, 15(4), 413. https://doi.org/10.3390/genes15040413






