Functional Differentiation of the Duplicated Gene BrrCIPK9 in Turnip (Brassica rapa var. rapa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification and Structural Analyses of BrrCIPK9 Genes in Turnip
2.2. Phylogenetic Analysis and Chromosomal Localization
2.3. Plant Material, Growth Conditions, and Stress Treatments
2.4. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.5. BrrCIPK9s-CBLs Yeast Two-Hybrid Assay
2.6. Subcellular Localization of BrrCIPK9s
2.7. Overexpression of BrrCIPK9 Genes in Arabidopsis
3. Results
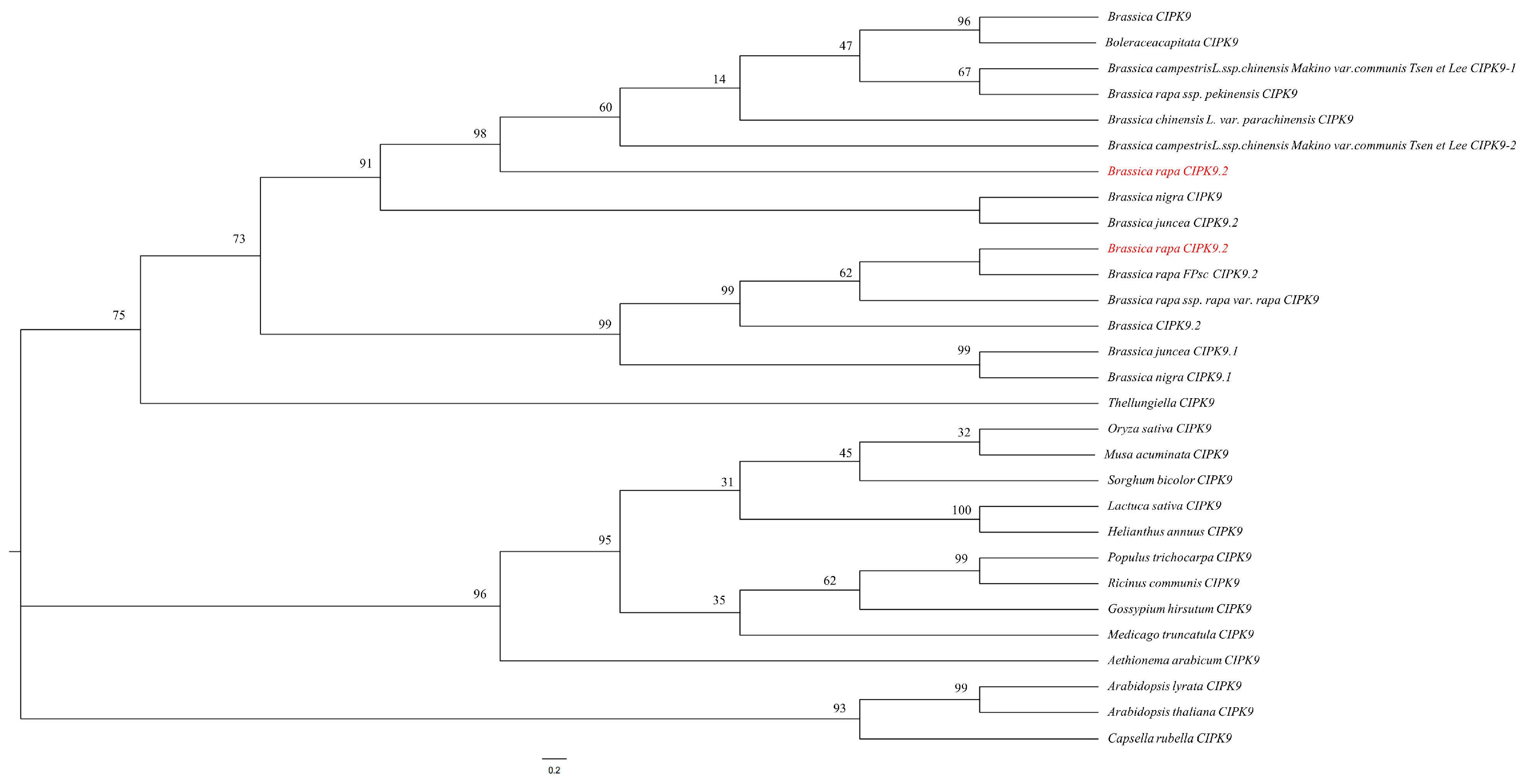
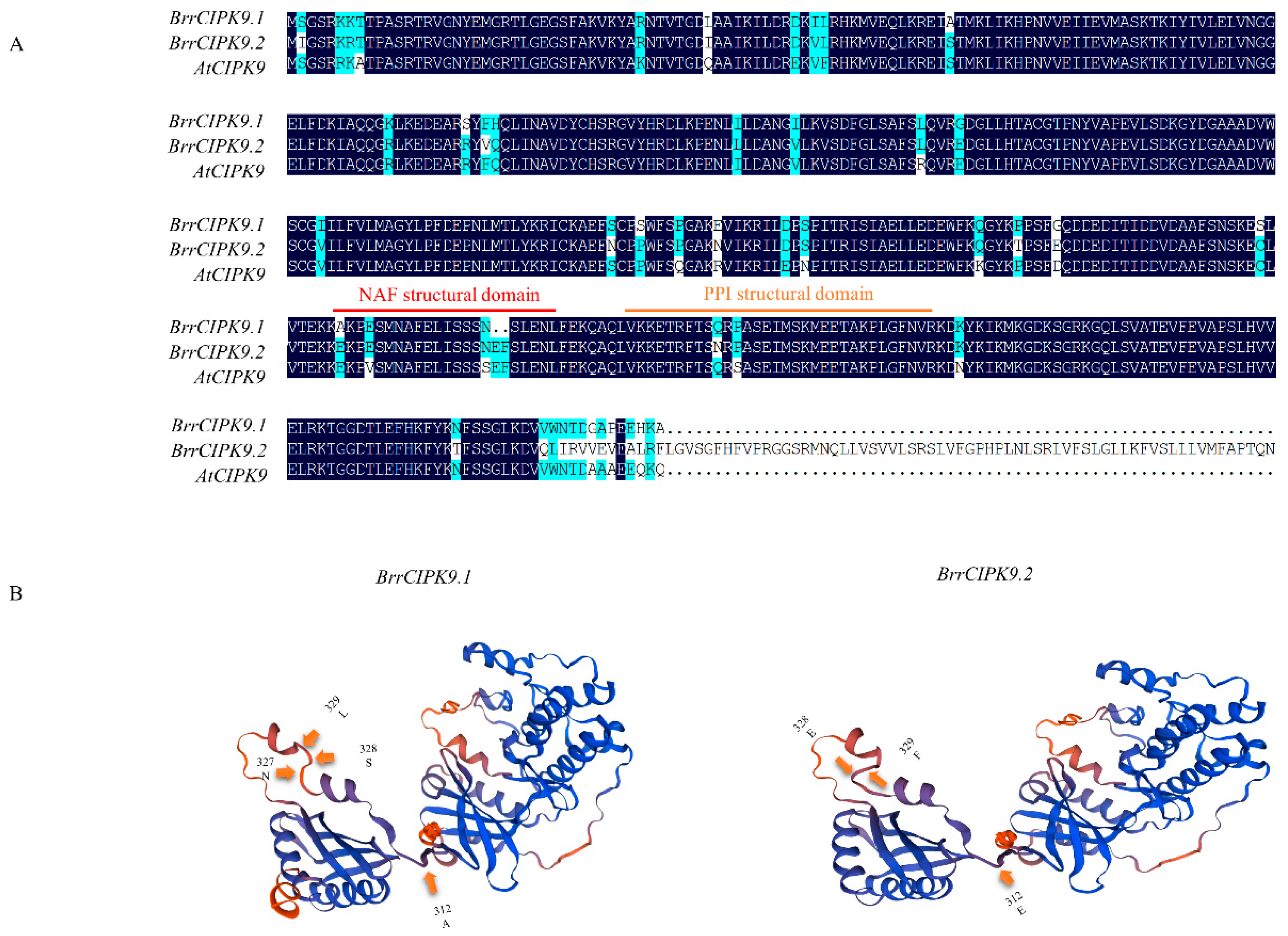
3.1. Characterization and Phylogenetic Relationships of BrrCIPK9 Genes
3.2. Interactions between BrrCIPK9s and BrrCBLs
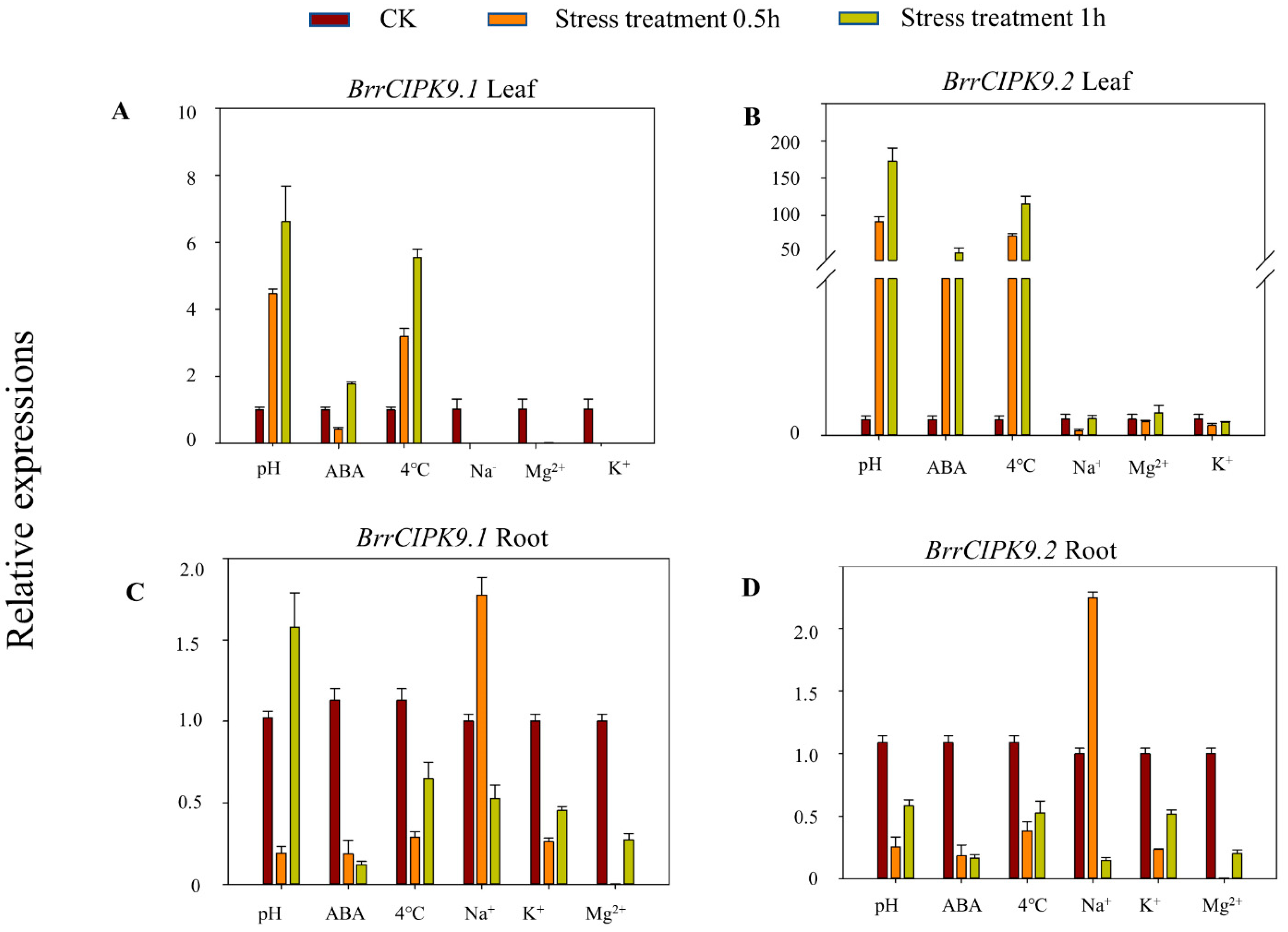
3.3. Transcriptional Profiles of BrrCIPK9 Genes in Turnip under Stress Treatments
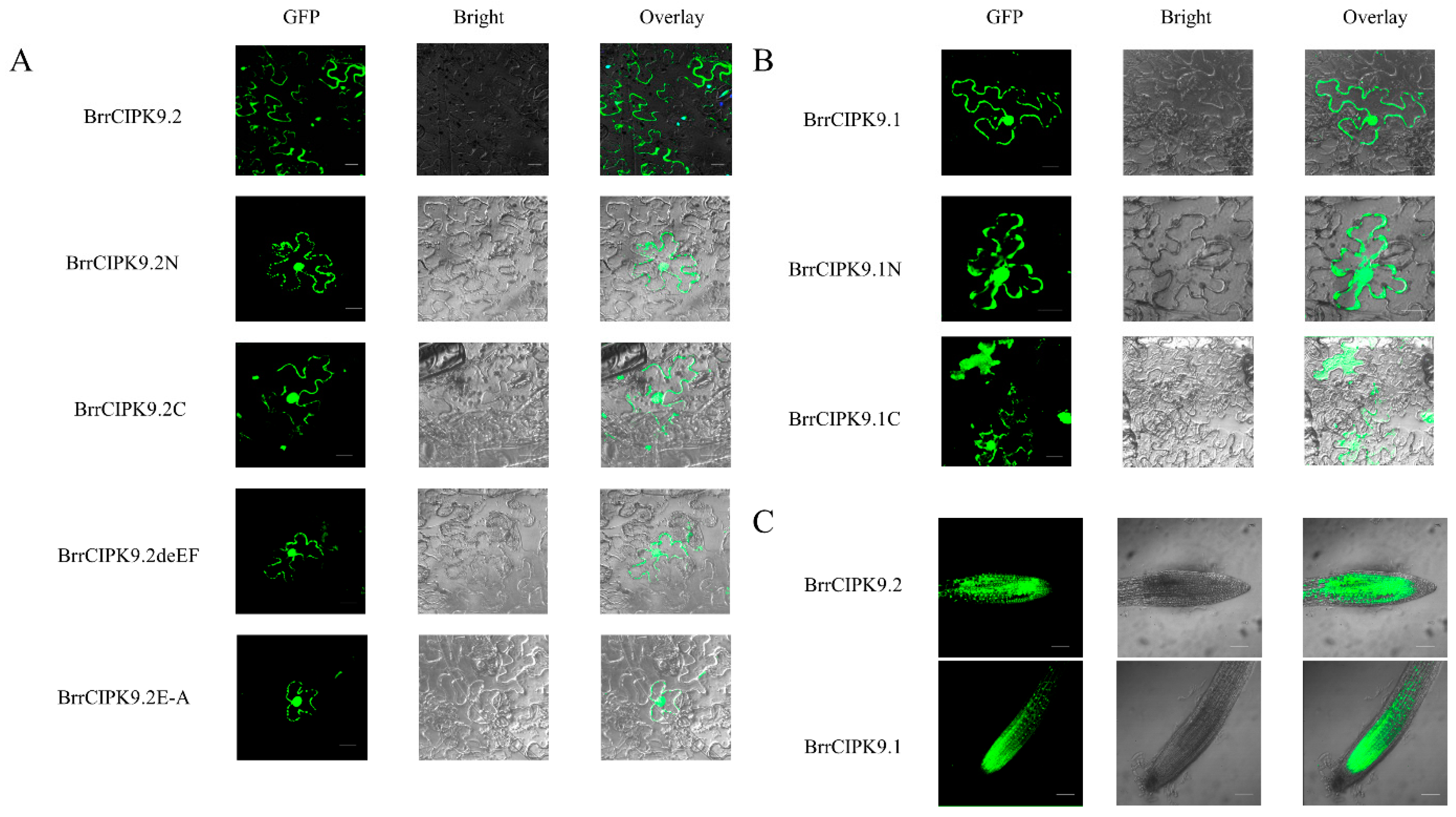
3.4. Subcellular Localization Analysis of BrrCIPK9s
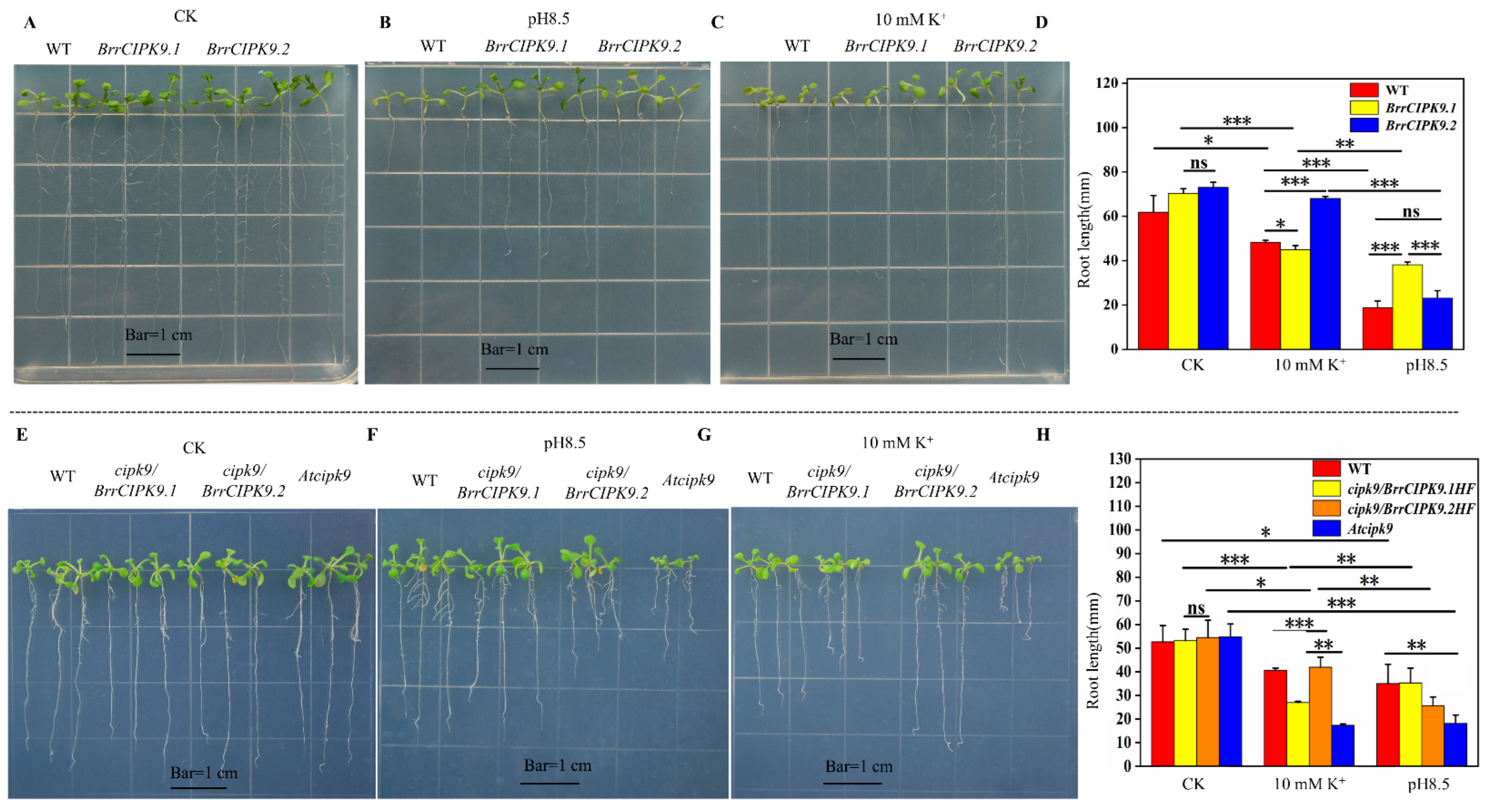
3.5. Functional Analysis of BrrCIPK9s under Different Abiotic Stresses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Z.; Schnable, J.C. Functional divergence between subgenomes and gene pairs after whole genome duplications. Mol. Plant 2018, 11, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, A.; Shirai, K.; Hanada, K. Degree of functional divergence in duplicates is associated with distinct roles in plant evolution. Mol. Biol. Evol. 2021, 38, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wu, J.; Wang, X. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014, 1, 14024. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.K.; Liu, Y.L.; Xia, E.H.; Gao, L.Z. Prevalent role of gene features in determining evolutionary fates of whole-genome duplication duplicated genes in flowering plants. Plant Physiol. 2013, 161, 1844–1861. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wang, B.; Zhang, W.; Shan, H.; Kong, H. Gains and losses of cis-regulatory elements led to divergence of the Arabidopsis apetala1 and cauliflower duplicate genes in the time, space, and level of expression and regulation of one paralog by the other. Plant Physiol. 2016, 171, 1055–1069. [Google Scholar] [PubMed]
- Wang, Z.; Zhou, Z.; Liu, Y.; Liu, T.; Li, Q.; Ji, Y.; Li, C.; Fang, C.; Wang, M.; Wu, M.; et al. Functional evolution of phosphatidylethanolamine binding proteins in Soybean and Arabidopsis. Plant Cell 2015, 27, 323–336. [Google Scholar] [CrossRef] [PubMed]
- De Smet, R.; Sabaghian, E.; Li, Z.; Saeys, Y.; Van de Peer, Y. Coordinated functional divergence of genes after genome duplication in Arabidopsis thaliana. Plant Cell 2017, 29, 2786–2800. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, K.; Gong, Y.; Yang, Y.; Yue, Y. Genome-wide identification and functional analysis of the calcineurin B-like protein and calcineurin B-like protein-interacting protein kinase gene families in chinese cabbage (Brassica rapa ssp. pekinensis). Genes 2022, 13, 795. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhang, H.; Xie, F.; Pan, Z.-Y.; Qiu, W.-M.; Tong, Z.; Wang, Z.-Q.; He, X.-J.; Xu, Y.-H.; Sun, Z.-H. Evolution, gene expression, and protein–protein interaction analyses identify candidate CBL-CIPK signalling networks implicated in stress responses to cold and bacterial infection in citrus. BMC Plant Biol. 2022, 22, 420. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Ma, L.; Su, Q.; Pang, Y. Identification and characterization of abiotic stress responsive cbl-cipk family genes in Medicago. Int. J. Mol. Sci. 2021, 22, 4634. [Google Scholar] [CrossRef]
- Ku, H.M.; Vision, T.; Liu, J.; Tanksley, S.D. Comparing sequenced segments of the tomato and Arabidopsis genomes: Large-scale duplication followed by selective gene loss creates a network of synteny. Proc. Natl. Acad. Sci. USA 2000, 97, 9121–9126. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.D.; Wu, M.; Eisen, J.A.; Salzberg, S.L. The age of the Arabidopsis thaliana genome duplication. Plant Mol. Biol. 2003, 51, 859–866. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Galvani, A.P.; Bush, R.M. Ecological and immunological determinants of influenza evolution. Nature 2003, 422, 428–433. [Google Scholar] [CrossRef]
- Town, C.D.; Cheung, F.; Maiti, R.; Crabtree, J.; Haas, B.J.; Wortman, J.R.; Hine, E.E.; Althoff, R.; Arbogast, T.S.; Tallon, L.J.; et al. Comparative genomics of Brassica oleracea and Arabidopsis thaliana reveal gene loss, fragmentation, and dispersal after polyploidy. Plant Cell 2006, 18, 1348–1359. [Google Scholar] [CrossRef]
- Moore, R.C.; Purugganan, M.D. The evolutionary dynamics of plant duplicate genes. Curr. Opin. Plant Biol. 2005, 8, 122–128. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhao, R.; Zhou, Y.; Jiao, Y. Evolutionary strategies drive a balance of the interacting gene products for the CBL and CIPK gene families. New Phytol. 2020, 226, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Wang, J.; Sun, R.; Wu, J.; Liu, S.; Bai, Y.; Mun, J.-H.; Bancroft, I.; Cheng, F.; et al. The genome of the mesopolyploid crop species Brassica rapa. Nat. Genet. 2011, 43, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, Q.; Chen, Q.; Xiang, N.; Yang, Y.; Yang, Y. Genome-wide identification and functional analysis of the calcineurin B-like protein and calcineurin B-like protein-interacting protein kinase gene families in turnip (Brassica rapa var. rapa). Front. Plant Sci. 2017, 8, 1191. [Google Scholar] [CrossRef]
- Song, X.-M.; Wang, J.-P.; Sun, P.-C.; Ma, X.; Yang, Q.-H.; Hu, J.-J.; Sun, S.-R.; Li, Y.-X.; Yu, J.-G.; Feng, S.-Y.; et al. Preferential gene retention increases the robustness of cold regulation in Brassicaceae and other plants after polyploidization. Hortic. Res. 2020, 7, 20. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, J.; Dong, C.; Cheng, Z.M. The CBL and CIPK gene family in grapevine (Vitis vinifera): Genome-wide analysis and expression profiles in response to various abiotic stresses. Front. Plant Sci. 2017, 8, 978. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, Q.; Cheng, Z.; Li, H.; Chang, Y.; Lin, J. Identification of important physiological traits and moderators that are associated with improved salt tolerance in CBL and CIPK overexpressors through a meta-analysis. Front. Plant Sci. 2017, 8, 856. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wang, Y.; Wang, M.; Li, T.; Zhou, Y.; Wang, X.; Wei, S.; He, G.; Yang, G. Identification and comprehensive analyses of the CBL and CIPK gene families in wheat (Triticum aestivum L.). BMC Plant Biol. 2015, 15, 269. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, S.K.; Pandey, A.; Pandey, G.K. The CBL-CIPK signaling module in plants: A mechanistic perspective. Physiol. Plant 2015, 155, 89–108. [Google Scholar] [CrossRef]
- Albrecht, V.; Ritz, O.; Linder, S.; Harter, K.; Kudla, J. The naf domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 2001, 20, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Sanjuan, A.; Sanchez-Barrena, M.J.; Gonzalez-Rubio, J.M.; Moreno, M.; Ragel, P.; Jimenez, M.; Pardo, J.M.; Martinez-Ripoll, M.; Quintero, F.J.; Albert, A. Structural basis of the regulatory mechanism of the plant CIPK family of protein kinases controlling ion homeostasis and abiotic stress. Proc. Natl. Acad. Sci. USA 2014, 111, E4532–E4541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dong, Q.; Zhao, P.; Eickelkamp, A.; Ma, C.; He, G.; Li, F.; Wallrad, L.; Becker, T.; Li, Z. The potassium channel GhAKT2bD is regulated by CBL-CIPK calcium signalling complexes and facilitates K+ allocation in cotton. FEBS Lett. 2022, 596, 1904–1920. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Greaves, J.G.; Jakada, B.H.; Fakher, B.; Wang, X.; Qin, Y. AcCIPK5, a pineapple cbl-interacting protein kinase, confers salt, osmotic and cold stress tolerance in transgenic Arabidopsis. Plant Sci. 2022, 320, 111284. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Zhang, Q.; Wang, Y.; Guan, Y.; Wang, R.; Shi, F.; Zeng, F.; Wang, Y.; Chen, M.; et al. Integrative gene duplication and genome-wide analysis as an approach to facilitate wheat reverse genetics: An example in the TaCIPK family. J. Adv. Res. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Berger, B.; Flügge, U.-I. Specific and coordinated control of indolic and aliphatic glucosinolate biosynthesis by R2R3-myb transcription factors in Arabidopsis thaliana. Phytochem. Rev. 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Yang, T.J.; Kim, J.S.; Kwon, S.J.; Lim, K.B.; Choi, B.S.; Kim, J.A.; Jin, M.; Park, J.Y.; Lim, M.-H.; Kim, H.-I. Sequence-level analysis of the diploidization process in the triplicated flowering locus C region of Brassica rapa. Plant Cell 2006, 18, 1339–1347. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the expasy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Cai, X.; Chang, L.; Zhang, T.; Chen, H.; Zhang, L.; Lin, R.; Liang, J.; Wu, J.; Freeling, M.; Wang, X. Impacts of allopolyploidization and structural variation on intraspecific diversification in Brassica rapa. Genome Biol. 2021, 22, 166. [Google Scholar] [CrossRef] [PubMed]
- Mogami, J.; Fujita, Y.; Yoshida, T.; Tsukiori, Y.; Nakagami, H.; Nomura, Y.; Fujiwara, T.; Nishida, S.; Yanagisawa, S.; Ishida, T.; et al. Two distinct families of protein kinases are required for plant growth under high external Mg2+ concentrations in Arabidopsis. Plant Physiol. 2015, 167, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xiong, L.; Song, C.P.; Gong, D.; Halfter, U.; Zhu, J.K. A calcium sensor and its interacting protein kinase are global regulators of abscisic acid signaling in Arabidopsis. Dev. Cell 2002, 3, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang, A.T.; Guo, Y.; Cuin, T.A.; Qiu, Q.; Song, C.; Kristiansen, K.A.; Bych, K.; Schulz, A.; Shabala, S.; Schumaker, K.S.; et al. Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-atpase by preventing interaction with 14-3-3 protein. Plant Cell 2007, 19, 1617–1634. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Hall, A.; Millar, A.J.; Darrah, C.; Davis, S.J. Protocol: Streamlined sub-protocols for floral-dip transformation and selection of transformants in Arabidopsis thaliana. Plant Methods 2009, 5, 3. [Google Scholar] [CrossRef]
- Kanwar, P.; Sanyal, S.K.; Tokas, I.; Yadav, A.K.; Pandey, A.; Kapoor, S.; Pandey, G.K. Comprehensive structural, interaction and expression analysis of CBL and CIPK complement during abiotic stresses and development in rice. Cell Calcium 2014, 56, 81–95. [Google Scholar] [CrossRef]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of duplicate genes in exon-intron structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef]
- Yamada, T.; Bork, P. Evolution of biomolecular networks: Lessons from metabolic and protein interactions. Nat. Rev. Mol. Cell Biol. 2009, 10, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.A.; Bowers, J.E.; Feltus, F.A.; Paterson, A.H. Buffering of crucial functions by paleologous duplicated genes may contribute cyclicality to angiosperm genome duplication. Proc. Natl. Acad. Sci. USA 2006, 103, 2730–2735. [Google Scholar] [CrossRef]
- Gu, X. Evolution of duplicate genes versus genetic robustness against null mutations. Trends Genet. 2003, 19, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Ren, H.M.; Chen, L.Q.; Wang, Y.; Wu, W.H. A protein kinase, calcineurin B-like protein-interacting protein kinase9, interacts with calcium sensor calcineurin B-like protein3 and regulates potassium homeostasis under low-potassium stress in Arabidopsis. Plant Physiol. 2013, 161, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tao, B.; Mai, J.; Guo, Y.; Li, R.; Chen, R.; Zhao, L.; Wen, J.; Yi, B.; Tu, J.; et al. Kinase CIPK9 integrates glucose and abscisic acid signaling to regulate seed oil metabolism in rapeseed. Plant Physiol. 2023, 191, 1836–1856. [Google Scholar] [CrossRef]
- Basnet, H.; Su, X.B.; Tan, Y.; Meisenhelder, J.; Merkurjev, D.; Ohgi, K.A.; Hunter, T.; Pillus, L.; Rosenfeld, M.G. Tyrosine phosphorylation of histone H2A by CK2 regulates transcriptional elongation. Nature 2014, 516, 267–271. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.; Yang, Y.; Meng, Y. Functional Differentiation of the Duplicated Gene BrrCIPK9 in Turnip (Brassica rapa var. rapa). Genes 2024, 15, 405. https://doi.org/10.3390/genes15040405
Kang H, Yang Y, Meng Y. Functional Differentiation of the Duplicated Gene BrrCIPK9 in Turnip (Brassica rapa var. rapa). Genes. 2024; 15(4):405. https://doi.org/10.3390/genes15040405
Chicago/Turabian StyleKang, Haotong, Yunqiang Yang, and Ying Meng. 2024. "Functional Differentiation of the Duplicated Gene BrrCIPK9 in Turnip (Brassica rapa var. rapa)" Genes 15, no. 4: 405. https://doi.org/10.3390/genes15040405
APA StyleKang, H., Yang, Y., & Meng, Y. (2024). Functional Differentiation of the Duplicated Gene BrrCIPK9 in Turnip (Brassica rapa var. rapa). Genes, 15(4), 405. https://doi.org/10.3390/genes15040405




