Long-Term Tissue Preservation at Ambient Temperature for Post-Mass Fatality Incident DNA-Based Victim Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- Non-iodized kitchen salt (Pagoda, Beijing, China) at ambient temperature (∼25 °C);
- Allprotect™ Tissue Reagent (Qiagen, Germantown, MD, USA) at ambient temperature;
- Vodka, 40% ethanol by volume (Absolut, Stockholm, Sweden) at ambient temperature;
- Freezing at −20 °C without cryoprotectants.
2.2. DNA Processing and Comparisons
2.3. Statistical Analyses
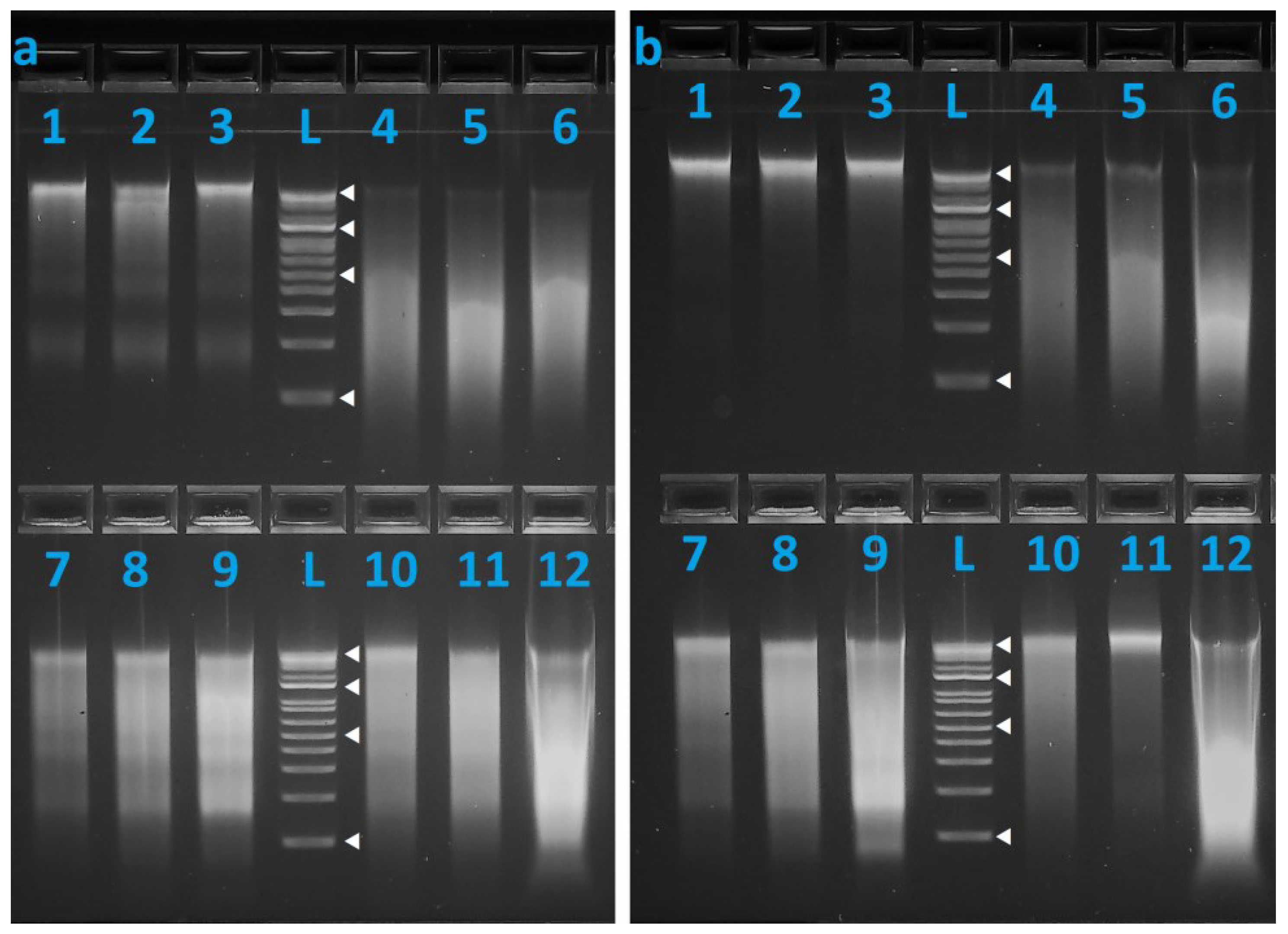
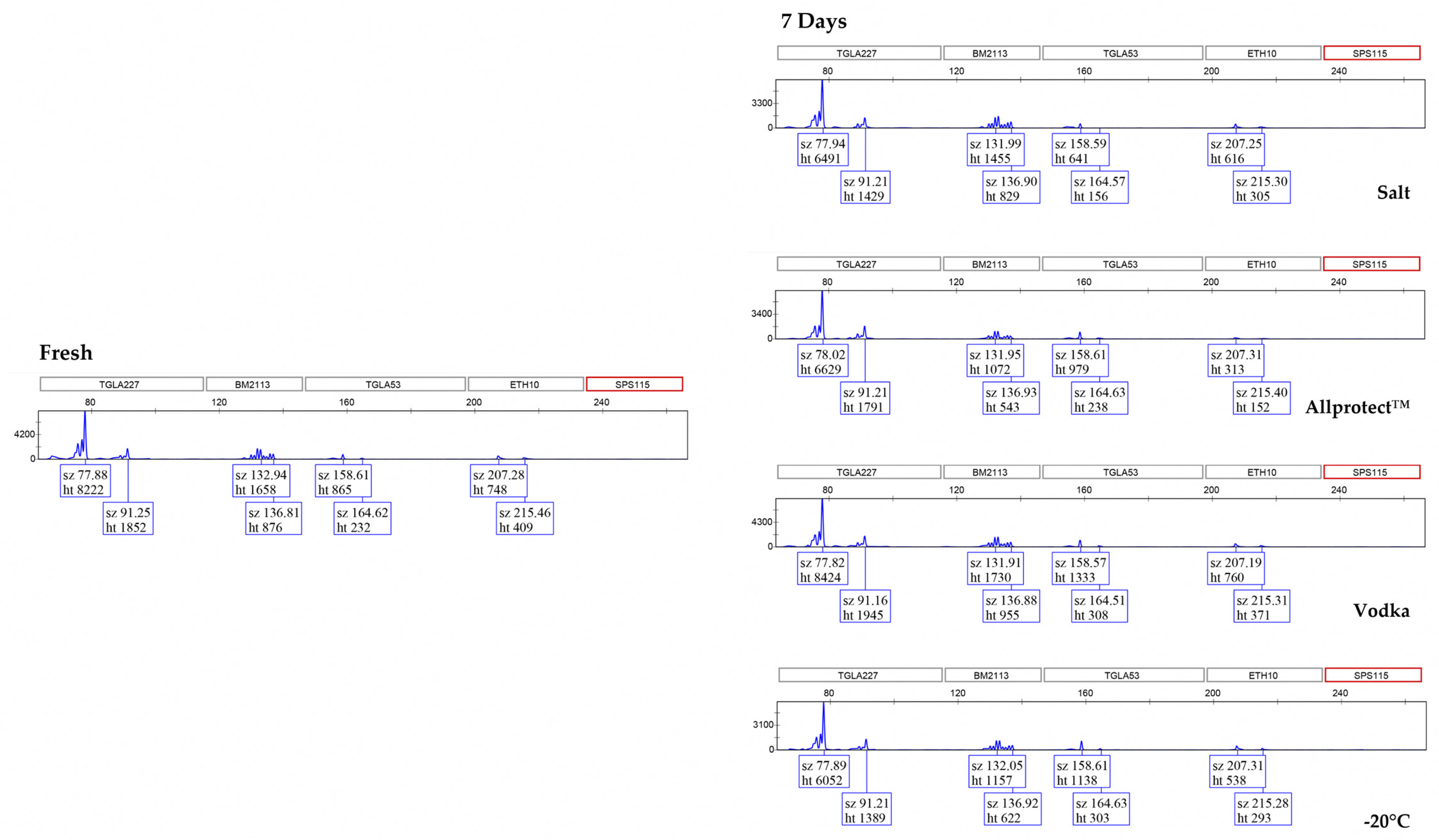
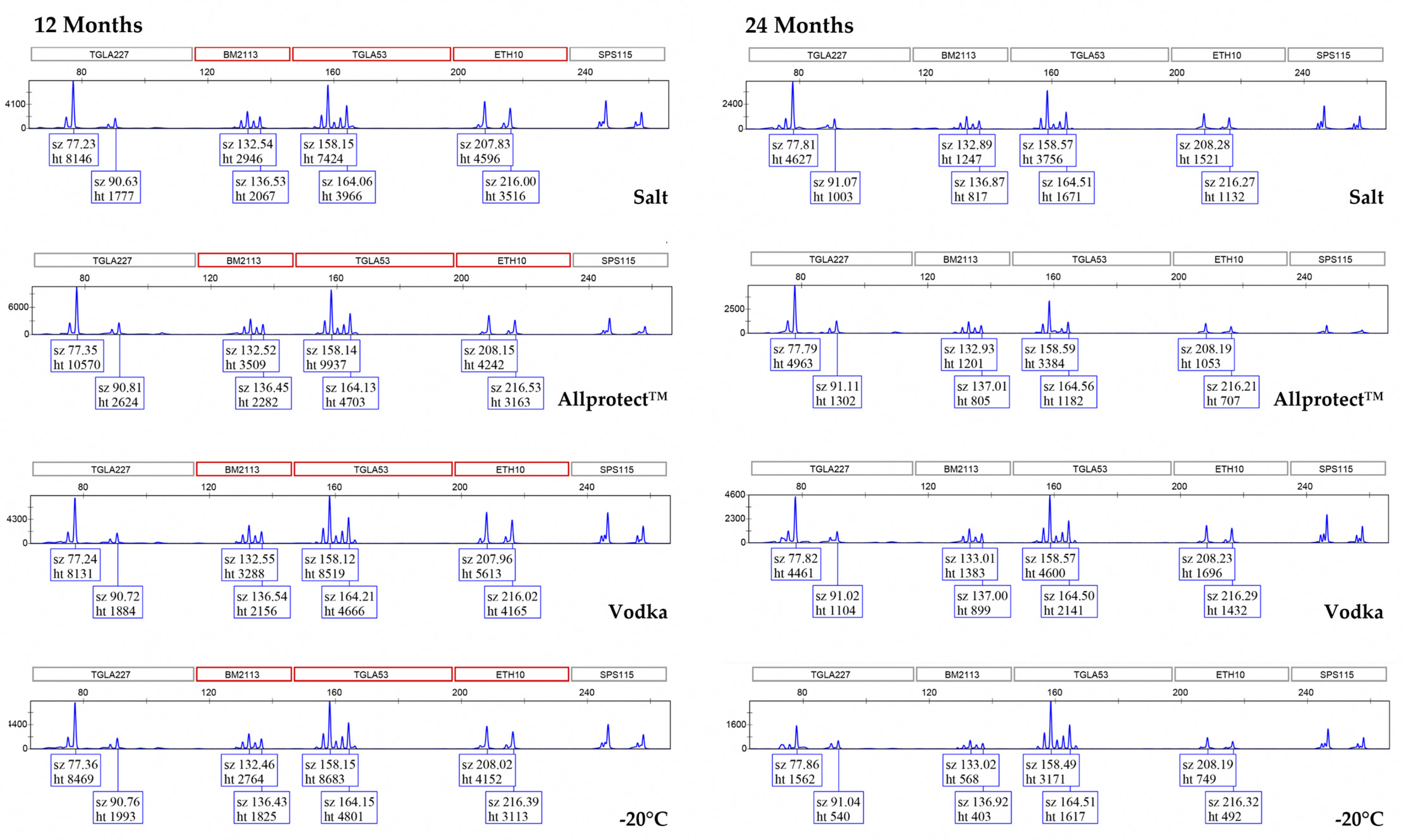
3. Results
3.1. DNA Recovery and Stability
3.2. Genotyping Allele Recovery
3.3. Statistical Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fresh | 7 Days | 1 Month | 4 Months | 12 Months | 16 Months | 24 Months | |
|---|---|---|---|---|---|---|---|
| Mean DNA Yield (ng) | |||||||
| Fresh control | 1922 ± 331 | ||||||
| Salt | 1892 ± 547 | 1983 ± 264 | 1850 ± 316 | 1917 ± 234 | 2000 ± 192 | 2750 ± 453 | |
| Allprotect™ | 2446 ± 426 | 2550 ± 491 | 2267 ± 662 | 1392 ± 501 | 1075 ± 272 | 1579 ± 695 | |
| Vodka | 3283 ± 609 | 2792 ± 357 | 1533 ± 434 | 1200 ± 182 | 626 ± 407 | 686 ± 440 | |
| Freezing | 2767 ± 489 | 2442 ± 540 | 1758 ± 357 | 1708 ± 267 | 171 ± 172 | 58 ± 54 | |
| Mean Input Amount of DNA Used for PCR-STR (ng) | |||||||
| Fresh Control | 34 | ||||||
| Salt | 34 | 35 | 33 | 34 | 36 | 49 | |
| Allprotect™ | 44 | 46 | 40 | 25 | 19 | 28 | |
| Vodka | 59 | 50 | 27 | 21 | 11 | 12 | |
| Freezing | 49 | 44 | 31 | 31 | 3 | 1 | |
| Percentage Allele Recovery (%) | |||||||
| Fresh Control | 100 | ||||||
| Salt | 100 | 100 | 100 | 100 | 100 | 100 | |
| Allprotect™ | 100 | 100 | 100 | 100 | 100 | 100 | |
| Vodka | 100 | 100 | 100 | 100 | 100 | 100 | |
| Freezing | 100 | 100 | 100 | 100 | 100 | 97 | |
| Brown–Forsythe Test Statistic | p-Value | |
|---|---|---|
| Salt | 0.994 | 0.442 |
| Allprotect™ | 0.699 | 0.652 |
| Vodka | 1.511 | 0.199 |
| Freezing | 2.931 | 0.018 |
| Shapiro–Wilk Test Statistic | p-Value | |
|---|---|---|
| Salt | 0.937 | 0.040 |
| Allprotect™ | 0.972 | 0.494 |
| Vodka | 0.940 | 0.046 |
| Freezing | 0.901 | 0.004 |

References
- Disaster Victim Identification Guide, Annexure 8. Available online: https://www.interpol.int/content/download/589/file/18Y1344%20E%20DVI_Guide.pdf (accessed on 2 February 2024).
- Mann, R.W.; Bass, W.M.; Meadows, L. Time since death and decomposition of the human body: Variables and observations in case and experimental field studies. J. Forensic Sci. 1990, 35, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lessig, R.; Thiele, K.; Edelmann, J. Tsunami 2004—Experiences, challenges and strategies. Int. Congr. Ser. 2006, 1288, 747–749. [Google Scholar] [CrossRef]
- Chan, X.L.; Kua, G.W.; Lai, S.; Lim, H.W.; Wee, M.X.; Syn, C.K. Ambient temperature storage of tissue samples (bovine) in readily available media during mass fatality incidents. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 227–228. [Google Scholar] [CrossRef]
- Allen-Hall, A.; McNevin, D. Human tissue preservation for disaster victim identification (DVI) in tropical climates. Forensic Sci. Int. Genet. 2012, 6, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Corach, D.; Sala, A.; Bobillo, C.; Caputo, M.; Alechine, E.; Irisarri, M.; Marino, M.; Canonaco, E.; Rodriguez, C. Optimized mass fatalities victim identification: An airplane crash as a test case. Forensic Sci. Int. Genet. Suppl. Ser. 2013, 4, e222–e223. [Google Scholar] [CrossRef]
- Caputo, M.; Bosio, L.A.; Corach, D. Long-term room temperature preservation of corpse soft tissue: An approach for tissue sample storage. Investig. Genet. 2011, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.; Chaseling, J.; Page, M.; Wright, K. Tissue preservation in extreme temperatures for rapid response to military deaths. Forensic Sci. Int. Genet. 2018, 36, 86–94. [Google Scholar] [CrossRef] [PubMed]
- De Arellano Sánchez, J.A.; Ortiz, J.M.; Quintero, A.L.; Pfeiffer, H.; Vennemann, M.; Bauer, H. Comparing preservation substrates under field conditions for efficient DNA recovery in bone. Int. J. Legal Med. 2023, 137, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C. Facts and ideas from anywhere. Proc. Bayl. Univ. Med. Cent. 2001, 14, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Michaud, C.L.; Foran, D.R. Simplified field preservation of tissues for subsequent DNA analyses. J. Forensic Sci. 2011, 56, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Osei-Asare, C.; Oppong, E.E.; Apenteng, J.A.; Adi-Dako, O.; Kumadoh, D.; Akosua, A.A.; Ohemeng, K.A. Managing Vibrio cholerae with a local beverage: Preparation of an affordable ethanol based hand sanitizer. Heliyon 2020, 6, e03105. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, M.; Lachenmeier, D.W.; Ferreira-Borges, C.; Rehm, J. Is alcohol an “Essential Good” during COVID-19? Yes, but only as a disinfectant! Alcohol Clin. Exp. Res. 2020, 44, 1906. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.V.; Goodwin, W. DNA persistence in soft tissue comparing vodka and absolute ethanol. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e46–e48. [Google Scholar] [CrossRef]
- Applied Biosystems®, StockMarks® Kits for Horses, Cattle, and Dogs; Equine, Bovine and Canine Genotyping Kit. Available online: https://www.thermofisher.com/document-connect/document-connect.html?url=https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2F4346135_Stockmarks_Kits_UG.pdf (accessed on 4 March 2024).
- Walsh, P.S.; Fildesm, N.J.; Reynolds, R. Sequence analysis and characterization of stutter products at the tetranucleotide repeat locus vWA. Nucleic Acids Res. 1996, 24, 2807–2812. [Google Scholar] [CrossRef] [PubMed]
- Kuffel, A.; Gray, A.; Daeid, N.N. Impact of metal ions on PCR inhibition and RT-PCR efficiency. Int. J. Legal Med. 2021, 135, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; Page, M.; Connell, J.; Chaseling, J. Improved methods for transport and preservation of DNA samples for victim identification in a military environment. Aust. Army J. 2019, 15, 133–147. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, X.L.S.; Lai, S.M.; bin Hamdan, D.A.; Ng, Y.B.; Yim, O.S.; Syn, C.K.C. Long-Term Tissue Preservation at Ambient Temperature for Post-Mass Fatality Incident DNA-Based Victim Identification. Genes 2024, 15, 373. https://doi.org/10.3390/genes15030373
Chan XLS, Lai SM, bin Hamdan DA, Ng YB, Yim OS, Syn CKC. Long-Term Tissue Preservation at Ambient Temperature for Post-Mass Fatality Incident DNA-Based Victim Identification. Genes. 2024; 15(3):373. https://doi.org/10.3390/genes15030373
Chicago/Turabian StyleChan, Xavier Liang Shun, Shumei Michelle Lai, Danial Asyraaf bin Hamdan, Yee Bin Ng, Onn Siong Yim, and Christopher Kiu Choong Syn. 2024. "Long-Term Tissue Preservation at Ambient Temperature for Post-Mass Fatality Incident DNA-Based Victim Identification" Genes 15, no. 3: 373. https://doi.org/10.3390/genes15030373
APA StyleChan, X. L. S., Lai, S. M., bin Hamdan, D. A., Ng, Y. B., Yim, O. S., & Syn, C. K. C. (2024). Long-Term Tissue Preservation at Ambient Temperature for Post-Mass Fatality Incident DNA-Based Victim Identification. Genes, 15(3), 373. https://doi.org/10.3390/genes15030373






