Genome-Wide Identification and Expression Analysis of the DMP and MTL Genes in Sweetpotato (Ipomoea batatas L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Identification of the IbDMP and IbpPLA Protein Family Members in Sweetpotato
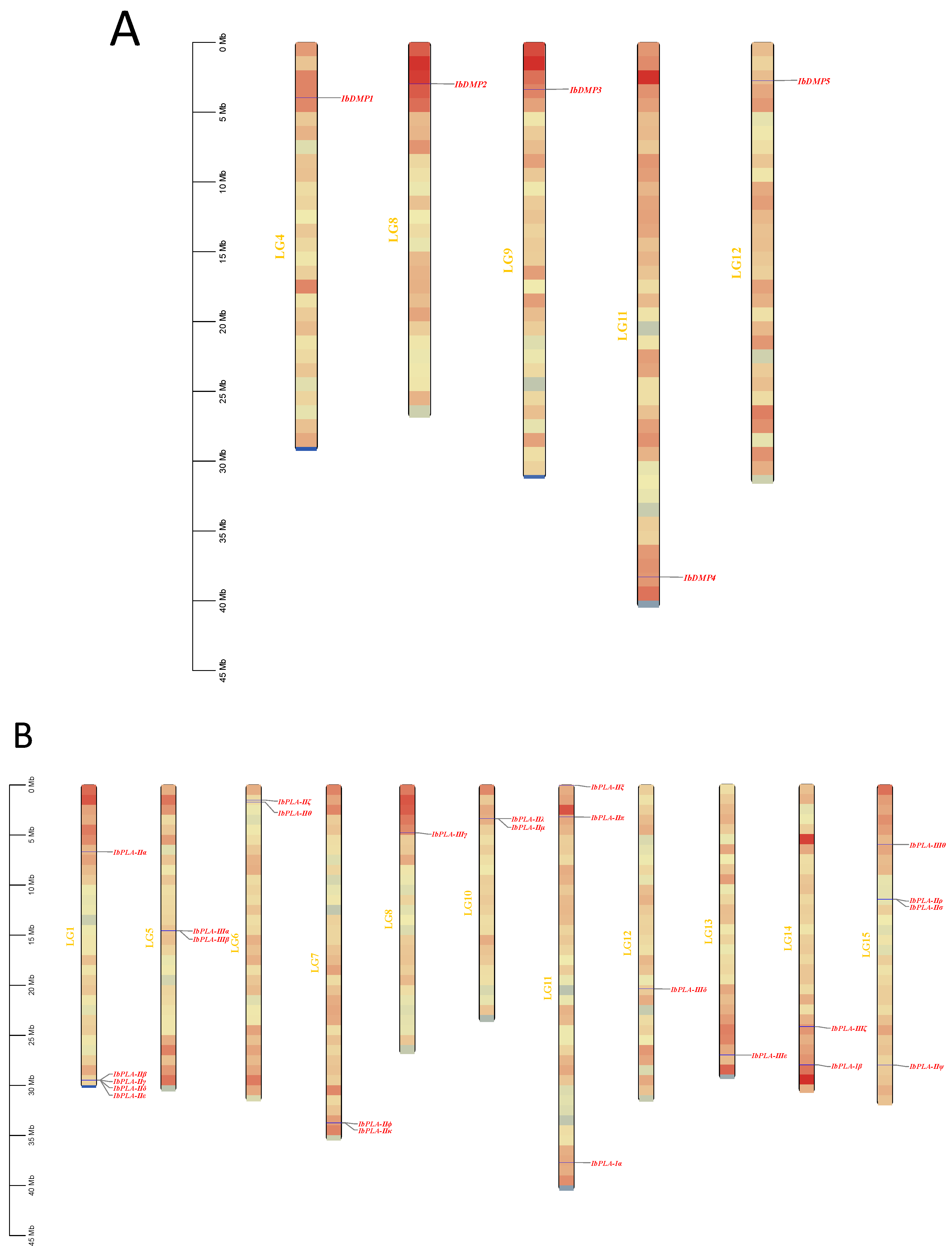
2.3. Protein Properties Prediction and Chromosomal Distribution of Genes
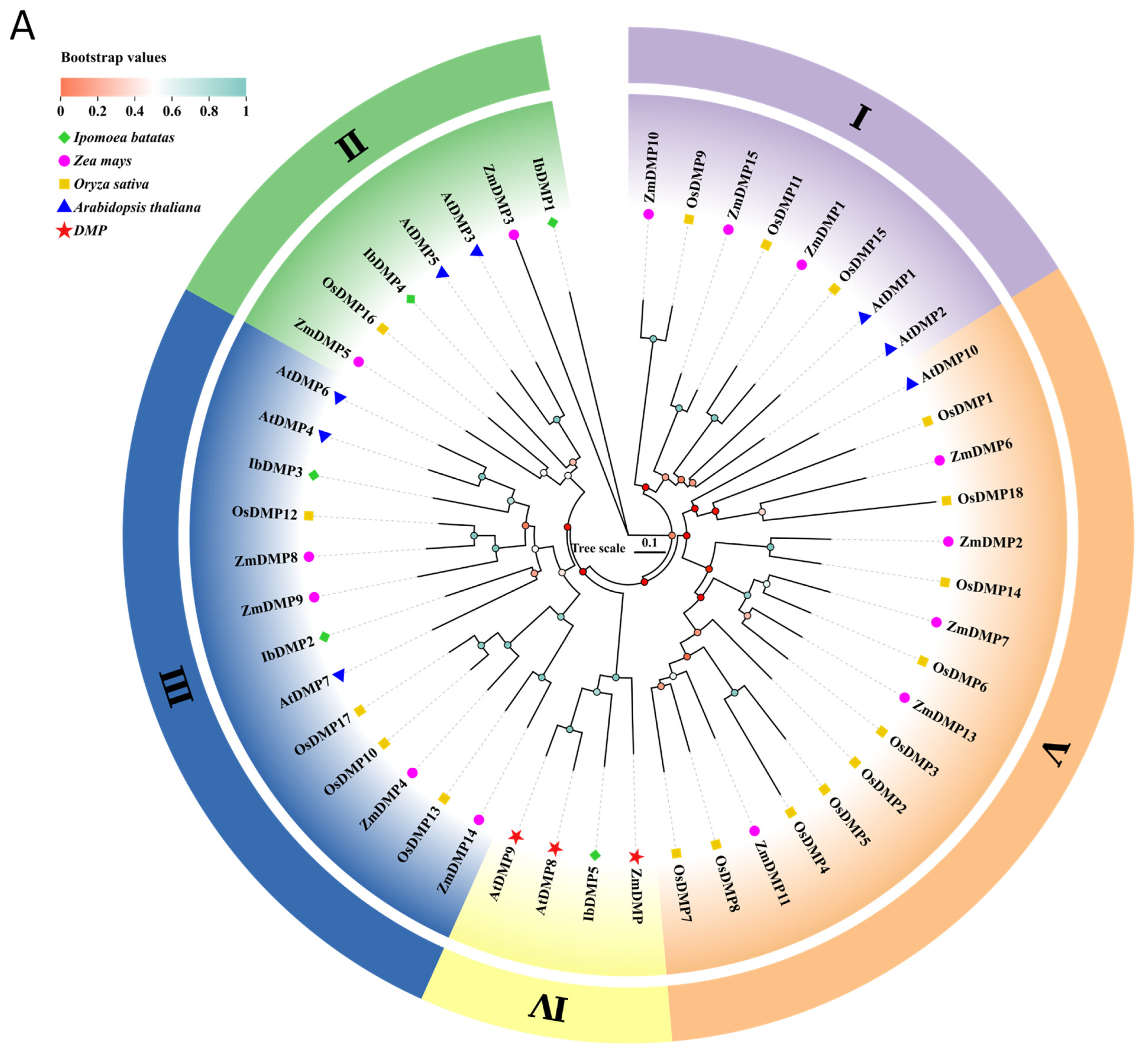
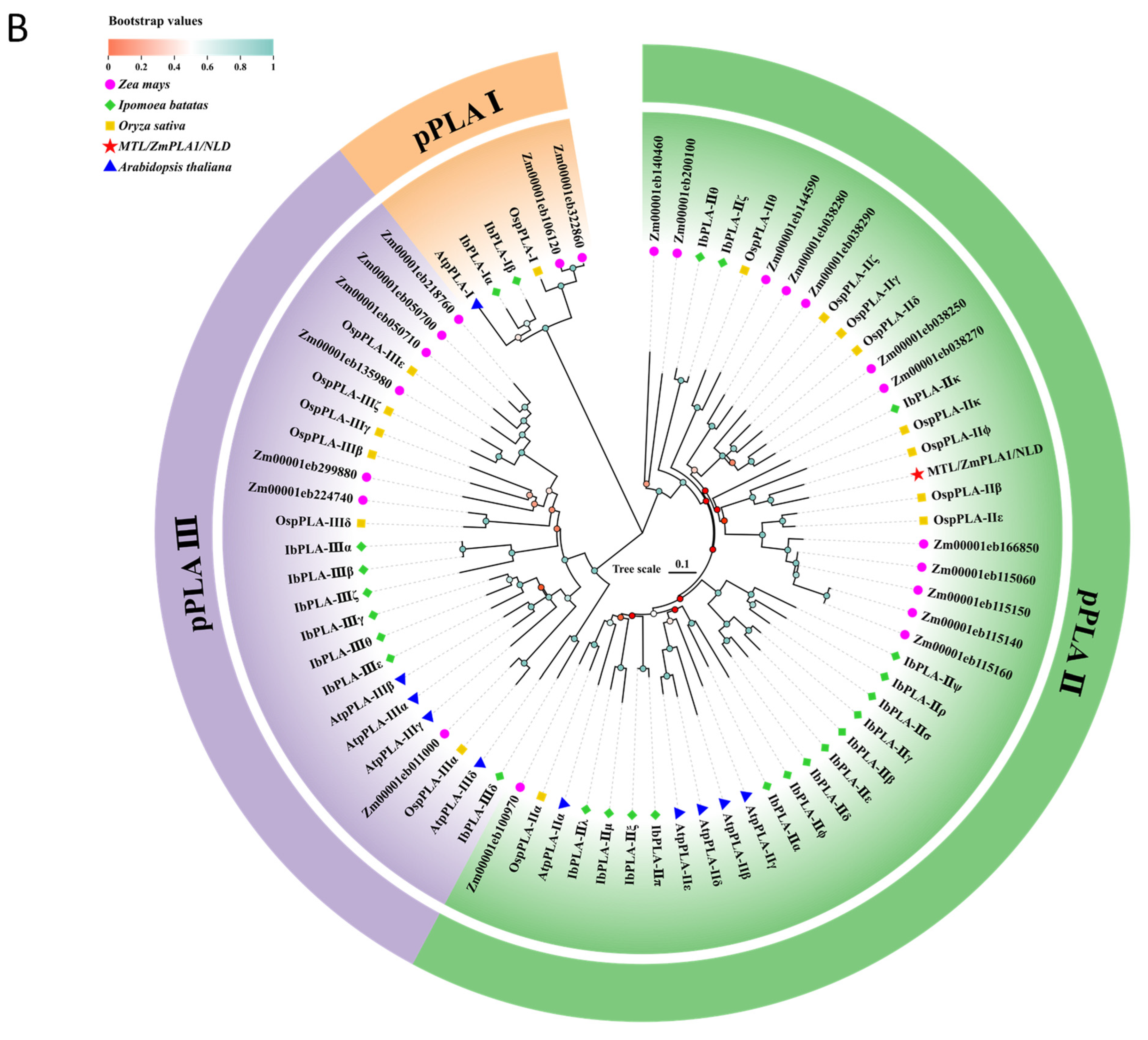
2.4. Amino Acid Sequence Comparison and Phylogenetic Analysis
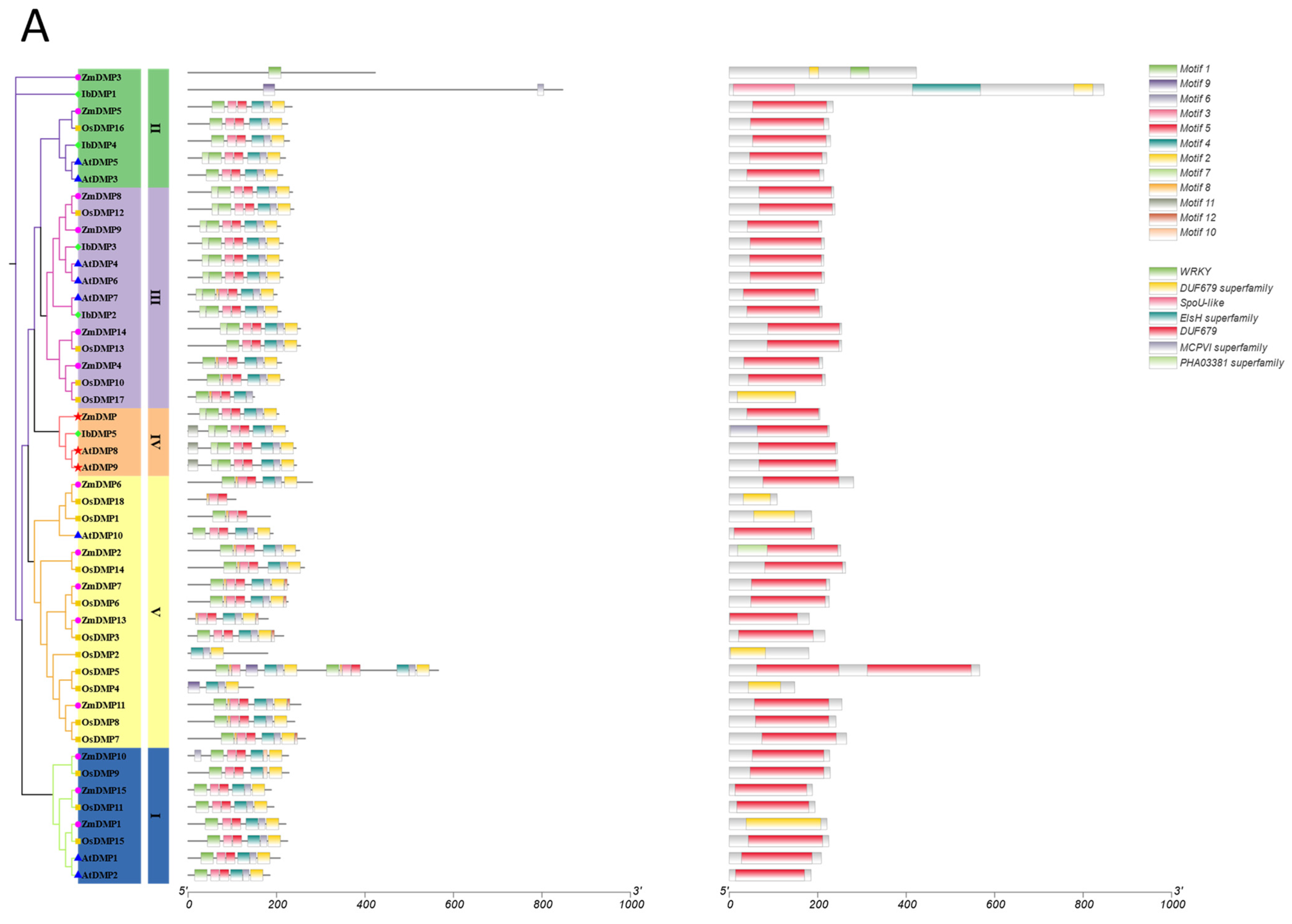
2.5. Analysis of Motif, Gene Structure, and Conserved Domain
2.6. Analysis of the Gene Promoter in Sweetpotato
2.7. Gene Interaction Network of the Proteins
2.8. qRT–PCR of Genes
3. Results
3.1. Identification of the IbDMP and IbpPLA Protein Family Members in Sweetpotato
3.2. Characteristics of the IbDMP and IbpPLA Protein Family Members in Sweetpotato
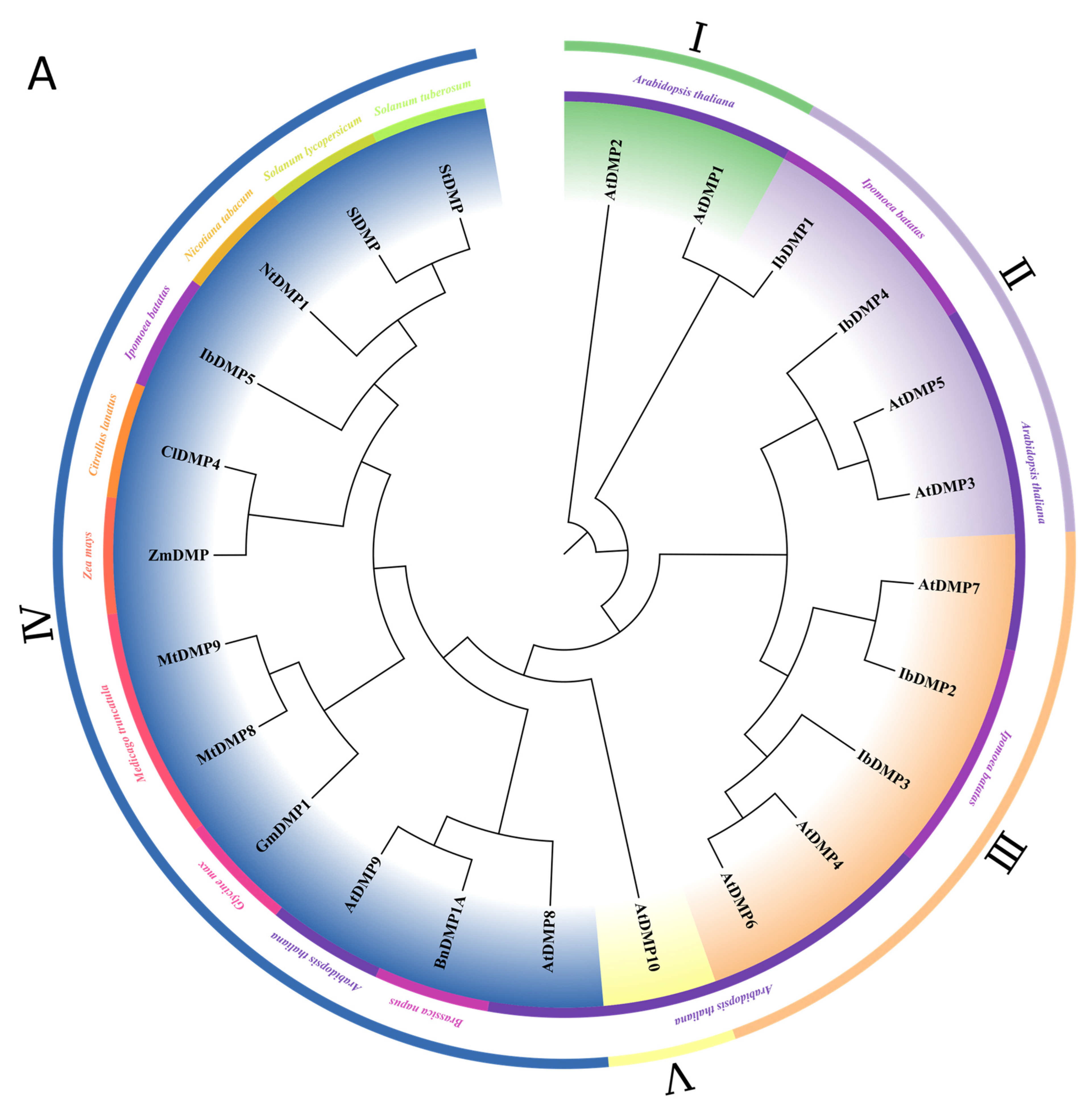
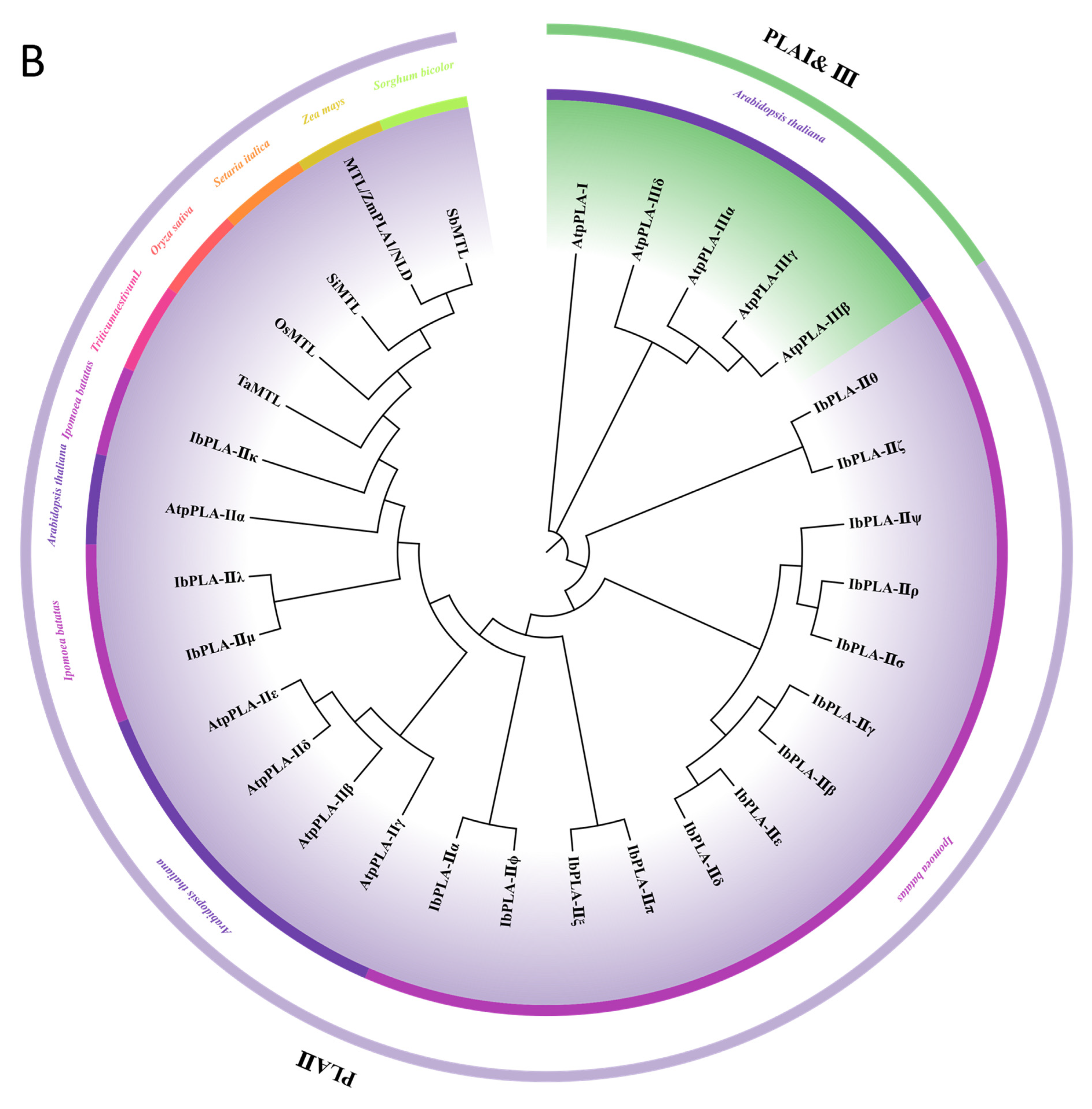
3.3. Phylogenetic Analysis of the IbDMP and IbpPLA Protein Family Members in Sweetpotato
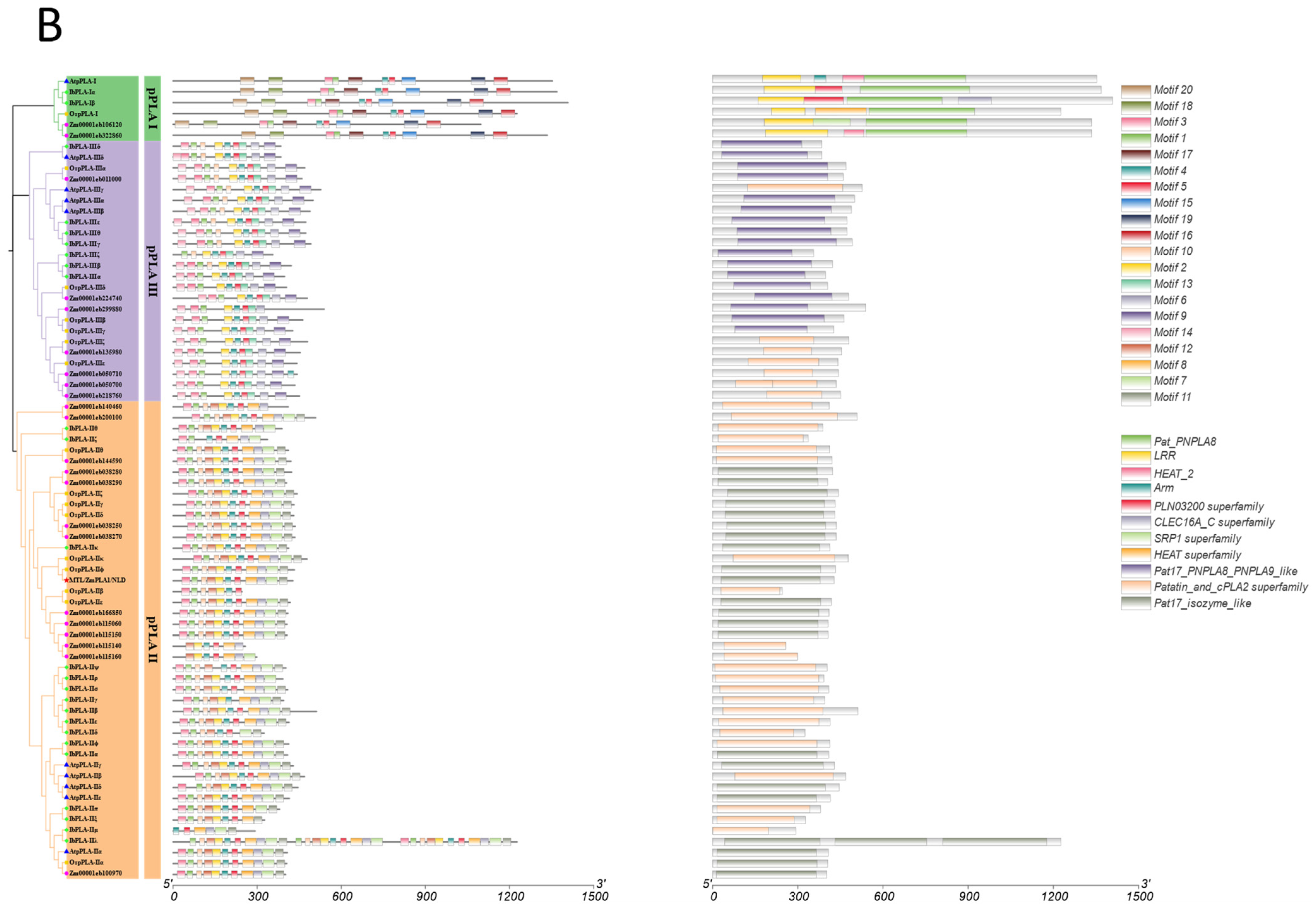
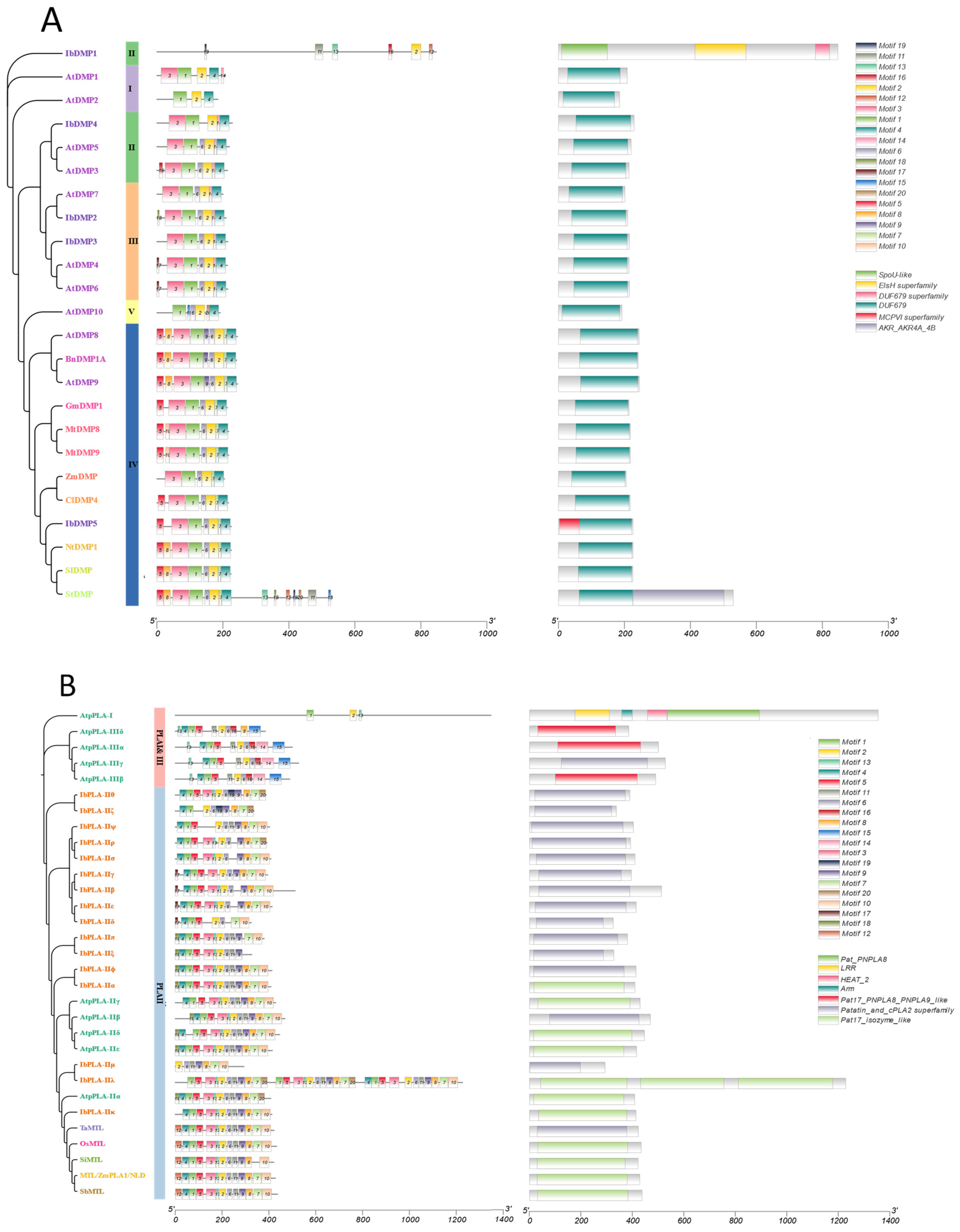
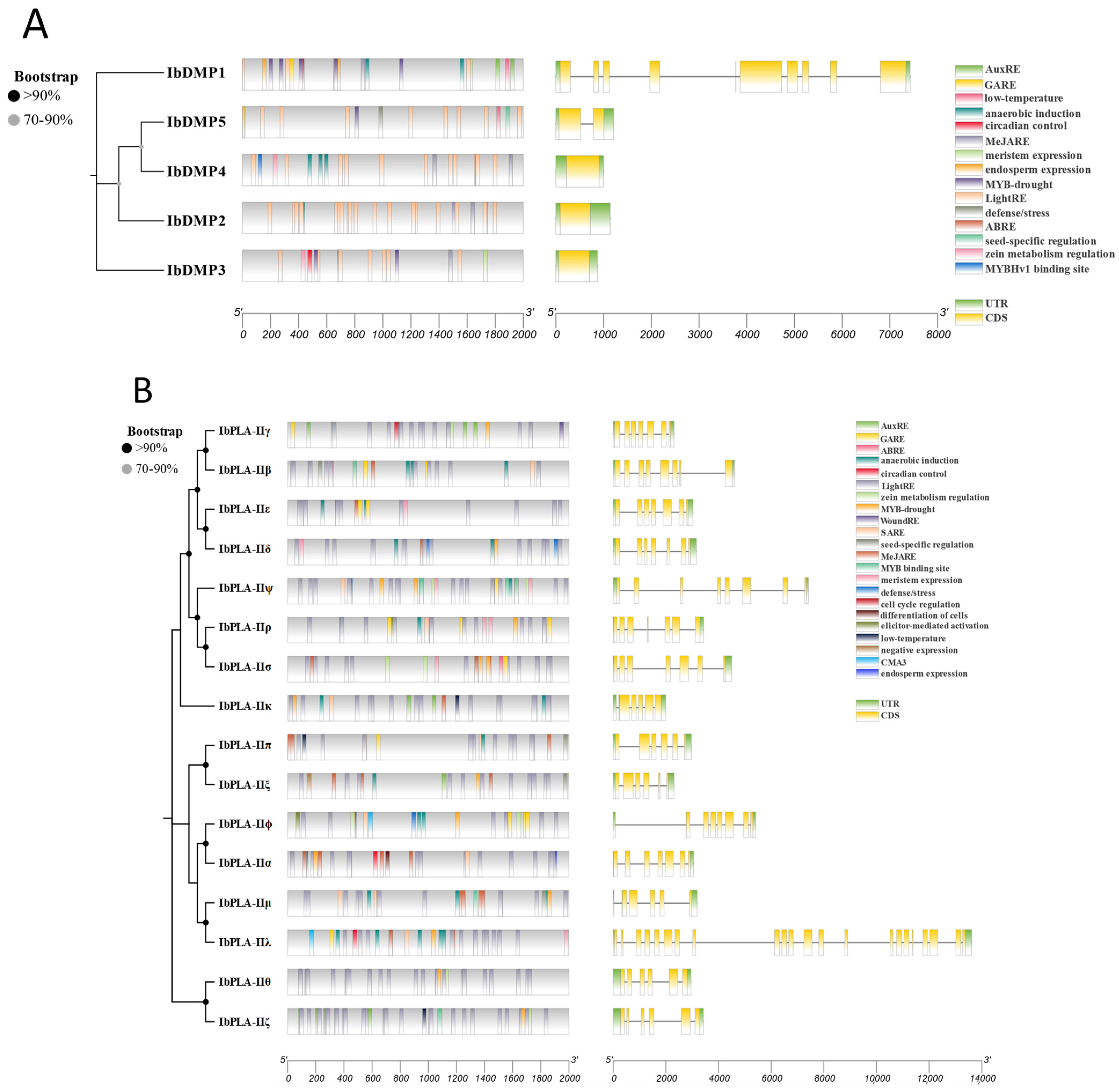
3.4. Conserved Motif and Domain Analysis of the IbDMP and IbpPLA Protein Family Members in Sweetpotato
3.5. Identification of Potential HI Genes in Sweetpotato
3.6. Gene Structure and Promoter Region Cis-Acting Regulatory Elements Analysis
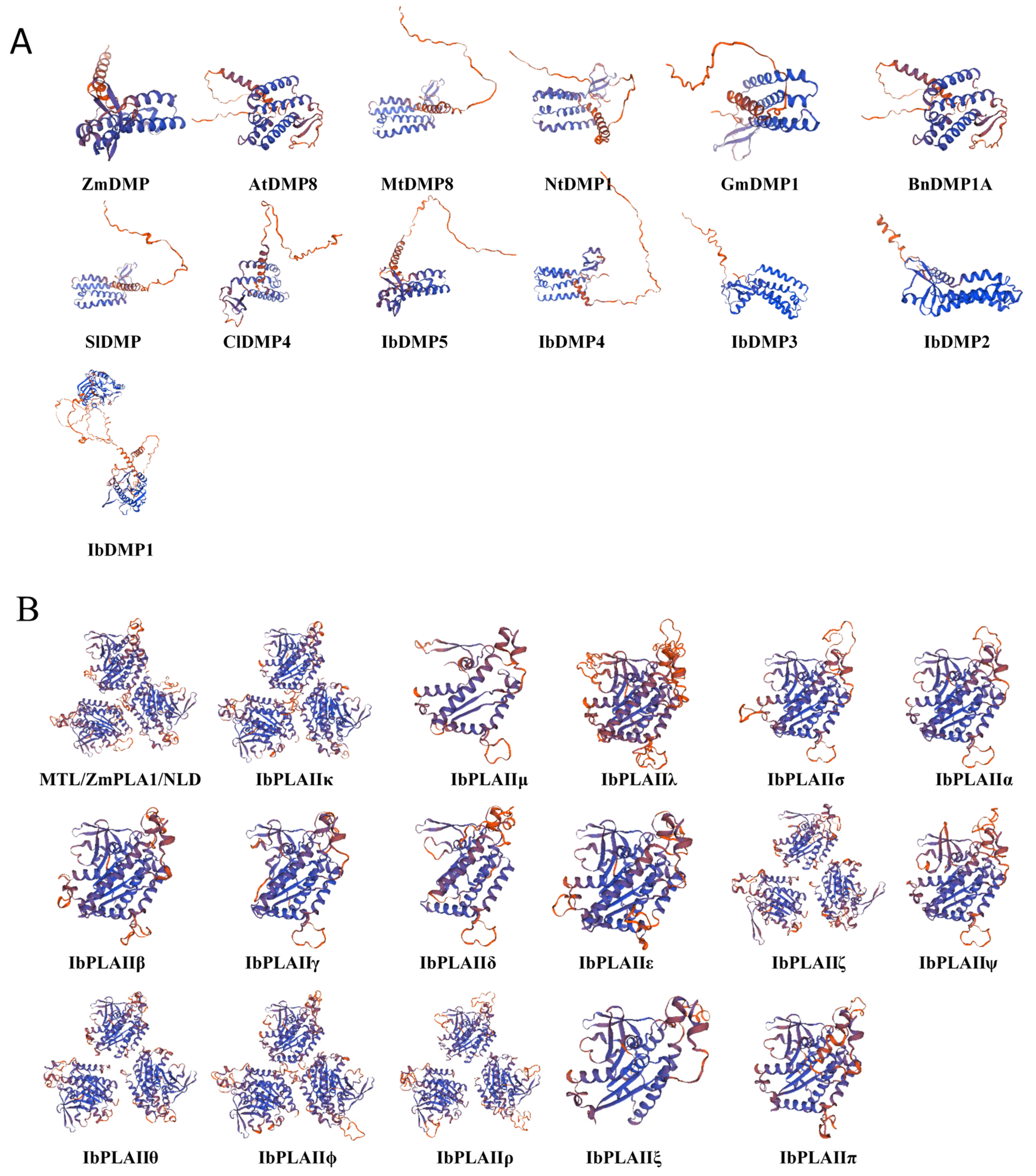
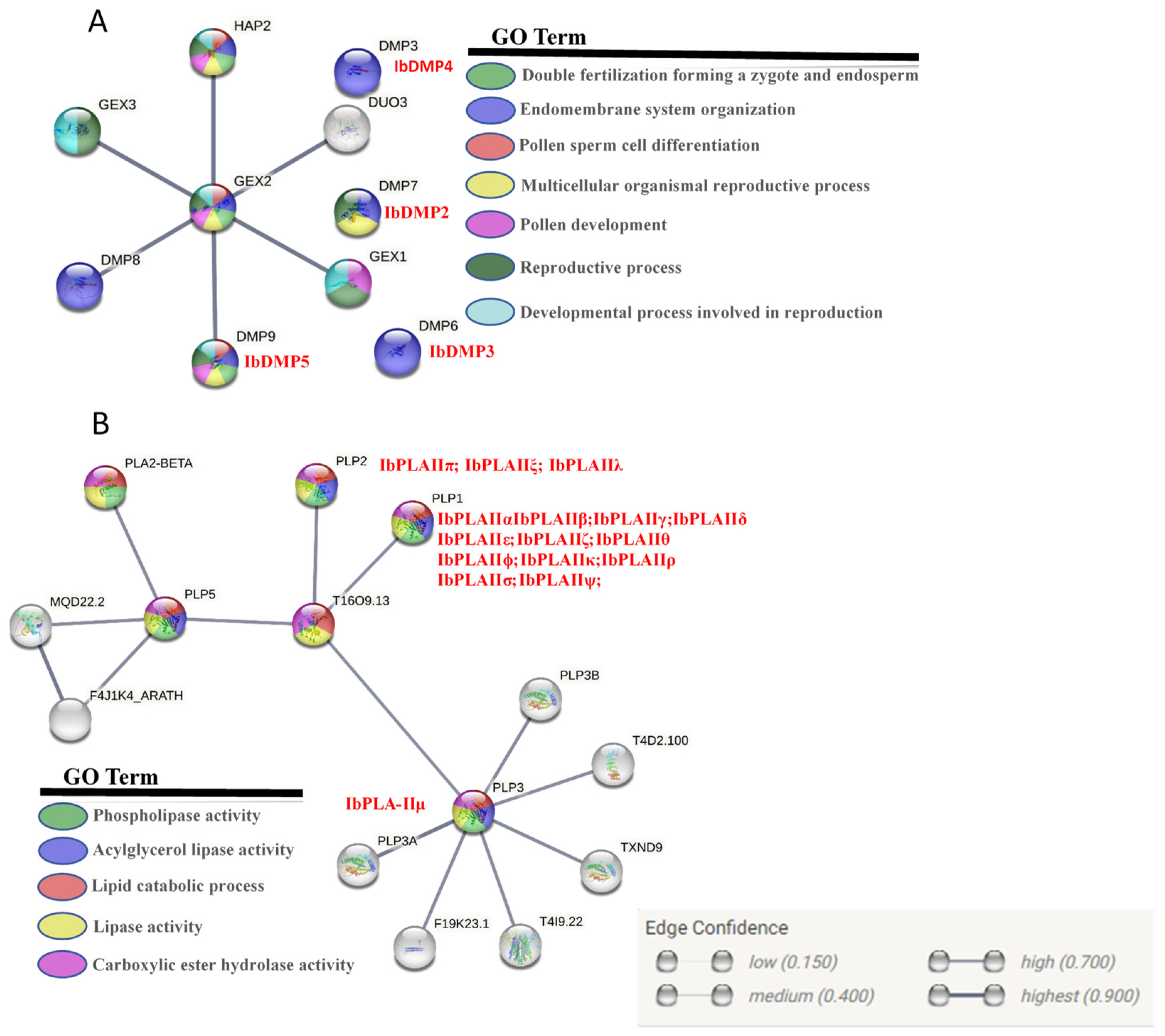
3.7. Protein Tertiary Structure and Potential Regulatory Network Analysis
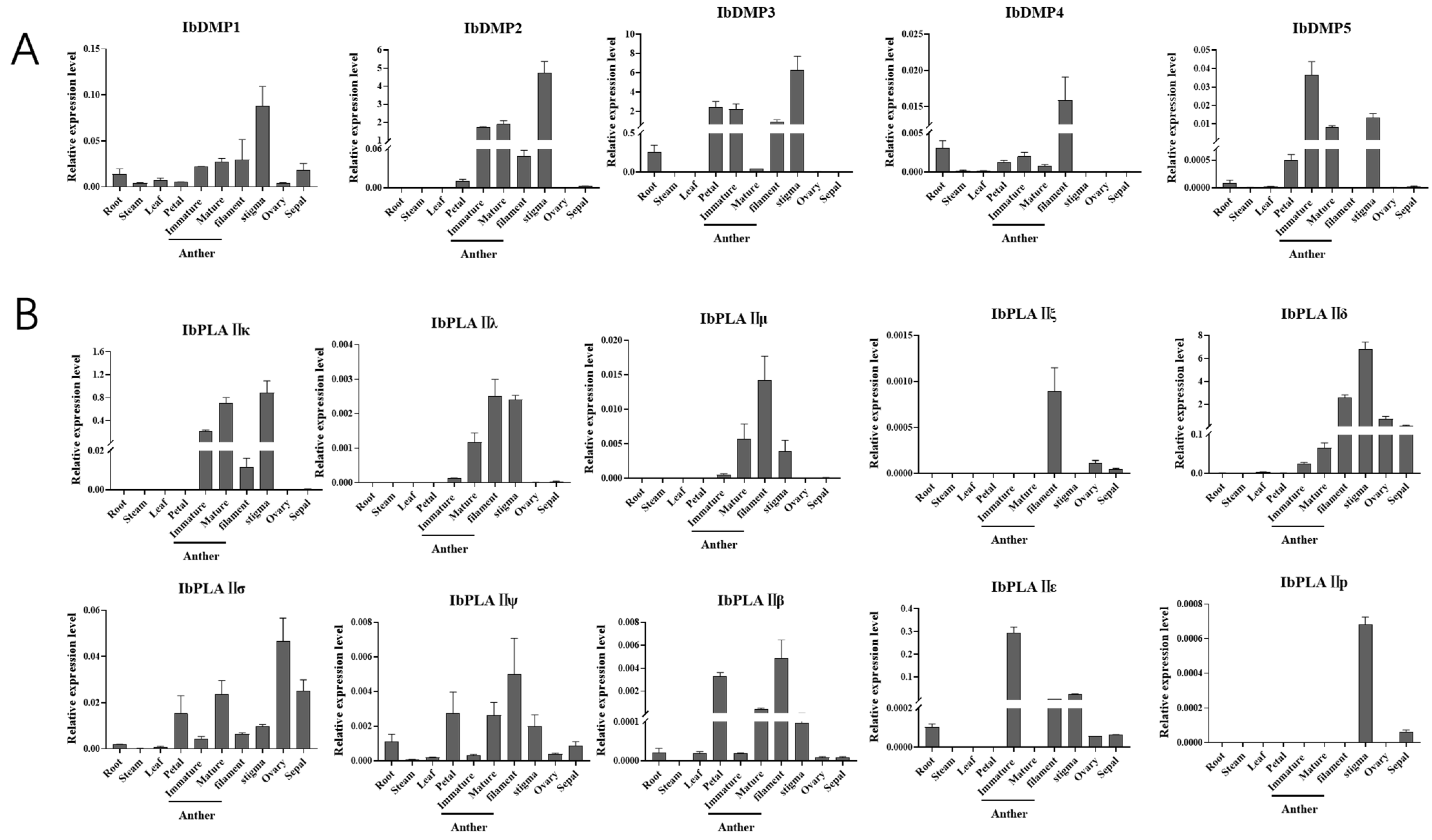
3.8. Tissue Expression Patterns Analysis of Potential HI Genes
4. Discussion
4.1. Identification and Characterization of the DMP Gene Family and Its Potential HI Genes in Sweetpotato
4.2. Identification and Characterization of the pPLA Gene Family and MTL Genes in Sweetpotato
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Tang, Z.; Pan, Z.; Wang, R.; Wang, X.; Zhao, P.; Liu, M.; Zhu, Y.; Liu, C.; Wang, W.; et al. Calcium-Mobilizing Properties of Salvia miltiorrhiza-Derived Carbon Dots Confer Enhanced Environmental Adaptability in Plants. ACS Nano 2022, 16, 4357–4370. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Nie, H.; Wang, Y.; Wang, X.; Jarret, R.; Zhao, J.; Wang, H.; Yang, J. Exploring and exploiting genetics and genomics for sweetpotato improvement: Status and perspectives. Plant Commun. 2022, 3, 100332. [Google Scholar] [CrossRef] [PubMed]
- Impens, L.; Lorenzo, C.D.; Vandeputte, W.; Wytynck, P.; Debray, K.; Haeghebaert, J.; Herwegh, D.; Jacobs, T.B.; Ruttink, T.; Nelissen, H.; et al. Combining multiplex gene editing and doubled haploid technology in maize. New Phytol. 2023, 239, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Gilles, L.M.; Pyott, D.E.; Martinant, J.P.; Rogowsky, P.M.; Widiez, T. Puzzling out plant reproduction by haploid induction for innovations in plant breeding. Nat. Plants 2020, 6, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liu, C.; Qi, X.; Jiao, Y.; Wang, D.; Wang, Y.; Liu, Z.; Chen, C.; Chen, B.; Tian, X.; et al. Mutation of ZmDMP enhances haploid induction in maize. Nat. Plants 2019, 5, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Gilles, L.M.; Khaled, A.; Laffaire, J.B.; Chaignon, S.; Gendrot, G.; Laplaige, J.; Berges, H.; Beydon, G.; Bayle, V.; Barret, P.; et al. Loss of pollen-specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J. 2017, 36, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, T.; Starr, D.; Richbourg, L.; Chintamanani, S.; Delzer, B.; Nuccio, M.L.; Green, J.; Chen, Z.; Mccuiston, J.; Wang, W.; et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 2017, 542, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.; Meng, D.; Zhong, Y.; Chen, C.; Dong, X.; Xu, X.; Chen, B.; Li, W.; Li, L.; et al. A 4-bp Insertion at ZmPLA1 Encoding a Putative Phospholipase A Generates Haploid Induction in Maize. Mol. Plant 2017, 10, 520–522. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, J.; Jia, M.; Cao, L.; Yu, J.; Zhao, D. Haploid induction in allotetraploid tobacco using DMPs mutation. Planta 2022, 255, 98. [Google Scholar] [CrossRef]
- Cyprys, P.; Lindemeier, M.; Sprunck, S. Gamete fusion is facilitated by two sperm cell-expressed DUF679 membrane proteins. Nat. Plants 2019, 5, 253–257. [Google Scholar] [CrossRef]
- Trentin, H.U.; Krause, M.D.; Zunjare, R.U.; Almeida, V.C.; Peterlini, E.; Rotarenco, V.; Frei, U.K.; Beavis, W.D.; Lubberstedt, T. Genetic basis of maize maternal haploid induction beyond MATRILINEAL and ZmDMP. Front. Plant Sci. 2023, 14, 1218042. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, B.; Wang, D.; Zhu, X.; Li, M.; Zhang, J.; Chen, M.; Wang, M.; Riksen, T.; Liu, J.; et al. In vivo maternal haploid induction in tomato. Plant Biotechnol. J. 2022, 20, 250–252. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Luo, J.; Tang, D.; Zhu, X.; Wang, J.; Liu, Z.; Wang, P.; Zhong, Y.; Liu, C.; et al. Construction of homozygous diploid potato through maternal haploid induction. Abiotech 2022, 3, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Ai, G.; Chen, J.; Guo, D.; Zhu, Z.; Zhu, X.; Tian, S.; Wang, J.; Liu, M.; et al. Creation of a watermelon haploid inducer line via ClDMP3-mediated single fertilization of the central cell. Hortic. Res. 2023, 10, uhad081. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, Y.; Chen, B.; Liu, J.; Wang, D.; Li, M.; Qi, X.; Liu, C.; Boutilier, K.; Chen, S. Establishment of a dmp based maternal haploid induction system for polyploid Brassica napus and Nicotiana tabacum. J. Integr. Plant Biol. 2022, 64, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhang, Y.; Liu, C.; Liu, Y.; Wang, Y.; Liang, D.; Liu, J.; Sahoo, G.; Kelliher, T. OsMATL mutation induces haploid seed formation in indica rice. Nat. Plants 2018, 4, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhong, Y.; Qi, X.; Chen, M.; Liu, Z.; Chen, C.; Tian, X.; Li, J.; Jiao, Y.; Wang, D.; et al. Extension of the in vivo haploid induction system from diploid maize to hexaploid wheat. Plant Biotechnol. J. 2020, 18, 316–318. [Google Scholar] [CrossRef]
- Jang, J.H.; Seo, H.S.; Widiez, T.; Lee, O.R. Loss-of-function of gynoecium-expressed phospholipase pPLAIIgamma triggers maternal haploid induction in Arabidopsis. New Phytol. 2023, 238, 1813–1824. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, T.; Yu, J.; Hong, H.; Liu, S.; Guo, F.; Ma, H.; Zhang, J.; Zhu, M.; Meng, X. Genome-wide survey and expression analysis of Dof transcription factor family in sweetpotato shed light on their promising functions in stress tolerance. Front. Plant Sci. 2023, 14, 1140727. [Google Scholar] [CrossRef]
- Jalili, V.; Afgan, E.; Gu, Q.; Clements, D.; Blankenberg, D.; Goecks, J.; Taylor, J.; Nekrutenko, A. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2020 update. Nucleic Acids Res. 2020, 48, W395–W402. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Gonzales, N.R.; Gwadz, M.; Lu, S.; Marchler, G.H.; Song, J.S.; Thanki, N.; Yamashita, R.A.; et al. The conserved domain database in 2023. Nucleic Acids Res. 2023, 51, D384–D388. [Google Scholar] [CrossRef]
- Sigrist, C.J.; Cerutti, L.; Hulo, N.; Gattiker, A.; Falquet, L.; Pagni, M.; Bairoch, A.; Bucher, P. PROSITE: A documented database using patterns and profiles as motif descriptors. Brief. Bioinform. 2002, 3, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, 587–592. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Mathura, S.R.; Sutton, F.; Bowrin, V. Genome-wide identification, characterization, and expression analysis of the sweet potato (Ipomoea batatas [L.] Lam.) ARF, Aux/IAA, GH3, and SAUR gene families. BMC Plant Biol. 2023, 23, 622. [Google Scholar] [CrossRef]
- Yu, Y.; Xuan, Y.; Bian, X.; Zhang, L.; Pan, Z.; Kou, M.; Cao, Q.; Tang, Z.; Li, Q.; Ma, D.; et al. Overexpression of phosphatidylserine synthase IbPSS1 affords cellular Na+ homeostasis and salt tolerance by activating plasma membrane Na+/H+ antiport activity in sweet potato roots. Hortic. Res. 2020, 7, 131. [Google Scholar] [CrossRef]
- Qin, C.; Wang, X. The Arabidopsis phospholipase D family. Characterization of a calcium-independent and phosphatidylcholine-selective PLD zeta 1 with distinct regulatory domains. Plant Physiol. 2002, 128, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Zhou, F.; Zhang, L.; Gong, J.; Cheng, C.; Chen, J.; Lou, Q. Genome-wide characterization, phylogenetic and expression analysis of Histone gene family in cucumber (Cucumis sativus L.). Int. J. Biol. Macromol. 2023, 230, 123401. [Google Scholar] [CrossRef]
- Wei, Y.; Chong, Z.; Lu, C.; Li, K.; Liang, C.; Meng, Z.; Wang, Y.; Guo, S.; He, L.; Zhang, R. Genome-wide identification and expression analysis of the cotton patatin-related phospholipase A genes and response to stress tolerance. Planta 2023, 257, 49. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yu, J.; Kong, L.; Zhang, W.; Hou, X.; Cui, G. Genome-wide investigation of the PLD gene family in alfalfa (Medicago sativa L.): Identification, analysis and expression. BMC Genom. 2022, 23, 243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, X.; Chen, W.; Yao, J.; Li, Y.; Fang, S.; Lv, Y.; Li, X.; Pan, J.; Liu, C.; et al. Cotton DMP gene family: Characterization, evolution, and expression profiles during development and stress. Int. J. Biol. Macromol. 2021, 183, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Nawade, B.; Bosamia, T.C.; Lee, J.H.; Jang, J.H.; Lee, O.R. Genome-wide characterization of the soybean DOMAIN OF UNKNOWN FUNCTION 679 membrane protein gene family highlights their potential involvement in growth and stress response. Front. Plant Sci. 2023, 14, 1216082. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xia, X.; Jiang, T.; Li, L.; Zhang, P.; Niu, L.; Cheng, H.; Wang, K.; Lin, H. In planta haploid induction by genome editing of DMP in the model legume Medicago truncatula. Plant Biotechnol. J. 2022, 20, 22–24. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.; Zhao, H.; Zong, M.; Li, M.; Gong, G.; Wang, J.; Zhang, J.; Ren, Y.; Zhang, H.; et al. Production of double haploid watermelon via maternal haploid induction. Plant Biotechnol. J. 2023, 21, 1308–1310. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, J.; Li, R.; Yan, S.; Chen, W.; Guo, L.; Qin, G.; Wang, P.; Luo, C.; Huang, W.; et al. A reactive oxygen species burst causes haploid induction in maize. Mol. Plant. 2022, 15, 943–955. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, Y.; Yang, S.; Zhi, H.; Yin, T.; Ma, X.; Zhang, H.; Diao, X.; Guo, Y.; Li, X.; et al. Establishing in planta haploid inducer line by edited SiMTL in foxtail millet (Setaria italica). Plant Biotechnol. J. 2021, 19, 1089–1091. [Google Scholar] [CrossRef]
- Lv, J.; Kelliher, T. Recent Advances in Engineering of In Vivo Haploid Induction Systems. Methods Mol. Biol. 2023, 2653, 365–383. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.; Kolapo, K. Drought Resistance in Rice from Conventional to Molecular Breeding: A Review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, L.; Zhao, B.; Zhao, Y.; Xie, Y.; Zheng, Z.; Li, Y.; Sun, J.; Wang, H. Development of a Haploid-Inducer Mediated Genome Editing System for Accelerating Maize Breeding. Mol. Plant 2019, 12, 597–602. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Zhong, Y.; Dong, X.; Hu, H.; Tian, X.; Wang, L.; Chen, B.; Chen, C.; Melchinger, A.E.; et al. Fine mapping of qhir8 affecting in vivo haploid induction in maize. Theor. Appl. Genet. 2015, 128, 2507–2515. [Google Scholar] [CrossRef]
- Prigge, V.; Xu, X.; Li, L.; Babu, R.; Chen, S.; Atlin, G.N.; Melchinger, A.E. New insights into the genetics of in vivo induction of maternal haploids, the backbone of doubled haploid technology in maize. Genetics 2012, 190, 781–793. [Google Scholar] [CrossRef]
- Scherer, G.F.; Ryu, S.B.; Wang, X.; Matos, A.R.; Heitz, T. Patatin-related phospholipase A: Nomenclature, subfamilies and functions in plants. Trends Plant Sci. 2010, 15, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wu, S.; Li, Z.; An, X.; Tian, Y. Lipid Metabolism: Critical Roles in Male Fertility and Other Aspects of Reproductive Development in Plants. Mol. Plant 2020, 13, 955–983. [Google Scholar] [CrossRef]
- Gao, X.R.; Zhang, H.; Li, X.; Bai, Y.W.; Peng, K.; Wang, Z.; Dai, Z.R.; Bian, X.F.; Zhang, Q.; Jia, L.C.; et al. The B-box transcription factor IbBBX29 regulates leaf development and flavonoid biosynthesis in sweet potato. Plant Physiol. 2023, 191, 496–514. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Abouelnasr, H.; Mohamed Hamed, A.; Kou, M.; Tang, W.; Yan, H.; Wang, X.; Wang, X.; Zhang, Y.; et al. Inhibition of miR397 by STTM technology to increase sweetpotato resistance to SPVD. J. Integr. Agric. 2022, 21, 2865–2875. [Google Scholar] [CrossRef]
- Chen, H.Q.; Liu, H.Y.; Wang, K.; Zhang, S.X.; Ye, X.G. Development and innovation of haploid induction technologies in plants. Yi Chuan 2020, 42, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Feng, Y.M.; Shang, S.Z.; Zhao, J.R.; Hu, G.Y.; Xu, F.C.; Song, C.P.; Jin, S.X.; Gao, W. In vivo maternal haploid induction system in cotton. Plant Physiol. 2023, 194, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhong, Y.; Qi, X.; An, T.; Guo, S.; Wang, D.; Wang, Y.; Feng, B.; Zhu, Z.; Chen, S.; et al. Haploids can be induced in knockout mutants of OsPLA1, but not OsDMP3 or OsDMP6, in rice. Crop J. 2023, 12, 213–221. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, C.; Li, S.; Zhang, B.; Luo, P.; Peng, X.; Zhao, P.; Dresselhaus, T.; Sun, M. A female in vivo haploid-induction system via mutagenesis of egg cell-specific peptidases. Mol. Plant 2023, 16, 471–480. [Google Scholar] [CrossRef]
- Khanday, I.; Skinner, D.; Yang, B.; Mercier, R.; Sundaresan, V. A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 2019, 565, 91–95. [Google Scholar] [CrossRef]
- Ahmadli, U.; Kalidass, M.; Khaitova, L.C.; Fuchs, J.; Cuacos, M.; Demidov, D.; Zuo, S.; Pecinkova, J.; Mascher, M.; Ingouff, M.; et al. High temperature increases centromere-mediated genome elimination frequency and enhances haploid induction in Arabidopsis. Plant Commun. 2023, 4, 100507. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Sun, H.; Liang, Q.; Wang, W.; Zhang, C.; Bian, X.; Cao, Q.; Li, Q.; Xie, Y.; et al. Improving CRISPR-Cas-mediated RNA targeting and gene editing using SPLCV replicon-based expression vectors in Nicotiana benthamiana. Plant Biotechnol. J. 2020, 18, 1993–1995. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, Z.; Wang, X.; Bian, X.; Wang, W.; Liang, Q.; Kou, M.; Ji, H.; Li, Y.; Ma, D.; et al. Targeting of SPCSV-RNase3 via CRISPR-Cas13 confers resistance against sweet potato virus disease. Mol. Plant Pathol. 2022, 23, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Liu, L.; Yan, J.; Hu, Y.; Han, M.; Gao, Z.; Chen, J.; Wang, Y.; Liu, Y.; Ma, Z. The correlation between microspore development period and flower appearance of sweet potato. J. Jiangsu Norm. Univ. 2023, 41, 24–28. [Google Scholar]
| Name | Gene Locus ID | AA | MW (Da) | pI | Instability Index | Aliphatic Index | Gravy | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| IbDMP5 | g47273.t1 | 225 | 24,123 | 8.19 | 30.38 | 87.87 | 0.341 | plas: 4, E.R.: 4, cyto: 3, nucl: 2, vacu: 1 |
| IbPLAⅡκ | g29928.t1 | 412 | 45,105 | 9.08 | 29.87 | 87.48 | −0.177 | chlo: 11, nucl: 2, vacu: 1 |
| IbPLAⅡλ | g38666.t1 | 1226 | 136,725 | 6.58 | 40.92 | 91.14 | −0.282 | nucl: 3.5, chlo: 3, plas: 3, cyto_nucl: 3, cyto: 1.5, mito: 1, E.R.: 1, golg: 1 |
| IbPLAⅡμ | g38668.t1 | 292 | 32,779 | 8.25 | 46.3 | 87.47 | −0.305 | chlo: 4, nucl: 3, cyto: 3, cysk: 2, plas: 1, E.R. vacu: 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Li, Z.; Han, Y.; Sun, J. Genome-Wide Identification and Expression Analysis of the DMP and MTL Genes in Sweetpotato (Ipomoea batatas L.). Genes 2024, 15, 354. https://doi.org/10.3390/genes15030354
Pan Z, Li Z, Han Y, Sun J. Genome-Wide Identification and Expression Analysis of the DMP and MTL Genes in Sweetpotato (Ipomoea batatas L.). Genes. 2024; 15(3):354. https://doi.org/10.3390/genes15030354
Chicago/Turabian StylePan, Zhiyuan, Zongyun Li, Yonghua Han, and Jian Sun. 2024. "Genome-Wide Identification and Expression Analysis of the DMP and MTL Genes in Sweetpotato (Ipomoea batatas L.)" Genes 15, no. 3: 354. https://doi.org/10.3390/genes15030354
APA StylePan, Z., Li, Z., Han, Y., & Sun, J. (2024). Genome-Wide Identification and Expression Analysis of the DMP and MTL Genes in Sweetpotato (Ipomoea batatas L.). Genes, 15(3), 354. https://doi.org/10.3390/genes15030354






