Metabolomic and Transcriptomic Analyses Reveal the Molecular Mechanism Underlying the Massive Accumulation of Secondary Metabolites in Fenugreek (Trigonella foenum-graecum L.) Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Pathogenic Microorganism Materials
2.3. Metabolomic Analysis
2.4. Liquid Chromatography–Mass Spectrometry (LC–MS)
2.5. Metabolic Information Analysis
2.6. Library Construction and Sequencing
2.7. Data Filtering
2.8. De Novo Assembly
2.9. Coding Sequence (CDS) Prediction
2.10. Gene Annotation
2.11. Gene Quantification
2.12. Differentially Expressed Gene (DEG) Analyses
2.13. Integrated Analysis of the Transcriptome and Metabolome
2.14. Preparation of the Crude Ethanol Extract of Fenugreek Seeds
2.15. Antifungal Activity Test of Fenugreek Seed Extracts
3. Results
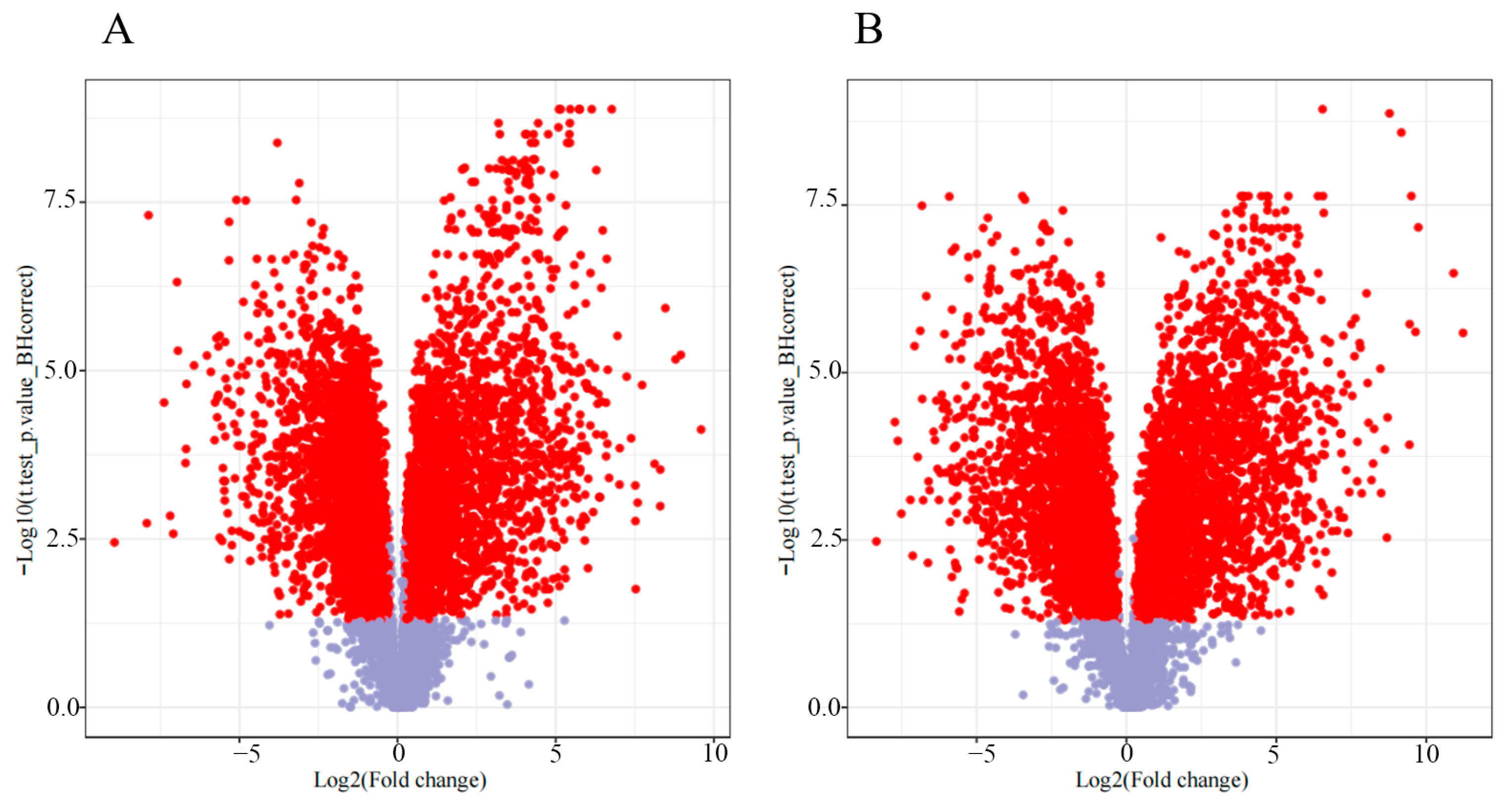
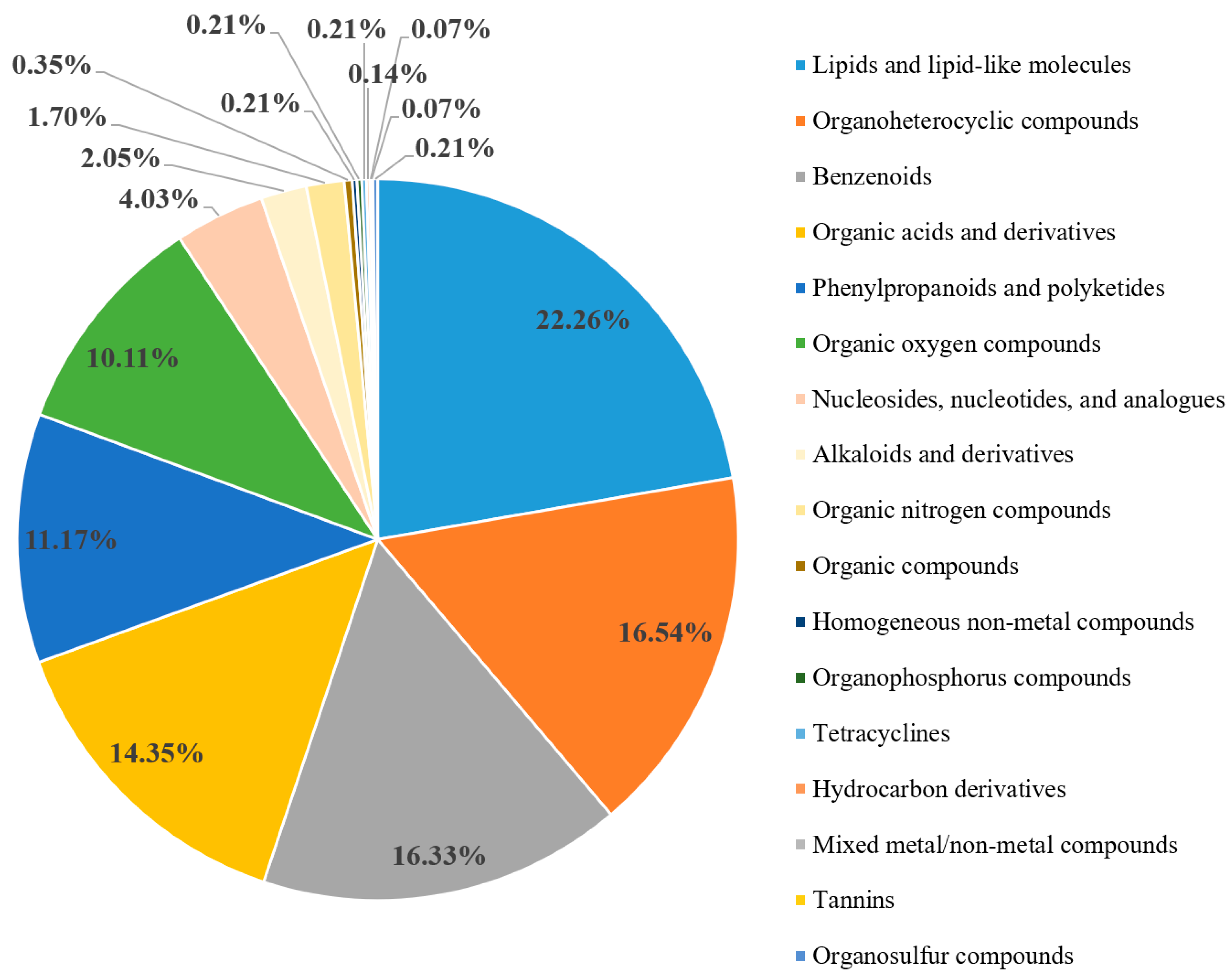
3.1. Fenugreek Metabolome Analysis
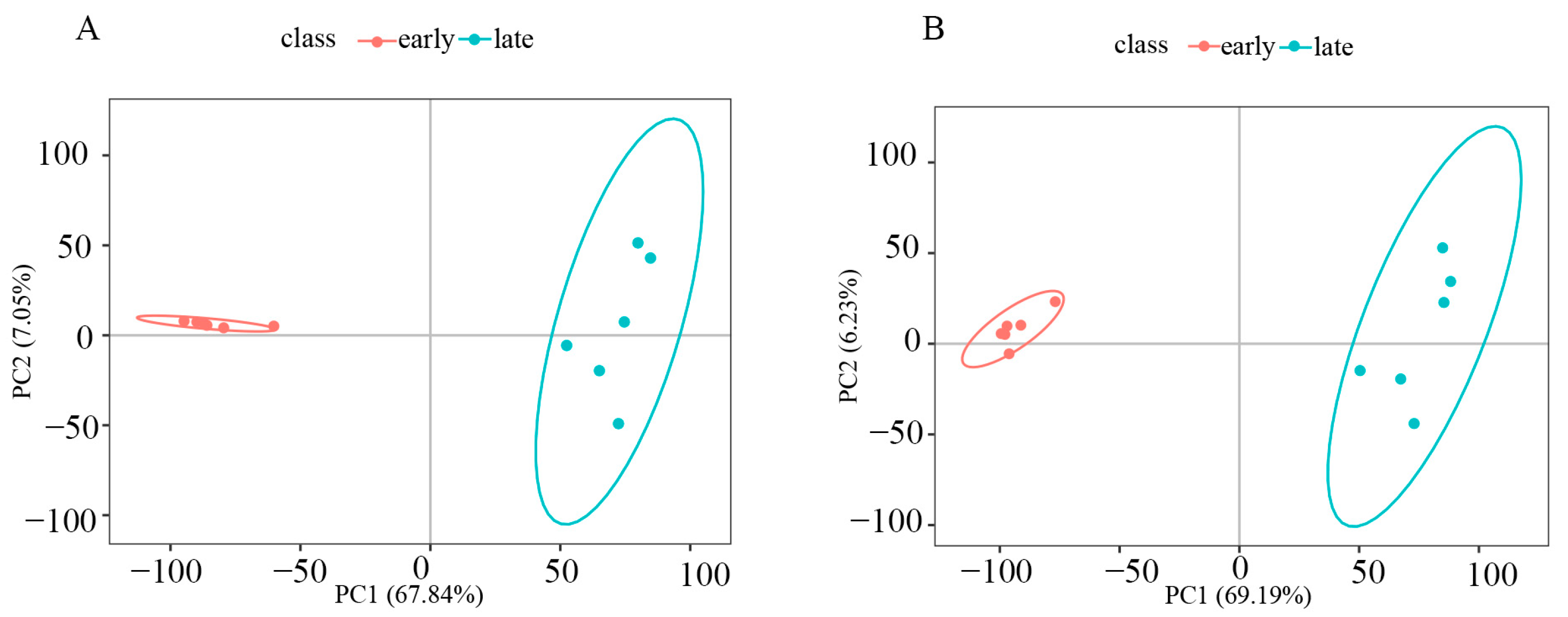
3.2. Principal Component Analysis (PCA)
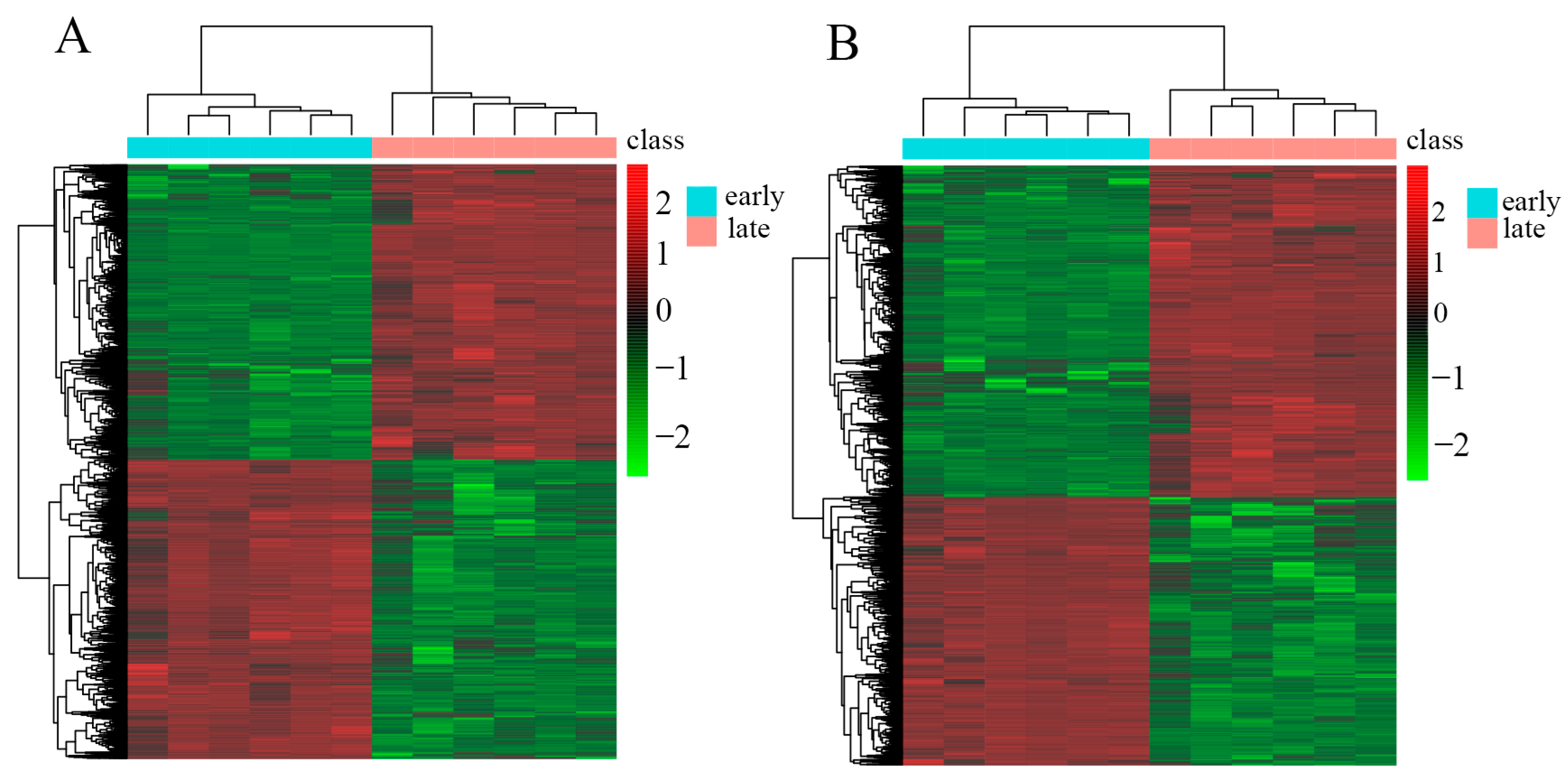
3.3. Cluster Heatmap Analysis
3.4. Enrichment Analyses of Differentially Accumulated Metabolites (DAMs) and Metabolites in Pathways Related to Flavonoids and Alkaloids
3.5. Analysis of Fenugreek Seed Transcriptome Data
3.6. Differential Expression Gene (DEG) Analysis
3.7. KEGG and GO Enrichment Analysis of DEGs
3.8. Integrated Metabolome and Transcriptome Analysis of Flavonoids and Alkaloids
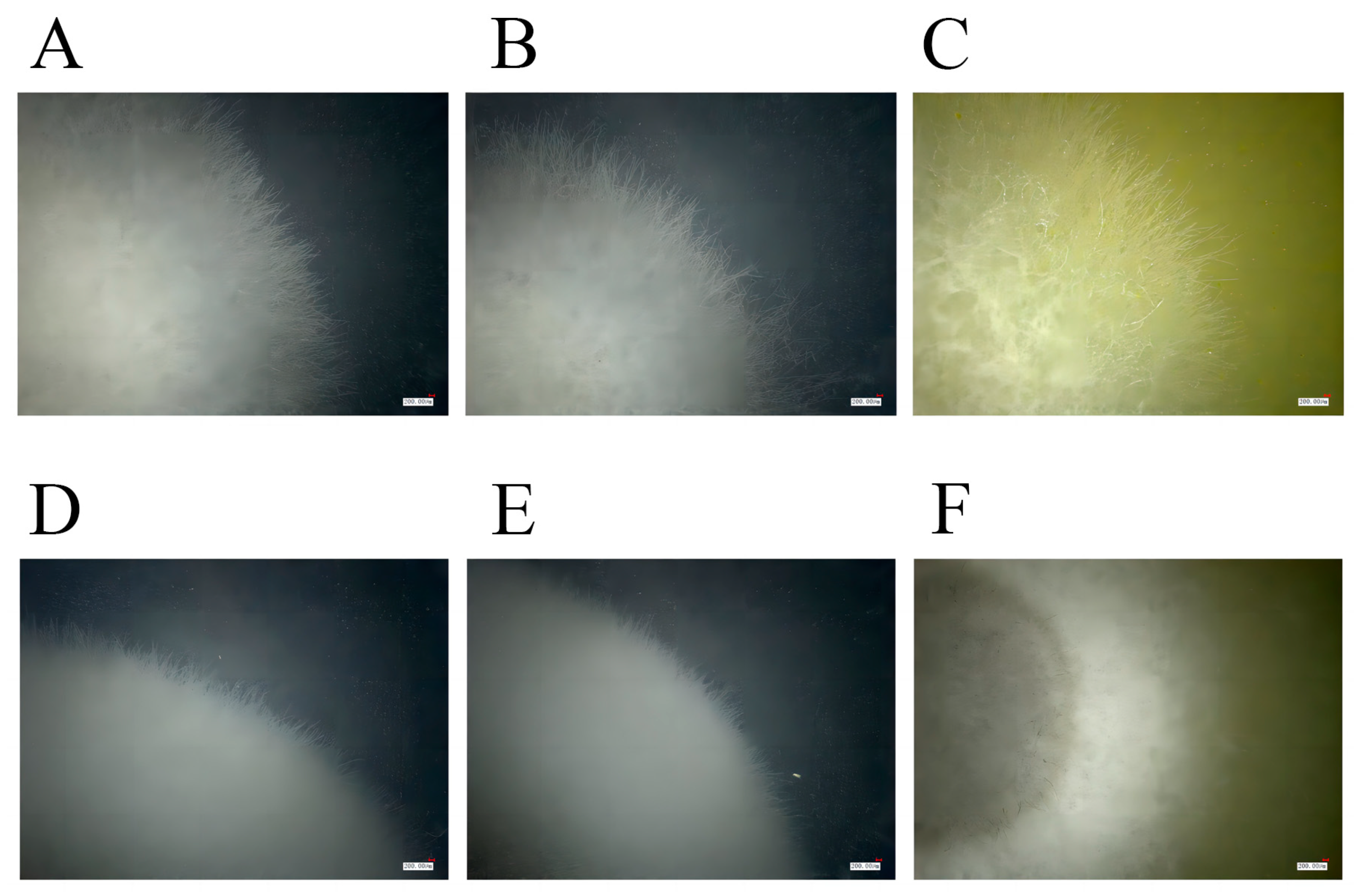
3.9. Antifungal Activity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.-Z.; Chen, Y.; Wu, Y.; Shan, F.-B.; Du, R.-X.; Yan, W.-Z.; Liu, C.-H. Fenugreek transcriptome sequencing and bioinformatics analysis. Mol. Plant Breed. 2021, 1–16. [Google Scholar]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek Cultivation with Emphasis on Historical Aspects and its uses in Traditional Medicine and Modern Pharmaceutical Science. Mini Rev. Med. Chem. 2021, 21, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Varshney, H.; Siddique, Y.H. Pharmacological Attributes of Fenugreek with Special Reference to Alzheimer’s Disease. Curr. Alzheimer Res. 2023, 20, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, X.; Yang, L.; He, W.-H.; Meng, T.-T.; Zheng, K.; Xia, X.; Zhou, Y.-J.; He, J.-H.; Liu, C.-M.; et al. The Effects of Fenugreek Extract on Growth Performance, Serum Biochemical Indexes, Immunity and NF-κB Signaling Pathway in Broiler. Front. Vet. Sci. 2022, 9, 882754. [Google Scholar] [CrossRef] [PubMed]
- Benayad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seeds by HPLC-DAD-ESI/MS analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef]
- Visuvanathan, T.; Than, L.T.L.; Stanslas, J.; Chew, S.Y.; Vellasamy, S. Revisiting Trigonella foenum-graecum L. Pharmacology and Therapeutic Potentialities. Plants 2022, 11, 1450. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef]
- Faghfoori, Z.; Javadivala, Z.; Khalili, Y.; Mahdavi, A.M. Effects of Trigonella foenum graecum (fenugreek) on rheumatoid arthritis: A systematic review. Immunopharmacol. Immunotoxicol. 2023, 45, 626–634. [Google Scholar] [CrossRef]
- Anwar, L.; Ali, S.A.; Khan, S.; Uzairullah, M.M.; Mustafa, N.; Ali, U.A.; Siddiqui, F.; Bhatti, H.A.; Rehmani, S.J.; Abbas, G. Fenugreek seed ethanolic extract inhibited formation of advanced glycation end products by scavenging reactive carbonyl intermediates. Heliyon 2023, 9, e16866. [Google Scholar] [CrossRef]
- Safarpour, S.; Mirzavi, F.; Rahmani, F.; Forouzanfar, F.; Sadeghnia, H.R.; Mashkani, B.; Alamdari, D.H.; Soukhtanloo, M. Fenugreek Seed Extract Regulates Human Umbilical Vein Endothelial Cell Angiogenesis and Proliferation via the PI3K/Akt/Cyclin D1 Pathway, Alternatives to laboratory animals. Altern. Lab. Anim. 2023, 51, 249–257. [Google Scholar] [CrossRef]
- Khoja, K.K.; Howes, M.R.; Hider, R.; Sharp, P.A.; Farrell, I.W.; Dada, G.O. Latunde- Cytotoxicity of Fenugreek Sprout and Seed Extracts and Their Bioactive Constituents on MCF-7 Breast Cancer Cells. Nutrients 2022, 14, 784. [Google Scholar] [CrossRef]
- Ilavenil, S.; Arasu, M.V.; Lee, J.-C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, G.J.; Mayakrishnan, V.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef]
- Li, Y.-H.; Sun, Q.-H.; Li, H.; Yang, B.; Wang, M. Vitexin suppresses renal cell carcinoma by regulating mTOR pathways. Transl. Androl. Urol. 2020, 9, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Naika, M.B.N.; Sathyanarayanan, N.; Sajeevan, R.S.; Bhattacharyya, T.; Ghosh, P.; Iyer, M.S.; Jarjapu, M.; Joshi, A.G.; Harini, K.; Shafi, K.M.; et al. Exploring the medicinally important secondary metabolites landscape through the lens of transcriptome data in fenugreek (Trigonella foenum graecum L.). Sci. Rep. 2022, 12, 13534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Extraction and Isolation of Total Flavonoids from Fenugreek Seeds and Research on Their Biological Activities; Anhui Agricultural University: Hefei, China, 2021. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-K.; Feng, Z.-X.; Wang, X.; Wang, X.-W.; Zhang, X.-G. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Murakami, T.; Komatsu, H.; Murakami, N.; Yamahara, J.; Matsuda, H. Medicinal foodstuffs. IV. Fenugreek seed. (1): Structures of trigoneosides Ia, Ib, IIa, IIb, IIIa, and IIIb, new furostanol saponins from the seeds of Indian Trigonella foenum-graecum L. Chem. Pharm. Bull. 1997, 45, 81–87. [Google Scholar] [CrossRef]
- Joshi, J.G.; Handler, P. Biosynthesis of trigonelline. J. Biol. Chem. 1960, 235, 2981–2983. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Jha, H.N.; Sanduja, S.K.; Sanduja, R. Trigocoumarin—A new coumarin from Trigonella foenum-graecum. Zeitschrift Für Naturforschung B 1982, 37, 521–523. [Google Scholar] [CrossRef]
- Shaukat, T.M.; Omer, M.O.; Javeed, A.; Rehman, H.U.; Shaukat, T.M. Isolation of alkaloidal and glycosidal fractions from leaves of Trigonella foenum-graecum L. cv. Desi indigenous to Pakistan for antiprostaglandin evaluation as substitute of nonsteroidal anti-inflammatory drugs. J. Ethnopharmacol. 2023, 317, 116730. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.-Z.; Wu, Q.-M.; Ding, X.-Y.; Yin, C.-Y.; Yang, E.D.; Sun, D.-D.; Wang, W.-Y.; Yang, Y.-Q.; Guo, F. Multiple responses optimization of antioxidative components extracted from Fenugreek seeds using response surface methodology to identify their chemicalingredients. Food Sci. Nutr. 2022, 10, 3475–3484. [Google Scholar] [CrossRef]
- Wang, R.; Wei, Y.-M.; Deng, W.-J.; Teng, J.-F. Pratensein Mitigates Oxidative Stress and NLRP3 Inflammasome Activation in OGD/R-Injured HT22 Cells by Activating Nrf2-Antioxidant Signaling. Neurotox. Res. 2022, 40, 384–394. [Google Scholar] [CrossRef]
- Yang, M.-Z.; Zhang, B.-B.; Liang, Z.-Q.; Cheng, N.-N.; Lü, A.-Q.; Yang, J.-Y.; Guo, X.-Z.; Bai, X.-Y.; Huang, Y.-J.; Jiao, A.-J.; et al. Sanguinarine suppresses cell proliferation, migration and invasion in nasopharyngeal carcinoma inhibiting mTOR signaling. J. Tradit. Chin. Med. Chung I Tsa Chih Ying Wen Pan 2022, 42, 687–692. [Google Scholar]
- Shawky, E.; Nassra, R.A.; El-Alkamy, A.M.T.; Sallam, S.M.; Sohafy, S.M.E. Unraveling the mechanisms of Fenugreek seed for managing different gynecological disorders: Steroidal saponins and isoflavones revealed as key bioactive metabolites. J. Pharm. Biomed. Anal. 2024, 238, 115865. [Google Scholar] [CrossRef]
- Tak, Y.; Kaur, M.; Chitranashi, A.; Samota, M.K.; Verma, P.; Bali, M.; Kumawat, C. Fenugreek derived diosgenin as an emerging source for diabetic therapy. Front. Nutr. 2024, 11, 128010. [Google Scholar] [CrossRef]
- Xia, Y.-Y.; Yang, J.-F.; Ma, L.; Yan, S.; Pang, Y.-Z. Genome-Wide Identification and Analyses of Drought/Salt-Responsive Cytochrome P450 Genes in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 9957. [Google Scholar] [CrossRef]
- Czemmel, S.; Stracke, R.; Weisshaar, B.; Cordon, N.; Harris, N.N.; Walker, A.R.; Robinson, S.P.; Bogs, J. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 2009, 151, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Ning, G.-G.; Wang, Z.; Shen, Y.-X.; Jin, H.-N.; Li, P.-H.; Huang, S.-S.; Zhao, J.; Bao, M.-Z. Disequilibrium of Flavonol Synthase and Dihydroflavonol-4-Reductase Expression Associated Tightly to White vs. Red Color Flower Formation in Plants. Front. Plant Sci. 2015, 6, 1257. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Li, C.; Song, W.-H.; Shen, Y.-F.; Tang, W.; Zhang, Y.-G.; Wang, X.; Yan, H.; Gao, R.-F.; Ahmad, M.-Q.; et al. Identification and functional characterization of a flavonol synthase gene from sweet potato [Ipomoea batatas (L.) Lam.]. Front. Plant Sci. 2023, 14, 1181173. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-C.; Zhang, X.-D.; Gao, Z.-Q.; Hu, T.; Liu, Y. The Research Progress of Chalcone Isomerase (CHI) in Plants. Mol. Biotechnol. 2019, 61, 32–52. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, Q.-H.; Zhao, K.-K.; Zhai, W.-H.; Zhang, F.; Dong, H.-Z.; Lin, L.-K.; Xing, C.-H.; Su, Z.-Y.; Pan, Z.-J.; et al. PbWRKY18 promotes resistance against black spot disease by activation of the chalcone synthase gene PbCHS3 in pear. Plant Sci. Int. J. Exp. Plant Biol. 2024, 341, 112015. [Google Scholar] [CrossRef]
- Haouala, R.; Hawala, S.; El-Ayeb, A.; Khanfir, R.; Boughanmi, N. Aqueous and organic extracts of Trigonella foenum-graecum L. inhibit the mycelia growth of fungi. J. Environ. Sci. 2008, 20, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Gajbar, T.D.; Kamble, M.; Adhikari, S.; Konappa, N.; Satapute, P.; Jogaiah, S. γ-irradiated fenugreek extracts mediates resistance to rice blast disease through modulating histochemical and biochemical changes. Anal. Biochem. 2021, 618, 114121. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.-L.; Ye, H.-J.; Shen, Q.; Jiang, X.-Y.; Cui, G.-B.; Gu, W.-X.; Zhang, H.-L.; Naqvi, N.I.; Deng, Y.-Z. Tangeretin inhibits fungal ferroptosis to suppress rice blast. J. Integr. Plant Biol. 2021, 63, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
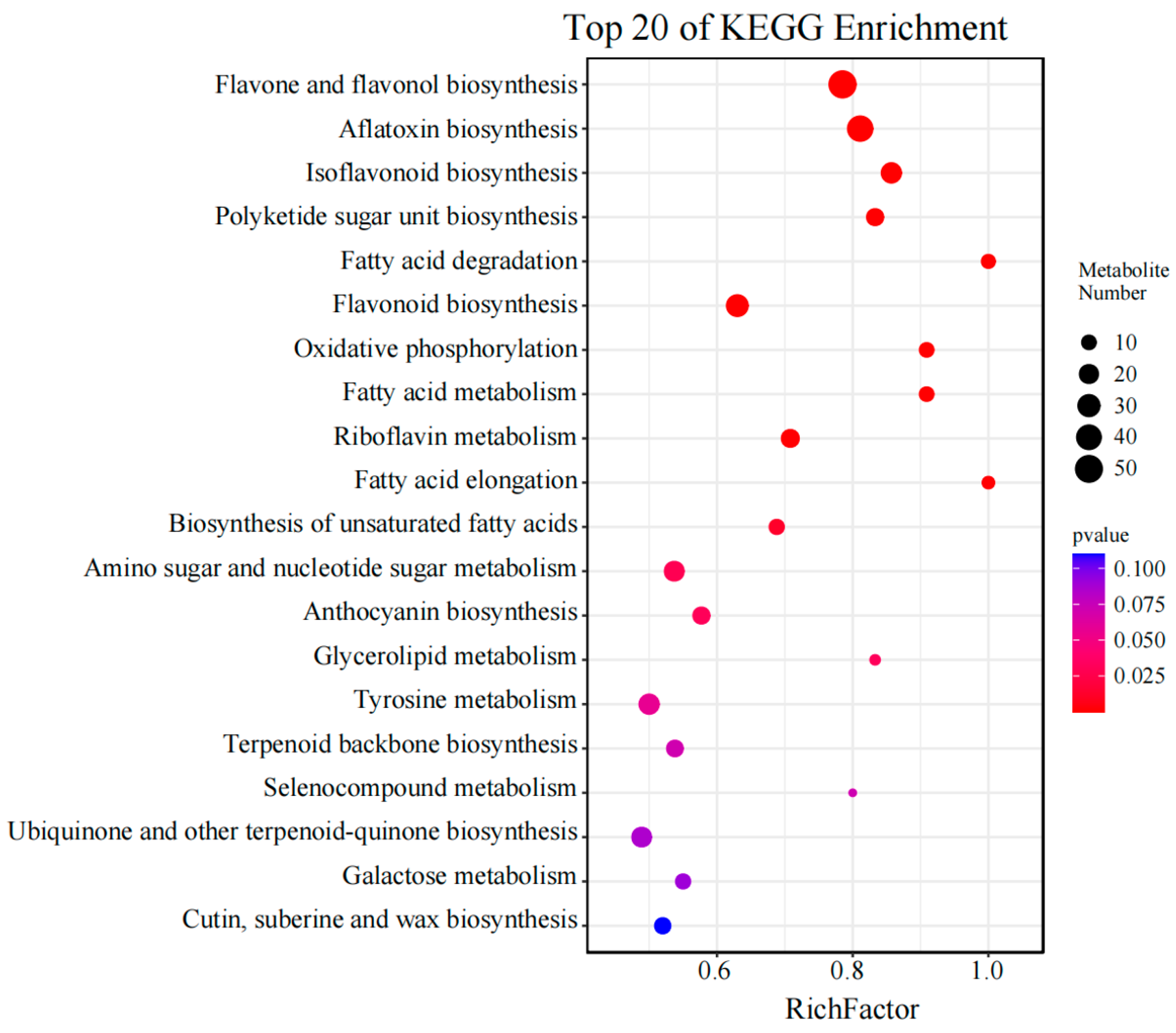
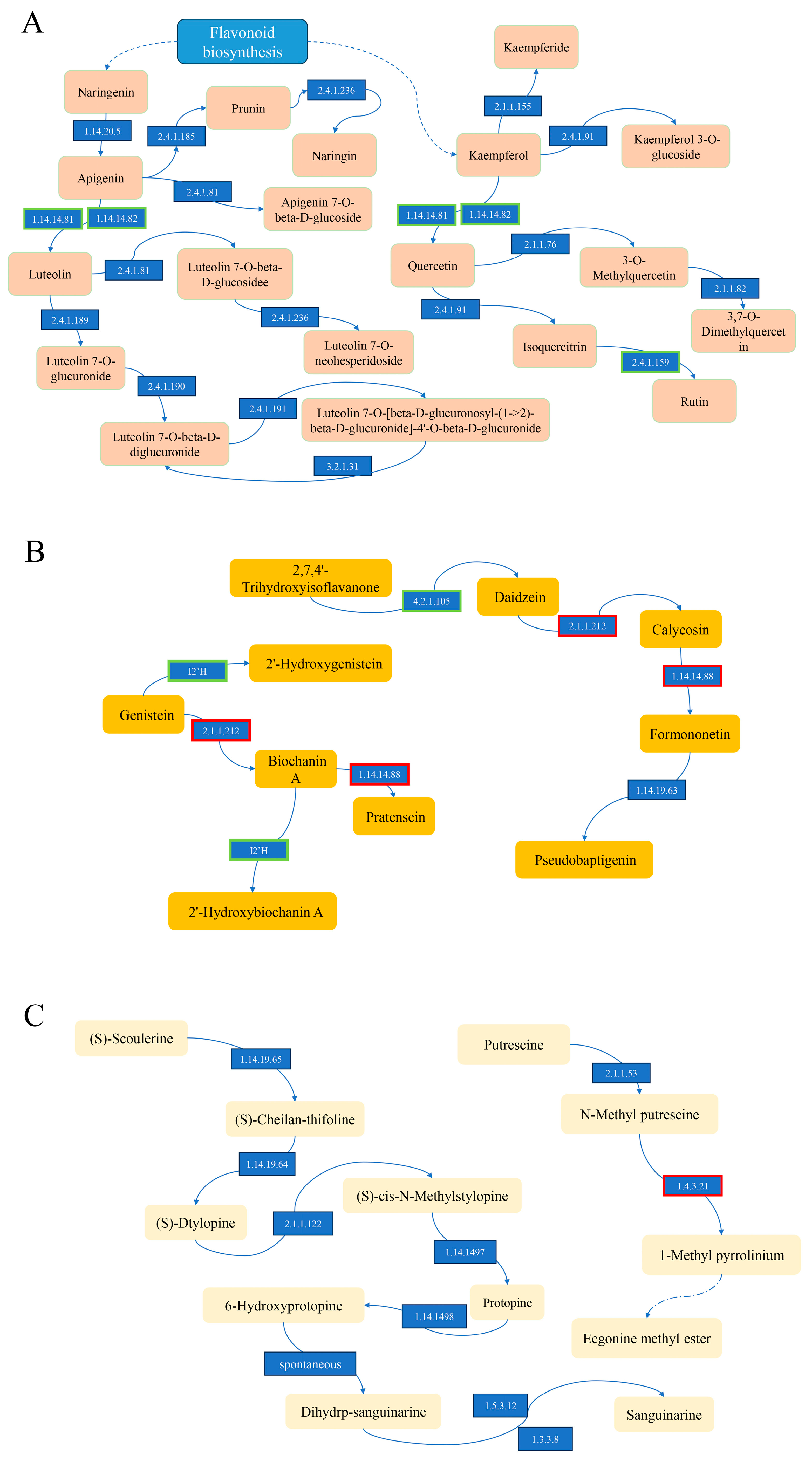
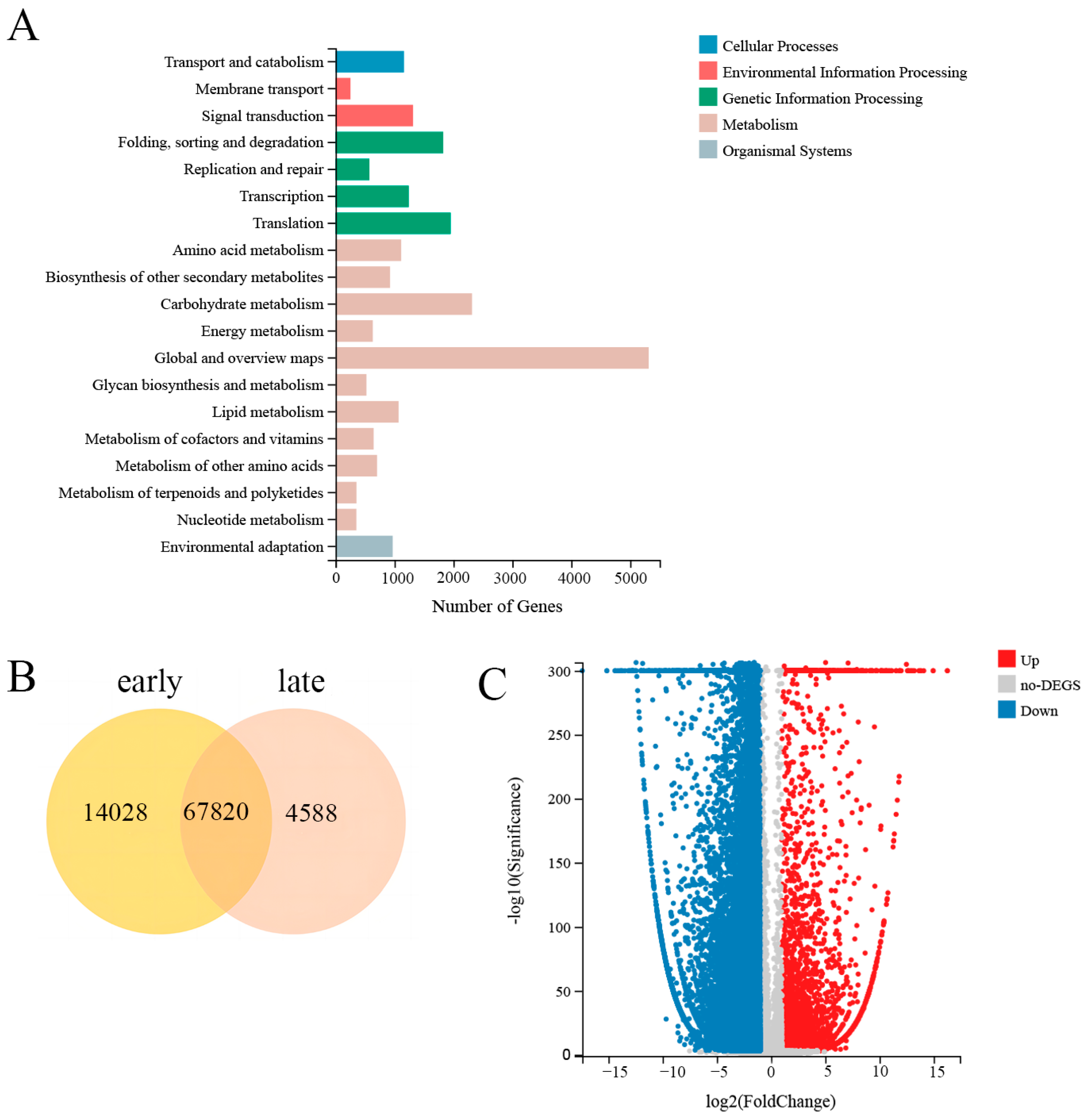
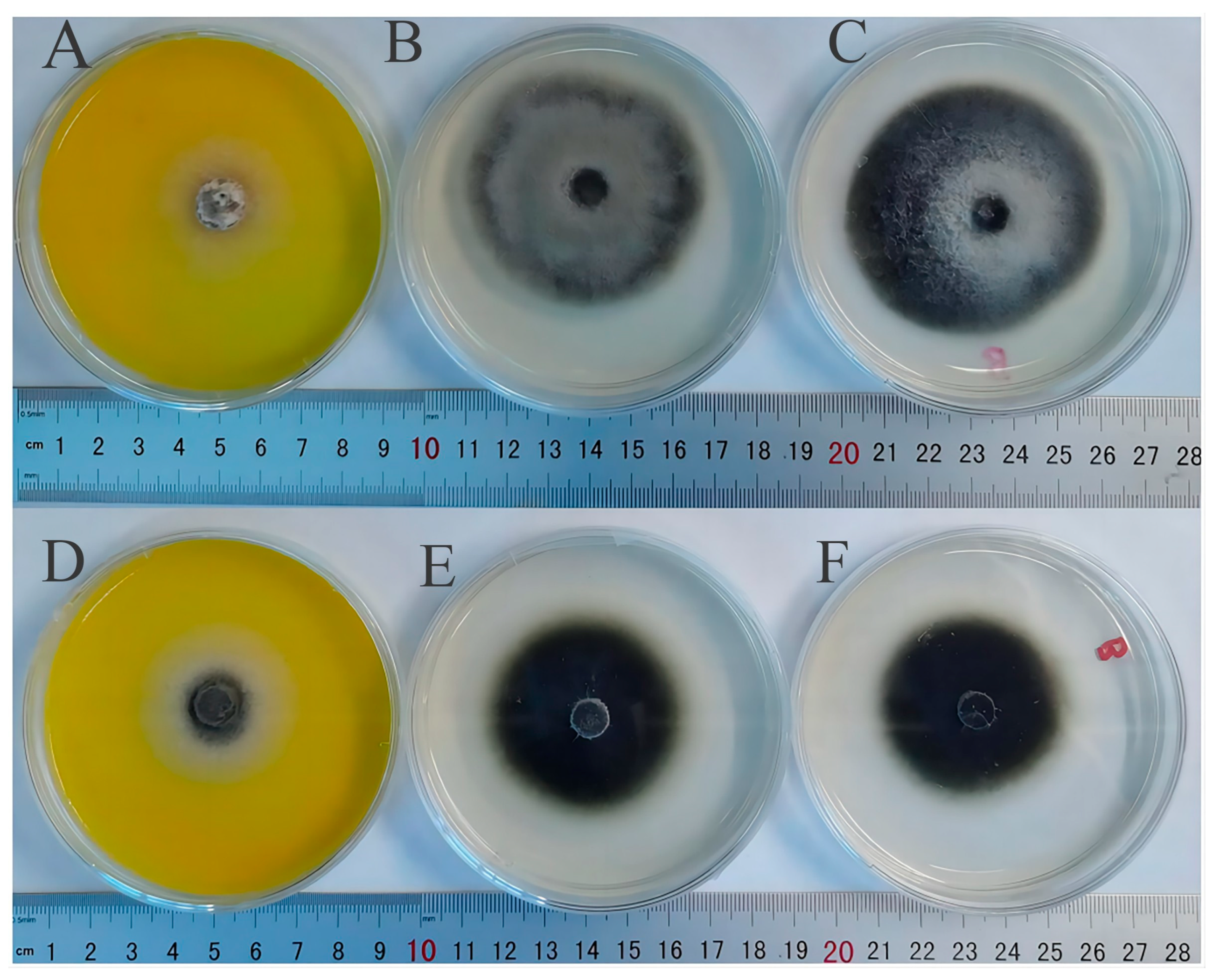
| Compounds | Fold Change | Pathway |
|---|---|---|
| Flavonoids: | ||
| 3,7-Di-O-methylquercetin | 36.862 | map00944 Flavone and flavonol biosynthesis |
| Luteolin 7-O-β-D-diglucuronide | 27.739 | map00944 Flavone and flavonol biosynthesis |
| Apigenin | 24.697 | map00941 Flavonoid biosynthesis |
| Apigenin 7-O-β-D-glucoside | 21.996 | map00941 Flavonoid biosynthesis |
| Naringin | 18.806 | map00941 Flavonoid biosynthesis |
| Chrysoeriol | 16.954 | map00944 Flavone and flavonol biosynthesis |
| Rutin | 16.662 | map00944 Flavone and flavonol biosynthesis |
| Kaempferol 3-O-glucoside | 14.202 | map00944 Flavone and flavonol biosynthesis |
| Luteolin 7-O-β-D-glucoside | 11.333 | map00944 Flavone and flavonol biosynthesis |
| Dihydrokaempferol | 8.712 | map00941 Flavonoid biosynthesis |
| Luteolin 7-O-[β-D-glucuronosyl-(1->2)-β-D-glucuronide]-4′-O-β-D-glucuronide | 8.27 | map00944 Flavone and flavonol biosynthesis |
| Epigallocatechin 3-gallate | 7.897 | map04152 AMPK signaling pathway |
| Phlorizin | 7.408 | map00941 Flavonoid biosynthesis |
| Kaempferide | 6.491 | map00944 Flavone and flavonol biosynthesis |
| (+)-Catechin | 2.936 | map00941 Flavonoid biosynthesis |
| Naringenin | 2.463 | map00941 Flavonoid biosynthesis |
| Isoflavonoids: | ||
| Pseudobaptigenin | 36.652 | map00941 Flavonoid biosynthesis |
| 2′-Hydroxybiochanin A | 30.126 | map00943 Isoflavonoid biosynthesis |
| 2′-Hydroxygenistein | 19.097 | map00943 Isoflavonoid biosynthesis |
| Pratensein | 16.954 | map00943 Isoflavonoid biosynthesis |
| Alkaloids and derivatives: | ||
| Ecgonine methyl ester | 24.183 | map00960 Tropane, piperidine, and pyridine alkaloid biosynthesis |
| Sanguinarine | 19.123 | map00950 Isoquinoline alkaloid biosynthesis |
| Compound | Gene Name | Correlation Coefficients | p-Values |
|---|---|---|---|
| Flavonoids: Apigenin | legumin B | 0.999397805 | 0.000000544 |
| Chrysosplenol D | legumin B | 0.999574004 | 0.000000272 |
| Cosmosiin | legumin J | 0.999622259 | 0.000000214 |
| Resokaempherol | legumin J | 0.999758896 | 0.0000000872 |
| seed linoleate 9S-lipoxygenase-3 | 0.9991967 | 0.000000968 | |
| Kaempferitrin | H/ACA ribonucleoprotein complex | 0.999758896 | 0.0000000872 |
| subunit 4 | |||
| HMG (high mobility group) box protein | 0.9991967 | 0.000000968 | |
| with ARID | |||
| Isoflavonoids: 3,7-Di-O-methylquercetin | legumin J | 0.999412712 | 0.000000517 |
| Apigenin dimethylether; | aldehyde decarbonylase | 0.999397805 | 0.000000544 |
| Astragalin | legumin B | 0.999583385 | 0.00000026 |
| Tangeretin | UPSTREAM OF FLC protein | 0.999001756 | 0.00000149 |
| Pratensein | legumin J | 0.999724414 | 0.000000114 |
| Dehydroferreirin; | legumin J | 0.999650167 | 0.000000184 |
| Alkaloids and derivatives: | |||
| Harmalol | (R)-mandelonitrile β-glucosyltransferase | 0.999715273 | 0.000000122 |
| (+/−)-6-Acetonyldihydrosanguinarine | tetratricopeptide repeat (TPR)-containing protein | 0.99925841 | 0.000000825 |
| Phytopathogenic Fungi | Treat (Diameter/cm) | CK (Diameter/cm) | Blank (Diameter/cm) | Inhibition Rate |
|---|---|---|---|---|
| M. oryzae | 3.85 ± 0.18 b | 6.13 ± 0.08 a | 5.63 ± 0.63 | 37.22% |
| A. tenuissima (Kunze)Wiltshire | 3.77 ± 0.28 b | 6.52 ± 0.25 a | 6.67 ± 0.16 | 42.16% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Wu, G.; Yang, P.; Shi, Y.; Fu, Z.; Mo, H.; Shi, C.; Yu, S. Metabolomic and Transcriptomic Analyses Reveal the Molecular Mechanism Underlying the Massive Accumulation of Secondary Metabolites in Fenugreek (Trigonella foenum-graecum L.) Seeds. Genes 2024, 15, 343. https://doi.org/10.3390/genes15030343
Zhao Q, Wu G, Yang P, Shi Y, Fu Z, Mo H, Shi C, Yu S. Metabolomic and Transcriptomic Analyses Reveal the Molecular Mechanism Underlying the Massive Accumulation of Secondary Metabolites in Fenugreek (Trigonella foenum-graecum L.) Seeds. Genes. 2024; 15(3):343. https://doi.org/10.3390/genes15030343
Chicago/Turabian StyleZhao, Qiuyu, Guoxing Wu, Pu Yang, Yuanchong Shi, Zuoyi Fu, Haifeng Mo, Chunlan Shi, and Shuhui Yu. 2024. "Metabolomic and Transcriptomic Analyses Reveal the Molecular Mechanism Underlying the Massive Accumulation of Secondary Metabolites in Fenugreek (Trigonella foenum-graecum L.) Seeds" Genes 15, no. 3: 343. https://doi.org/10.3390/genes15030343
APA StyleZhao, Q., Wu, G., Yang, P., Shi, Y., Fu, Z., Mo, H., Shi, C., & Yu, S. (2024). Metabolomic and Transcriptomic Analyses Reveal the Molecular Mechanism Underlying the Massive Accumulation of Secondary Metabolites in Fenugreek (Trigonella foenum-graecum L.) Seeds. Genes, 15(3), 343. https://doi.org/10.3390/genes15030343






