Abstract
Gambling Disorder (GD) is characterised by a harmful, enduring, and recurrent involvement in betting-related behaviours. Therefore, GD shares similar biological mechanisms and symptoms to substance use disorders (SUD). Therefore, in this study, we chose the behavioural addictions group. During the examination and recruitment to the study, it turned out that all the people undergoing treatment for gambling addiction were also addicted to amphetamines, which is consistent with the biological mechanism related to cerebral neurotransmission. The aim of the study was to investigate the association of the COMT gene polymorphism with behavioral addiction. The study group consisted of 307 participants: 107 men with gambling disorder and amphetamine dependency (mean age = 27.51, SD = 5.25) and 200 non-addicted, nor dependent, free from neuro-psychiatric disorders control group men (mean age = 20.20, SD = 4.51). Both groups were subjected to psychometric evaluation using the State-Trait Anxiety Inventory and the NEO Five-Factor Personality Inventory. Genomic DNA was extracted from venous blood following standard protocols. Determination of the rs4680 polymorphism in the COMT gene was performed using the real-time PCR technique. Statistically significant differences in the frequency of rs4680 genotypes were found in the tested sample of subjects compared with the control group (p = 0.03543). Subjects with gambling disorder and amphetamine use disorder compared to the control group obtained higher scores in the assessment of the STAI trait scale (p = 0.0019), state scale (p < 0.0000), and NEO-FFI Neuroticism scale (p < 0.0000). Significantly lower results were obtained for the NEO-FFI Agreeability scale (p < 0.0000). Additionally, a significant statistical impact of gambling disorder and amphetamine use disorder, and the COMT rs4680 genotype was demonstrated for the score of the STAI trait (p = 0.0351) and state (p = 0.0343) and the NEO-FFI Conscientiousness scale (p = 0.0018). We conclude that COMT and its polymorphic variant influence the development of addiction. Still, considering its multifactorial and polygenic nature, it should be combined with other factors such as personality.
1. Introduction
Behavioral addictions have an identical neurobiological basis as substance addictions. Gambling Disorder (GD) is characterised by a harmful, enduring, and recurrent involvement in betting-related behaviours [1]. Hence, Gambling Disorder exhibits biological processes and symptoms that are akin to those found in Substance Use Disorders (SUD) [2]. Therefore, in the present study, a homogeneous subgroup of people with behavioural addiction was selected. This choice was dictated by the literature but also by the fact that in rehab facilities, groups of behavioural addicts are formed as separate, homogeneous subgroups. Previous research has shown that GD and SUD often co-occur [3,4], and both of these conditions may be very different in a population with only one of these diagnoses [4]. Specifically, symptoms of SUD and coexisting GD are typically complex [5,6], and treatment can be particularly challenging [7]. The relative time of onset of substance use disorder and gambling disorder is problematic. Specifically, SUD may precede GD in some individuals, whereas it might follow GD in another group, while the co-occurring disorders are more often present before GD [8,9]. Therefore, in this study, we chose the behavioural addictions group. During the examination and recruitment to the study, it turned out that all people undergoing treatment for gambling addiction were also addicted to amphetamines, which is consistent with the biological mechanism related to cerebral neurotransmission. It was also important for us to select a gene related to brain neurotransmission, which was described in detail in the introduction.
Impairments in many post-cognitive areas, including inhibition, working memory, decision-making, cognitive flexibility and executive planning, have been reported in studies regarding adults with gambling problems [10,11]. Dopamine is crucial for cognitive functions that rely on the frontostriatal circuit. It is believed that dopamine imbalances can significantly influence impulsive behaviours, especially those associated with decision-making and inhibitory control [12]. A unique role in the regulation of dopamine in the prefrontal cortex is played by the enzyme catechol-O-methyltransferase (COMT) [13] and is recognised in many psychiatric disorders, particularly those characterised by high impulsivity, as a potential pharmacological target for the treatment of cognitive dysfunction [14,15].
As the dopaminergic system undoubtedly influences the development and course of addiction, the present study focused on the gene encoding one of the enzymes involved in the metabolism of dopamine, catechol-o-methyltransferase, which is a postsynaptic enzyme that degrades catecholamines (epinephrine, dopamine and norepinephrine) [16]. In the COMT gene, mapped to chromosome 22q11.1-q11.2, with a size of approximately 27 Kbp, up to 345 polymorphisms have been identified. One functional single nucleotide polymorphism (rs4680) is caused by guanine to adenine substitution at codon 158, resulting in a change of valine (Val) to methionine (Met). This polymorphism may affect dopamine levels, particularly in the prefrontal cortex [17]. Carriers of the Val158 allele synthesise a thermostable form of the enzyme [18], with 40% higher brain activity than the Met158 allele at normal body temperature. As these two alleles are additive, heterozygotes show intermediate activity [19]. Higher extracellular dopamine levels in prefrontal cortex areas and better performance in cognitive tasks have been reported for the low-activity Met158 allele [20]. The Val158 allele, on the other hand, has been associated with positive processing of the signals related to aversive stimuli [21]. This polymorphism has been identified as a risk factor for several neuropsychiatric disorders, including substance use and addiction, obsessive-compulsive disorder (OCD) [22] and attention-deficit/hyperactivity disorder (ADHD) [23]. The low-activity COMT allele or genotype has been linked to alcohol problems in several studies [24,25,26]. It has also been shown that the highly active COMT allele (Val158) is more common in polysubstance users [27] and heroin abusers [28].
In the present study, the gene encoding COMT and its functional polymorphic variant were selected due to their connection with the functioning of the dopaminergic system and the possibility of interaction with the environment. Research on this gene suggests that the effect of the COMT Val158Met polymorphism on behaviour should be considered in the context of gene-environment interactions rather than a direct effect. Interestingly, carriers of the methionine allele are more susceptible to stress and environmental factors in some studies of general population samples [29]. The methionine allele is associated with increased anxiety, decreased extraversion and decreased novelty seeking [30,31]. Psychostimulants have different effects in people with different COMT gene variants. In Val/Val subjects, amphetamine improves PFC function and performance on tasks measuring working memory or attention [32,33]. Met/Met individuals show better PFC function and are reported to have higher baseline PFC dopamine concentrations than Val/Val carriers under normal conditions [34]. However, amphetamine exposure has been shown to impair PFC function, working memory performance and attention in Met/Met carriers [32,33]. The Val158Met substitution has been shown to have sex-specific consequences. In vitro cell studies have shown that physiological levels of 17-β-estradiol can downregulate COMT gene transcription and COMT protein expression [35,36]. In another study, an association was found between Met alleles with low levels of activity and obsessive-compulsive disorder in men, but not in women [37]. Studies in mice have shown that homozygous COMT-knockout females develop increased anxiety in a light-dark model compared to COMT-knockout males. In the same study, heterozygous COMT knockout males showed increased aggressive behaviour compared to other male genotypes [38].
The ‘endophenotypic’ approach [39,40,41,42] is a recent conceptual approach that may help reduce the heterogeneity of substance use disorder phenotypes and provide a framework for identifying general and specific factors influencing SUD [39,40,41,42]. Considered genetically ‘simpler’ than SUDs, endophenotypes are measurable traits that lie between the clinical phenotype and the disease susceptibility genotype [39,40,41]. Neurocognitive function is particularly well suited as an endophenotype. It is more objective than self-reported measures. According to researchers, impulsivity has a significant relationship with addiction. The neurocognitive dimensions of impulsivity have received the strongest support as a potential SUD endophenotype among the various neurocognitive functions associated with SUD [40,43,44,45]. Several dimensions characterise neurocognitive impulsivity. These are typically measured using tasks that fall into one of two categories [46]: (1) decision/choice impulsivity, referring to the tendency to choose immediate but smaller rewards over delayed but larger rewards; may involve deficits in delayed gratification and self-control [41], as assessed by decision making tasks involving different risk, reward and delay events [41,47]; (2) motor/action impulsivity, referring to the ability to fail to inhibit inappropriate actions, as assessed by response inhibition tasks [48,49]. People addicted to different classes of drugs, such as opiates and stimulants, may differ significantly in these dimensions of impulsivity [50,51,52,53].
The factor related to impulsivity and other traits also seems important, as described by Boscutti et al. in 2022, considering various genetic factors [54]. Another study supporting our approach to analysis was presented by Fang et al., who examined COMT in a clinical context [55].
In a comprehensive and holistic approach to addiction and dependency as a dysfunction of the dopaminergic system in the brain, personality-related factors cannot be forgotten or omitted. Therefore, in the presented study we analysed personality dimensions measured by the NEO-Five Factor Inventory, and anxiety measured by the State-Trait Anxiety Inventory together with COMT rs4680. The aim of the study was to investigate the association of the COMT gene polymorphism with behavioral addiction.
We emphasise that this is the first study of its kind to consider the simultaneous analysis of psychological and genetic factors, also taking into account interactions. The study included a group of men as a homogeneous group, not only biologically but also psychologically.
2. Materials and Methods
The study group consisted of 307 subjects: 107 men with gambling disorder and amphetamine dependence, during three months of abstinence in an addiction treatment facility (mean age = 27.51, SD = 5.25) and 200 non-addicted, nor dependent, free from neuropsychiatric disorders control group men (mean age = 20.20, SD = 4.51). The study was approved by the Bioethics Committee of the Pomeranian Medical University in Szczecin (KB-0012/106/16 (17 October 2016)). All participants gave written informed consent before participating in the study. The study was conducted in the Independent Laboratory of Health Promotion at the Pomeranian Medical University in Szczecin.
2.1. Psychometric Tests
In the study group and in the control group, the NEO-FFI personality test and the State-Trait Anxiety Inventory (STAI) were performed. NEO-FFI defines five main traits—extraversion, openness, conscientiousness, agreeableness and neuroticism. The STAI inventory, on the other hand, describes anxiety as a trait and/or as a state [56]. A psychologist interpreted the results of the psychometric tests. The results were converted to the sten scale. The interpretation included Polish standards for adults, which assume a meagre rating for sten 1–2, a low rating for sten 3–4, an average rating for sten 5–6, a high rating for sten 7–8 and a very high rating for sten 9–10.
The MINI International Neuropsychiatric Interview was used to evaluate the eligibility for inclusion into the control group [57]. It is a structured diagnostic interview assessing mental disorders. Mini focuses on the patient’s current condition. Past diagnoses are analysed to determine if they are clinically significant for the present.
2.2. Genotyping
Standard procedures were used to isolate genomic DNA from venous blood.
The isolation of genetic material was carried out according to ROCHE standards and procedures. The selection of reagents and primers can be found in the description of the ROCHE real-time PCR methodology.
Determination of the rs4680 polymorphism in the COMT gene was performed using the real-time PCR technique. Melting curves were generated for each sample by plotting the fluorescence signal as a function of temperature. The peaks of the COMT rs4680 polymorphic site were read at 53.29 °C for the A allele and at 59.93 °C for the G allele.
2.3. Statistical Analysis
The HWE software was used to test the concordance of the alleles frequency distribution with the Hardy–Weinberg equilibrium (https://wpcalc.com/en/equilibrium-hardy-weinberg/ (accessed on 3 December 2023).
A multivariate analysis of factor effects ANOVA was used to analyse the relations between COMT rs4680 variants, gambling disorder and amphetamine dependency, and control subjects, as well as the NEO Five-Factor Inventory [NEO-FFI/scale STAI/ × genetic feature × control and gambling disorder and amphetamine dependency × (genetic feature × control and gambling disorder and amphetamine dependency)]. The homogeneity of variance condition was met (Levene test p > 0.05). The variables under analysis did not follow a normal distribution. The U Mann–Whitney test was used to compare sten scores for the NEO Five-Factor Inventory (Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness). The association of the COMT rs4680 genotype and alleles in both groups was tested using the chi-squared test (n = 307, φ = 0.15; α = 0.05; statistical power 0.646). The computations were performed using STATISTICA 13 (Tibco Software Inc., Palo Alto, CA, USA) for Windows (Microsoft Corporation, Redmond, WA, USA).
3. Results
The frequency distributions were consistent with the Hardy–Weinberg equilibrium (HWE) in both the group with a gambling disorder and amphetamine use disorder, as well as the control group (Table 1).

Table 1.
Hardy-Weinberg’s equilibrium for the COMT rs4680 polymorphism.
Statistically significant differences in the frequency of COMT rs4680 genotypes were found in the tested sample of subjects with gambling disorder and amphetamine use disorder compared with the control group (G/A 0.57 vs. G/A 0.42; A/A 0.24 vs. A/A 0.36; G/G 0.19 vs. G/G 0.22, χ2 = 6.681, p = 0.03543). No statistically significant differences in the frequency of COMT rs4680 alleles were found between subjects with gambling disorder and amphetamine use disorder and the control group (A 0.53 vs. A 0.57; G 0.47 vs. G 0.43, χ2 = 0.990, p = 0.3187) (Table 2).

Table 2.
Frequency of the genotypes and alleles of the COMT rs4680 polymorphism in the subjects with gambling disorder and amphetamine use disorder and control subjects.
The means and standard deviations of the NEO-FFI and STAI state and trait scores for subjects with gambling and amphetamine use disorders and controls are shown in Table 3.

Table 3.
STAI and NEO Five-Factor Inventory sten scores in subjects with gambling disorder and amphetamine use disorder, and controls.
Subjects with gambling disorder and amphetamine use disorder compared to the control group obtained higher scores in the assessment of STAI trait scale (6.98 vs. 5.33; Z = 3.106; p = 0.0019), and state scale (5.60 vs. 4.77; Z = 5.575; p < 0.0000), and NEO-FFI Neuroticism scale (6.58 vs. 4.76; Z = 6.657; p < 0.0000). Significantly lower results were obtained for the NEO-FFI Agreeability scale (4.28 vs. 5.54; Z = −4.941; p < 0.0000).
The results of the 2 × 3 factorial ANOVA of the NEO Five-Factor Personality Inventory and the State-Trait Anxiety Inventory sten scales are summarised in Table 4. The significant statistical impact of gambling disorder and amphetamine use disorder and the COMT rs4680 genotype was demonstrated for the score of the STAI trait scale. There was a statistically significant effect of the COMT rs4680 genotype interaction and gambling disorder and amphetamine use disorder or not using (control group), on the STAI trait scale (F2,301 = 3.39; p = 0.0351; η2 = 0.022; Figure 1). The power observed for this factor was 64%, and approximately 2% was explained by the polymorphism of COMT rs4680 and gambling disorder and amphetamine use disorder, or lack thereof, on STAI trait score variance. There was also a statistically significant effect of gambling disorder and amphetamine use disorder or the control group on the STAI state scale score (F2,301 = 3.41; p = 0.0343; η2 = 0.022; Figure 2). The power observed for this factor was 64%, and approximately 2% was explained by the polymorphism of COMT rs4680 and gambling disorder and amphetamine use disorder, or lack thereof, on the variance in the STAI state scale score.

Table 4.
The results of 2 × 3 factorial ANOVA for gambling and amphetamine use disorder subjects and controls, NEO-FFI, STAI and COMT rs4680.
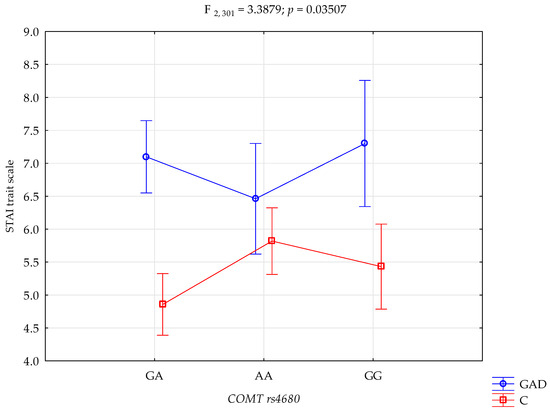
Figure 1.
Interaction between gambling disorder and amphetamine use disorder (GAD); n = 107/control (C), and COMT rs4680, and STAI trait scale.
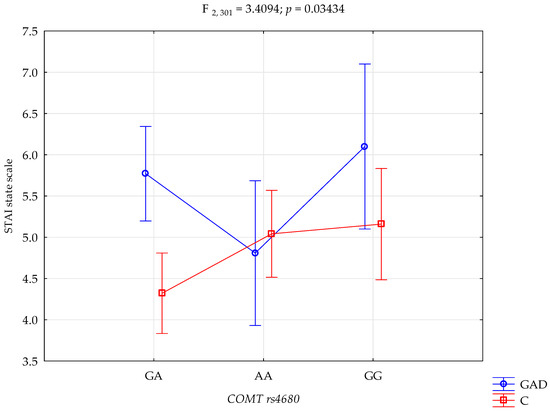
Figure 2.
Interaction between gambling disorder and amphetamine use disorder (GAD); n = 107/control (C), and COMT rs4680, and STAI state scale.
A significant statistical impact of gambling disorder and amphetamine use disorder and the COMT rs4680 genotype was demonstrated for the score of the NEO-FFI Conscientiousness scale. There was a statistically significant effect of the COMT rs4680 genotype interaction and gambling disorder and amphetamine use disorder or not using (control group) on the Conscientiousness scale (F2,301 = 6.47; p = 0.0018; η2 = 0.041; Figure 3). The power observed for this factor was 90%, and approximately 4% was explained by the polymorphism of COMT rs4680, gambling disorder and amphetamine use disorder, or lack thereof, on the trait of the Conscientiousness score variance. Table 5 shows the results of the post hoc test.
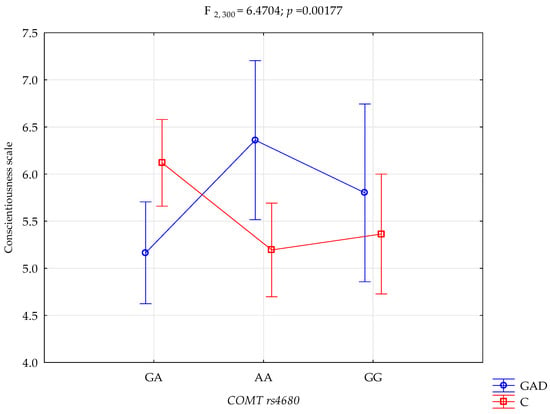
Figure 3.
Interaction between gambling disorder and amphetamine use disorder (GAD)/control (C) and COMT rs4680 and Conscientiousness scale.

Table 5.
Post hoc test (Bonferroni) analysis of interactions between gambling disorder and amphetamine use disorder, control and COMT rs4680 and Conscientiousness scale, anxiety as a state and as a trait.
There is a significant interaction between gambling disorder and amphetamine use disorder, and the COMT gene rs4680 polymorphism in the outcome score level of anxiety as a trait. Subjects with gambling disorder and amphetamine use disorder with the GA genotype have significantly higher levels of anxiety as a trait compared to the control group with the GA, AA and GG genotypes. Similarly, gambling disorder and amphetamine use disorder subjects with the GG genotype have significantly higher levels of anxiety as a trait compared to the control group with the GA, AA and GG genotypes. Subjects with gambling disorder and amphetamine use disorder with the AA genotype have significantly higher levels of anxiety as a trait compared to the control group with the GA genotype. The control group with the GA genotype has significantly lower anxiety as a trait compared to the control group with the AA genotype (Table 5).
There is a significant interaction between gambling disorder and amphetamine use disorder, and the COMT gene rs4680 polymorphism in the outcome score level of anxiety as a state. Gambling disorder and amphetamine use disorder subjects with the GA genotype have significantly higher levels of anxiety as a state compared to the control group with the GA genotype. Gambling disorder and amphetamine use disorder subjects with the GG genotype have significantly higher levels of anxiety as a state compared to the control group with the GA genotype The control group with the GA genotype has significantly lower levels of anxiety as a state compared to the control group with the AA and GG genotype (Table 5).
There is a significant interaction between gambling disorder, amphetamine use disorder, and COMT gene polymorphism in the outcome of Conscientiousness level. Gambling disorder and amphetamine use disorder subjects with the GA genotype have significantly lower scores of Conscientiousness compared to the control group with the GA genotype. Conversely, gambling disorder and amphetamine use disorder subjects with the AA genotype have significantly higher scores of Conscientiousness compared to the control group with the AA genotype. Furthermore, Gambling disorder and amphetamine use disorder subjects with the GA genotype had significantly lower scores of Conscientiousness compared to the Gambling disorder and amphetamine use disorder subjects with the AA genotype. Also, the control group with the GA genotype has significantly higher scores of Conscientiousness compared to the control group with the AA genotype (Table 5).
4. Discussion
The aim of the presented study was the case-control analysis of over one hundred male subjects with amphetamine use disorder and gambling disorder. We analysed the catechol-o-methyl transferase single nucleotide polymorphism rs4680; personality was measured with the NEO-FFI inventory and anxiety was measured as a state and trait by the STAI questionnaire. Additionally, we analysed the interactions between the COMT genotypes, personality traits and anxiety measures. The main findings of the analyses are as follows: the GA genotype is statistically significantly more frequent in the study group compared to the control group; the study group had higher scores on the anxiety as a trait and as a state scale and higher scores on the neuroticism scale compared to the control group and lower scores on the agreeableness scale. We also showed an interaction between anxiety as a trait and the rs4680 genotypes of the COMT gene and conscientiousness and the rs4680 genotypes of the COMT gene.
In the presented study, we analysed subjects burdened with both behavioural addiction, gambling disorder and substance use disorder, amphetamines. A high degree of concordance between substance use disorders and other potentially addictive behaviours has been demonstrated in epidemiological studies [48,58,59,60,61,62,63]. There also appears to be an overlap in the psychological mechanisms at the basis of these behaviours. Specific personality traits [49], e.g., impulsivity [64], and motivational factors [65] appear to play an important role in both substance use and other potential behavioural addictions. Research also suggests a strong neurobiological link between substance use disorders and behavioural addictions from biochemical, neuroimaging, genetic and treatment perspectives [66,67,68,69]. Individuals with those disorders derive pleasure, stimulation and satisfaction from their impulsive behaviour (e.g., gambling addiction, compulsive shopping). In addition, a common psychological and molecular pathway underlying impulsive, compulsive and addictive behaviours is suggested by the Reward Deficiency Syndrome hypothesis [70]. It is often the case that methamphetamine use disorder and gambling disorder cooccur. People with both of these disorders tend to be more difficult to treat than people with only one of these disorders [9]. Compared to non-gamblers, adolescent gamblers were more likely to drink alcohol, smoke tobacco and use illicit drugs in an earlier study [62]. Similarly, the study of a sample of young people found that men who were nicotine, alcohol or cannabis users were almost twice as likely to be problem gamblers than those who were not [48]. In another study [58], it was found that people with alcohol use disorders had significantly higher scores on scales for gambling disorder, compulsive buying and sex addiction when compared to control subjects. Higher levels of impulsivity and alcohol craving were also found in people with alcohol use disorder and co-occurring behavioural addictions. The main findings of this study suggest that there is an association between the use of certain substances (in particular, the regular use of alcohol) and the severity of certain potentially addictive behaviours. In addition, some potentially addictive behaviours (problematic internet use, gambling and eating disorders) appear to be more related to substance use than others (e.g., hair pulling), suggesting that addictions may be divided into different homogeneous subgroups [71].
In our study, we analysed only male subjects since we did not encounter female sub-jects with both gambling disorder and amphetamine use disorder, as both of these disorders are far more frequent in males than in females. Knowing its sex-dependent action, we chose the COMT gene for the analysis. The selection of the group was also justified by the analysis of the group of men as a homogeneous subgroup of addicts. This research model is justified due to psychological factors and the course of addiction.
The effect of COMT on sex may result from a number of possible mechanisms. In both men and women, the Met allele is associated with lower levels of COMT enzyme activity (relative to Val/Val). However, women have lower levels of COMT enzyme activity in the dorsolateral PFC [72] and blood [19] for each genotype (Val/Val, Val/Met, Met/Met) compared to men. Estrogen regulation of COMT may also be a contributor to its sex-specific effects [73]. In addition, significant sex differences have been shown in the dopaminergic systems affected by COMT, which are associated with smoking and addiction more generally [74,75,76]. Functional neuroimaging studies indicate that in contrast to men, women have higher basal synaptic levels of dopamine [77] and may show lower amphetamine-induced dopamine release in the striatum [78]. In smokers, the smoking-induced striatal dopamine release regional location differs between sexes as well, i.e., in men we observed increased activation of the ventral striatum, and in women of the dorsal striatum [79]. Women have been shown to experience a greater decrease in dopamine in the nucleus accumbens following nicotine withdrawal [80]. Additionally, studies have shown sex differences in the cognitive impact of dopaminergic interventions [81,82]. Furthermore, the sex-specific effects of the COMT genotype on cortical development and morphology have been documented [83,84,85]. The psychiatric phenotypes affected by the COMT genotype exhibit sex-specificity [73], including smoking behavior, depression, and anxiety-related phenotypes where we observe a stronger association with the Val allele in women [86,87,88,89].
Our analysis began with an examination of the frequencies of genotypes and alleles of COMT rs4680. Statistically significant differences were found in the frequencies of genotypes in the tested sample of subjects with gambling disorder and amphetamine use disorder compared to the control group. The GA genotype was more frequent in cases, and the AA genotype was more frequent in controls. For the alleles, we did not find significant differences. Chmielowiec et al. [90] found no statistically significant differences under the co-dominant model of genotype frequencies for rs4680 in their study regarding patients diagnosed with other-than-cocaine stimulant dependence. Allelic frequencies were also not statistically significant. Zhang et al. [91], whose study showed reduced prefrontal fractional anisotropy only in Met/Met homozygotes who were also drug users, found a significant genotype×drug use status interaction. These data suggest that Met/Met homozygotes may have an increased susceptibility to white matter structural alterations in the context of addiction, which may contribute to previously identified structural and functional prefrontal cortical deficits in addiction.
The personality and anxiety measures were the second analysis we conducted. Subjects with amphetamine use disorder and gambling disorder scored higher on the STAI trait and state scales and the NEO-FFI Neuroticism scale compared to the control group. Significantly lower scores were obtained on the NEO-FFI Agreeableness scale. While comparing the controls and the group of patients with a diagnosis of other-than-cocaine stimulants dependence, for the latter, Chmielowiec et al. [90] observed significantly higher scores on the STAI trait and state scale, and the NEO Five-Factor Inventory scale of Neuroticism and Openness. The study group had significantly lower results on the NEO Five-Factor Inventory scale of Extraversion, Agreeability, and Conscientiousness than the control group. More than half (60%) of participants were classified as having moderate or severe anxiety and/or depression in a study of correlates of anxiety and depression in people who smoke methamphetamine. In multivariate models, being in poor/very poor health, being dependent on methamphetamine and being unemployed were associated with higher odds of both moderate to severe depression and moderate to severe anxiety. Lower odds of moderate or severe depression were associated with living in a large rural town, identifying as Aboriginal and Torres Strait Islander and smoking methamphetamine. Higher odds of moderate or severe anxiety were associated with being female [92]. Anxious people may gamble to cope with negative effects, according to stress reduction theory. It is important to examine moderators, as the literature shows mixed associations between anxiety and gambling behaviour. The research investigated how impulsivity moderates anxiety and problem gambling, as well as gambling, to cope. Since sex differences are important, the moderation of impulsivity has been examined across sexes. Results showed that at both high and low levels of impulsivity, men with higher levels of anxiety scored higher on coping motives for gambling. However, the effect size was larger for men with high impulsivity. Women did not show this moderating effect [93].
The third and final step of the presented study was the interaction analysis. A significant statistical effect of gambling disorder and amphetamine use disorder, and the COMT rs4680 genotype was shown for the score on the STAI trait scale. Compared to controls with the GA genotype, dependent subjects with the GA genotype have significantly higher levels of anxiety as a trait. Similarly, compared to the control group with the GG genotype, dependent subjects with the GG genotype have significantly higher levels of anxiety as a trait. There was also a statistically significant effect between gambling disorder and amphetamine use disorder and the control group on the STAI state scale score. Compared to the control group with the GA genotype, people with an addiction with the GA genotype have a significantly higher level of anxiety as a state. For the NEO-FFI Conscientiousness scale score, a significant statistical effect of gambling disorder and amphetamine dependence and the COMT rs4680 genotype was demonstrated. Compared to the control group with the GA genotype, dependent subjects with the GA genotype have significantly lower conscientiousness scores. Conversely, compared to the control group with the AA genotype, dependent subjects with the AA genotype have significantly higher Conscientiousness scores. The analysis of the interactions between dependency on other-than-cocaine stimulants and COMT rs4680, the STAI trait scale, the STAI state scale, the NEO-FFI neuroticism scale and the NEO-FFI extraversion scale showed significant results. The G/G COMT rs4680 genotype polymorphism was associated with higher STAI trait and STAI state scores in patients dependent on other stimulants. However, there were no such interactions in the control group, suggesting that hypodopaminergic activity in these patients may more likely be a COMT function [90].
5. Conclusions
In the presented study, we see that addictions should be analysed multi-factorially. We can conclude that COMT and its polymorphic variant influence the development of addiction. Still, considering its multifactorial and polygenic nature, it should be combined with other factors such as personality. The presented group is also interesting, as it confirms the multithreadedness and combination of behavioural addiction with substance addiction. We hope that these, and similar discoveries, will translate into clinical practice in the future.
There are also limitations to the study. A similar analysis scheme should be carried out on a larger group of subjects and taking into account a larger number of tested genes.
Author Contributions
Conceptualisation, R.R. and A.G.; methodology, A.B. and A.G.; software, K.C.; validation, J.C. and A.S.; formal analysis, K.C.; investigation, A.B.; resources, A.S.-P.; data curation, A.S.; writing—original draft preparation, A.G., R.R., K.C., A.S., A.S.-P., J.C., A.B., M.T.K. and J.M.; writing—review and editing, A.G., G.T., K.C., A.S.-P., J.C., A.B. and J.M.; visualisation, A.S.; supervision, A.G.; project administration, A.G.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Center, Poland, grant number UMO2015/19/B/NZ7/03691.
Institutional Review Board Statement
Approval was obtained from the Bioethical Committee of the Pomeranian Medical University in Szczecin (KB-0012/106/16 (17 October 2016)).
Informed Consent Statement
All participants gave their written, informed consent prior to entering the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rash, C.J.; Weinstock, J.; Patten, R. Van A Review of Gambling Disorder and Substance Use Disorders. Subst. Abus. Rehabil. 2016, 7, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Kraus, S.W.; Etuk, R.; Potenza, M.N. Current Pharmacotherapy for Gambling Disorder: A Systematic Review. Expert Opin. Pharmacother. 2020, 21, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Chamberlain, S.R. Gambling and Substance Use: Comorbidity and Treatment Implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109852. [Google Scholar] [CrossRef] [PubMed]
- Lorains, F.K.; Cowlishaw, S.; Thomas, S.A. Prevalence of Comorbid Disorders in Problem and Pathological Gambling: Systematic Review and Meta-Analysis of Population Surveys. Addiction 2011, 106, 490–498. [Google Scholar] [CrossRef]
- Håkansson, A.; Karlsson, A. Suicide Attempt in Patients With Gambling Disorder—Associations With Comorbidity Including Substance Use Disorders. Front. Psychiatry 2020, 11, 593533. [Google Scholar] [CrossRef]
- Sundqvist, K.; Wennberg, P. The Association Between Problem Gambling and Suicidal Ideations and Attempts: A Case Control Study in the General Swedish Population. J. Gambl. Stud. 2022, 38, 319–331. [Google Scholar] [CrossRef]
- Grant, J.E.; Potenza, M.N.; Kraus, S.W.; Petrakis, I.L. Naltrexone and Disulfiram Treatment Response in Veterans With Alcohol Dependence and Co-Occurring Problem-Gambling Features. J. Clin. Psychiatry 2017, 78, 15673. [Google Scholar] [CrossRef]
- Kessler, R.C.; Hwang, I.; Labrie, R.; Petukhova, M.; Sampson, N.A.; Winters, K.C.; Shaffer, H.J. DSM-IV Pathological Gambling in the National Comorbidity Survey Replication. Psychol. Med. 2008, 38, 1351–1360. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, J.; Hao, W.; Wu, L.; Liu, F.; Wang, Q.; He, L.; Peng, P.; Zhou, Y.; Li, M.; et al. Relationships Between Impulsivity, Methamphetamine Use Disorder and Gambling Disorder. J. Gambl. Stud. 2023, 39, 1635–1650. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, D.M.; Orr, E.S.; Kaploun, K.A.; Milosevic, A.; Frisch, G.R.; Rupcich, N.; Lundahl, L.H. Executive Function in Pathological Gamblers and Healthy Controls. J. Gambl. Stud. 2011, 28, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, A.E.; Oosterlaan, J.; De Beurs, E.; Van Den Brink, W. Neurocognitive Functions in Pathological Gambling: A Comparison with Alcohol Dependence, Tourette Syndrome and Normal Controls. Addiction 2006, 101, 534–547. [Google Scholar] [CrossRef]
- Malloy-Diniz, L.F.; Lage, G.M.; Campos, S.B.; de Paula, J.J.; de Souza Costa, D.; Romano-Silva, M.A.; de Miranda, D.M.; Correa, H. Association between the Catechol O-Methyltransferase (COMT) Val158met Polymorphism and Different Dimensions of Impulsivity. PLoS ONE 2013, 8, e73509. [Google Scholar] [CrossRef]
- Tunbridge, E.M.; Bannerman, D.M.; Sharp, T.; Harrison, P.J. Catechol-o-Methyltransferase Inhibition Improves Set-Shifting Performance and Elevates Stimulated Dopamine Release in the Rat Prefrontal Cortex. J. Neurosci. 2004, 24, 5331–5335. [Google Scholar] [CrossRef]
- Grant, J.E.; Leppink, E.W.; Redden, S.A.; Odlaug, B.L.; Chamberlain, S.R. COMT Genotype, Gambling Activity, and Cognition. J. Psychiatr. Res. 2015, 68, 371–376. [Google Scholar] [CrossRef]
- Scheggia, D.; Sannino, S.; Luisa Scattoni, M.; Papaleo, F. COMT as a Drug Target for Cognitive Functions and Dysfunctions. CNS Neurol. Disord. Drug Targets 2012, 11, 209–221. [Google Scholar] [CrossRef]
- Pavlov, K.A.; Chistiakov, D.A.; Chekhonin, V.P. Genetic Determinants of Aggression and Impulsivity in Humans. J. Appl. Genet. 2011, 53, 61–82. [Google Scholar] [CrossRef]
- Akil, M.; Kolachana, B.S.; Rothmond, D.A.; Hyde, T.M.; Weinberger, D.R.; Kleinman, J.E. Catechol-O-Methyltransferase Genotype and Dopamine Regulation in the Human Brain. J. Neurosci. 2003, 23, 2008. [Google Scholar] [CrossRef]
- Lachman, H.M.; Papolos, D.F.; Saito, T.; Yu, Y.M.; Szumlanski, C.L.; Weinshilboum, R.M. Human Catechol-O-Methyltransferase Pharmacogenetics: Description of a Functional Polymorphism and Its Potential Application to Neuropsychiatric Disorders. Pharmacogenetics 1996, 6, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Weinshilboum, R.M.; Otterness, D.M.; Szumlanski, C.L. Methylation Pharmacogenetics: Catechol O-Methyltransferase, Thiopurine Methyltransferase, and Histamine N-Methyltransferase. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Dumontheil, I.; Roggeman, C.; Ziermans, T.; Peyrard-Janvid, M.; Matsson, H.; Kere, J.; Klingberg, T. Influence of the COMT Genotype on Working Memory and Brain Activity Changes during Development. Biol. Psychiatry 2011, 70, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mier, D.; Kirsch, P.; Meyer-Lindenberg, A. Neural Substrates of Pleiotropic Action of Genetic Variation in COMT: A Meta-Analysis. Mol. Psychiatry 2009, 15, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rai, V. Catechol-O-Methyltransferase Gene Val158Met Polymorphism and Obsessive Compulsive Disorder Susceptibility: A Meta-Analysis. Metab. Brain Dis. 2020, 35, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Villemonteix, T.; De Brito, S.A.; Slama, H.; Kavec, M.; Balériaux, D.; Metens, T.; Baijot, S.; Mary, A.; Ramoz, N.; Septier, M.; et al. Structural Correlates of COMT Val158Met Polymorphism in Childhood ADHD: A Voxel-Based Morphometry Study. World J. Biol. Psychiatry 2015, 16, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Kauhanen, J.; Hallikainen, T.; Tuomainen, T.-P.; Koulu, M.; Karvonen, M.K.; Salonen, J.T.; Tiihonen, J. Association Between the Functional Polymorphism of Catechol-O-Methyltransferase Gene and Alcohol Consumption Among Social Drinkers. Alcohol. Clin. Exp. Res. 2000, 24, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Franke, P.; Neidt, H.; Cichon, S.; Knapp, M.; Lichtermann, D.; Maier, W.; Propping, P.; Nöthen, M.M. Association Study of the Low-Activity Allele of Catechol-O-Methyltransferase and Alcoholism Using a Family-Based Approach. Mol. Psychiatry 2001, 6, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Guillot, C.R.; Fanning, J.R.; Liang, T.; Berman, M.E. COMT Associations with Disordered Gambling and Drinking Measures. J. Gambl. Stud. Co-Spons. Natl. Counc. Probl. Gambl. Inst. Study Gambl. Commer. Gaming 2015, 31, 513. [Google Scholar] [CrossRef]
- Vandenbergh, D.J.; Rodriguez, L.A.; Miller, I.T.; Uhl, G.R.; Lachman, H.M. High-Activity Catechol-O-Methyltransferase Allele Is More Prevalent in Polysubstance Abusers. Am. J. Med. Genet. 1997, 74, 439–442. [Google Scholar] [CrossRef]
- Horowitz, R.; Kotler, M.; Shufman, E.; Aharoni, S.; Kremer, I.; Cohen, H.; Ebstein, R.P. Rapid Publication Confirmation of an Excess of the High Enzyme Activity COMT Val Allele in Heroin Addicts in a Family-Based Haplotype Relative Risk Study. Am. J. Med. Genet. 2000, 96, 599–603. [Google Scholar] [CrossRef]
- Oswald, L.M.; McCaul, M.; Choi, L.; Yang, X.; Wand, G.S. Catechol-O-Methyltransferase Polymorphism Alters Hypothalamic-Pituitary-Adrenal Axis Responses to Naloxone: A Preliminary Report. Biol. Psychiatry 2004, 55, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Nennicioglu, Y.; Kaya, H.; Eraybar, S.; Atmaca, S.; Gorukmez, O.; Armagan, E. An Investigation of the COMT Gene Val158Met Polymorphism in Patients Admitted to the Emergency Department Because of Synthetic Cannabinoid Use. Balk. J. Med. Genet. 2020, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Fallin, M.D.; Schork, N.J.; Gelernter, J. COMT Polymorphisms and Anxiety-Related Personality Traits. Neuropsychopharmacology 2005, 30, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Mattay, V.S.; Goldberg, T.E.; Fera, F.; Hariri, A.R.; Tessitore, A.; Egan, M.F.; Kolachana, B.; Callicott, J.H.; Weinberger, D.R. Catechol O-Methyltransferase Val158-Met Genotype and Individual Variation in the Brain Response to Amphetamine. Proc. Natl. Acad. Sci. USA 2003, 100, 6186–6191. [Google Scholar] [CrossRef] [PubMed]
- Hamidovic, A.; Dlugos, A.; Palmer, A.A.; De Wit, H. Catechol-O-Methyltransferase Val158met Genotype Modulates Sustained Attention in Both the Drug-Free State and in Response to Amphetamine. Psychiatr. Genet. 2010, 20, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Bilder, R.M.; Volavka, J.; Lachman, H.M.; Grace, A.A. The Catechol-O-Methyltransferase Polymorphism: Relations to the Tonic-Phasic Dopamine Hypothesis and Neuropsychiatric Phenotypes. Neuropsychopharmacology 2004, 29, 1943–1961. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Ho, S.L.; Ramsden, D. Characterization and Implications of Estrogenic Down-Regulation of Human Catechol-O-Methyltransferase Gene Transcription. Mol. Pharmacol. 1999, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xie, T.; Ramsden, D.B.; Ho, S.L. Human Catechol-O-Methyltransferase down-Regulation by Estradiol. Neuropharmacology 2003, 45, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Karayiorgou, M.; Sobin, C.; Blundell, M.L.; Galke, B.L.; Malinova, L.; Goldberg, P.; Ott, J.; Gogos, J.A. Family-Based Association Studies Support a Sexually Dimorphic Effect of COMT and MAOA on Genetic Susceptibility to Obsessive-Compulsive Disorder. Biol. Psychiatry 1999, 45, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Gogos, J.A.; Morgan, M.; Luine, V.; Santha, M.; Ogawa, S.; Pfaff, D.; Karayiorgou, M. Catechol-O-Methyltransferase-Deficient Mice Exhibit Sexually Dimorphic Changes in Catecholamine Levels and Behavior. Proc. Natl. Acad. Sci. USA 1998, 95, 9991. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D. The Endophenotype Concept in Psychiatry: Etymology and Strategic Intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef]
- Frederick, J.A.; Iacono, W.G. Beyond the DSM: Defining Endophenotypes for Genetic Studies of Substance Abuse. Curr. Psychiatry Rep. 2006, 8, 144–150. [Google Scholar] [CrossRef]
- Fineberg, N.A.; Potenza, M.N.; Chamberlain, S.R.; Berlin, H.A.; Menzies, L.; Bechara, A.; Sahakian, B.J.; Robbins, T.W.; Bullmore, E.T.; Hollander, E. Probing Compulsive and Impulsive Behaviors, from Animal Models to Endophenotypes: A Narrative Review. Neuropsychopharmacology 2010, 35, 591. [Google Scholar] [CrossRef]
- Gilmore, C.S.; Malone, S.M.; Iacono, W.G. Brain Electrophysiological Endophenotypes for Externalizing Psychopathology: A Multivariate Approach. Behav. Genet. 2010, 40, 186. [Google Scholar] [CrossRef]
- Bickel, W.K. Discounting of Delayed Rewards as an Endophenotype. Biol. Psychiatry 2015, 77, 846–847. [Google Scholar] [CrossRef]
- Mackillop, J. Integrating Behavioral Economics and Behavioral Genetics: Delayed Reward Discounting as an Endophenotype for Addictive Disorders. J. Exp. Anal. Behav. 2013, 99, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Kreek, M.J.; Nielsen, D.A.; Butelman, E.R.; LaForge, K.S. Genetic Influences on Impulsivity, Risk Taking, Stress Responsivity and Vulnerability to Drug Abuse and Addiction. Nat. Neurosci. 2005, 8, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.A.; Olausson, P.; Taylor, J.R.; Jentsch, J.D. Insight Into the Relationship Between Impulsivity and Substance Abuse From Studies Using Animal Models. Alcohol. Clin. Exp. Res. 2010, 34, 1306. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.R.; Mitchell, M.R.; Wing, V.C.; Balodis, I.M.; Bickel, W.K.; Fillmore, M.; Lane, S.D.; Lejuez, C.W.; Littlefield, A.K.; Luijten, M.; et al. Choice Impulsivity: Definitions, Measurement Issues, and Clinical Implications. Pers. Disord. 2015, 6, 182. [Google Scholar] [CrossRef]
- Van Rooij, A.J.; Kuss, D.J.; Griffiths, M.D.; Shorter, G.W.; Schoenmakers, T.M.; Van De Mheen, D. The (Co-)Occurrence of Problematic Video Gaming, Substance Use, and Psychosocial Problems in Adolescents. J. Behav. Addict. 2014, 3, 157. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, C.S.; Griffiths, M.D.; Gjertsen, S.R.; Krossbakken, E.; Kvam, S.; Pallesen, S. The Relationships between Behavioral Addictions and the Five-Factor Model of Personality. J. Behav. Addict. 2013, 2, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Serrano, M.J.; Pérez-García, M.; Verdejo-García, A. What Are the Specific vs. Generalized Effects of Drugs of Abuse on Neuropsychological Performance? Neurosci. Biobehav. Rev. 2011, 35, 377–406. [Google Scholar] [CrossRef]
- Ersche, K.D.; Sahakian, B.J. The Neuropsychology of Amphetamine and Opiate Dependence: Implications for Treatment. Neuropsychol. Rev. 2007, 17, 317. [Google Scholar] [CrossRef]
- Rogers, R.D.; Everitt, B.J.; Baldacchino, A.; Blackshaw, A.J.; Swainson, R.; Wynne, K.; Baker, N.B.; Hunter, J.; Carthy, T.; Booker, E.; et al. Dissociable Deficits in the Decision-Making Cognition of Chronic Amphetamine Abusers, Opiate Abusers, Patients with Focal Damage to Prefrontal Cortex, and Tryptophan-Depleted Normal Volunteers: Evidence for Monoaminergic Mechanisms. Neuropsychopharmacology 1999, 20, 322–339. [Google Scholar] [CrossRef]
- Badiani, A.; Belin, D.; Epstein, D.; Calu, D.; Shaham, Y. Opiate versus Psychostimulant Addiction: The Differences Do Matter. Nat. Rev. Neurosci. 2011, 12, 685. [Google Scholar] [CrossRef]
- Boscutti, A.; Pigoni, A.; Delvecchio, G.; Lazzaretti, M.; Mandolini, G.M.; Girardi, P.; Ferro, A.; Sala, M.; Abbiati, V.; Cappucciati, M.; et al. The Influence of 5-HTTLPR, BDNF Rs6265 and COMT Rs4680 Polymorphisms on Impulsivity in Bipolar Disorder: The Role of Gender. Genes 2022, 13, 482. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Tan, C.H.; Tu, S.C.; Liu, C.Y.; Yu, R.L. More than an “Inverted-U”? An Exploratory Study of the Association between the Catechol-o-Methyltransferase Gene Polymorphism and Executive Functions in Parkinson’s Disease. PLoS ONE 2019, 14, e0214146. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.T.; McCrae, R.R. The Revised NEO Personality Inventory (NEO-PI-R). In The SAGE Handbook of Personality Theory and Assessment: Volume 2—Personality Measurement and Testing; Sage: Newcastle, UK, 2008; pp. 179–198. [Google Scholar] [CrossRef]
- Lecrubier, Y.; Sheehan, D.V.; Weiller, E.; Amorim, P.; Bonora, I.; Sheehan, K.H.; Janavs, J.; Dunbar, G.C. Mini International Neuropsychiatric Interview. APA PsycTests. 1997. Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft18597-000 (accessed on 6 January 2024).
- Di Nicola, M.; Tedeschi, D.; De Risio, L.; Pettorruso, M.; Martinotti, G.; Ruggeri, F.; Swierkosz-Lenart, K.; Guglielmo, R.; Callea, A.; Ruggeri, G.; et al. Co-Occurrence of Alcohol Use Disorder and Behavioral Addictions: Relevance of Impulsivity and Craving. Drug Alcohol. Depend. 2015, 148, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Mancebo, M.C.; Pinto, A.; Eisen, J.L.; Rasmussen, S.A. Impulse Control Disorders in Adults with Obsessive Compulsive Disorder. J. Psychiatr. Res. 2006, 40, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Potenza, M.N. Tobacco Use and Pathological Gambling. Ann. Clin. Psychiatry 2005, 17, 237–241. [Google Scholar] [CrossRef]
- Griffiths, M.; Sutherland, I. Adolescent Gambling and Drug Use. J. Community Appl. Soc. Psychol. 1998, 8, 423–427. [Google Scholar] [CrossRef]
- Griffiths, M.; Wardle, H.; Orford, J.; Sproston, K.; Erens, B. Gambling, Alcohol, Consumption, Cigarette Smoking and Health: Findings from the 2007 British Gambling Prevalence Survey. Addict. Res. Theory 2010, 18, 208–223. [Google Scholar] [CrossRef]
- Sussman, S.; Lisha, N.; Griffiths, M. Prevalence of the Addictions: A Problem of the Majority or the Minority? Eval. Health Prof. 2011, 34, 3. [Google Scholar] [CrossRef]
- Walther, B.; Morgenstern, M.; Hanewinkel, R. Co-Occurrence of Addictive Behaviours: Personality Factors Related to Substance Use, Gambling and Computer Gaming. Eur. Addict. Res. 2012, 18, 167–174. [Google Scholar] [CrossRef]
- Ream, G.L.; Elliott, L.C.; Dunlap, E. Patterns of and Motivations for Concurrent Use of Video Games and Substances. Int. J. Environ. Res. Public Health 2011, 8, 3999. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Febo, M.; Mclaughlin, T.; Cronjé, F.J.; David, H.A.N.; Gold, M.S. Hatching the Behavioral Addiction Egg: Reward Deficiency Solution System (RDSS)TM as a Function of Dopaminergic Neurogenetics and Brain Functional Connectivity Linking All Addictions under a Common Rubric. J. Behav. Addict. 2014, 3, 149. [Google Scholar] [CrossRef]
- Blum, K.; Febo, M.; Badgaiyan, R.D.; Demetrovics, Z.; Simpatico, T.; Fahlke, C.; Oscar-Berman, M.; Li, M.; Dushaj, K.; Gold, M.S. Common Neurogenetic Diagnosis and Meso-Limbic Manipulation of Hypodopaminergic Function in Reward Deficiency Syndrome (RDS): Changing the Recovery Landscape. Curr. Neuropharmacol. 2017, 15, 184. [Google Scholar] [CrossRef]
- Grant, J.E.; Brewer, J.A.; Potenza, M.N. The Neurobiology of Substance and Behavioral Addictions. CNS Spectr. 2006, 11, 924–930. [Google Scholar] [CrossRef]
- Leeman, R.F.; Potenza, M.N. A Targeted Review of the Neurobiology and Genetics of Behavioral Addictions: An Emerging Area of Research. Can. J. Psychiatry 2013, 58, 260. [Google Scholar] [CrossRef]
- Blum, K.; Sheridan, P.J.; Wood, R.C.; Braverman, E.R.; Chen, T.J.H.; Cull, J.G.; Comings, D.E. The D2 Dopamine Receptor Gene as a Determinant of Reward Deficiency Syndrome. J. R. Soc. Med. 1996, 89, 396. [Google Scholar] [CrossRef] [PubMed]
- Kotyuk, E.; Magi, A.; Eisinger, A.; Király, O.; Vereczkei, A.; Barta, C.; Griffiths, M.D.; Székely, A.; Kökönyei, G.; Farkas, J.; et al. Co-Occurrences of Substance Use and Other Potentially Addictive Behaviors: Epidemiological Results from the Psychological and Genetic Factors of the Addictive Behaviors (PGA) Study. J. Behav. Addict. 2020, 9, 272. [Google Scholar] [CrossRef]
- Chen, J.; Lipska, B.K.; Halim, N.; Ma, Q.D.; Matsumoto, M.; Melhem, S.; Kolachana, B.S.; Hyde, T.M.; Herman, M.M.; Apud, J.; et al. Functional Analysis of Genetic Variation in Catechol-O-Methyltransferase (COMT): Effects on MRNA, Protein, and Enzyme Activity in Postmortem Human Brain. Am. J. Hum. Genet. 2004, 75, 807. [Google Scholar] [CrossRef] [PubMed]
- Tunbridge, E.M.; Harrison, P.J. Importance of the COMT Gene for Sex Differences in Brain Function and Predisposition to Psychiatric Disorders. Curr. Top. Behav. Neurosci. 2011, 8, 119–140. [Google Scholar] [CrossRef]
- Loke, H.; Harley, V.; Lee, J. Biological Factors Underlying Sex Differences in Neurological Disorders. Int. J. Biochem. Cell Biol. 2015, 65, 139–150. [Google Scholar] [CrossRef]
- Bobzean, S.A.M.; DeNobrega, A.K.; Perrotti, L.I. Sex Differences in the Neurobiology of Drug Addiction. Exp. Neurol. 2014, 259, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Chartoff, E. Sex Differences in Neural Mechanisms Mediating Reward and Addiction. Neuropsychopharmacology 2019, 44, 166. [Google Scholar] [CrossRef] [PubMed]
- Laakso, A.; Vilkman, H.; Bergman, J.; Haaparanta, M.; Solin, O.; Syvälahti, E.; Salokangas, R.K.R.; Hietala, J. Sex Differences in Striatal Presynaptic Dopamine Synthesis Capacity in Healthy Subjects. Biol. Psychiatry 2002, 52, 759–763. [Google Scholar] [CrossRef]
- Munro, C.A.; McCaul, M.E.; Wong, D.F.; Oswald, L.M.; Zhou, Y.; Brasic, J.; Kuwabara, H.; Kumar, A.; Alexander, M.; Ye, W.; et al. Sex Differences in Striatal Dopamine Release in Healthy Adults. Biol. Psychiatry 2006, 59, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, K.P.; Wang, S.; Kim, S.J.; McGovern, E.; Nabulsi, N.; Gao, H.; Labaree, D.; Tagare, H.D.; Sullivan, J.M.; Morris, E.D. Sex Differences in the Brain’s Dopamine Signature of Cigarette Smoking. J. Neurosci. 2014, 34, 16851. [Google Scholar] [CrossRef] [PubMed]
- Carcoba, L.M.; Flores, R.J.; Natividad, L.A.; O’Dell, L.E. Amino Acid Modulation of Dopamine in the Nucleus Accumbens Mediates Sex Differences in Nicotine Withdrawal. Addict. Biol. 2018, 23, 1046. [Google Scholar] [CrossRef]
- De Wit, S.; Standing, H.R.; Devito, E.E.; Robinson, O.J.; Ridderinkhof, K.R.; Robbins, T.W.; Sahakian, B.J. Reliance on Habits at the Expense of Goal-Directed Control Following Dopamine Precursor Depletion. Psychopharmacology 2012, 219, 621. [Google Scholar] [CrossRef]
- Robinson, O.J.; Standing, H.R.; Devito, E.E.; Cools, R.; Sahakian, B.J. Dopamine Precursor Depletion Improves Punishment Prediction during Reversal Learning in Healthy Females but Not Males. Psychopharmacology 2010, 211, 187. [Google Scholar] [CrossRef][Green Version]
- Sannino, S.; Padula, M.C.; Managò, F.; Schaer, M.; Schneider, M.; Armando, M.; Scariati, E.; Sloan-Bena, F.; Mereu, M.; Pontillo, M.; et al. Adolescence Is the Starting Point of Sex-Dichotomous COMT Genetic Effects. Transl. Psychiatry 2017, 7, e1141. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qin, W.; Li, Q.; Li, W.; Liu, F.; Liu, B.; Jiang, T.; Yu, C. Prefrontal Volume Mediates Effect of COMT Polymorphism on Interference Resolution Capacity in Healthy Male Adults. Cereb. Cortex. 2017, 27, 5211–5221. [Google Scholar] [CrossRef]
- Sannino, S.; Gozzi, A.; Cerasa, A.; Piras, F.; Scheggia, D.; Managò, F.; Damiano, M.; Galbusera, A.; Erickson, L.C.; De Pietri Tonelli, D.; et al. COMT Genetic Reduction Produces Sexually Divergent Effects on Cortical Anatomy and Working Memory in Mice and Humans. Cereb. Cortex 2015, 25, 2529. [Google Scholar] [CrossRef] [PubMed]
- Domschke, K.; Deckert, J.; O’Donovan, M.C.; Glatt, S.J. Meta-Analysis of COMT Val158met in Panic Disorder: Ethnic Heterogeneity and Gender Specificity. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 667–673. [Google Scholar] [CrossRef]
- Hettema, J.M.; An, S.S.; Bukszar, J.; van den Oord, E.J.C.G.; Neale, M.C.; Kendler, K.S.; Chen, X. COMT Contributes to Genetic Susceptibility Shared Among Anxiety Spectrum Phenotypes. Biol. Psychiatry 2008, 64, 302. [Google Scholar] [CrossRef][Green Version]
- Hill, L.D.; Lorenzetti, M.S.; Lyle, S.M.; Fins, A.I.; Tartar, A.; Tartar, J.L. Catechol-O-methyltransferase Val158Met Polymorphism Associates with Affect and Cortisol Levels in Women. Brain Behav. 2018, 8, e00883. [Google Scholar] [CrossRef]
- DeVito, E.E.; Sofuoglu, M. Catechol-O-Methyltransferase Effects on Smoking: A Review and Proof of Concept of Sex-Sensitive Effects. Curr. Behav. Neurosci. Rep. 2022, 9, 113. [Google Scholar] [CrossRef]
- Chmielowiec, K.; Chmielowiec, J.; Masiak, J.; Strońska-Pluta, A.; Śmiarowska, M.; Boroń, A.; Grzywacz, A. Associations between the COMT Rs4680 Gene Polymorphism and Personality Dimensions and Anxiety in Patients with a Diagnosis of Other Stimulants Dependence. Genes 2022, 13, 1768. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, M.R.; Salmeron, B.J.; Stein, D.J.; Hong, L.E.; Geng, X.; Ross, T.J.; Li, N.; Hodgkinson, C.; Shen, P.H.; et al. Prefrontal White Matter Impairment in Substance Users Depends upon the Catechol-o-Methyl Transferase (COMT) Val158met Polymorphism. Neuroimage 2013, 69, 62. [Google Scholar] [CrossRef]
- Duncan, Z.; Kippen, R.; Sutton, K.; Ward, B.; Agius, P.A.; Quinn, B.; Dietze, P. Correlates of Anxiety and Depression in a Community Cohort of People Who Smoke Methamphetamine. Aust. N. Z. J. Psychiatry 2022, 56, 964–973. [Google Scholar] [CrossRef]
- Rapinda, K.K.; Edgerton, J.D.; Keough, M.T. Impulsivity Moderates the Association Between Anxiety and Problem Gambling Among Canadian Undergraduates. J. Gambl. Stud. 2023, 39, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).