FGF23 Expression Is a Promising Immunohistochemical Diagnostic Marker for Undifferentiated Pleomorphic Sarcoma of Bone (UPSb)
Abstract
1. Introduction
1.1. Undifferentiated Pleomorphic Sarcoma of Bone (UPSb) Features
1.2. Osteosarcoma (OS) Features
1.3. Dedifferentiated Chondrosarcoma (DDSC) Features
1.4. Diagnostic Procedures, Markers, and Challenges
1.5. FGF23 Expression in UPSb
2. Materials and Methods
2.1. Study Samples
2.2. FGF23 Immunohistochemistry
2.3. Statistical Analysis
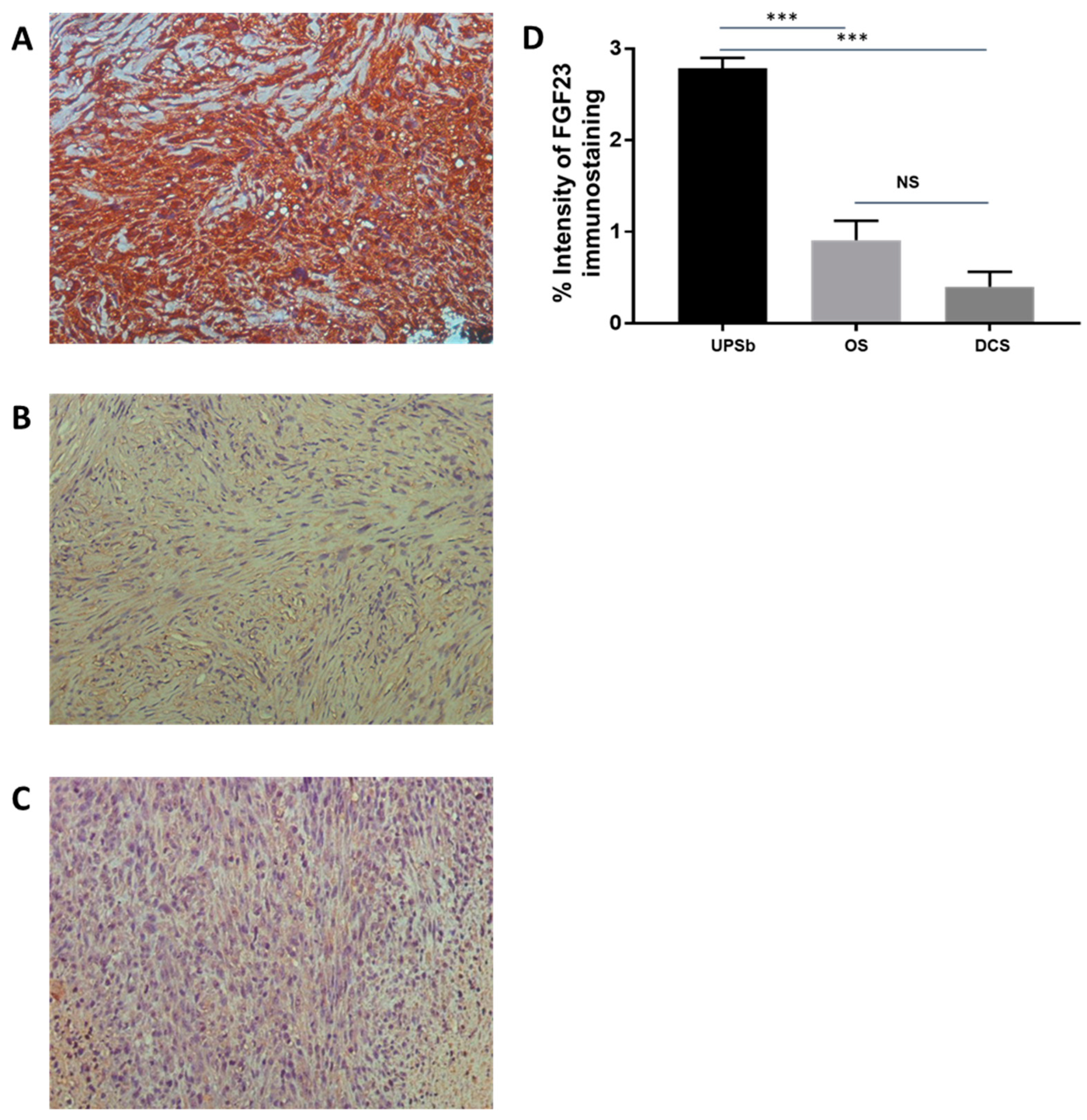
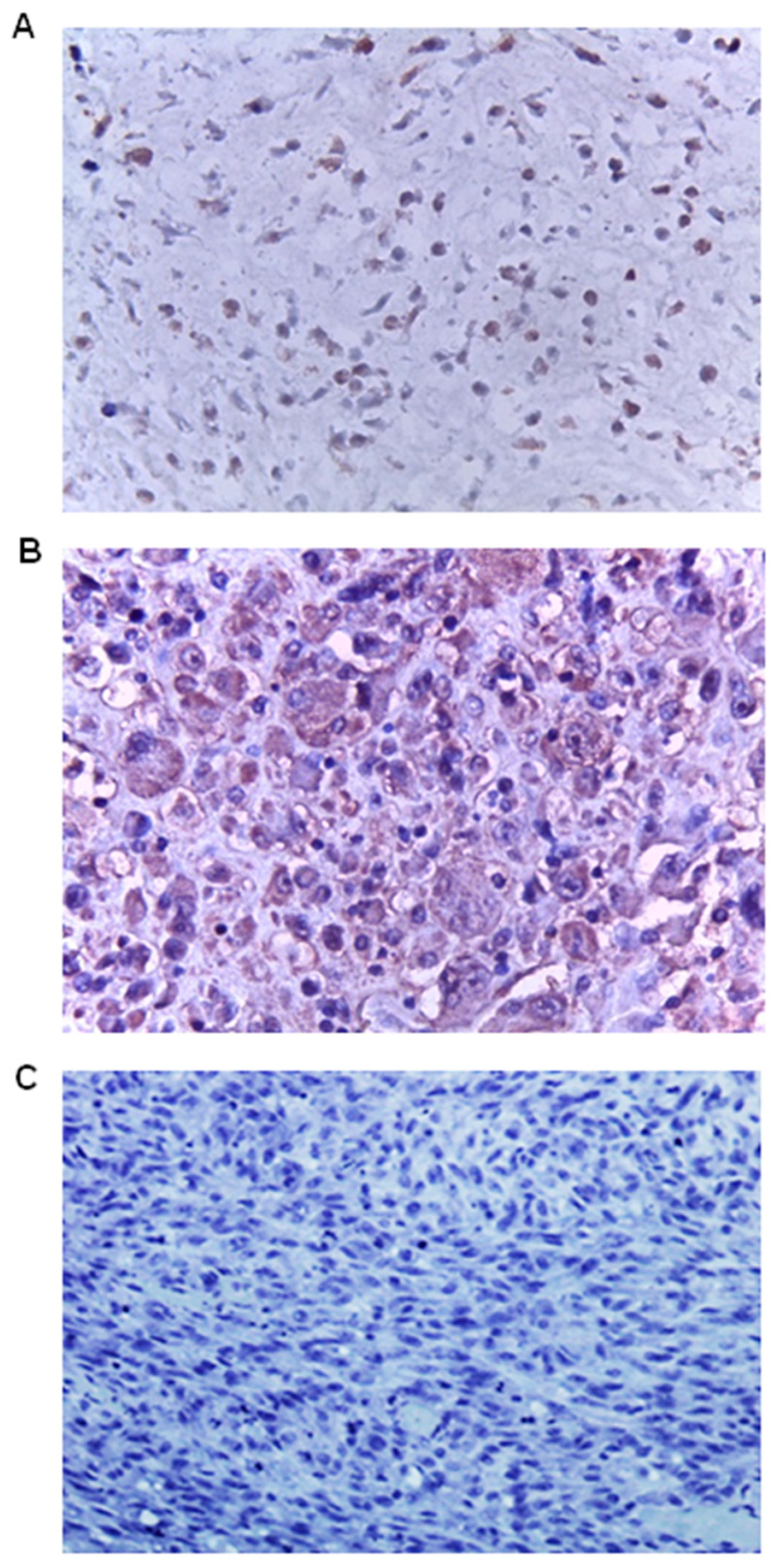
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, A.T.; Baek, J.; Alexander, J.H.; Voskuil, R.T.; Khan, S.N.; Scharschmidt, T.J. Malignant fibrous histiocytoma of bone: A survival analysis from the National Cancer Database. J. Surg. Oncol. 2020, 121, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Christopher, D.; Fletcher, J.; Bridge, P. WHO Classification of Tumours of Soft Tissue and Bone, 4th ed.; Centre International de Recherche sur le Cancer, Ed.; WHO: Lyon, France, 2013. [Google Scholar]
- Chen, S.; Fritchie, K.; Wei, S.; Ali, N.; Curless, K.; Shen, T.; Brini, A.T.; Latif, F.; Sumathi, V.; Siegal, G.P.; et al. Diagnostic utility of IDH1/2 mutations to distinguish dedifferentiated chondrosarcoma from undifferentiated pleomorphic sarcoma of bone. Hum. Pathol. 2017, 65, 239–246. [Google Scholar] [CrossRef]
- Lindsey, B.A.; Markel, J.E.; Kleinerman, E.S. Osteosarcoma Overview. Rheumatol. Ther. 2017, 4, 25–43. [Google Scholar] [CrossRef]
- Gerrand, C.; Athanasou, N.; Brennan, B.; Grimer, R.; Judson, I.; Morland, B.; Peake, D.; Seddon, B.; Whelan, J. UK guidelines for the management of bone sarcomas. Clin. Sarcoma Res. 2016, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, H.D.; Czerniak, B. Bone cancers. Cancer 1995, 75, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Pritchard, D.J. Etiology of osteosarcoma. Clin. Orthop. Relat. Res. 2002, 397, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.E.; Kopeć, S.; Szostakowski, B.; Spałek, M.J.; Fiedorowicz, M.; Bylina, E.; Filipowicz, P.; Szumera-Ciećkiewicz, A.; Tysarowski, A.; Czarnecka, A.M.; et al. Chondrosarcoma-from Molecular Pathology to Novel Therapies. Cancers 2021, 13, 2390. [Google Scholar] [CrossRef]
- Zając, W.; Dróżdż, J.; Kisielewska, W.; Karwowska, W.; Dudzisz-Śledź, M.; Zając, A.E.; Borkowska, A.; Szumera-Ciećkiewicz, A.; Szostakowski, B.; Rutkowski, P.; et al. Dedifferentiated Chondrosarcoma from Molecular Pathology to Current Treatment and Clinical Trials. Cancers 2023, 15, 3924. [Google Scholar] [CrossRef]
- Gusho, C.A.; Lee, L.; Zavras, A.; Seikel, Z.; Miller, I.; Colman, M.W.; Gitelis, S.; Blank, A.T. Dedifferentiated Chondrosarcoma: A Case Series and Review of the Literature. Orthop. Rev. 2022, 14, 35448. [Google Scholar] [CrossRef]
- Papagelopoulos, P.J.; Galanis, E.C.; Sim, F.H.; Unni, K.K. Clinicopathologic features, diagnosis, and treatment of malignant fibrous histiocytoma of bone. Orthopedics 2000, 23, 59–65. [Google Scholar] [CrossRef]
- Ali, N.M.; Niada, S.; Brini, A.T.; Morris, M.R.; Kurusamy, S.; Alholle, A.; Huen, D.; Antonescu, C.R.; Tirode, F.; Sumathi, V.; et al. Genomic and transcriptomic characterisation of undifferentiated pleomorphic sarcoma of bone. J. Pathol. 2019, 247, 166–176. [Google Scholar] [CrossRef]
- Yokota, K.; Sakamoto, A.; Matsumoto, Y.; Matsuda, S.; Harimaya, K.; Oda, Y.; Iwamoto, Y. Clinical outcome for patients with dedifferentiated chondrosarcoma: A report of 9 cases at a single institute. J. Orthop. Surg. Res. 2012, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Zhang, L.; Hirai, T.; Tsuda, Y.; Ikegami, M.; Tanaka, S. Clinical characteristics of undifferentiated pleomorphic sarcoma of bone and the impact of adjuvant chemotherapy on the affected patients: A population-based cohort study. Jpn. J. Clin. Oncol. 2022, 52, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kosti, I.; Jain, N.; Aran, D.; Butte, A.J.; Sirota, M. Cross-tissue Analysis of Gene and Protein Expression in Normal and Cancer Tissues. Sci. Rep. 2016, 6, 24799. [Google Scholar] [CrossRef] [PubMed]
- Shiba, E.; Matsuyama, A.; Shibuya, R.; Yabuki, K.; Harada, H.; Nakamoto, M.; Kasai, T.; Hisaoka, M. Immunohistochemical and molecular detection of the expression of FGF23 in phosphaturic mesenchymal tumors including the non-phosphaturic variant. Diagn. Pathol. 2016, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Ewendt, F.; Feger, M.; Föller, M. Role of Fibroblast Growth Factor 23 (FGF23) and αKlotho in Cancer. Front. Cell Dev. Biol. 2020, 8, 601006. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Mizutani, S.; Muto, T.; Yoneya, T.; Hino, R.; Takeda, S.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Yamashita, T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. USA 2001, 98, 6500–6505. [Google Scholar] [CrossRef] [PubMed]
- Larsson, T.; Zahradnik, R.; Lavigne, J.; Ljunggren, O.; Jüppner, H.; Jonsson, K.B. Immunohistochemical detection of FGF-23 protein in tumors that cause oncogenic osteomalacia. Eur. J. Endocrinol. 2003, 148, 269–276. [Google Scholar] [CrossRef][Green Version]
- Boland, J.M.; Tebben, P.J.; Folpe, A.L. Phosphaturic mesenchymal tumors: What an endocrinologist should know. J. Endocrinol. Investig. 2018, 41, 1173–1184. [Google Scholar] [CrossRef]
- Tebben, P.J.; Kalli, K.R.; Cliby, W.A.; Hartmann, L.C.; Grande, J.P.; Singh, R.J.; Kumar, R. Elevated fibroblast growth factor 23 in women with malignant ovarian tumors. Mayo Clin. Proc. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Wang, H.; Yoshiko, Y.; Yamamoto, R.; Minamizaki, T.; Kozai, K.; Tanne, K.; Aubin, J.E.; Maeda, N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J. Bone Miner. Res. 2008, 23, 939–948. [Google Scholar] [CrossRef]
- Guo, Y.C.; Yuan, Q. Fibroblast growth factor 23 and bone mineralisation. Int. J. Oral Sci. 2015, 7, 8–13. [Google Scholar] [CrossRef]
- Shalhoub, V.; Ward, S.C.; Sun, B.; Stevens, J.; Renshaw, L.; Hawkins, N.; Richards, W.G. Fibroblast growth factor 23 (FGF23) and α-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif. Tissue Int. 2011, 89, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Sitara, D.; Kim, S.; Razzaque, M.S.; Bergwitz, C.; Taguchi, T.; Schüler, C.; Erben, R.G.; Lanske, B. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008, 4, e1000154. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Viswanathan, V.K. Lytic Bone Lesions. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Liu, C.; Xi, Y.; Li, M.; Jiao, Q.; Zhang, H.; Yang, Q.; Yao, W. Dedifferentiated chondrosarcoma: Radiological features, prognostic factors and survival statistics in 23 patients. PLoS ONE 2017, 12, e0173665. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.E.; Bligh, R.C.; Mirams, M.; Gill, A.; Au, A.; Clarkson, A.; Jüppner, H.; Ruff, S.; Stalley, P.; Scolyer, R.A.; et al. Clinical case seminar: Fibroblast growth factor 23: A new clinical marker for oncogenic osteomalacia. J. Clin. Endocrinol. Metab. 2003, 88, 4088–4094. [Google Scholar] [CrossRef] [PubMed]
- Rothzerg, E.; Xu, J.; Wood, D. Different Subtypes of Osteosarcoma: Histopathological Patterns and Clinical Behaviour. J. Mol. Pathol. 2023, 4, 99–108. [Google Scholar] [CrossRef]
- Yuan, L.L.; Chen, Z.; Qin, J.; Qin, C.J.; Bian, J.; Dong, R.F.; Yuan, T.B.; Xu, Y.T.; Kong, L.Y.; Xia, Y.Z. Single-cell sequencing reveals the landscape of the tumor microenvironment in a skeletal undifferentiated pleomorphic sarcoma patient. Front. Immunol. 2022, 13, 1019870. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, W.; Dai, Y.; Bao, M.; Yuan, Z.; He, M.; Qin, Z.; Liao, S.; He, J.; Huang, Q.; et al. Single-Cell Transcriptomics Reveals the Complexity of the Tumor Microenvironment of Treatment-Naive Osteosarcoma. Front. Oncol. 2021, 11, 709210. [Google Scholar] [CrossRef]
- He, M.; Jiang, X.; Miao, J.; Feng, W.; Xie, T.; Liao, S.; Qin, Z.; Tang, H.; Lin, C.; Li, B.; et al. A new insight of immunosuppressive microenvironment in osteosarcoma lung metastasis. Exp. Biol. Med. 2023, 248, 1056–1073. [Google Scholar] [CrossRef]
- Thomas, D.D.; Lacinski, R.A.; Lindsey, B.A. Single-cell RNA-seq reveals intratumoral heterogeneity in osteosarcoma patients: A review. J. Bone Oncol. 2023, 39, 100475. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Van Tine, B.A. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. J. Clin. Med. 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Wang, J.; Zhang, Y.; Creighton, C.J.; Ittmann, M. FGF23 promotes prostate cancer progression. Oncotarget 2015, 6, 17291–17301. [Google Scholar] [CrossRef] [PubMed]
- Suvannasankha, A.; Tompkins, D.R.; Edwards, D.F.; Petyaykina, K.V.; Crean, C.D.; Fournier, P.G.; Parker, J.M.; Sandusky, G.E.; Ichikawa, S.; Imel, E.A.; et al. FGF23 is elevated in multiple myeloma and increases heparanase expression by tumor cells. Oncotarget 2015, 6, 19647–19660. [Google Scholar] [CrossRef]
- Jan de Beur, S.M.; Miller, P.D.; Weber, T.J.; Peacock, M.; Insogna, K.; Kumar, R.; Rauch, F.; Luca, D.; Cimms, T.; Roberts, M.S.; et al. Burosumab for the Treatment of Tumor-Induced Osteomalacia. J. Bone Miner. Res. 2021, 36, 627–635. [Google Scholar] [CrossRef]
- Imanishi, Y.; Ito, N.; Rhee, Y.; Takeuchi, Y.; Shin, C.S.; Takahashi, Y.; Onuma, H.; Kojima, M.; Kanematsu, M.; Kanda, H.; et al. Interim Analysis of a Phase 2 Open-Label Trial Assessing Burosumab Efficacy and Safety in Patients With Tumor-Induced Osteomalacia. J. Bone Miner. Res. 2021, 36, 262–270. [Google Scholar] [CrossRef]
- Crotti, C.; Zucchi, F.; Alfieri, C.; Caporali, R.; Varenna, M. Long-term use of burosumab for the treatment of tumor-induced osteomalacia. Osteoporos. Int. 2023, 34, 201–206. [Google Scholar] [CrossRef]


| Characteristics | UPSb (n = 10) | OS (n = 10) | DDSC (n = 10) |
|---|---|---|---|
| Age | |||
| Mean ± SD | 61.0 ± 20.2 | 36.4 ± 20.3 | 64.5 ± 20.2 |
| Range | 20–86 | 9–69 | 51–78 |
| Sex | |||
| Female | 7 (70%) | 5 (50%) | 3 (30%) |
| Male | 3 (30%) | 5 (50%) | 7 (70%) |
| Primary site | |||
| Femur | 7 (70%) | 5 (50%) | 7 (63%) |
| Tibia | 1 | 4 (40%) | - |
| Fibula | 1 | - | - |
| Humerus | 1 | 1 | - |
| Pelvis/Scapula/Sternum | - | - | 1/1/1 |
| Size (range in mm) | 60–132 | 75–160 | 70–310 |
| Recurrence | |||
| Yes | 0 | 0 | 6 (60%) |
| No | 9 (90%) | 7 (70%) | 4 (40%) |
| na | 1 | 3 | - |
| Metastasis | |||
| Yes | 5 (50%) | 6 (60%) | 6 (64%) |
| Lung | 3 (30%) | 3 (30%) | 4 (40%) |
| Chest | 1 | 3 (30%) | 1 |
| Other sites | 1 (bone, liver) | - | 3 |
| No | 5 (50%) | 3 (30%) | 3 (30%) |
| na | - | 1 | - |
| Chemotherapy | 4 (40%) | 9 (90%) | 1 |
| 5-Year Survival | |||
| Yes | 8 (80%) | 4 (40%) | 3 (27%) |
| No | 2 (20%) | 5 (50%) | 3 (27%) |
| na | - | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hassi, H.O.; Ali, N.M.; Cooke, H.; De Silva, S.; Brini, A.T.; Babu, P.; Sumathi, V.; Morris, M.R.; Niada, S. FGF23 Expression Is a Promising Immunohistochemical Diagnostic Marker for Undifferentiated Pleomorphic Sarcoma of Bone (UPSb). Genes 2024, 15, 242. https://doi.org/10.3390/genes15020242
Al-Hassi HO, Ali NM, Cooke H, De Silva S, Brini AT, Babu P, Sumathi V, Morris MR, Niada S. FGF23 Expression Is a Promising Immunohistochemical Diagnostic Marker for Undifferentiated Pleomorphic Sarcoma of Bone (UPSb). Genes. 2024; 15(2):242. https://doi.org/10.3390/genes15020242
Chicago/Turabian StyleAl-Hassi, Hafid O., Naser M. Ali, Hannah Cooke, Shamini De Silva, Anna T. Brini, Pavithra Babu, Vaiyapuri Sumathi, Mark R. Morris, and Stefania Niada. 2024. "FGF23 Expression Is a Promising Immunohistochemical Diagnostic Marker for Undifferentiated Pleomorphic Sarcoma of Bone (UPSb)" Genes 15, no. 2: 242. https://doi.org/10.3390/genes15020242
APA StyleAl-Hassi, H. O., Ali, N. M., Cooke, H., De Silva, S., Brini, A. T., Babu, P., Sumathi, V., Morris, M. R., & Niada, S. (2024). FGF23 Expression Is a Promising Immunohistochemical Diagnostic Marker for Undifferentiated Pleomorphic Sarcoma of Bone (UPSb). Genes, 15(2), 242. https://doi.org/10.3390/genes15020242






