Abstract
Paeonia lactiflora (P. lactiflora), a perennial plant renowned for its medicinal roots, provides a unique case for studying the phylogenetic relationships of species based on organelle genomes, as well as the transference of DNA across organelle genomes. In order to investigate this matter, we sequenced and characterized the mitochondrial genome (mitogenome) of P. lactiflora. Similar to the chloroplast genome (cpgenome), the mitogenome of P. lactiflora extends across 181,688 base pairs (bp). Its unique quadripartite structure results from a pair of extensive inverted repeats, each measuring 25,680 bp in length. The annotated mitogenome includes 27 protein-coding genes, 37 tRNAs, 8 rRNAs, and two pseudogenes (rpl5, rpl16). Phylogenetic analysis was performed to identify phylogenetic trees consistent with Paeonia species phylogeny in the APG Ⅳ system. Moreover, a total of 12 MTPT events were identified and 32 RNA editing sites were detected during mitogenome analysis of P. lactiflora. Our research successfully compiled and annotated the mitogenome of P. lactiflora. The study provides valuable insights regarding the taxonomic classification and molecular evolution within the Paeoniaceae family.
1. Introduction
Paeonia lactiflora Pall. (P. lactiflora), belonging to the genus Paeonia L. (Garl von Linne, 1935) of the Paeoniaceae Raf. family, is an iconic floral emblem of the Tanabata festival. The germplasm of Paeonia species holds significant value to both the floral and pharmaceutical industries [1]. Yangzhou, Zhongjiang, Bozhou and Heze have emerged as the four leading producers of P. lactiflora in China, with the plant being cultivated in nearly every corner of the aforementioned cities [2]. Moreover, these plants boast substantial medicinal potential. Paeoniaceae species are rich in compounds like monoterpene glycosides, flavonoids, tannins, stilbenes, and triterpenoids, among others [3]. According to traditional Chinese medicine, P. lactiflora aligns with yin, and is considered effective for nourishing yin [4], alleviating heat, and mitigating pain [5]. As a perennial plant, its dried roots serve as a popular ingredient in traditional remedies.
Mitochondria, widely recognized as the powerhouses of the cell, are critical for eukaryotic life [6]. While they have their own genome, most mitochondrial proteins are imported from the nuclear genome. Contrary to animal mitogenomes, plant mitogenomes demonstrate significant differences in size and genomic organization [7]. Mitogenomes typically take the form of naked, double-stranded DNA molecules, occurring predominantly as loops but also as linear molecules, with sizes varying across species [8]. Repetitive sequences, which make up the majority of the genome in many species [9], and their complex structures complicate the sequencing and assembly of mitogenomes compared to plastid genomes. The transmission patterns of mitogenomes are distinct from those of nuclear genomes [10]. The lack of histone protection and a DNA damage repair system make the mitogenome more susceptible to mutations [11]. Additionally, assembling repetitive regions and obtaining complete mitogenome sequences are challenging tasks with short-read sequencing data from platforms like Illumina [12,13,14]. With the advent of long-read sequencing technologies by PacBio and Oxford Nanopore in recent years, sequencing plant mitogenomes has become more feasible and cost-effective [15,16].
A few years ago, the pioneering sequencing of the genus Paeonia’s genome was undertaken, with several genomic resources now available for species including Paeonia jishanensis T. Hong and W. Z. Zhao (P. jishanensis), Paeonia delavayi Franch. (P. delavayi), Paeonia sinjiangensis K. Y. Pan (P. sinjiangensis), and several native variants [17]. This endeavor significantly enriched the genetic resources for Paeonia varieties, offering a wealth of data to breeders. Despite these advancements, systematic studies on the mitogenome of P. lactiflora have been scarce. Only one mitogenome resource of a Paeonia species has been published, with its details yet to be extensively examined [18], thereby limiting our understanding of mitogenome evolution in the Paeoniaceae family. Homologous recombination, a common occurrence in plant mitogenomes, often leads to frequent duplication and replacement of spacer regions [19]. This process enables the merging of certain genetic information from different species, paving the way for innovative methodologies to study the origins and evolution of angiosperms, DNA repair, the maintenance of genetic diversity, and the emergence of heterosis [20].
RNA editing, particularly in the form of C to U base conversions, is a frequent phenomenon in organelles like mitochondria and chloroplasts [21,22,23]. This process in plant mitochondria necessitates the involvement of a multitude of nuclear-encoded factors [21]. Many C→U (and U→C in some plants) conversions modify the coding sequence of numerous organellar transcripts [24], facilitating translatable mRNA production through the generation of an AUG start site or elimination of premature stop codons, or altering RNA structure, splicing, and stability. By generating new start or stop codons or modifying the amino acid sequence, RNA editing fosters the formation of the correct secondary structure of proteins, enabling their functionality and ensuring the precise expression of genetic information within cells [25,26,27].
Organelle DNA transfer into the nucleus occurs in the form of ongoing transfer of mitogenome or cpgenome fragments into the nuclear genome. Transferred organelle genome mainly forms non-coding sequences or pseudogenes in the nuclear genome, and these fragments are called nuclear mitochondrial DNA (NUMT) [28] and nuclear chloroplast DNA (NUPT) [29], respectively. At present, NUMT and NUPT have been found in the nuclear genomes of most eukaryotes, and a large part of them are associated with nuclear genes [30]. DNA transfer between organelles is another type of intracellular gene transfer. Mitochondrial plastid DNAs (MTPTs) refers to the chloroplast-derived DNA that is contained in the mitogenome of angiosperms, representing 0.1% to 10.3% of the mitogenome [31]. The analysis of MTPTs will lead to complex mitogenome results, as most mtpts evolution is relatively unconstrained by function; the MTPT’s sequence evolution reveals the possibility of mutations in the mitogenome of angiosperms [32].
In this study, we sequenced the mitogenome of a P. lactiflora cultivar to elucidate its genome structure, analyzed repeat sequences and investigated genomic recombination events. Furthermore, we aimed to conduct a comparative analysis with other mitogenomes within the Paeoniaceae family. These findings enhance our understanding of organelle genome evolution in P. lactiflora and lay a solid foundation for future breeding research, helping to unravel the phylogenetic relationships of species based on organelle genomes and the transference of DNA between organelle genomes [33]. Hybridization among diverse P. lactiflora species can overcome barriers, thereby augmenting genetic diversity [34].
2. Materials and Methods
2.1. Plant Materials, DNA and RNA Extraction, and Sequencing
The fresh juvenile leaves were provided using plants housed in the Botanical Garden of Medicinal Plants in Beijing, China (E116°25′, N39°47′). The samples were rinsed and cleaned with DEPC water, then preserved at −80 °C until further use. Total genomic DNA was extracted using the Cetytrimethylammonium Bromide (CTAB) method [35], followed by the utilization of a plant genomic DNA kit (Tiangen Biotech, Beijing, Co., Ltd., Beijing, China). The same DNA sample was employed for both Illumina sequencing and Oxford Nanopore sequencing. As part of the study, 350 bp fragments were incorporated into a DNA library and sequenced on the Illumina HiSeq X platform (Benagen Technology Co., Ltd., Wuhan, China) for short-read sequencing. For Oxford Nanopore sequencing, the DNA library was constructed using the DNA Library Kit with the catalog number SQK-LSK110.
2.2. Mitochondrial Genome Assembly and Annotation
To extract the complete sequence and investigate the potential structure of the mitogenome of P. lactiflora, a hybrid assembly strategy was employed. Short mitogenome reads were extended using Getorganelle (v1.6.4) [36], and the resulting readings were assembled into a unitig graph using SPAdes software included in Unicycler (v0.4.9). The repeat region in the unitig graph was resolved using Unicycler with Nanopore long reads. The assembled mitogenome of P. lactiflora was annotated using the GeSeq web server [37] and IPMGA. Any issues with the protein-coding genes (PCGs) were checked and rectified using Apollo, and the genome map was constructed with OGDRAW [38]. All tRNA genes were verified using the default parameters of tRNAscan-SE [39].
2.3. Repeat Sequence Analysis and MTPT Prediction
The parameters for identifying repeat sequences were specified by using MISA [40] with “1-10 2-5 3-4 4-3 5-3 6-3”. Additionally, Tandem Repeats Finder (TRF) [41], and ROUS Finder were also used [42]. TRF was employed to identify tandem repeats with the parameters “2 7 7 80 10 50 500 –f -d –m”, while ROUS Finder, with the default parameters “-o input_repeat m 24 -b /usr/bin/ -k false -gb false”, was used to identify dispersed repeats.
For an accurate analysis of mitochondrial plastid DNA (MTPTs), the cpgenome was assembled using Illumina reads. The GetOrganelle software [23], with default parameters “get_organelle_from_reads.py -1 forward.fq -2 reverse.fq -o plastome_output -R 15 -k 21,45,65,85,105 -F embplant_pt”, was used to assemble the cpgenome from the same mitogenome assembly data. The cpgenome was annotated using CPGAVAS2 [43], visualized using CPGView [44], and any annotation errors flagged via CPGView were manually corrected with Apollo. MTPTs were identified using BLASTN based on the assembled cpgenome that is longer than 100 bp.
2.4. Phylogenetic Analysis
Given the large non-conservation of non-coding regions in plant mitogenomes, we constructed trees using common genes. First, 28 mitogenomes were downloaded from the NCBI database, and common genes (atp6, atp8, ccmC, ccmFC-e2, ccmFN, cox3, matR-e1, matR-e2, nad1-e1, nad1-e5, nad3, nad4-e1, nad4-e2, nad4-e3, nad4-e4, nad5-e2, nad6, nad7-e1, nad7-e3, nad7-e4, nad7-e5, nad9, adh4) were extracted using PhyloSuite [45]. These common protein-coding regions underwent multiple sequence alignment using MAFFT v7.505 software [46]. The resulting sequences were linked in tandem with PhyloSuite and analyzed using IQTREE [47] for phylogenetic purposes. The final phylogenetic analysis results were visualized using ITOL v6.8.1 software [48].
2.5. RNA Editing Site Identification and Validation
To identify RNA editing sites, we extracted both DNA and RNA from the whole genome. We used PhyloSuite [45] software to extract the protein-coding genes (PCGs) of the studied taxa. For our prediction of C-to-U RNA editing events in plant mitochondria, we employed Deepred-Mt [49], a program that uses deep characterization learning. This program used default parameters to predict each PCG for the selected species.
3. Results
3.1. Elucidating Mitogenome Structure and Broad Genomic Features through Graph-Based Techniques
In order to assemble the mitogenome of P. lactiflora, we implemented a hybrid assembly strategy that capitalized on both long and short-read sequencing technologies. From the total DNA, we sequenced and assembled the mitogenome length of 181,688 bp. Leveraging the GetOrganelle v1.7.7.0 software, we isolated and expanded the mitogenome short reads. The genome was assembled into a graph-based mitogenome (Figure 1A); these reads were then assembled into a unitig graph with the help of the SPAdes software embedded within the Unicycler software suite, thus establishing the graph model of P. lactiflora’s mitogenome (Figure 1D).
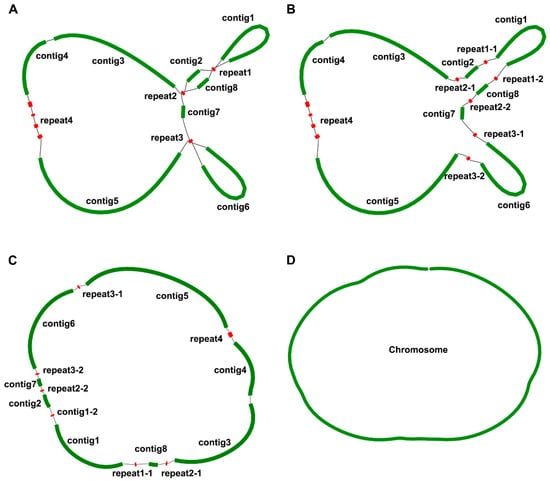
Figure 1.
Schematic representation of the assembly results of P. lactiflora mitogenome. The green contigs represent a single copy of the mitogenome fragment, and the red contigs represent the repeat sequences. (A). The second generation data assembly of the De Bruijn Graph; (B). Third Generation Data processing repeat sequences; (C). Adjust the position of each fragment sequence; (D). Graphical representation of the mitogenome of P. lactiflora.
The constructed graph consists of fifteen contigs, three of which exhibit a double bifurcation (Figure 1). Contig 5 stands out as the longest, stretching to 51,364 bp, while repeat2-1 and repeat2-2 remain the shortest, amounting to only 181 bp (Table 1). Importantly, the complex double bifurcating structures were resolved using the Nanopore long-reads, and all contigs were subsequently merged with the Bandage software. In conclusion, the mitogenome of P. lactiflora is encapsulated within a single chromosome spanning 181,688 bp, with a GC content of 55.7%. The GC content of its related species was calculated; the GC content of Heuchera parviflora var. Saurensis R.A.Folk and Sedum plumbizincicola X.H.Guo and S.B.Zhou ex L.H.Wu were 47.18% and 45.93%, respectively, while the GC content of Rhodiola crenulata (Hook.f. and Thomson) H.Ohba was 46.64%, Ribes nigrum L. was 47.25%, Loropetalum chinense (R.Br.) Oliv. was 47.35%, Rhodiola tangutica (Maxim.) S.H.Fu was 45.67%, and the GC content of Rhodiola juparensis (Fröd.) S.H.Fu was 46.01%.

Table 1.
The length, bifurcation structures, and chromosome attribution of each assembled contig of P. lactiflora mitogenome.
3.2. Genome Annotation
We conducted a comprehensive annotation of the mitogenome of P. lactiflora, which includes 27 protein-coding genes (PCGs), 17 transfer RNA genes (tRNA), and 3 ribosomal RNA genes (rRNA).
The classification of these PCGs based on their function is elaborated in Table 2. The mitogenome of P. lactiflora was confirmed to possess annotations for all core genes, including five small subunit ribosomal genes (rps3, rps4, rps10, rps12, rps14), two large subunit ribosomal genes (rpl5, rpl16), and a solitary succinate dehydrogenase subunit gene (sdh4) as shown in Figure 2.

Table 2.
Gene composition in the mitogenome of P. lactiflora.
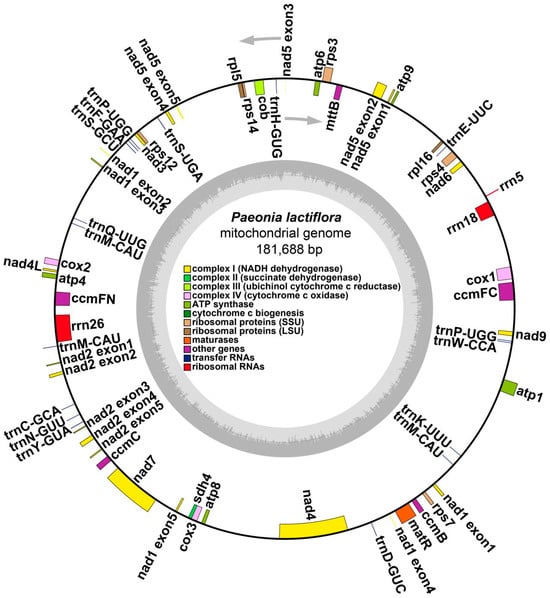
Figure 2.
Schematic of the mitogenome of the P. lactiflora. Genes inside and outside the circle are transcribed clockwise and counterclockwise, respectively. Genes were shown in different colors based on their functional classification, which is shown in the center of the map.
3.3. Repeat Element Analysis
Simple Sequence Repeats (SSRs), also known as tandem repeats, are prevalent components in all known genomes [50]. They are considered among the most significant genetic markers and can be found in the majority of species [51]. In this investigation, we used the MISA software to predict SSRs in the mitogenome of P. lactiflora. As a result, we identified a total of 55 SSRs on the mitogenome’s chromosome, which included 15 monomers and dimers, 12 trimers, 8 tetramers, 17 pentamers, and 3 hexamers (Table S1). These SSR loci have the potential to serve as valuable molecular markers in future research.
Tandem Repetitive Sequences (TRS) are contiguous, repeating units with a high degree of sequence identity [52]. They have been implicated in numerous genetic processes, such as chromosomal organization, gene regulation, and disease diagnosis [53]. Using Tandem Repeats Finder software, we predicted tandem repetitive sequences in the mitogenome of P. lactiflora. As shown in Table S2, we identified a total of 26 TRS, with TRS18 possessing the longest repeat unit at 45 bp and TRS4, TRS18, and TRS22 exhibiting the greatest cumulative lengths at 98 bp.
Dispersed Repetitive Sequences are DNA sequences that are repeated throughout the genome and play crucial roles in genome rearrangement, evolution, and stress resistance [10]. We used ROUSFinder software to predict dispersed repetitive sequences in the mitogenome of P. lactiflora, identifying two types of matching directions (Table S3). The chromosome with the longest dispersed repetitive sequence, named DRS1, measured 224 bp in length. However, further analysis and experimental evidence are required to determine whether these repetitive sequences can mediate homologous recombination (Figure 3).
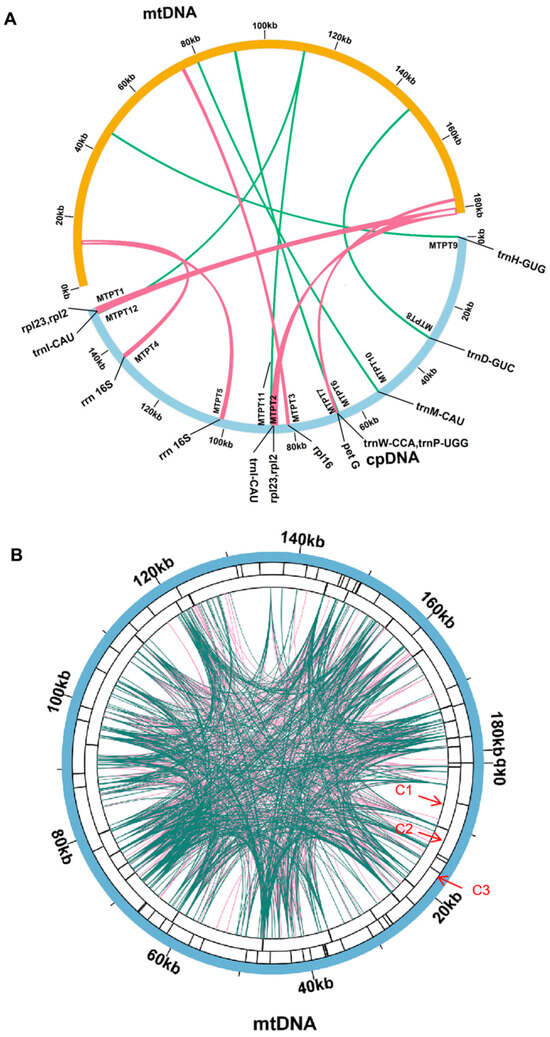
Figure 3.
The repeat of the P. lactiflora organelle genome. (A) The yellow color represents the mitogenome, the blue color represents the cpgenome, and the inside connecting red and green color lines represent similar DNA. (B) The repeat sequences identified in the mitogenome. The C1 circle shows the dispersed repeats connected with blue arcs from the center from outward. The C2 circle shows the tandem repeats as short bars. The C3 circle shows the microsatellite sequences identified using MISA. The scale is shown on the C3 circle, with intervals of 20 kb.
3.4. Mitochondrial Plastid DNAs Prediction
MTPTs are remnants of plastid-derived DNA in mitogenomes [54], which means that they are fragments of DNA that originated from the cpgenome and have been incorporated into the mitogenome. In this study, the researchers assembled the cpgenome of P. lactiflora (PP049084) using the same dataset as the mitogenome assembly. The cpgenome spanned 152,731 bp and had a GC content of 38.00%; it contained 85 genes, 112 of which were unique, including 87 protein-coding genes (79 unique), 37 tRNA genes (29 unique), and 8 rRNA genes (4 unique). According to the cpgenome, we identified nine MTPTs between the mitogenome (NC_070189.1) and cpgenome of P. lactiflora via sequence similarity calculations, as delineated in Table S4. These MTPTs constituted 2.2% of the total mitogenome. MTPT1, MTPT2 (1683 bp) was the longest, while MTPT10 (77 bp) was the shortest identified MTPT. The genomic positions of these MTPTs in the cpgenomes and mitogenomes are presented in Table 3 and Figure 3. To delve into their functions, we annotated the DNA fragments of the 12 MTPTs, identifying 10 unique genes in total. Two protein genes and six tRNA genes (rps19, petG, trnW-CCA, trnP-UGG, trnD-GUC, trnH-GUG, trnM-CAU, and trnI-CAU) were fully intact, while three genes (rpl2, rpl16, and rrn16s) became pseudogenes during migration. Among the MTPTs identified in the mitogenome of P. lactiflora, MTPT1, MTPT2, MTPT4, MTPT5, and MTPT11 are located in repeat sequences, including genes such as rpl23 and rpl2. These genes are hotspots for gene transfer [55,56,57].

Table 3.
Plastid homologous sequences identified in mitogenome (MTPTs) of P.lactiflora.
To explore the MTPTs pattern in P. lactiflora and its homologous species, we searched the NCBI database and found that the mitogenome data of seven closely-related species within the Saxifragales Order have been published, including Loropetalum chinense var. rubrum Yieh, Heuchera parviflora var. saurensis R.A.Folk, Sedum plumbizincicola, Rhodiola crenulata (Hook. f. et Thoms.) H. Ohba, Ribes nigrum L., Rhodiola tangutica (Maximowicz) S. H. Fu, and Rhodiola coccinea (Royle) Borissova (Tables S4–S10). In the results of the MTPTs analysis of these seven closely-related species, fragments were found in repeat sequences, including the species with the most gene numbers, H. parviflora [58,59]. The number of MTPTs in Saxifragales, which belongs to the same order as P. lactiflora, is the least with only 11, particularly compared to the most in L. chinense, which has a significant difference. This may suggest that external factors such as the environment, human intervention, and metabolic influences have affected the evolutionary process of closely-related species [60].
3.5. Phylogenetic Relationships
We conducted phylogenetic analyses by aligning 25 common genes from 29 angiosperm species, including a broad spectrum of species such as Vitis vinifera L. (NC_012119.1), Zygophyllum fabago L. (MK431827.1), Tribulus terrestris Muhl. (MK431825.1), Eucalyptus grandis W.Hill ex Maiden (NC_040010.1), Oenothera biennis strain suaveolens Grado (MZ934756.1), Lagerstroemia indica L. (NC_035616.1), Oenothera elata subsp. hookeri strain johansen Standard (MZ934757.1), Oenothera villaricae strain berteriana Schwemmle (MZ934755.1), Medinilla magnifica Lindl. (MT043351.1), Geranium maderense voucher (NC_027000.1), Acacia ligulata A.Cunn. ex Benth. (NC_040998.1), Medicago truncatula Gaertn. (NC_029641.1), Gleditsia sinensis Lam. (NC_058235.1), Glycine soja Siebold and Zucc. (NC_039768.1), Glycyrrhiza uralensis Fisch. ex DC. (NC_053919.1), Haematoxylum brasiletto H.Karst. (NC_045040.1), Ormosia boluoensis Y.Q.Wang and P.Y.Chen (NC_059804.1), Phaseolus vulgaris L. (NC_045135.1), Pisum abyssinicum A.Braun (NC_059791.1), Senna tora (L.) Roxb. (NC_038053.1), Glycine max (L.) Merr. (NC_020455.1), Vigna radiata (L.) R.Wilczek (NC_015121.1), Sophora flavescens Aiton (NC_043897.1), Stylosanthes pilosa M.B.Ferreira and Sousa Costa (NC_063516.1), Tamarindus indica L. (NC_045038.1), Trifolium grandiflorum Hook. and Arn. (NC_048501.1), β macrocarpa Guss. (NC_015994.1), Mirabilis jalapa L. (NC_056991.1), and, of course, P. lactiflora. By utilizing these common PCGs within the mitogenomes, we generated a phylogenetic tree using IQTREE2 (v1.2.3). Figure 4 clearly demonstrates a distinct clade formed by the relationship between P. lactiflora and V. vinifera. The complete mitogenome of P. lactiflora serves as a valuable asset in developing molecular markers and enhancing our understanding of the evolutionary history and cultivation strategies of Paeoniae.
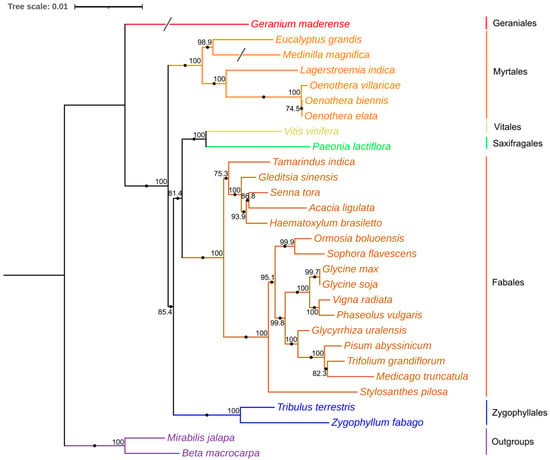
Figure 4.
Phylogenetic tree based on 25 homologous protein-coding genes (atp6, atp8, ccmC, ccmFC-e2, ccmFN, cox3, matR-e1, matR-e2, nad1-e1, nad1-e5, nad3, nad4-e1, nad4-e2, nad4-e3, nad4-e4, nad5-e2, nad6, nad7-e1, nad7-e3, nad7-e4, nad7-e5, nad9, adh4) in 29 plants’ mitogenomes using maximum likelihood (ML) analysis. Numbers above nodes are support values with ML bootstrap values and branch lengths. The red color represents Geraniales, the orange color represents Myrtales, the yellow color represents Vitales, the green color represents Saxifragales, the brown color represents Fabales, the blue color represents Zygophyllales, the purple color represents Outgroups.
3.6. Prediction of RNA-Editing Sites
By mapping transcriptome data onto the mitogenomes, we were able to identify 32 sites of RNA modification within the spacer and protein-coding regions [61]. These modifications were found within a diverse range of genes, including atp1, atp4, atp6, atp8, atp9, ccmB, ccmC, ccmFC, ccmFN, cob, cox1, cox2, cox3, matR, mttB, nad1, nad2, nad3, nad4, nad4L, nad5, nad6, nad7, nad9, rpl16, rpl5, rps12, rps4, and sdh4 (Figure 5). A total of 569 RNA editing events occurred, with the most editing events occurring in nad4, with 47 edits, and the fewest in rps7 and rps14, with 2 edits each. The proportion of editing events with more than 20 edits was 34.4%. Out of the identified modifications, there were 747 instances with a frequency greater than 0.01, with 289 of these boasting a certainty of 1 [62]. The proportion of editing events with a frequency greater than 0.5 is 99.07%, indicating that there are many gene-editing events. There was a total of 13 types of amino acid change events, with the most frequent being the conversion of proline to leucine, which occurred 147 times. The majority of RNA editing events that did not result in changes in amino acid type were synonymous mutations, where the base substitution did not alter the amino acid. There were three events with more than 100 times, with the other two types being the conversion of serine to phenylalanine and phenylalanine to leucine [63].
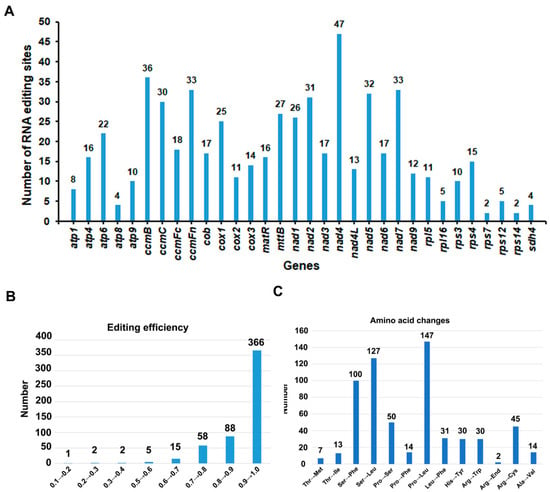
Figure 5.
The characteristics of the RNA editing sites identified in protein-coding genes (PCGs). The “X” axis shows the name of protein-coding genes, and the “Y” axis shows the number of predicted RNA editing sites. (A) The number of RNA editing sites identified in each PCGs of mitogenome; (B) RNA editing efficiency; (C) Frequency of amino acid changes caused by RNA editing.
4. Discussion
The recent publication of our study on the mitogenome of P. lactiflora, utilizing both Nanopore long reads [62] and Illumina short reads [64], marked the first-ever confirmation that the mitogenome of this species primarily comprises circular chromosomes. To gain a comprehensive understanding of the diversity and evolution of P. lactiflora’s mitogenome, we conducted extensive sequencing, assembly, and detailed characterization. This involved the determining the gene content, simple sequence repeats (SSRs), tandem repeats, dispersed repeats, mitochondrial plastid DNAs (MTPTs), and RNA-editing events within P. lactiflora’s mitogenome. The insights gained from this study are crucial for further exploration of the diversity and evolutionary dynamics of the P. lactiflora mitogenome.
In this study, we sequenced and assembled the mitogenome of P. lactiflora, which has a circular genome structure of 181,688 bp. The mitogenome of another species in the Paeoniaceae family, Paeonia suffruticosa Andrews, has been published with a size of 203,077 bp (https://www.ncbi.nlm.nih.gov/nuccore/OR551751.1 (accessed on 13 February 2024)). These two species are currently the only ones in the Paeoniaceae family to have their mitogenomes sequenced, and their sizes are relatively small compared to the range of 66 kb to 11 Mb for mitogenomes in eudicots. According to the data on NCBI, the whole genome size of Paeonia suffruticosa Andrews is 12.28 Gb [65], while the mitogenome is relatively small, indicating that the gene fragment interaction between the mitogenome and the nuclear genome is relatively small.
Mitochondrial plastid DNA (MTPTs) refers to DNA fragments that have migrated from the plastid genome to the mitochondrial genome, belonging to a type of horizontal gene transfer (HGT). In this study, 11 MTPTs were discovered in P. lactiflora through sequence similarity between the cpgenome and mitogenome, with a total insertion length accounting for 3.6% of the mitogenome. Among the seven closely-related species analyzed, the cpgenome found in Heuchera parviflora var. saurensis was the most abundant, accounting for 8.5%, while the cpgenome found in Loropetalum chinense was the least abundant, accounting for 1.4%.
In this study, we discovered 569 potential RNA editing sites in the mitogenome of P. lactiflora, leading to 13 distinct types of amino acid substitutions. The RNA editing events were least prevalent in ribosomal protein genes like rps4 and rps14, whereas genes such as nad4, ccmB, and ccmFn exhibited a higher frequency of RNA editing events. These findings suggest that RNA editing plays a crucial role in plant adaptation to environmental changes and signal transduction, particularly in mitochondrial and plastid biogenesis [66].
Repetitive sequences are widely distributed in plant mitogenomes and have relatively complex structures. These sequences can cause changes in the structure of plant genomes through recombination, thereby influencing plant evolution. In the mitogenome of P. lactiflora, various repetitive sequences were observed, including 55 pairs of SSRs, 26 pairs of Tandem repeat sequences, 623 pairs of Palindromic repeats, and 641 pairs of Forward repeats. The recombination of these sequences has a significant impact on the size, gene arrangement, and evolution of plant mitogenomes and may also lead to phenotypic mutations in plants. For instance, in Pisum sativum L., the amplification of repetitive sequences results in seed mutations that affect subsequent growth and development [67]. In Zea mays L., repetitive sequences influence the expression of chloroplast genes, thereby affecting photosynthesis and growth [68]. In Oryza sativa L., variations in repetitive sequences can impact traits such as plant height and tiller number [69]. Furthermore, repetitive sequences play an important role in cytoplasmic male sterility. Some plant mitogenomes contain numerous repetitive sequences that can cause gene amplification, deletion, or rearrangement, thereby affecting pollen development. For example, in certain Saccharum officinarum varieties, the amplification of a mitogenome repeat sequence is associated with male sterility [70]. The analysis of the mitogenome of P. lactiflora provides a theoretical basis for understanding subsequent seedling breeding and phenotypic changes in P. lactiflora.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes15020239/s1, Table S1: SSRs in the mitochondrial genome of Paeonia lactiflora; Table S2: Tandem repeat sequences in the mitochondrial genome of Paeonia lactiflora; Table S3: Dispersed repeats sequences in the mitochondrial genome of Paeonia lactiflora; Table S4: Plastid homologous sequences identified in mitogenome (MTPTs) of Loropetalum chinense var. rubrum Yieh (R.Br.) Oliv.; Table S5: Plastid homologous sequences identified in mitogenome (MTPTs) of Heuchera parviflora var. Saurensis Bartl.; Table S6: Plastid homologous sequences identified in mitogenome (MTPTs) of Sedum plumbizincicola X.H.Guo and S.B.Zhou ex L.H.Wu; Table S7: Plastid homologous sequences identified in mitogenome (MTPTs) of Rhodiola crenulata (Hook.f. and Thomson) H.Ohba; Table S8: Plastid homologous sequences identified in mitogenome (MTPTs) of Ribes nigrum L.; Table S9: Plastid homologous sequences identified in mitogenome (MTPTs) of Rhodiola tangutica L.; Table S10: Plastid homologous sequences identified in mitogenome (MTPTs) of Rhodiola juparensis (Fröd.) S.H.Fu.
Author Contributions
J.G., C.L. designed the experiments; Y.N., J.L., Q.L. and P.T. performed the experiments and analyzed the data; J.G. and C.L. provided advice and assistance during experiments and data analysis; P.T. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Sciences Foundation of China (grant numbers 81872959, 81373920). “Xinglin Scholar” Talent Research Promotion Plan of Chengdu University of Traditional Chinese Medicine (grant number ZYTS2019022). Young Science and Technology Innovation Team of Sichuan Province (grant number 2019CXTD0055).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The assembled mitochondrial genome sequences have been deposited in NCBI (https://www.ncbi.nlm.nih.gov/ (accessed on 25 January 2023)) with accession number: NC_070189.1; The assembled chloroplast genome sequences have been deposited in NCBI with accession number: PP049084.1; The BioProject ID: PRJNA905540 (https://www.ncbi.nlm.nih.gov/bioproject/905540 (accessed on 10 February 2024)); The BioSample ID: SAMN37366985 (https://www.ncbi.nlm.nih.gov/biosample/37366985 (access on 10 February 2024)); SRA ID: SRR15412863 (https://trace.ncbi.nlm.nih.gov/Traces/index.html?view=run_browser&acc=SRR15412863&display=metadata (access on 10 February 2024)).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
mtDNA: Mitochondrial genome; cpDNA: Chloroplast genome; PCG: Protein-coding genes; DNA: DeoxyriboNucleic Acid; RNA: Ribonucleic Acid; tRNA: transfer RNA; rRNA: ribosomal RNA; ATP: Adenosine triphosphate; mRNA: messenger RNA; CMS: Cytoplasmic male sterility; DEPC: diethyl pyrocarbonate; CTAB: Cetyltrimethylammonium Bromide; TRF: Tandem Repeats Finder; SSR: Simple Sequence Repeat; TRS: Tandem Repeats Sequences; DRS: Dispersed Repeats Sequences; NUMT: Nuclear Mitochondrial DNA; MTPT: Mitochondrial Plastid DNA.
References
- Wang, Y.; He, J.; Wu, Z.; Huang, L.; Gao, M.; Li, M.; Feng, J. The Complete Mitogenome and Phylogeny Analysis of Pseudohemiculter Hainanensis (Boulenger, 1900) (Cyprinidae: Cultrinae). Mitochondrial DNA Part B 2022, 7, 2056–2059. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Q.; Ying, Z.; Ge, Y.; Cheng, R. Molecular Structure and Phylogenetic Analysis of Complete Chloroplast Genomes of Medicinal Species Paeonia Lactiflora from Zhejiang Province. Mitochondrial DNA Part B 2020, 5, 1077–1078. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Guo, L.; Li, Y.; Yang, H.; Hu, X.; Song, C.; Hou, X. Development and Characterization of Microsatellite Markers Based on the Chloroplast Genome of Tree Peony. Genes 2022, 13, 1543. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.-Y.; Chang, H.-W.; Lin, C.-F.; Liu, C.-J.; Hsieh, C.-F.; Horng, J.-T. Characterization of the Anti-Influenza Activity of the Chinese Herbal Plant Paeonia lactiflora. Viruses 2014, 6, 1861–1875. [Google Scholar] [CrossRef]
- Pan, H.-T.; Xi, Z.-Q.; Wei, X.-Q.; Wang, K. A Network Pharmacology Approach to Predict Potential Targets and Mechanisms of “Ramulus Cinnamomi (Cassiae)—Paeonia Lactiflora” Herb Pair in the Treatment of Chronic Pain with Comorbid Anxiety and Depression. Ann. Med. 2022, 54, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef]
- Cole, L.W.; Guo, W.; Mower, J.P.; Palmer, J.D. High and Variable Rates of Repeat-Mediated Mitochondrial Genome Rearrangement in a Genus of Plants. Mol. Biol. Evol. 2018, 35, 2773–2785. [Google Scholar] [CrossRef]
- Yang, H.; Chen, H.; Ni, Y.; Li, J.; Cai, Y.; Ma, B.; Yu, J.; Wang, J.; Liu, C. De Novo Hybrid Assembly of the Salvia Miltiorrhiza Mitochondrial Genome Provides the First Evidence of the Multi-Chromosomal Mitochondrial DNA Structure of Salvia Species. Int. J. Mol. Sci. 2022, 23, 14267. [Google Scholar] [CrossRef]
- Lower, S.E.; Dion-Côté, A.-M.; Clark, A.G.; Barbash, D.A. Special Issue: Repetitive DNA Sequences. Genes 2019, 10, 896. [Google Scholar] [CrossRef]
- Bi, C.; Qu, Y.; Hou, J.; Wu, K.; Ye, N.; Yin, T. Deciphering the Multi-Chromosomal Mitochondrial Genome of Populus Simonii. Front. Plant Sci. 2022, 13, 914635. [Google Scholar] [CrossRef]
- Muller, H.; Ogereau, D.; Da Lage, J.-L.; Capdevielle, C.; Pollet, N.; Fortuna, T.; Jeannette, R.; Kaiser, L.; Gilbert, C. Draft Nuclear Genome and Complete Mitogenome of the Mediterranean Corn Borer, Sesamia Nonagrioides, a Major Pest of Maize. G3 Genes|Genomes|Genet 2021, 11, jkab155. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, W.; Xu, J.; Li, M.; Zhang, Y. Chloroplast Genome Evolution and Species Identification of Styrax (Styracaceae). BioMed Res. Int. 2022, 2022, 5364094. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Z.; Cai, D.; Song, L.; Bai, J. The Chloroplast Genome Sequence and Phylogenetic Analysis of Apocynum venetum L. PLoS ONE 2022, 17, e0261710. [Google Scholar] [CrossRef] [PubMed]
- Skuza, L.; Gastineau, R.; Sielska, A. The Complete Chloroplast Genome of Secale Sylvestre (Poaceae: Triticeae). J. Appl. Genet. 2022, 63, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Shearman, J.R.; Sonthirod, C.; Naktang, C.; Sangsrakru, D.; Yoocha, T.; Chatbanyong, R.; Vorakuldumrongchai, S.; Chusri, O.; Tangphatsornruang, S.; Pootakham, W. Assembly of the Durian Chloroplast Genome Using Long PacBio Reads. Sci. Rep. 2020, 10, 15980. [Google Scholar] [CrossRef]
- Suzuki, Y. Informatics for PacBio Long Reads. Adv. Exp. Med. Biol. 2019, 1129, 119–129. [Google Scholar] [CrossRef]
- Sheng, M.; She, J.; Xu, W.; Hong, Y.; Su, Z.; Zhang, X. HpeNet: Co-Expression Network Database for de Novo Transcriptome Assembly of Paeonia Lactiflora Pall. Front. Genet. 2020, 11, 570138. [Google Scholar] [CrossRef]
- Ren, X.; Shi, Y.; Xue, Y.; Xue, J.; Tian, Y.; Wang, S.; Zhang, X. Seed Proteomic Profiles of Three Paeonia Varieties and Evaluation of Peony Seed Protein as a Food Product. BioMed Res. Int. 2020, 2020, 5271296. [Google Scholar] [CrossRef]
- Štorchová, H.; Stone, J.D.; Sloan, D.B.; Abeyawardana, O.A.J.; Müller, K.; Walterová, J.; Pažoutová, M. Homologous Recombination Changes the Context of Cytochrome b Transcription in the Mitochondrial Genome of Silene Vulgaris KRA. BMC Genom. 2018, 19, 874. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, P.; Tong, C.; Bi, C.; Xu, L.A. Assembly and Analysis of the Populus Deltoides Mitochondrial Genome: The First Report of a Multicircular Mitochondrial Conformation for the Genus Populus. J. For. Res. 2023, 34, 717–733. [Google Scholar] [CrossRef]
- Small, I.D.; Schallenberg-Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant Organellar RNA Editing: What 30 Years of Research Has Revealed. Plant J. 2020, 101, 1040–1056. [Google Scholar] [CrossRef]
- Swoboda, P.; Hohn, B.; Gal, S. Somatic Homologous Recombination in Planta: The Recombination Frequency Is Dependent on the Allelic State of Recombining Sequences and May Be Influenced by Genomic Position Effects. Mol. Genet. Genom. 1993, 237, 33–40. [Google Scholar] [CrossRef]
- Van Norden, M.; Falls, Z.; Mandloi, S.; Segal, B.; Baysal, B.; Samudrala, R.; Elkin, P.L. The Role of C-to-U RNA Editing in Human Biodiversity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Q.; Yin, P. RNA Editing Machinery in Plant Organelles. Sci. China Life Sci. 2018, 61, 162–169. [Google Scholar] [CrossRef]
- Zanlungo, S.; Moenne, A.; Holuigue, L.; Jordana, X.; Quiñones, V. Splicing and Editing of Rps10 Transcripts in Potato Mitochondria. Curr. Genet. 1995, 27, 565–571. [Google Scholar] [CrossRef]
- Quinones, V.; Zanlungo, S.; Holuigue, L.; Litvak, S.; Jordana, X. The Cox1 Initiation Codon Is Created by RNA Editing in Potato Mitochondria. Plant Physiol. 1995, 108, 1327–1328. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, S.F.; Dias, S.G.; Hardouin, P.; Pereira, F.R.; Lejeune, B.; de Souza, A.P. Transcription of Succinate Dehydrogenase Subunit 4 (Sdh4) Gene in Potato: Detection of Extensive RNA Editing and Co-Transcription with Cytochrome Oxidase Subunit III (Cox3) Gene. Curr. Genet. 2002, 41, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Moreira, J.D.; Smith, K.K.; Fetterman, J.L. The Mighty NUMT: Mitochondrial DNA Flexing Its Code in the Nuclear Genome. Biomolecules 2023, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.L.; Yuyama, P.M.; Sartori, D.; Fungaro, M.H.P.; Vanzela, A.L.L. Occurrence and Chromosome Distribution of Retroelements and NUPT Sequences in Copaifera Langsdorffii Desf. (Caesalpinioideae). Chromosom. Res. 2010, 18, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Hatmaker, E.A.; Wadl, P.A.; Rinehart, T.A.; Carroll, J.; Lane, T.S.; Trigiano, R.N.; Staton, M.E.; Schilling, E.E. Complete chloroplast genome comparisons for Pityopsis (Asteraceae). PLoS ONE 2020, 15, e0241391. [Google Scholar] [CrossRef]
- Gandini, C.L.; Sanchez-Puerta, M.V. Foreign Plastid Sequences in Plant Mitochondria are Frequently Acquired Via Mitochondrion-to-Mitochondrion Horizontal Transfer. Sci. Rep. 2017, 7, 43402. [Google Scholar] [CrossRef] [PubMed]
- Sloan, D.B.; Wu, Z. History of Plastid DNA Insertions Reveals Weak Deletion and AT Mutation Biases in Angiosperm Mitochondrial Genomes. Genome Biol. Evol. 2014, 6, 3210–3221. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Li, J.; Zhao, Q.; Liang, Y.; Yu, J. Assembly of the Complete Mitochondrial Genome of Chinese Plum (Prunus salicina): Characterization of Genome Recombination and RNA Editing Sites. Genes 2021, 12, 1970. [Google Scholar] [CrossRef]
- Lv, S.; Cheng, S.; Wang, Z.; Li, S.; Jin, X.; Lan, L.; Yang, B.; Yu, K.; Ni, X.; Li, N.; et al. Draft Genome of the Famous Ornamental Plant Paeonia suffruticosa. Ecol. Evol. 2020, 10, 4518–4530. [Google Scholar] [CrossRef]
- Adamovic, D.; Djalovic, I.; Mitrovic, P.; Kojic, S.; Pivic, R.; Josic, D. First Report on Natural Infection of Paeonia tenuifolia by ‘Candidatus Phytoplasma Solani’ in Serbia. Plant Dis. 2014, 98, 565. [Google Scholar] [CrossRef]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; Depamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A Fast and Versatile Toolkit for Accurate de Novo Assembly of Organelle Genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—Versatile and Accurate Annotation of Organelle Genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) Version 1.3.1: Expanded Toolkit for the Graphical Visualization of Organellar Genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. In Gene Prediction; Methods in Molecular Biology; Kollmar, M., Ed.; Springer: New York, NY, USA, 2019; Volume 1962, pp. 1–14. ISBN 978-1-4939-9172-3. [Google Scholar]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-Web: A Web Server for Microsatellite Prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Benson, G. Tandem Repeats Finder: A Program to Analyze DNA Sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Kozik, A.; Rowan, B.A.; Lavelle, D.; Berke, L.; Schranz, M.E.; Michelmore, R.W.; Christensen, A.C. The Alternative Reality of Plant Mitochondrial DNA: One Ring Does Not Rule Them All. PLOS Genet. 2019, 15, e1008373. [Google Scholar] [CrossRef]
- Shi, L.; Chen, H.; Jiang, M.; Wang, L.; Wu, X.; Huang, L.; Liu, C. CPGAVAS2, an Integrated Plastome Sequence Annotator and Analyzer. Nucleic Acids Res. 2019, 47, W65–W73. [Google Scholar] [CrossRef]
- Liu, S.; Ni, Y.; Li, J.; Zhang, X.; Yang, H.; Chen, H.; Liu, C. CPGVIEW: A Package for Visualizing Detailed Chloroplast Genome Structures. Mol. Ecol. Resour. 2023, 23, 694–704. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zhou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An Integrated and Scalable Desktop Platform for Streamlined Molecular Sequence Data Management and Evolutionary Phylogenetics Studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Edera, A.A.; Small, I.; Milone, D.H.; Sanchez-Puerta, M.V. Deepred-Mt: Deep Representation Learning for Predicting C-to-U RNA Editing in Plant Mitochondria. Comput. Biol. Med. 2021, 136, 104682. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Wu, K.; Pan, J.; Long, H.; Yan, Y. Characterization of Simple Sequence Repeats (SSRs) in Ciliated Protists Inferred by Comparative Genomics. Microorganisms 2020, 8, 662. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xing, J.; Nie, T.; Su, A.; Zhang, R.; Zhao, Y.; Song, W.; Zhao, J. Comparative Analysis of Mitochondrial Genomes of Maize CMS-S Subtypes Provides New Insights into Male Sterility Stability. BMC Plant Biol. 2022, 22, 469. [Google Scholar] [CrossRef]
- Balzano, E.; Pelliccia, F.; Giunta, S. Genome (in)Stability at Tandem Repeats. Semin. Cell Dev. Biol. 2021, 113, 97–112. [Google Scholar] [CrossRef]
- Mehrotra, S.; Goyal, V. Repetitive Sequences in Plant Nuclear DNA: Types, Distribution, Evolution and Function. Genom. Proteom. Bioinform. 2014, 12, 164–171. [Google Scholar] [CrossRef]
- Wang, X.-C.; Chen, H.; Yang, D.; Liu, C. Diversity of Mitochondrial Plastid DNAs (MTPTs) in Seed Plants. Mitochondrial DNA Part A 2018, 29, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Massenet, O.; Dome, A.-M.; Briat, J.-F.; Mache, R. Expression of the Rpl23, Rpl2 and Rps19 Genes in Spinach Chloroplasts. Nucleic Acids Res. 1988, 16, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Ong, H.C.; Palmer, J.D. Mitochondrial Gene Transfer in Pieces: Fission of the Ribosomal Protein Gene Rpl2 and Partial or Complete Gene Transfer to the Nucleus. Mol. Biol. Evol. 2001, 18, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Haberhausen, G.; Zetsche, K.; Bömmer, D. A Large Deletion in the Plastid DNA of the Holoparasitic Flowering Plant Cuscuta Reflexa Concerning Two Ribosomal Proteins (Rpl2, Rpl23), One Transfer RNA (trnI) and an ORF 2280 Homologue. Curr. Genet. 1993, 24, 171–176. [Google Scholar] [CrossRef]
- Dong, W.; Xu, C.; Liu, Y.; Shi, J.; Li, W.; Suo, Z. Chloroplast Phylogenomics and Divergence Times of Lagerstroemia (Lythraceae). BMC Genom. 2021, 22, 434. [Google Scholar] [CrossRef] [PubMed]
- Filipenko, E.A.; Sidorchuk, Y.V.; Deineko, E.V. Spontaneous spectinomycin resistance mutations of the chloroplast rrn16 gene in Daucus carota callus lines. Genetika 2011, 47, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.-N.; Wu, C.-S.; Ye, L.-J.; Mo, Z.-Q.; Liu, J.; Chang, Y.-W.; Li, D.-Z.; Chaw, S.-M.; Gao, L.-M. Prevalence of Isomeric Plastomes and Effectiveness of Plastome Super-Barcodes in Yews (Taxus) Worldwide. Sci. Rep. 2019, 9, 2773. [Google Scholar] [CrossRef]
- Brennicke, A.; Marchfelder, A.; Binder, S. RNA Editing. FEMS Microbiol. Rev. 1999, 23, 297–316. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore Sequencing Technology, Bioinformatics and Applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef]
- Han, F.; Qu, Y.; Chen, Y.; Xu, L.; Bi, C. Assembly and Comparative Analysis of the Complete Mitochondrial Genome of Salix Wilsonii Using PacBio HiFi Sequencing. Front. Plant Sci. 2022, 13, 1031769. [Google Scholar] [CrossRef]
- Modi, A.; Vai, S.; Caramelli, D.; Lari, M. The Illumina Sequencing Protocol and the NovaSeq 6000 System. In Bacterial Pangenomics; Methods in Molecular Biology; Mengoni, A., Bacci, G., Fondi, M., Eds.; Springer: New York, NY, USA, 2021; Volume 2242, pp. 15–42. ISBN 978-1-07-161098-5. [Google Scholar]
- Yuan, J.; Jiang, S.; Jian, J.; Liu, M.; Yue, Z.; Xu, J.; Li, J.; Xu, C.; Lin, L.; Jing, Y.; et al. Genomic Basis of the Giga-Chromosomes and Giga-Genome of Tree Peony Paeonia Ostii. Nat. Commun. 2022, 13, 7328. [Google Scholar] [CrossRef]
- Giudice, C.L.; Hernández, I.; Ceci, L.R.; Pesole, G.; Picardi, E. RNA Editing in Plants: A Comprehensive Survey of Bioinformatics Tools and Databases. Plant Physiol. Biochem. 2019, 137, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, S.; Kumar, N.; Rana, R.S.; Sharma, P.; Kumar, P.; Rani, M. Morpho-Molecular Genetic Diversity and Population Structure Analysis in Garden Pea (Pisum sativum L.) Genotypes Using Simple Sequence Repeat Markers. PLoS ONE 2022, 17, e0273499. [Google Scholar] [CrossRef] [PubMed]
- Bedbrook, J.R.; Kolodner, R.; Bogorad, L. Zea Mays Chloroplast Ribosomal RNA Genes Are Part of a 22,000 Base Pair Inverted Repeat. Cell 1977, 11, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Long Terminal Repeat Retrotransposons of Oryza Sativa—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/12372141/ (accessed on 29 December 2023).
- Vilela, M.d.M.; Del-Bem, L.-E.; Van Sluys, M.-A.; de Setta, N.; Kitajima, J.P.; Cruz, G.M.Q.; Sforça, D.A.; de Souza, A.P.; Ferreira, P.C.G.; Grativol, C.; et al. Analysis of Three Sugarcane Homo/Homeologous Regions Suggests Independent Polyploidization Events of Saccharum officinarum and Saccharum spontaneum. Genome Biol. Evol. 2017, 9, 266–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).