KLHL40-Related Myopathy: A Systematic Review and Insight into a Follow-up Biomarker via a New Case Report
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Collection Process
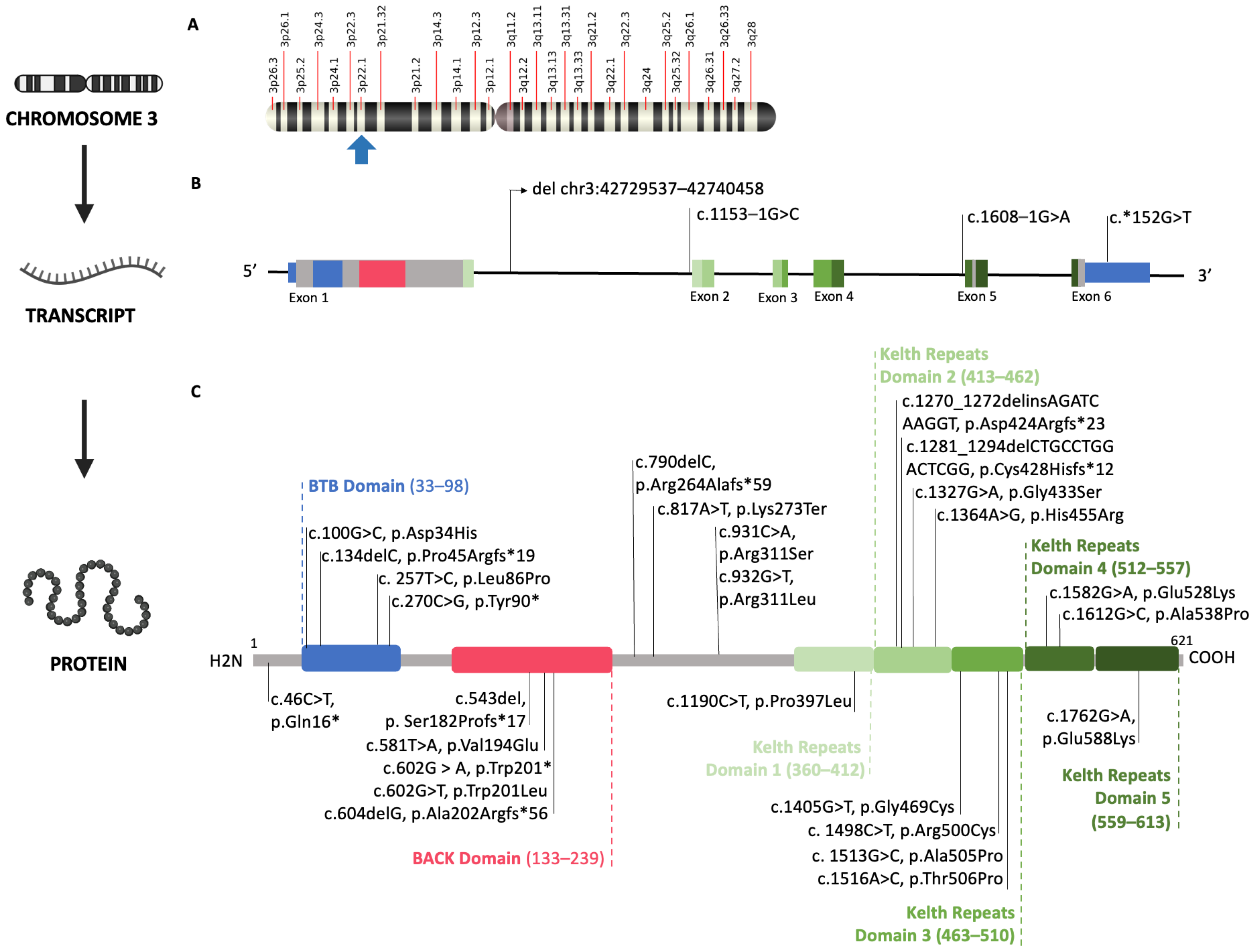
2.3. Genetic Analysis
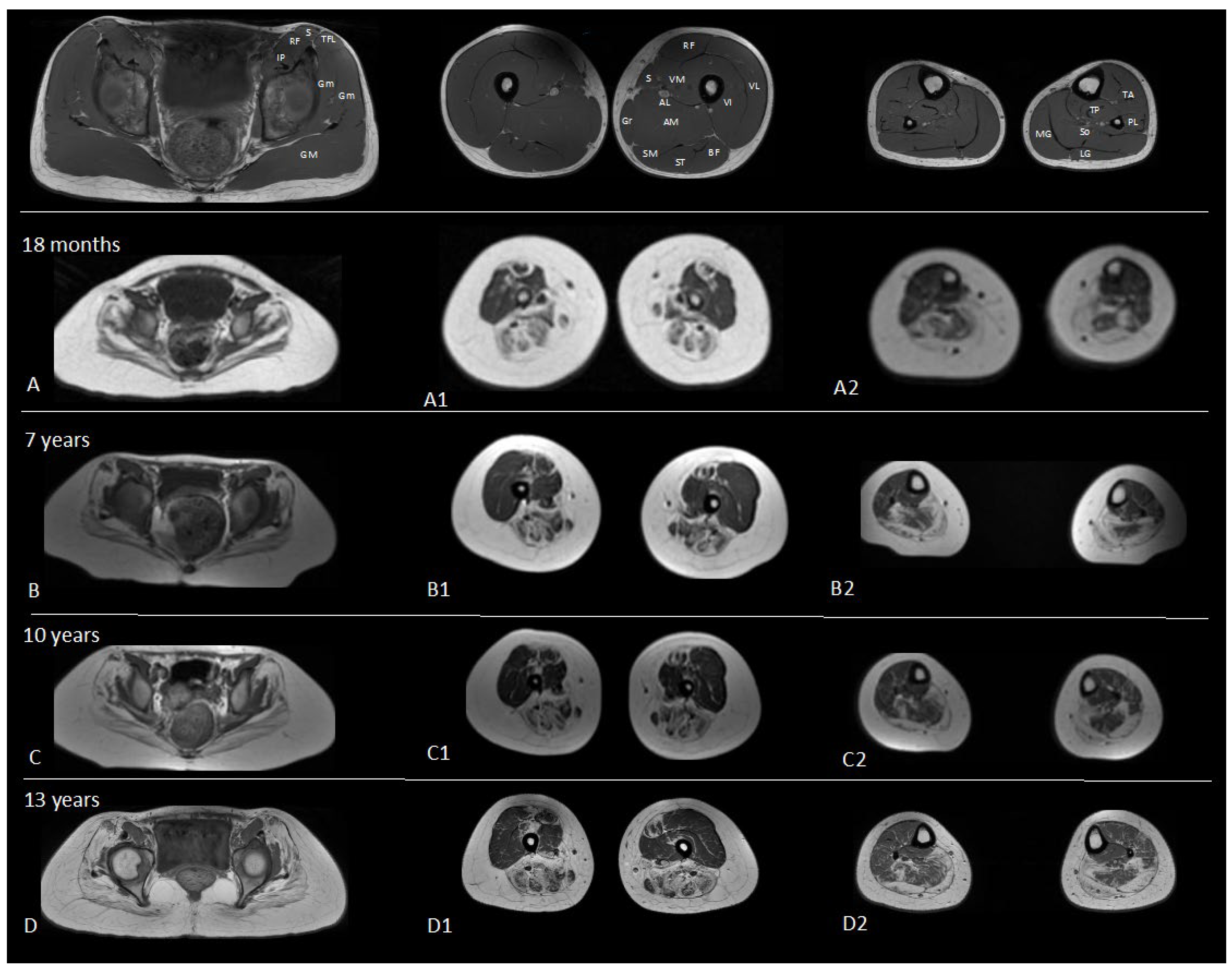
2.4. Case Report
3. Results
Descriptive Findings
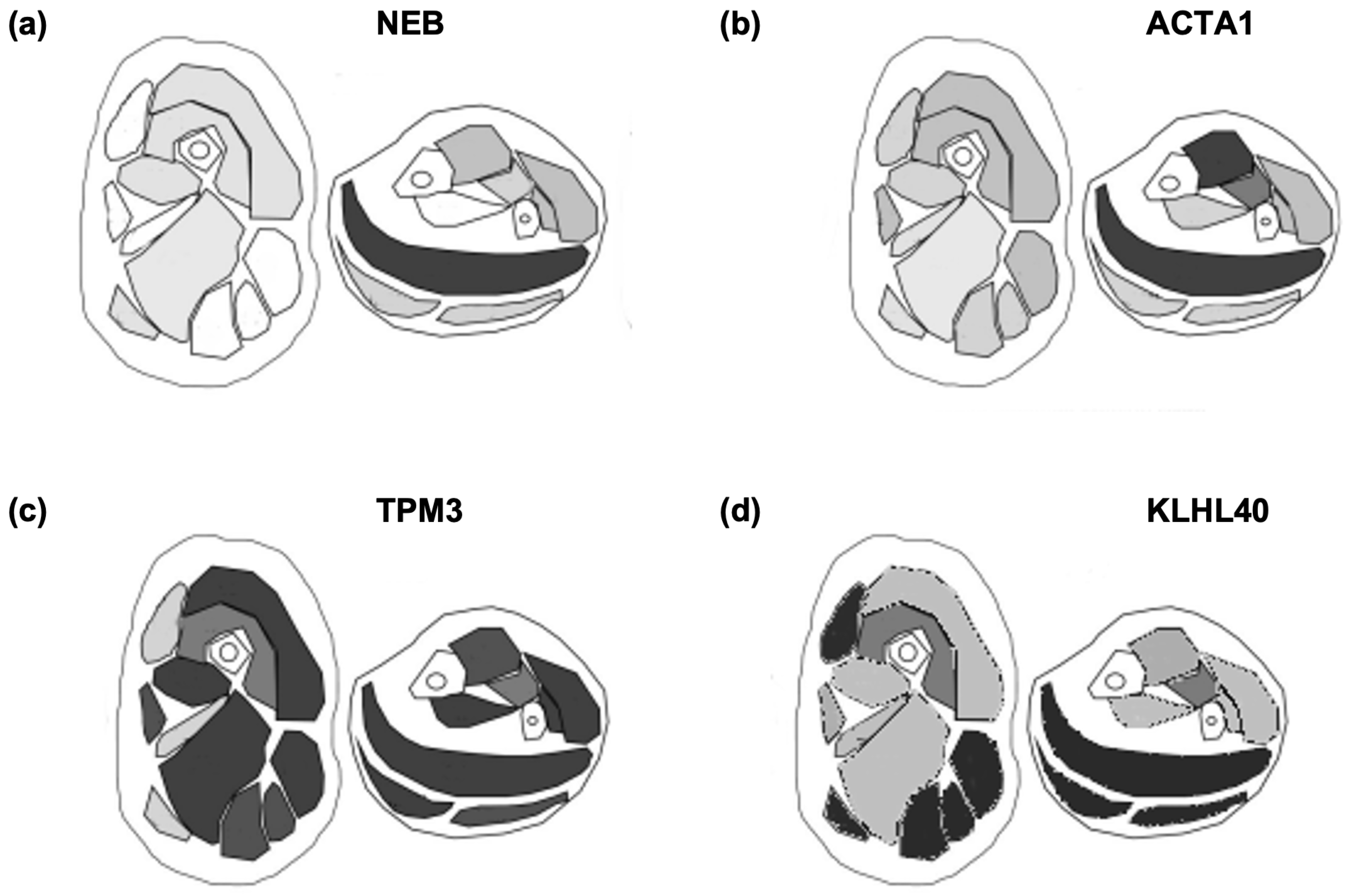
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowak, K.J.; Davis, M.R.; Wallgren-Pettersson, C.; Lamont, P.J.; Laing, N.G. Clinical utility gene card for: Nemaline myopathy–update 2015. Eur. J. Hum. Genet. 2015, 23, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Nance, J.R.; Dowling, J.J.; Gibbs, E.M.; Bönnemann, C.G. Congenital Myopathies: An Update. Curr. Neurol. Neurosci. Rep. 2012, 12, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Laitila, J.; Wallgren-Pettersson, C. Recent advances in nemaline myopathy. Neuromuscul. Disord. 2021, 31, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Nishino, I. A review of major causative genes in congenital myopathies. J. Hum. Genet. 2023, 68, 215–225. [Google Scholar] [CrossRef] [PubMed]
- North, K.N.; Wang, C.H.; Clarke, N.; Jungbluth, H.; Vainzof, M.; Dowling, J.J.; Amburgey, K.; Quijano-Roy, S.; Beggs, A.H.; Sewry, C.; et al. Approach to the diagnosis of congenital myopathies. Neuromuscul. Disord. 2014, 24, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.-X.; Zhen, L.; Lin, X.-M.; Wen, Y.-J.; Li, D.-Z. Clinical and molecular analysis of nine fetal cases with clinically significant variants causing nemaline myopathy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Stimpson, G.; Singh, L.; Morrow, J.M.; Shah, S.; Baranello, G.; Muntoni, F.; Sarkozy, A. Muscle magnetic resonance imaging involvement patterns in nemaline myopathies. Ann. Clin. Transl. Neurol. 2023, 10, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, G.; Miyatake, S.; Lehtokari, V.-L.; Todd, E.J.; Vornanen, P.; Yau, K.S.; Hayashi, Y.K.; Miyake, N.; Tsurusaki, Y.; Doi, H.; et al. Mutations in KLHL40 Are a Frequent Cause of Severe Autosomal-Recessive Nemaline Myopathy. Am. J. Hum. Genet. 2013, 93, 6–18. [Google Scholar] [CrossRef]
- Kawase, K.; Nishino, I.; Sugimoto, M.; Togawa, T.; Sugiura, T.; Kouwaki, M.; Kibe, T.; Koyama, N.; Yokochi, K. Nemaline myopathy with KLHL40 mutation presenting as congenital totally locked-in state. Brain Dev. 2015, 37, 887–890. [Google Scholar] [CrossRef]
- Anderson, S.L.; Ekstein, J.; Donnelly, M.C.; Keefe, E.M.; Toto, N.R.; LeVoci, L.A.; Rubin, B.Y. Nemaline myopathy in the Ashkenazi Jewish population is caused by a deletion in the nebulin gene. Hum. Genet. 2004, 115, 185–190. [Google Scholar] [CrossRef]
- Massalska, D.; Zimowski, J.G.; Bijok, J.; Kucińska-Chahwan, A.; Łusakowska, A.; Jakiel, G.; Roszkowski, T. Prenatal diagnosis of congenital myopathies and muscular dystrophies. Clin. Genet. 2016, 90, 199–210. [Google Scholar] [CrossRef] [PubMed]
- de Winter, J.M.; Ottenheijm, C.A. Sarcomere Dysfunction in Nemaline Myopathy. J. Neuromuscul. Dis. 2017, 4, 99–113. [Google Scholar] [CrossRef]
- Yin, X.; Pu, C.Q.; Wang, Q.; Liu, J.X.; Mao, Y.L. Clinical and pathological features of patients with nemaline myopathy. Mol. Med. Rep. 2014, 10, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.H.; Wong, S.; Leung, F.Y.-K.; Ho, L.-C.; Chan, S.-K.T.; Fung, T.-H.S.; Kwan, K.-F.; Yau, K.-C.E.; Li, K.-W.; Yau, W.-N.; et al. Founder mutation c.1516a>C in KLHL40 is a frequent cause of nemaline myopathy with hyponatremia in ethnic Chinese. J. Neuropathol. Exp. Neurol. 2019, 78, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Lammens, M.; Moerman, P.; Fryns, J.P.; Lemmens, F.; van de Kamp, G.M.; Goemans, N.; Dom, R. Fetal Akinesia Sequence Caused by Nemaline Myopathy. Neuropediatrics 1997, 28, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Benito, D.N.-D.; Nascimento, A.; Abicht, A.; Ortez, C.; Jou, C.; Müller, J.S.; Evangelista, T.; Töpf, A.; Thompson, R.; Jimenez-Mallebrera, C.; et al. KLHL40-related nemaline myopathy with a sustained, positive response to treatment with acetylcholinesterase inhibitors. J. Neurol. 2016, 263, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Kopanos, C.; Tsiolkas, V.; Kouris, A.; Chapple, C.E.; Aguilera, M.A.; Meyer, R.; Massouras, A. VarSome: The human genomic variant search engine. Bioinformatics 2019, 35, 1978–1980. [Google Scholar] [CrossRef]
- Boeckmann, B. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003, 31, 365–370. [Google Scholar] [CrossRef]
- Dosi, C.; Rubegni, A.; Baldacci, J.; Galatolo, D.; Doccini, S.; Astrea, G.; Berardinelli, A.; Bruno, C.; Bruno, G.; Comi, G.P.; et al. Using Cluster Analysis to Overcome the Limits of Traditional Phenotype–Genotype Correlations: The Example of RYR1-Related Myopathies. Genes 2023, 14, 298. [Google Scholar] [CrossRef]
- Bérard, C.; Payan, C.; Hodgkinson, I.; Fermanian, J.; The MFM Collaborative Study Group. A motor function measure scale for neuromuscular diseases. Construction and validation study. Neuromuscul. Disord. 2005, 15, 463–470. [Google Scholar] [CrossRef]
- Marinella, G.; Astrea, G.; Buchignani, B.; Cassandrini, D.; Doccini, S.; Filosto, M.; Galatolo, D.; Gallone, S.; Giannini, F.; Lopergolo, D.; et al. A Schematic Approach to Defining the Prevalence of COL VI Variants in Five Years of Next-Generation Sequencing. Int. J. Mol. Sci. 2022, 23, 14567. [Google Scholar] [CrossRef]
- Dofash, L.N.H.; Monahan, G.V.; Servián-Morilla, E.; Rivas, E.; Faiz, F.; Sullivan, P.; Oates, E.; Clayton, J.; Taylor, R.L.; Davis, M.R.; et al. A KLHL403′ UTR splice-altering variant causes milder NEM8, an under-appreciated disease mechanism. Hum. Mol. Genet. 2023, 32, 1127–1136. [Google Scholar] [CrossRef]
- Seferian, A.M.; Malfatti, E.; Bosson, C.; Pelletier, L.; Taytard, J.; Forin, V.; Gidaro, T.; Gargaun, E.; Carlier, P.; Fauré, J.; et al. Mild clinical presentation in KLHL40-related nemaline myopathy (NEM 8). Neuromuscul. Disord. 2016, 26, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.S.; Yu, F.N.Y.; Fung, C.W.; Wong, S.; Lee, H.H.C.; Fung, S.T.H.; Fung, G.P.G.; Leung, K.Y.; Chung, W.H.; Lee, Y.T.; et al. The KLHL40 c.1516A>C is a Chinese-specific founder mutation causing nemaline myopathy 8: Report of six patients with pre- and postnatal phenotypes. Mol. Genet. Genom. Med. 2020, 8, e1229. [Google Scholar] [CrossRef] [PubMed]
- Amburgey, K.; Acker, M.; Saeed, S.; Amin, R.; Beggs, A.H.; Bönnemann, C.G.; Brudno, M.; Constantinescu, A.; Dastgir, J.; Diallo, M.; et al. A Cross-Sectional Study of Nemaline Myopathy. Neurology 2021, 96, e1425–e1436. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.M.R.; Sewry, C.; Beeson, D.; Jayawant, S.; Squier, W.; McWilliam, R.; Palace, J. Congenital myopathies with secondary neuromuscular transmission defects; A case report and review of the literature. Neuromuscul. Disord. 2014, 24, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Vuillerot, C.; Rippert, P.; Kinet, V.; Renders, A.; Jain, M.; Waite, M.; Glanzman, A.M.; Girardot, F.; Hamroun, D.; Iwaz, J.; et al. Rasch Analysis of the Motor Function Measure in Patients With Congenital Muscle Dystrophy and Congenital Myopathy. Arch. Phys. Med. Rehabil. 2014, 95, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.; Tobler, P.; Stalder, A.; Schaedelin, S.; Deligianni, X.; Reinhardt, J.; Gloor, M.; Fischmann, D.F.A. Intra-rater and Inter-rater Reliability of Quantitative Thigh Muscle Magnetic Resonance Imaging. Imaging Med. 2019, 11, 3–12. Available online: https://api.semanticscholar.org/CorpusID:190867371 (accessed on 27 January 2023).
- Grimm, A.; Meyer, H.; Nickel, M.D.; Nittka, M.; Raithel, E.; Chaudry, O.; Friedberger, A.; Uder, M.; Kemmler, W.; Engelke, K.; et al. Repeatability of Dixon magnetic resonance imaging and magnetic resonance spectroscopy for quantitative muscle fat assessments in the thigh. J. Cachex-Sarcopenia Muscle 2018, 9, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Burakiewicz, J.; Sinclair, C.D.J.; Fischer, D.; Walter, G.A.; Kan, H.E.; Hollingsworth, K.G. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J. Neurol. 2017, 264, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Carlier, R.-Y.; Quijano-Roy, S. Myoimaging in Congenital Myopathies. Semin. Pediatr. Neurol. 2019, 29, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.J.; Yau, K.S.; Ong, R.; Slee, J.; McGillivray, G.; Barnett, C.P.; Haliloglu, G.; Talim, B.; Akcoren, Z.; Kariminejad, A.; et al. Next generation sequencing in a large cohort of patients presenting with neuromuscular disease before or at birth. Orphanet J. Rare Dis. 2015, 10, 148. [Google Scholar] [CrossRef]
- Chen, T.H.; Tian, X.; Kuo, P.L.; Pan, H.P.; Wong, L.C.; Jong, Y.J. Identification of KLHL40 mutations by targeted next-generation sequencing facilitated a prenatal diagnosis in a family with three consecutive affected fetuses with fetal akinesia deformation sequence. Prenat. Diagn. 2016, 36, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, M.A.; Pastore, M.; Schrader, R.; Sites, E.; Bartholomew, D.; Tsao, C.Y.; Flanigan, K.M. Diagnostic Utility of Whole Exome Sequencing in the Neuromuscular Clinic. Neuropediatrics 2019, 50, 96–102. [Google Scholar] [CrossRef]
- Avasthi, K.K.; Agarwal, S.; Panigrahi, I. KLHL40 Mutation Associated with Severe Nemaline Myopathy, Fetal Akinesia, and Cleft Palate. J. Pediatr. Neurosci. 2019, 14, 222–224. [Google Scholar] [CrossRef]
- Wu, Q.C.; Sun, L.; Xu, Y.S.; Yang, X.M.; Zheng, L.K. Diagnosis of fetal nemaline myopathy by whole-exome sequencing: Case report and review of literature. Clin. Exp. Obstet. Gynecol. 2020, 47, 915–919. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, Y.; Qin, Z.; Yi, S.; Zheng, H.; Luo, J.; Li, Q.; Wang, J.; Yang, Q.; Li, M.; et al. A novel and recurrent KLHL40 pathogenic variants in a Chinese family of multiple affected neonates with nemaline myopathy 8. Mol. Genet. Genomic Med. 2021, 9, e1683. [Google Scholar] [CrossRef]
- Lai , T.H.T.; Au, L.K.S.; Lau, Y.T.E.; Luo, J.; Lo, H.M.; Chan, K.Y.K.; Cheung, K.Y.K.; Ma, T.W.; Leung, W.C.; Kong, C.W.; et al. Application of Prenatal Whole Exome Sequencing for Structural Congenital Anomalies-Experience from a Local Prenatal Diagnostic Laboratory. Healthcare 2022, 10, 2521. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, H.; Ma, C.; Fu, F.; Cheng, K.; Wang, Y.; Li, R.; Lei, T.; Yu, Q.; Wang, D.; et al. Whole exome sequencing improves genetic diagnosis of fetal clubfoot. Hum Genet. 2023, 142, 407–418. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Q.; Zeng, X.; He, P.; Xu, W.; Guo, H.; Liu, Y.; Lin, Y. Clinical and molecular analysis of four unrelated Chinese families with pathogenic KLHL40 variants causing nemaline myopathy 8. Orphanet J. Rare Dis. 2022, 17, 149. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, J.; Wang, X.; Yang, Y.; Yu, L.; Zeng, S.; Zhang, M.; Xu, G. Case Report: Prenatal Diagnosis of Nemaline Myopathy. Front. Pediatr. 2022, 10, 937668. [Google Scholar] [CrossRef] [PubMed]
- Gurgel-Giannetti, J.; Souza, L.S.; Yamamoto, G.L.; Belisario, M.; Lazar, M.; Campos, W.; Pavanello, R.D.; Zatz, M.; Reed, U.; Zanoteli, E.; et al. Nemaline Myopathy in Brazilian Patients: Molecular and Clinical Characterization. Int. J. Mol. Sci. 2022, 23, 11995. [Google Scholar] [CrossRef] [PubMed]

| 7 Years | 11 Years | 12 Years | 13 Years | |
|---|---|---|---|---|
| MFM total | 77.08% | 77.08% | 78.13% | 78.12% |
| D1 (standing position and transfers) | 53.84% | 51.28% | 53.80% | 51.28% |
| D2 (axial and proximal motor function) | 94.44% | 94.22% | 91.67% | 94.44% |
| D3 (distal motor function) | 90.74% | 95.23% | 100% | 100% |
| Spirometry | ||||
| FVC %/mL | 93%/1710 | 83%/2030 | 85%/2340 | |
| PEF %/L/s | 95/3460 | 80/3760 | 90/4540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchignani, B.; Marinella, G.; Pasquariello, R.; Sgherri, G.; Frosini, S.; Santorelli, F.M.; Orsini, A.; Battini, R.; Astrea, G. KLHL40-Related Myopathy: A Systematic Review and Insight into a Follow-up Biomarker via a New Case Report. Genes 2024, 15, 208. https://doi.org/10.3390/genes15020208
Buchignani B, Marinella G, Pasquariello R, Sgherri G, Frosini S, Santorelli FM, Orsini A, Battini R, Astrea G. KLHL40-Related Myopathy: A Systematic Review and Insight into a Follow-up Biomarker via a New Case Report. Genes. 2024; 15(2):208. https://doi.org/10.3390/genes15020208
Chicago/Turabian StyleBuchignani, Bianca, Gemma Marinella, Rosa Pasquariello, Giada Sgherri, Silvia Frosini, Filippo Maria Santorelli, Alessandro Orsini, Roberta Battini, and Guja Astrea. 2024. "KLHL40-Related Myopathy: A Systematic Review and Insight into a Follow-up Biomarker via a New Case Report" Genes 15, no. 2: 208. https://doi.org/10.3390/genes15020208
APA StyleBuchignani, B., Marinella, G., Pasquariello, R., Sgherri, G., Frosini, S., Santorelli, F. M., Orsini, A., Battini, R., & Astrea, G. (2024). KLHL40-Related Myopathy: A Systematic Review and Insight into a Follow-up Biomarker via a New Case Report. Genes, 15(2), 208. https://doi.org/10.3390/genes15020208







