Abstract
Background: Avocado is an important economic fruit tree that requires a lot of nitrogen (N) to support growth and development. Nitrate transporter (NRT) gene family plays an essential role in N uptake and use in plants. However, no systematic identification of the NRT gene family has been reported in avocado. Methods: Bioinformatic analysis was used to identify and characterize the NRT gene family in avocado. The five N additions (29.75, 59.50, 119.00, 178.50, and 238.00 mg/L N) were used to identify the N requirement of avocado seedlings based on physiological indexes, while RNA-seq was conducted to analyze the response of PaNRTs under low-N and high-N conditions. Results: Sixty-one members of the NRT gene family were identified and dispersed on 12 chromosomes in avocado. Many cis-regulatory elements (CREs) related to phytohormonal and stress response were found in the PaNRTs promoter regions. The avocado leaves in N3 have the highest activities of N-assimilating enzymes and N content as well as the lowest activities of antioxidant enzymes. Thus, 29.75 mg/L and 119.00 mg/L were chosen as low-N supply and normal-N supply for transcriptome analysis. The transcriptome analysis showed that PaNRT1.11, PaNRT1.22, PaNRT1.32, PaNRT1.33, PaNRT1.38, and PaNRT1.52 and PaNRT1.56 among PaNRT1 members were up-regulated under normal-N condition in the leaves or roots, suggesting that these genes might affect N absorption under nitrate-sufficient conditions in avocado. RT-qPCR analysis found the relative expression patterns of selected genes among four samples were consistent with transcriptome data, suggesting that transcriptome data were reliable. Conclusions: This study would provide valuable information for identifying the functions of the NRT gene family in avocado.
1. Introduction
Nitrogen (N) is an essential mineral element involved in the plant growth and development process [1]. Insufficient N supply could inhibit photosynthesis as well as the synthesis of primary and secondary metabolites, consequently limiting plant growth and development [2]. Owing to low N availability in soil, N fertilizers have been widely applied to meet the N requirement of plant growth and development over the past half-century [3]. However, excessive use of N fertilizers, combined with N uptake and utilization by plants, leads to environmental pollution and increases production costs [4]. Therefore, it is essential to comprehensively understand the biological and molecular mechanism of N uptake and distribution to improve the efficiency of N uptake and use in plants.
Nitrate is the primary form of N taken by the higher plants [5], while nitrate reductase (NR), nitrite reductase (NiR), glutamine synthase (GS), glutamate synthase (GOGAT) play essential roles in N assimilation [6]. Superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT). Inappropriate N supply could induce oxidative stress to inhibit plant growth, while the antioxidant enzymes can protect plant tissue by inhibiting oxidative stress [7]. In plants, high-affinity (HATS) and low-affinity nitrate-transport systems (LATS) were developed to cope with low- or high-nitrate environments [8]. Nitrate transporters (NRTs), including NRT1, NRT2, and NRT3 families, have been proven to regulate N uptake and transport in plants [9,10]. The NRT1 family, belonging to the peptide transporters (PTR) family of the Major Facilitator Super (MFS) family, is a low-affinity transporter [11]. 91 NRT1 members were found in rice, while Arabidopis thaliana consists of 53 NRT1 members, of which AtNRT1.1-1.12 has been proven to be involved in nitrate transport [12]. As a high-affinity transporter, the NRT2 family belongs to the nitrate-nitrite-porter (NNP) family of the MFS family [13]. MeNRT2.2 among six NRT2 genes of Manihot esculenta was found to adapt to low N, while AcNRT2.1 and AcNRT2.2 among three NRT2 genes of pineapple responded to nitrate starvation [9]. The NRT3 family serves HATS by interacting with the NRT2 family [11,14]. The interaction between AtNRT3.1 and almost all members of the AtNRT2 gene family in Arabidopsis has been observed, with the exception of AtNRT2.7 [15]. Due to key NRT genes involved in N transport varying among various plants, clarifying the information on NRT genes is essential to improve N uptake and use in plants.
Avocado (Persea americana Mill.), a woody fruit tree of the Lauraceae family, is widely distributed in the tropical and subtropical regions of the world [16]. Owing to the high nutritional value and economic benefit of fruit, the worldwide fruit production and cultivation of avocado in 2022 exceeded 8.9 million tons and 884 thousand hectares, respectively [17]. Due to a lot of N being taken away with harvesting avocado fruit, avocado orchards require a large amount of N fertilizer to make up for the lost N each year [18]. Previous studies showed that long-term application of large amounts of N fertilizer resulted in loss of productivity, nitrogen loss, and groundwater pollution in avocado orchards [16,18,19]. Thus, clarifying the molecular mechanisms of N uptake and use is essential for achieving higher growth by reducing N supply in avocado. Although the chromosomal-level genome of avocado was published [20], the information on PaNRT genes has not yet been reported.
Thus, this study aimed to identify PaNRT genes, explore the chromosomal location, genetic structure, and phylogenetic relationship of the PaNRT gene family, and to investigate the expression profiles of PaNRT genes under low and normal N supply. These findings would facilitate clarification of the molecular regulatory network of N uptake and transportation in avocado.
2. Materials and Methods
2.1. Plant Materials
The experiment was conducted in the growth chamber under 25 °C/20 °C day/night cycle with a 14 h photoperiod. Seeds of avocado (cv. ‘Hass’) were germinated on non-woven with medium (coconut husk: peat soil: yellow soil = 4:4:2). Four months later, 54 uniform and healthy seedlings (seedling height approximately 35 cm and ground diameter approximately 4 mm) were selected and transferred to polypropylene containers (diameter and height are 10.6 cm and 9.5 cm, respectively) with coconut husk. 5 N supply concentrations were setup, including N1 (29.75 mg/L N), N2 (59.50 mg/L N), N3 (119.00 mg/L N), N4 (178.50 mg/L N), and N5 (238.00 mg/L N) (Table 1). N was supplied with the form of Ca(NO3)2·4H2O, KNO3 and NH4SO4. The concentrations of other elements were maintained at consistent levels in both the HN and LN treatments. Each treatment had three replicates and 3 seedlings for each replicate. 100 mL nutrient solutions (pH = 6.0, adjusted using NaOH and H2SO4) were added to the seedlings of the corresponding treatment. After 24 h, the leaves (on the third expanded leaf from the top) and roots for each treatment were collected and stored in liquid nitrogen. The leaves of 5 treatments were used to measure the activities of N-assimilating enzymes and antioxidant enzymes. Then, based on the activities of N-assimilating enzymes and antioxidant enzymes, the leaves and roots of low-N (LN) and normal-N treatments were chosen for transcriptome analysis.

Table 1.
Formulation of nutrient solutions with different nitrogen concentrations.
2.2. Identification and Characterization of NRT Genes in Avocado
The sequence file of Arabidopsis NRT was retrieved from the TAIR database (https://www.arabidopsis.org/, accessed on 14 June 2024). The genome data of Avocado (GCA_029852735.1) was downloaded from the custom database. The protein sequence of Arabidopsis was blasted against the proteinic sequence of the avocado whole genome for obtaining potential PaNRT proteins by the BLASTP algorithm with an E-value < 0.01. To obtain the PaNRT domains, the potential PaNRT sequences were submitted to the Pfam (http://pfam.sanger.ac.uk/search, accessed on 14 June 2024) and SMART (http://smart.embl-heidelberg.de/, accessed on 14 June 2024). The reliable PaNRT proteins were retained by removing the sequences without the peptide transporter 2 (PTR2), Major Facilitator Super (MFS1), or nitrate assimilation related 2 (NAR2) domain. The ExPASy program (https://web.expasy.org/protparam/, accessed on 14 June 2024) was used to calculate the number of amino acids, the isoelectric point (pI), molecular weight (MW), instability index (II), and grand average of hydropathicity (GRAVY).
2.3. Chromosomal Localization and Phylogenetic Analysis of PaNRT Proteins
The chromosome localization data of PaNRT genes were obtained from the avocado genome database, and then the map of the chromosomal position of PaNRT genes and their relative distances was performed using the TBtools software (v2.0906) [21]. For the phylogenetic analysis, the full-length NRT amino acid sequences of Arabidopsis and avocado were aligned by Clustal W. MEGA 6 was employed to construct the phylogenetic tree using the neighbor-joining method with p-distance, pairwise deletion, and 1000 bootstrap replicates. Finally, the phylogenetic tree was visualized using Evolview (http://www.evolgenius.info/evolview/, accessed on 14 June 2024) [22].
2.4. Gene Structure, Conserved Motif, and Cis-Regulatory Element (CRE) Analysis of PaNRT Proteins
The gene structure of PaNRTs was analyzed using the web-based gene structure display server (http://gsds.cbi.pku.edu.cn/, accessed on 14 June 2024). MEME (ver. 5.1.1, http://meme-suite.org, accessed on 14 June 2024) was employed to analyze the conversed motifs of PaNRT proteins (the maximum motifs number of 10 and the motifs width of 6–50 aa). The 2000 bp upstream sequence of each gene was extracted and then loaded to the PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 15 June 2024) to predict CRE. The TBtools software (v2.0906) was used to visualize this data information.
2.5. Measurement of the Physiological Indexes
The activities of N-assimilating enzymes (NR, NiR, GS, and GOGAT) and N content were measured following the method of Qin et al. [1]. The activities of antioxidant enzymes (Superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT)) were performed using a kit (comin, Suzhou, China), and all enzyme activities were expressed as units per kilogram soluble protein [23].
2.6. RNA Sequencing and Gene Expression Analysis
Total RNA was extracted using an RNA Extraction Kit (Tiangen, Beijing, China), while the integrity, purity, and concentration of total RNA were checked using an agarose gel and NanoDrop® ND-1000 portable UV-Vis Spectrophotometer (Thermo Scientific, Waltham, MA, USA). The Sequencing libraries were generated using NEBNext®Ultra™ RNA Library Prep Kit for Illumina® (NEB, San Diego, CA, USA) following the manufacturer’s recommendations. Then, 12 cDNA libraries (including leaves and roots of N1 and N3) were sequenced using Illumina Hiseq 2000 platform and generating reads with a length of 2 × 100 bp. Raw data of fastq format were performed using in-house perl scripts. Raw sequences were transformed into clean reads after data processing. These clean reads were then mapped to the reference genome sequence of Avocado (GCA_029852735.1). The unigenes function was annotated based on Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) databases. RSEM was used to estimate gene expression levels [24]. Differentially expressed genes (DEG) were assigned using false discovery rate (FDR) < 0.01 and fold change (FC) ≥ 2 as thresholds. The enrichment of DEGs was analyzed based on GO (p value Cut off = 0.05, p Adjust Method = “BH”) and KEGG (p value Cut off = 0.05, p Adjust Method = “BH”, q value Cut off = 0.2) pathway analysis using the clusterProfiler R package with default parameters [25]. Based on the transcriptome data, TBtools software was employed to generate a heatmap of expression profiles of PaNRT genes.
RNA was reverse transcribed cDNA using a HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China). The real-time quantitative polymerase chain reaction (RT-qPCR) analysis was performed using Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) on the BioRad CFX96Real-Time PCR platform (BioRad, Hercules, CA, USA). The reaction system was 20 μL, including 10 μL Taq Pro Universal SYBR qPCR Master Mix, 2 μL cDNA, 0.4 μL each prime, and 7.2 μL ddH2O. The RT-qPCR program was as follow: 95 °C for 30 s, followed by 39 cycles 95 °C for 5 s, and 60 °C for 30 s; 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s. Three biological replicates were conducted, while 2−∆∆CT method was used to calculate the relative expression levels with PaActin used as the internal control [26]. The primer for RT-qPCR is shown in Supplementary Table S1.
2.7. Statistical Analysis
One-way analysis of variance was performed to analyze the significance of difference (Duncan’s test, p < 0.05) using SPSS 19.0 statistical software (SPSS Inc, Chicago, IL, USA).
3. Results
3.1. Characterization of the PaNRT Gene Family
61 NRT genes were identified in the avocado genome using the Arabidopsis NRTs protein sequence as queries, including 57 PaNRT1, 3 PaNRT2, and 1 PaNRT3 (Table 2). These NRT genes encoded from 389 (PaNRT3) to 1130 (PaNRT1.19) amino acids, while MW varied from 42.20 kDa (PaNRT2.2) to 125.09 kDa (PaNRT1.19). The pI of PaNRT proteins varied from 5.33 (PaNRT1.29) to 9.87 (PaNRT2.2), with 50 PaNRTs classified as alkaline proteins (pI > 7). The II of PaNRT proteins varied from 22.86 (PaNRT1.53) to 53.51 (PaNRT1.12), while 21 PaNRTs exhibited instability with II exceeding 40. The PaNRT proteins belong to hydrophobic proteins except PaNRT3 (GRAVY < 0).

Table 2.
Characterization of PaNRT family genes in avocado.
In addition, the NRT genes (PaNRT1.1-PaNRT1.57/PaNRT2.1-PaNRT2.3/PaNRT3) were named based on the locations in the chromosomes (Figure 1). These NRT genes were mapped on 11 avocado chromosomes including Chr1 (9 PaNRTs), Chr2 (12 PaNRTs), Chr3 (8 PaNRTs), Chr4 (1 PaNRTs), Chr5 (8 PaNRTs), Chr6 (2 PaNRTs), Chr7 (5 PaNRTs), Chr8 (6 PaNRTs), Chr10 (3 PaNRTs), Chr11 (4 PaNRTs), and Chr12 (3 PaNRTs) (Table 2).

Figure 1.
Distribution for the PaNRT genes on twelve chromosomes. The chromosome number is represented at each bar top. Mb, megabase.
3.2. Phylogenetic Relationship and Conserved Motifs of PaNRTs
A Neighbor-Joining phylogenetic tree was performed using the full-length amino acid sequences of NRTs from avocado and Arabidopsis, which reflected the evolutionary history and functional association of avocado NRT proteins (Figure 2). The 61 PaNRTs were clustered into three groups. 57 PaNRT1 were clustered in Group Ⅰ, while 3 PaNRT2 and 1 PaNRT3 family were classified into Group Ⅱ and Group Ⅲ respectively.
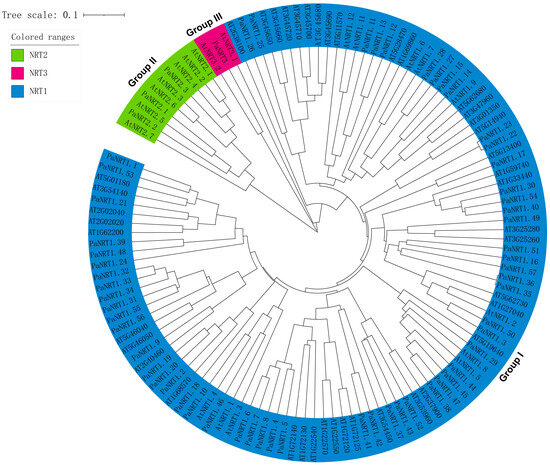
Figure 2.
Phylogenetic analysis of NRT protein involved 62 Arabidopsis NRT protein and 61 avocado NRT protein sequences. The tree is further clustered into 3 subfamilies, which are shown in different colors.
In addition, the relationships of the PaNRTs were exhibited deeply using phylogenetic analysis (Figure 3a). All PaNRT1 members contain motif 1, motif 2, motif 4, motif 7, and motif 16, indicating that these might be the most conserved motifs in the NRT1 family. However, the members of PaNRT2 and PaNRT3 only contained motif 18. Subsequently, the gene structure of the PaNRT family was constructed to investigate the differences in structure (Figure 3b). The PaNRT family gene sequences exhibited significant variation in length, ranging from the longest DNA sequence (PaNRT1.44) at nearly 70 kb to the shortest sequence (PaNRT1.9) at less than 10 kb. The number distribution of exon-intron structure of the PaNRT family showed an obvious difference, with the number of intron and exon varied 1–9 and 2–10, respectively.
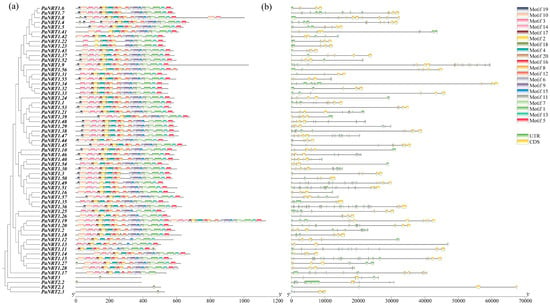
Figure 3.
Phylogenetic relationships, motif distribution, and gene structure of PaNRT genes. (a) The phylogenetic relationships of 61 PaNRT proteins using the neighbor-joining method and the conserved domain architecture of the PaNRT proteins. (b) Gene structure analysis of the PaNRT gene family. The bars of yellow and blue represent UTR and CDS, respectively.
3.3. Characterization of CREs in the Promoter Regions of PaNRTs
As shown in Figure 4a and Table S2, the characterization of CREs in the promoter regions of PaNRTs was analyzed. In the process of plant growth and development, phytohormones response, and stress response, some vital CREs were found in the promoter regions of PaNRTs, including auxin-responsive element, light responsiveness, salicylic acid responsiveness, abscisic acid responsiveness, MeJA-responsiveness, drought-inducibility, gibberellin-responsiveness, low-temperature responsiveness, and wound-responsive element. 54 PaNRT genes contained light responsiveness, including ACE and G-Box. Abscisic acid responsiveness (ABRE) was found in the promoter regions of 51 PaNRT genes, while MeJA-responsiveness (CGTCA and TGACG motif) was distributed in 41 PaNRT genes. Gibberellin-responsiveness (P-box, TATC-box, and GARE-motif), drought-inducibility (MBS), and salicylic acid responsiveness were identified in 39, 36, and 35 PaNRT genes, respectively. In addition, approximately 29.6%, 24.6%, and 9.8% of PaNRT genes contained low-temperature responsiveness (LTR), auxin-responsive element (TGA-element), and wound-responsive element (WUN motif), respectively (Figure 4b).
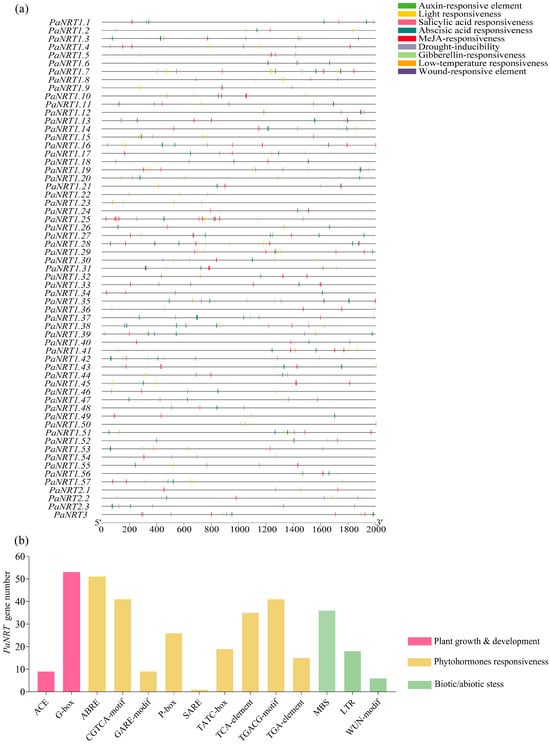
Figure 4.
Characterization of cis-elements in the promoter regions of PaNRT genes. (a) Distribution of cis-elements in different colored rectangles. The y-axis indicates the upstream length to the translation start site. (b) The number of PaNRT genes harboring different cis-elements.
3.4. Physiological Indexes in Avocado Leaves Under Different N Supply
As shown in Table 3, N supply significantly affected the activities of N-assimilating enzymes (NR, NiR, GS, and GOGAT) in the leaves of avocado (p < 0.05). As the N supply increased, the activities of NR, NiR, GS, and GOGAT showed an unimodal pattern, peaking at N3. Compared to N3, the activities of NR, NIR, GS, and GOGAT in avocado leaves decreased by 66.29%, 71.91%, 61.73%, and 70.59% in N1 treatment, 32.41%, 46.90%, 28.72%, and 38.66% in N2 treatment, 43.08%, 53.06%, 33.22%, and 44.54% in N4 treatment, and 64.35%, 77.86%, 68.72%, and 78.15% in N5 treatment.

Table 3.
Effects of N supply on physiological indexes in avocado leaves.
In addition, significant differences were observed in the activities of antioxidant enzymes (SOD, POD, and CAT) among N supply (Table 3, p < 0.05). The activities of SOD, POD, and CAT were all in the order of N5 > N1 >N4 > N2 > N3. Compared to N3, the activities of antioxidant enzymes improved by 250.05–294.00% in N1 treatment, 106.72–137.00% in N2 treatment, 132.60–180.00% in N4 treatment, and 313.74–402.00% in N5 treatment. The highest N content of leaves was achieved in N3 (Table 3, p < 0.05). Compares to N3, the N content in N1, N2, N4, and N5 reduced by 39.73%, 24.80%, 32.62%, and 47.29%, respectively.
3.5. RNA-Seq Analysis in Avocado Under Different N Supply
Based on the activities of N-assimilating enzymes and antioxidant enzymes in avocado leaves, N1 and N2 had low N levels for the growth of avocado seedlings, while N3 had a normal N level. The leaves and roots of N1 and N3 were used as low-N supply and normal-N supply for transcriptome analysis. In total, 72.97 gigabytes (Gb) of clean data from 12 samples were obtained. The GC% of the clean data ranged from 44.73% to 46.53%, and the percentage of Q30 from all the samples ranged from 96.21% to 96.93%, indicating that the quality and accuracy of sequencing data were sufficient for further analysis.
The mapped ratio of clean reads in each sample to the assembled Transcript or Unigene library ranged from 93.22% to 96.16%. Then, only mapped reads were used in the subsequent analysis. Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values were used to represent the abundance of each unigene. Moreover, Pearson correlation analysis for estimated gene expression levels showed that a strong correlation (R2 > 0.84) existed between the replicated of each treatment (Figure S1a). On the contrary, a weaker correlation was observed between different samples. As illustrated in Figure S1b, the PCA plot indicated a little intragroup variation for QC samples.
5862 DEGs were identified between PaLL (leaf under low-N supply) and PaHL (leaf under normal-N supply), in which 2262 DEGs were up-regulated and 3600 DEGs were down-regulated (Figure 5a,b). The comparison between PaLR (root under low N supply) and PaHR (root under normal-N supply) resulted in 551 up-regulated and 583 down-regulated DEGs (Figure 5a,c). For DEGs of the PaLL vs. PaHL and PaLR vs. PaHR groups, the metabolic process, cellular process, single-organism process, membrane, cell, cell part, membrane part, catalytic activity, and blinding were both the most enriched categories in GO enrichment analysis (Figure S2). In KEGG enrichment analysis, DEGs of the PaLL vs. PaHL group were mainly involved in ribosome, plant hormone signal transduction, phenylpropanoid biosynthesis, and plant-pathogen interaction, while DEGs of the PaLR vs. PaHR group were responsible for plant hormone signal transduction, plant-pathogen interaction, phenylpropanoid biosynthesis, carbon metabolism, and protein processing in endoplasmic reticulum (Figure S3).
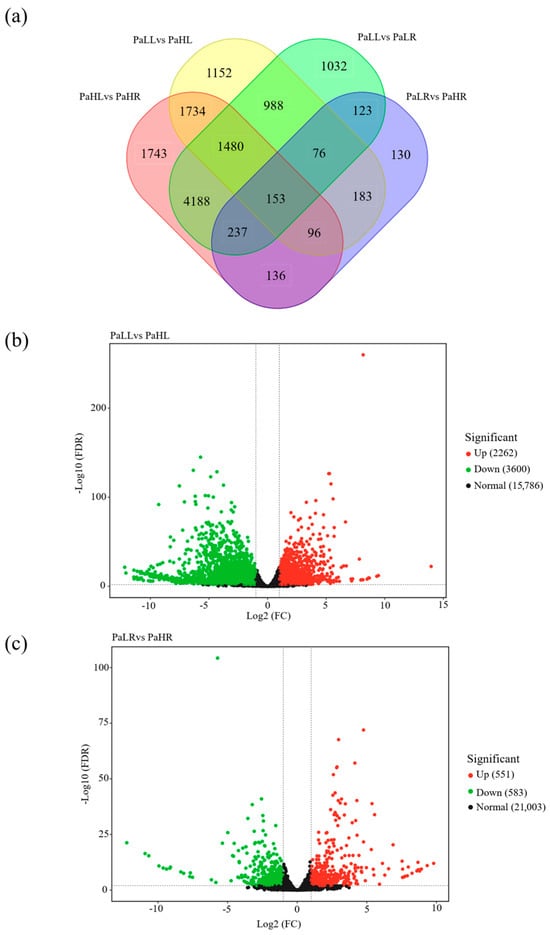
Figure 5.
Differentially expressed genes (DEGs) of leaf and root in avocado under different N supply conditions. (a) The display of DEGs in PaLL, PaLR, PaHL, and PaHR. (b) The up-regulated and downregulated DEGs in PaLL vs. PaHL group. (c) The up-regulated and downregulated DEGs in PaLR vs. PaHR group.
3.6. Expression Profiles of PaNRT Genes Under Different N Supply
Transcriptome data was used to analyze the expression profiles of PaNRT genes to explore their potential role and tissue-specific characteristics under normal-N and low-N supply. Figure 6a,b showed that no expression (FPKM < 1) was found in 11 PaNRTs genes of four samples. PaNRT1.6, PaNRT1.16, PaNRT1.19, PaNRT1.25, PaNRT1.26, PaNRT1.27, PaNRT1.35, PaNRT1.36, PaNRT1.43, PaNRT1.48, PaNRT1.51, PaNRT1.54, and PaNRT1.57 were mainly expressed in the root, while only one gene (PaNRT1.37) was mainly expressed in the leaf. Compared to low-N supply in the leaf, PaNRT1.11, PaNRT1.22, PaNRT1.32, PaNRT1.33, PaNRT1.38, PaNRT1.52, and PaNRT2.1 were highly expressed under normal-N supply, while PaNRT1.3, PaNRT1.4, PaNRT1.5, PaNRT1.10, PaNRT1.18, PaNRT1.31, PaNRT1.39, and PaNRT1.56 were lowly expressed. Compared to low-N supply, the expressions of PaNRT3 and PaNRT1.56 in the root were highly expressed, while the expression levels of PaNRT1.4, PaNRT1.16, PaNRT1.18, PaNRT1.24, and PaNRT1.48 were down-regulated. To verify the reliability of transcriptome data, eight PaNRT genes (PaNRT1.11, PaNRT1.16, PaNRT1.18, PaNRT1.28, PaNRT1.48, PaNRT1.56, PaNRT2.1, and PaNRT2.3) were selected for RT-qPCR analysis (Figure 6c). Pearson correlation analysis showed that the relative expression of eight PaNRT genes was positively associated with FPKM values (Figure 6).
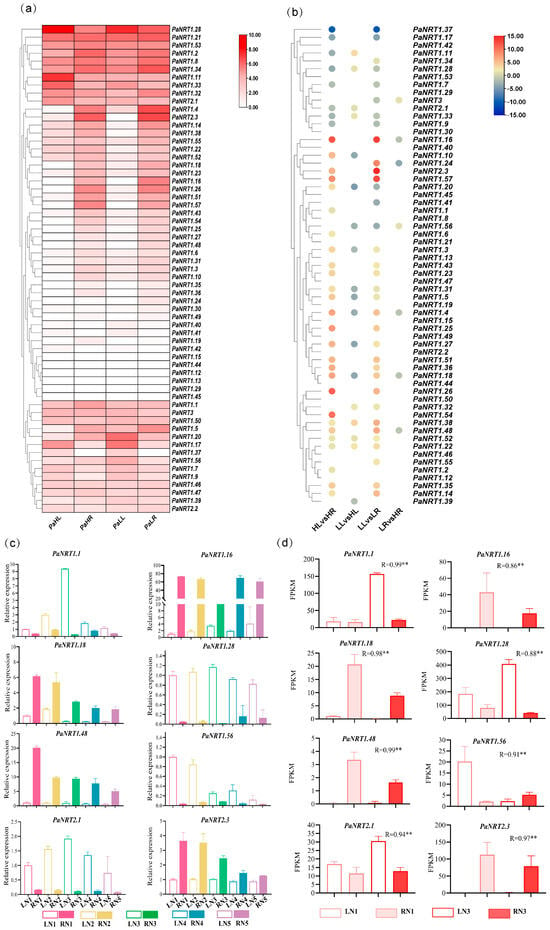
Figure 6.
The expression profiles of PaNRT genes in the leaf and root under different N conditions. (a) Heatmap illustrates the expression levels of PaNRT genes. (b) Fold change of PaNRT genes. (c) Expression data from RNA-Seq by RT-qPCR. (d) Validation of 8 PaNRT genes’ FPKM. Different letters indicate significant differences among different samples (p < 0.05). R value indicates Pearson correlation coefficients between RNA-seq and RT-qPCR data, ** indicates a significance at p < 0.05.
4. Discussion
Nitrate is one of the primary N forms absorbed by plants, while NRT genes are responsible for the uptake and transportation of nitrate in plants [27]. The critical roles of NRT genes have been identified in some woody plants, such as Eucalyptus grandis [21], apple [27], and poplar [28]. However, the identification and functional roles of the PaNRT gene family have not been characterized until now. In the present work, a total of 61 PaNRTs were identified at the genome level in avocado (Table 2). In addition, gene structure, phylogenetic relationship, and expression profiles of these PaNRT genes were characterized (Table 2). To the best of our knowledge, this study represents the first comprehensive report on the characterization of the NRT gene family in avocado. Compared to Arabidopsis, the number of PaNRT genes slightly decreased. However, the number of NRT family members varied greatly among species, such as 75 members in E. grandis [21], 39 members in radish [2], and 84 members in apple [27]. The number of NRT genes in avocado was less than in apple and E. grandis, suggesting the contraction of the NRT family during evolution. The species-specific adaptations may be the reason for this contraction [29], while avocado might not require as many NRT genes for N transport.
An uneven pattern was found in the distribution of PaNRT genes in 11 avocado chromosomes (Figure 1), supported by other reports on E. grandis [21] and apple [27]. This result suggested that the chromosomes with relatively more genes (like Chr 2) have undergone the duplication event or contributed more to N transport [27]. The structure (the number of intron and exon as well as the number and order of motifs) varied among NRT genes in avocados (Figure 3), consistent with the reports from radish [2] and E. grandis [21]. The structure difference may affect the transport efficiency of NRT genes [29]. In a word, understanding the distribution pattern of chromosomes and gene structure would contribute to identifying genomic regions and genes involved in the N transport of avocado.
CREs in the PaNRTs promoter were associated with phytohormonal response and various stresses (including light, drought, low temperature, and wound) (Figure 4). This finding was consistent with the results from radish [2] and E. grandis [21], indicating that PaNRT genes play an essential role in regulating the growth, development, and stress adaption of plants. Interestingly, the PaNRTs promoter found many CREs related to the phytohormonal response (Figure 4 and Table S2). Moreover, KEGG analysis confirmed that DEGs in avocado under different N conditions were associated with plant hormone signal transduction (Figure S2). Other plants also reported that NRT gene expression can be modulated by phytohormone signaling, while phytohormone biosynthesis and transportation (such as gibberellin, salicylic acid, and abscisic acid) can be regulated by NRT genes [30,31,32,33]. However, as shown in GO and KEGG analysis (Figures S2 and S3), the regulatory mechanisms of PaNRTs expression are complex and need more research work.
N-assimilating enzymes are essential for N uptake in plants, which is influenced by N additions [31]. The N content and activities of N-assimilating enzymes in N3 were significantly higher than those of other treatments (Table 3), suggesting that 119.00 mg/L was the optimal N supply for avocado seedling growth in this study. In addition, the antioxidant enzymes can mitigate damage of plant tissue in low-N and high-N stresses [8]. Our results showed that the activities of SOD, POD, and CAT were the lowest in N3 (Table 3), supported the above conclusion. Thus, N1 and N3 were chosen as low-N supply and normal-N supply of avocado seedlings growth for transcriptome analysis. Previous studies showed that NRT gene expressions differed concerning the tissue [28,34]. Based on transcriptome data, this study chose eight genes among DEGs of tissue-specific under different N conditions. The relative expression patterns of these genes among four samples were consistent with transcriptome data (Figure 6), suggesting that the expression profiles of transcriptome data were reliable. Here, the transcriptome and RT-qPCR analysis demonstrated that the PaNRT genes exhibited leaf- or root-specific expression patterns (Figure 6). In addition, the NRT family members play diverse roles in the absorption and allocation of nitrate [35]. The subfamily NRT1 belonging to LATS proteins was highly expressed in the normal nitrate environment, while NRT2 and NRT3 subfamilies belonging to LATS proteins were opposite [21]. In this study, PaNRT1.11, PaNRT1.22, PaNRT1.32, PaNRT1.33, PaNRT1.38, and PaNRT1.52, and PaNRT1.56 among PaNRT1 members were up-regulated under normal-N conditions in the leaves or roots, while the NRT2 and NRT3 genes did not show high expressions either in the leaves or in the roots at the low N supply (Figure 6a,b). These results suggested that PaNRT1.11, PaNRT1.22, PaNRT1.32, PaNRT1.33, PaNRT1.38, PaNRT1.52, and PaNRT1.56 might be involved in N absorption under nitrate-sufficient conditions in avocado (Figure 6c). Further studies are necessary to determine the specific role of these PaNRT genes in avocado N absorption.
5. Conclusions
In this study, 61 NRT genes were identified in avocado. Many cis-regulatory elements in the PaNRTs promoter were associated with phytohormonal response and various stresses. Expression pattern analysis showed that PaNRT1.11, PaNRT1.22, PaNRT1.32, PaNRT1.33, PaNRT1.38, and PaNRT1.52, and PaNRT1.56 were significantly up-regulated under normal-N conditions in both leaves or roots, indicating that these genes are crucial for N absorption in avocado. This study would provide valuable information for identifying the functions of NRT gene family in avocado. In addition, it contributes to the breeding of avocado varieties with high N uptake and utilization for reducing the use and loss of N fertilizer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes15121600/s1, Figure S1: Correlation analysis (a) and principal component analysis (PCA) (b) of the samples in transcriptome results; Figure S2: GO analysis of DEGs in PaLL vs. PaHL and PaLR vs. PaHR groups; Figure S3: KEGG-enrichment analysis of DEGs in PaLL vs. PaHL and PaLR vs. PaHR groups; Table S1: Primers used in this study; Table S2: The potential cis-elements in the promoter region of 61 PaNRT genes; Table S3: Statistics of the RNA-seq data; Table S4: The annotation rate of avocado genes in 8 different databases.
Author Contributions
Conceptualization, Y.T. and R.J.; methodology, Y.T.; software, Y.T.; validation, Y.T. and R.J.; formal analysis, R.J.; investigation, Y.T.; resources, J.Q.; data curation, Y.T.; writing—original draft preparation, Y.T.; writing—review and editing, J.Q. and R.J.; visualization, Y.T.; supervision, R.J.; project administration, J.Q.; funding acquisition, J.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Introduction of Scientific and Technological Talents of Guangdong Academy of Agricultural Sciences, grant number R2022YJ-YB3027.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data reported in this paper were deposited in the Genome Sequence Archive (Genomics, Proteomics, and Bioinformatics 2021) at the National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (Bioproject: PRJCA029897), and are publicly accessible at https://ngdc.cncb.ac.cn/gsa, accessed on 5 September 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qin, J.; Yue, X.L.; Shang, X.L.; Fang, S.Z. Response of nitrogen use efficiency, N-assimilating enzymes and growth in Cyclocarya paliurus seedlings to different nitrogen nutrition. J. Plant Nutr. 2023, 46, 3547–3556. [Google Scholar] [CrossRef]
- Ding, M.C.; He, M.; Zhang, W.L.; Han, Y.; Zhang, X.Y.; Zhang, X.L.; Zhu, Y.L.; Wang, Y.; Liu, L.W.; Xu, L. Genome-wide identification and expression analysis of RsNRT gene family reveals their potential roles in response to low-nitrogen condition in radish (Raphanus sativus L.). Sci. Hortic. 2023, 321, 112273. [Google Scholar] [CrossRef]
- Swarbreck, S.M.; Wang, M.; Wang, Y.; Kindred, D.; Sylvester-Bradley, R.; Shi, W.M.; Varinderpal-Singh; Bentley, A.R.; Griffiths, H. A roadmap for lowering crop nitrogen requirement. Trends Plant Sci. 2019, 24, 892–904. [Google Scholar] [CrossRef]
- Stevens, C.J. Nitrogen in the environment. Science 2019, 363, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Gaudinier, A.; Rodriguez-Medina, J.; Zhang, L.; Olson, A.; LiseronMonfils, C.; Bagman, A.M.; Foret, J.; Abbitt, S.; Tang, M.; Li, B.; et al. Transcriptional regulation of nitrogen associated metabolism and growth. Nature 2018, 563, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Gojon, A.; Krouk, G.; Perrine, W.F.; Laugier, E. Nitrate transceptor(s) in plants. J. Exp. Bot. 2011, 62, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.X.; Ling, F.L.; Jia, D.S.; Cui, J.J.; Zhang, Z.A.; Xu, C.; Yu, L.T.; Guan, C.L.; Wang, Y.; Zhang, M.R.; et al. Effects of low nitrogen on seedling growth, photosynthetic characteristics and antioxidant system of rice varieties with different nitrogen efficiencies. Sci. Rep. 2023, 13, 19780. [Google Scholar] [CrossRef] [PubMed]
- Li, W.M.; Yan, M.K.; Hu, B.Y.; Priyadarshani, S.V.G.N.; Hou, Z.M.; Ojolo, P.S.; Xiong, J.J.; Zhao, H.M.; Qin, Y. Characterization and the expression analysis of nitrate transporter (NRT) gene family in pineapple. Trop. Plant Biol. 2018, 11, 177–191. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, P.F.; Liu, P.; Song, Y.P.; Zhang, D.Q. Genetic effects and expression patterns of the nitrate transporter (NRT) gene family in Populus tomentosa. Front. Plant Sci. 2021, 12, 661635. [Google Scholar] [CrossRef] [PubMed]
- Bellegarde, F.; Gojon, A.; Martin, A. Signals and players in the transcriptional regulation of root responses by local and systemic N signaling in Arabidopsis thaliana. J. Exp. Bot. 2017, 68, 2553–2565. [Google Scholar] [PubMed]
- Wang, Y.; Cheng, Y.; Chen, K.; Tsay, Y.F. Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 2018, 69, 85–122. [Google Scholar] [PubMed]
- You, L.L.; Wang, Y.; Zhang, T.T.; Zhu, Y.F.; Ren, N.; Jiang, X.Y.; Zhou, Y. Genome-wide identification of nitrate transporter 2 (NRT2) gene family and functional analysis of MeNRT2.2 in cassava (Manihot esculenta Crantz). Gene 2022, 809, 146038. [Google Scholar] [CrossRef]
- Okamoto, M.; Kumar, A.; Li, W.; Wang, Y.; Siddiqi, M.Y.; Crawford, N.M.; Glass, A.D.M. High-affinity nitrate transport in roots of Arabidopsis depends on expression of the NAR2-like gene AtNRT3.1. Plant Physiol. 2006, 140, 1036–1046. [Google Scholar] [PubMed]
- Kotur, Z.; Mackenzie, N.; Ramesh, S.; Tyerman, S.D.; Kaiser, S.N.; Glass, A.D. Nitrate transport capacity of the Arabidopsis thaliana NRT2 family members and their interactions with AtNAR2.1. New Phytol. 2012, 194, 724–731. [Google Scholar] [PubMed]
- Gallart, M.; Paungfoo-Lonhienne, C.; Trueman, S.J. Effects of a growth-promoting Paraburkholderia species on nitrogen acquisition by avocado seedlings. Sci. Hortic. 2022, 295, 110767. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAOSTAT). Cultivos. 2024. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 5 June 2024).
- Selladurai, R.; Awachare, C.M. Nutrient management for avocado (Persea americana miller). J. Plant Nutr. 2020, 43, 138–147. [Google Scholar] [CrossRef]
- Lovatt, C.J. Properly timed soil applied nitrogen fertilizer increases yield and fruit size of ‘Hass’ avocado. J. Am. Soc. Hortic. Sci. 2001, 126, 555–559. [Google Scholar]
- Nath, O.; Fletcher, S.J.; Hayward, A.; Shaw, L.M.; Masouleh, A.K.; Furtado, A.; Henry, R.J.; Mitter, N. A haplotype resolved chromosomal level avocado genome allows analysis of novel avocado genes. Hortic. Res. 2022, 9, uhac157. [Google Scholar] [CrossRef]
- Li, G.Y.; Yang, D.M.; Hu, Y.; Xu, J.M.; Lu, Z.H. Genome-wide identification and expression analysis of nitrate transporter (NRT) gene family in Eucalyptus grandis. Genes 2024, 15, 930. [Google Scholar] [CrossRef] [PubMed]
- Landrein, B.; Formosa-Jordan, P.; Malivert, A.; Schuster, C.; Melnyk, W.C.; Yang, W.B.; Turnbull, C.; Meyerowitz, M.E.; Locke, C.W.J.; Jönsson, H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. USA 2018, 115, 201718670. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, X.; Tang, X.H.; Shao, X.H.; Lai, D.; Xiao, W.Q.; Zhuang, Q.L.; Wang, W.L.; Dong, T. Near-freezing temperature suppresses avocado (Persea americana Mill.) fruit softening and chilling injury by maintaining cell wall and reactive oxygen species metabolism during storage. Plant Physiol. Biochem. 2024, 210, 108621. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Jeffryes, M.; Bateman, A.; Finn, R.D. The HMMER web server for protein sequence similarity search. Curr. Protoc. Bioinform. 2017, 60, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Rangel, A.; Li, R.; Mauk, P.; Santiago, L.; Lovatt, C.J. Effects of temperature, soil moisture and light intensity on the temporal pattern of floral gene expression and flowering of avocado buds (Persea americana cv. Hass). Sci. Hortic. 2021, 280, 109940. [Google Scholar] [CrossRef]
- Tahir, M.M.; Wang, H.; Ahmad, B.; Liu, Y.; Fan, S.; Li, K.; Lei, C.; Shah, K.; Li, S.H.; Zhang, D. Identification and characterization of NRT gene family reveals their critical response to nitrate regulation during adventitious root formation and development in apple rootstock. Sci. Hortic. 2021, 275, 109642. [Google Scholar] [CrossRef]
- Bai, H.; Euring, D.; Volmer, K.; Janz, D.; Polle, A. The nitrate transporter (NRT) gene family in poplar. PLoS ONE 2013, 8, e72126. [Google Scholar] [CrossRef]
- Li, G.Y.; Yang, D.M.; Hu, Y.; Xu, J.M.; Li, J.; Lu, Z.H. Genome-wide identification, expression analysis, and transcriptome analysis of the NPF gene family under various nitrogen conditions in Eucalyptus grandis. Forests 2024, 15, 1697. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yue, X.L.; Fang, S.Z.; Qian, M.Y.; Zhou, S.T.; Shang, X.L.; Yang, W.X. Responses of nitrogen metabolism, photosynthetic parameter and growth to nitrogen fertilization in Cyclocarya paliurus. For. Ecol. Manag. 2021, 502, 119715. [Google Scholar] [CrossRef]
- Lezhneva, L.; Kiba, T.; Feriabourrelier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2015, 80, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, Y.N.; Yu, M.; Su, B.D.; Gong, W.; Baluška, F.; Komis, G.; Šamaj, J.; Shan, X.Y.; Li, J.X. Phosphorylation-mediated dynamics of nitrate transceptor NRT1.1 regulate auxin flux and nitrate signaling in lateral root growth. Plant Physiol. 2019, 181, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Migocka, M.; Warzybok, A.; Kłobus, G. The genomic organization and transcriptional pattern of genes encoding nitrate transporters 1 (NRT1) in cucumber. Plant Soil 2012, 364, 254–260. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).