Klrb1 Loss Promotes Chronic Hepatic Inflammation and Metabolic Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hematoxylin and Eosin Staining
2.3. Routine Blood Test
2.4. Total RNA Isolation, Bulk RNA-Seq, and RT-PCR
2.5. Data Statistics
3. Results
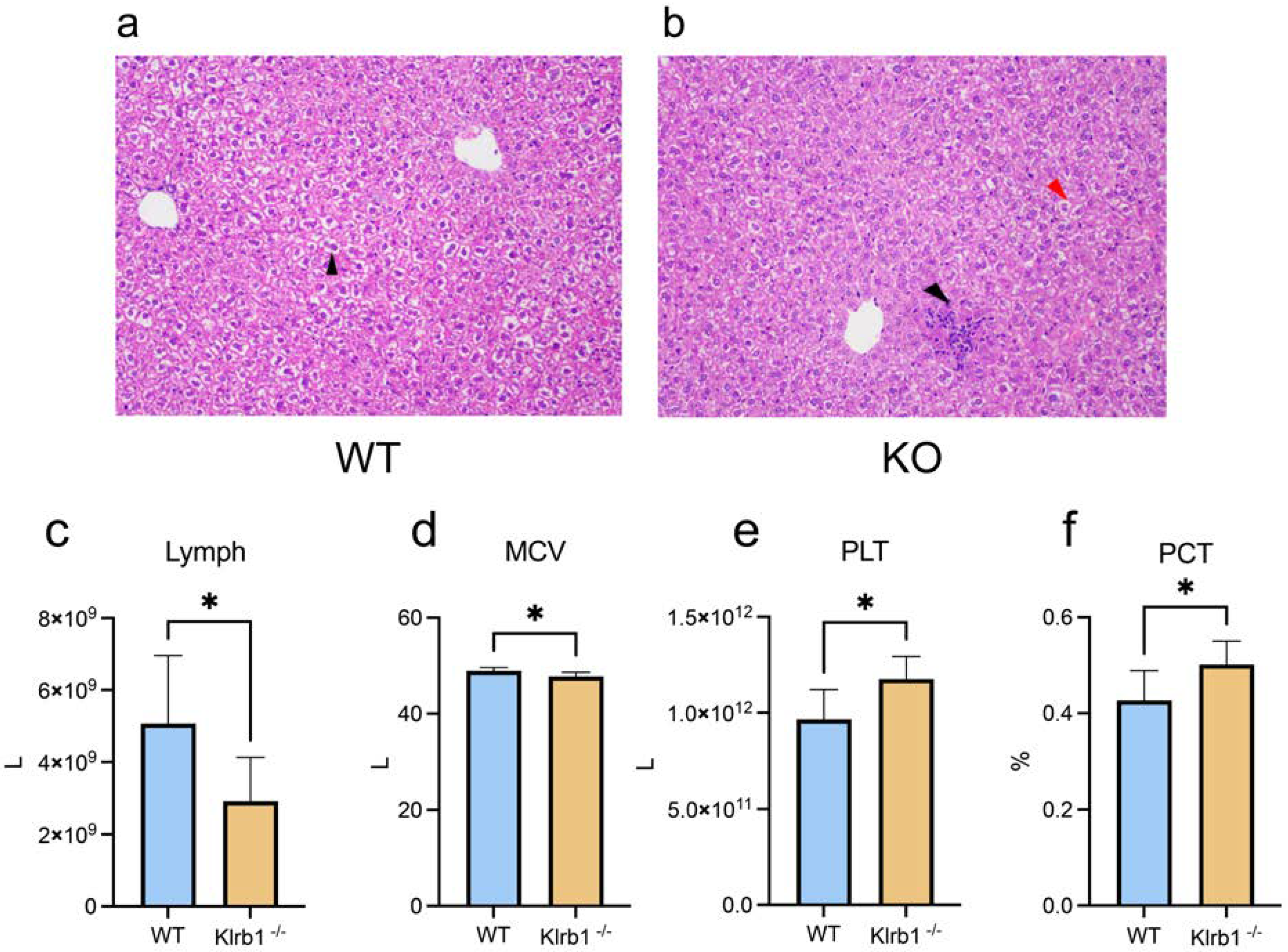
3.1. Inflammatory Response upon Klrb1 Knockout
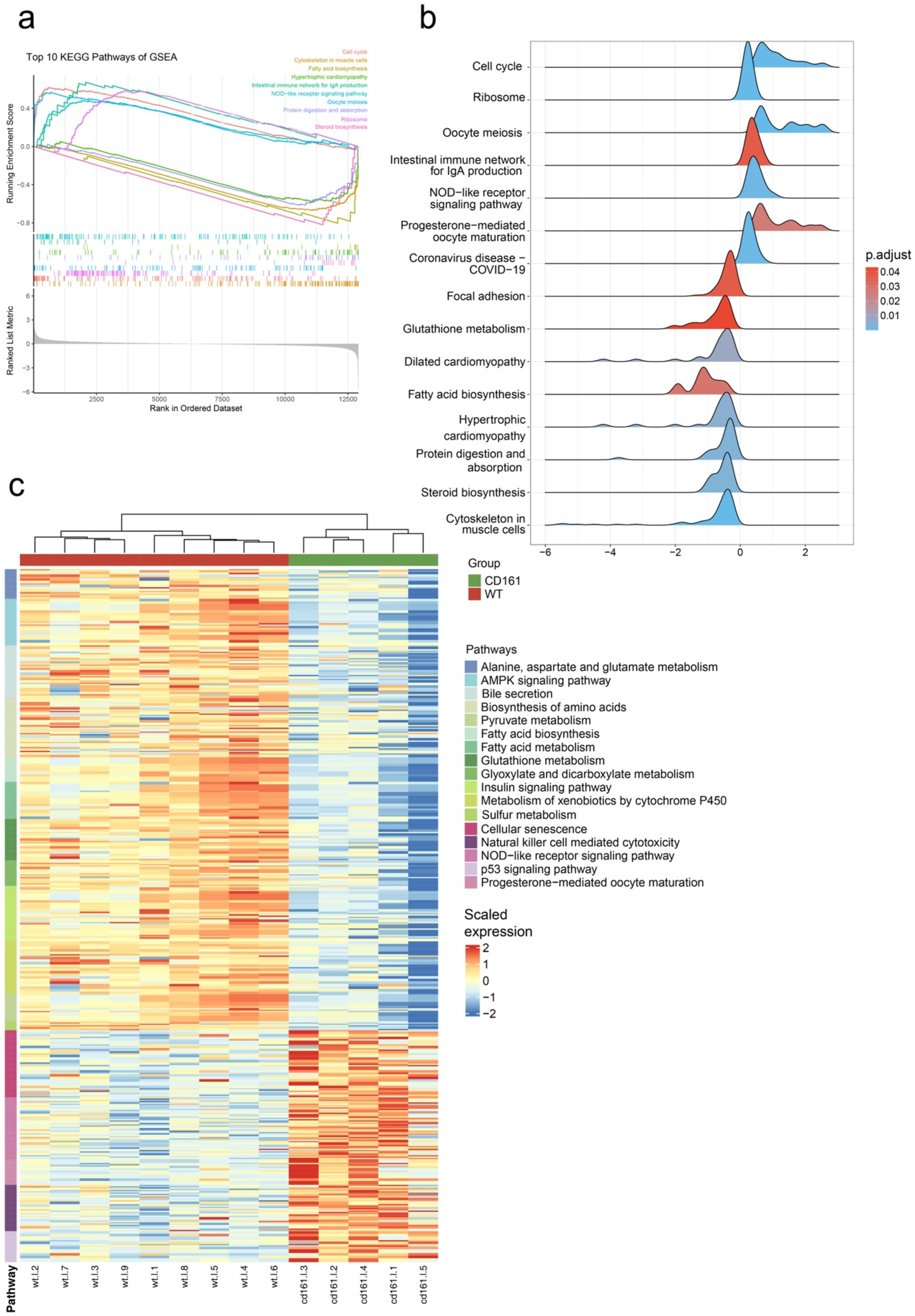
3.2. Transcriptome Analysis and Gene Expression
3.3. Gene Expression Changes Caused by the Deletion of Klrb1
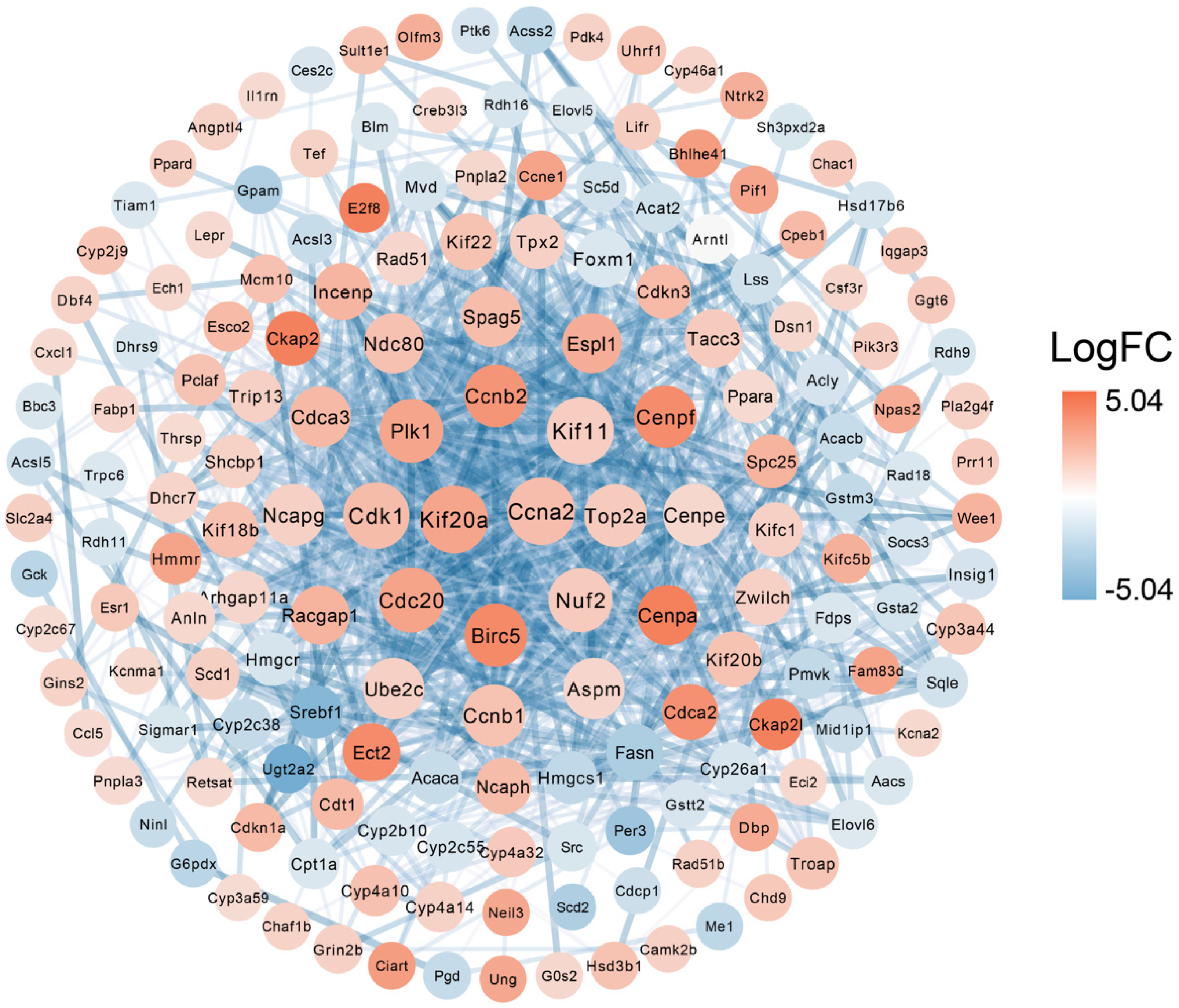
3.4. Protein–Protein Interaction of DEGs
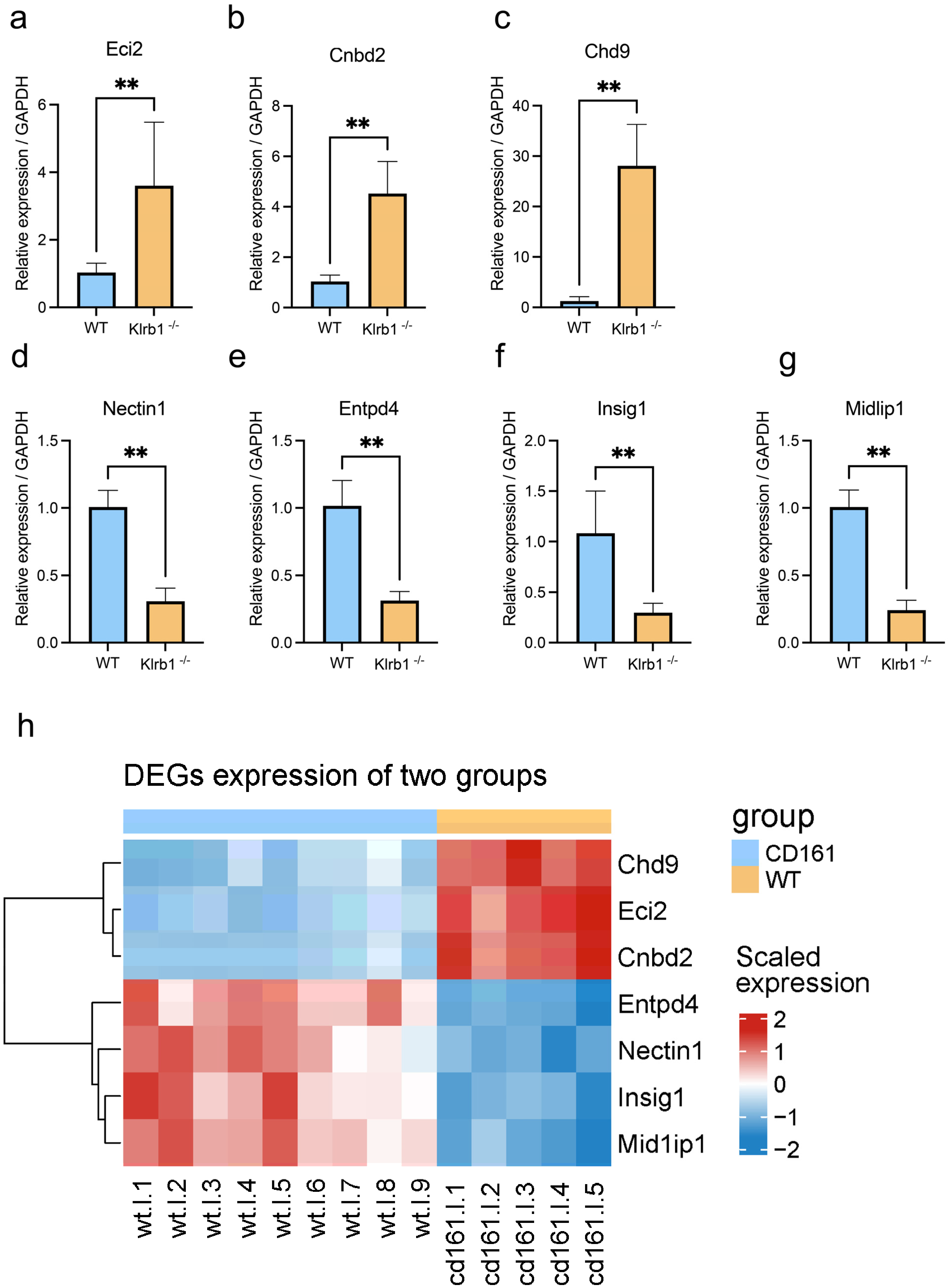
3.5. Validation of DEGs by qPCR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aldemir, H.; Prod’homme, V.; Dumaurier, M.-J.; Retiere, C.; Poupon, G.; Cazareth, J.; Bihl, F.; Braud, V.M. Cutting Edge: Lectin-Like Transcript 1 Is a Ligand for the CD161 Receptor. J. Immunol. 2005, 175, 7791–7795. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.B.; Bettadapura, J.; Alsharifi, M.; Mathew, P.A.; Warren, H.S.; Lanier, L.L. Cutting Edge: Lectin-Like Transcript-1 Is a Ligand for the Inhibitory Human NKR-P1A Receptor. J. Immunol. 2005, 175, 7796–7799. [Google Scholar] [CrossRef] [PubMed]
- Boles, K.S.; Barten, R.; Kumaresan, P.R.; Trowsdale, J.; Mathew, P.A. Cloning of a New Lectin-like Receptor Expressed on Human NK Cells. Immunogenetics 1999, 50, 1–7. [Google Scholar] [CrossRef]
- Braud, V.M.; Meghraoui-Kheddar, A.; Elaldi, R.; Petti, L.; Germain, C.; Anjuère, F. LLT1-CD161 Interaction in Cancer: Promises and Challenges. Front. Immunol. 2022, 13, 847576. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. T Cell Atlas Reveals Route to Glioma Immunotherapy. Nat. Rev. Drug Discov. 2021, 20, 261. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, Y.; Gao, B. Natural Killer Cells in Liver Disease. Hepatology 2013, 57, 1654–1662. [Google Scholar] [CrossRef]
- Liu, P.; Chen, L.; Zhang, H. Natural Killer Cells in Liver Disease and Hepatocellular Carcinoma and the NK Cell-Based Immunotherapy. J. Immunol. Res. 2018, 2018, 1206737. [Google Scholar] [CrossRef]
- Kirkham, C.L.; Carlyle, J.R. Complexity and Diversity of the NKR-P1: Clr (Klrb1: Clec2) Recognition Systems. Front. Immunol. 2014, 5, 214. [Google Scholar] [CrossRef]
- Jameson, G.; Robinson, M.W. Insights into Human Intrahepatic NK Cell Function From Single Cell RNA Sequencing Datasets. Front. Immunol. 2021, 12, 649311. [Google Scholar] [CrossRef]
- Li, L.; Hu, Y.; Li, X.; Ju, B. A Comprehensive Analysis of the KLRB1 Expression and Its Clinical Implication in Testicular Germ Cell Tumors: A Review. Medicine 2024, 103, E37688. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, H.; Jiang, Z.; He, T.; Xie, Y.; Mao, W.; Han, J.; Liu, S.; Lou, W.; Wu, W.; et al. Poor Clinical Outcomes and Immunoevasive Contexture in CD161+CD8+ T Cells Barren Human Pancreatic Cancer. J. Immunother. Cancer 2024, 12, e008694. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Kim, D.H.; Kim, G.; Cho, J.W.; Sung, E.; Baek, S.; Hong, M.H.; Kim, C.G.; Sim, N.S.; Hong, H.J.; et al. Single-Cell Analysis Reveals Cellular and Molecular Factors Counteracting HPV-Positive Oropharyngeal Cancer Immunotherapy Outcomes. J. Immunother. Cancer 2024, 12, e008667. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Li, Y.; Wu, B.; Shen, Q.; Chen, L.; Sun, W.; Wang, B.; Ying, L.; Wu, L.; Zhou, H.; et al. CD161+CD127+CD8+ T Cell Subsets Can Predict the Efficacy of Anti-PD-1 Immunotherapy in Non-Small Cell Lung Cancer with Diabetes Mellitus. OncoImmunology 2024, 13, 2371575. [Google Scholar] [CrossRef]
- Nasr Azadani, H.; Nassiri Toosi, M.; Shahmahmoodi, S.; Nejati, A.; Rahimi, H.; Farahmand, M.; Keshavarz, A.; Ghorbani Motlagh, F.; Samimi-Rad, K. New Insights into Potential Biomarkers and Their Roles in Biological Processes Associated with Hepatitis C-Related Liver Cirrhosis by Hepatic RNA-Seq-Based Transcriptome Profiling. Virus Res. 2024, 349, 199457. [Google Scholar] [CrossRef]
- Fang, S.; Zhou, Y. Deciphering the Role of KLRB1: A Novel Prognostic Indicator in Hepatocellular Carcinoma. BMC Gastroenterol. 2024, 24, 210. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, N.D.; Ashenberg, O.; Tirosh, I.; Gritsch, S.; Perez, E.M.; Marx, S.; Jerby-Arnon, L.; Chanoch-Myers, R.; Hara, T.; Richman, A.R.; et al. Inhibitory CD161 Receptor Identified in Glioma-Infiltrating T Cells by Single-Cell Analysis. Cell 2021, 184, 1281–1298.e26. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Zhang, N.; Yang, R.; Chen, K.; Kong, D. Whole Transcriptome Analysis of Chemically Induced Hepatocellular Carcinoma Using RNA-Sequencing Analysis. FEBS Open Bio 2019, 9, 1900–1908. [Google Scholar] [CrossRef]
- Zhu, M.; Hao, S.; Liu, T.; Yang, L.; Zheng, P.; Zhang, L.; Ji, G. Lingguizhugan Decoction Improves Non-Alcoholic Fatty Liver Disease by Altering Insulin Resistance and Lipid Metabolism Related Genes: A Whole Trancriptome Study by RNA-Seq. Oncotarget 2017, 8, 82621–82631. [Google Scholar] [CrossRef]
- Van Der Maaten, L.; Hinton, G. Visualizing Data Using T-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2625. [Google Scholar]
- Boettcher, S.; Manz, M.G. Regulation of Inflammation- and Infection-Driven Hematopoiesis. Trends Immunol. 2017, 38, 345–357. [Google Scholar] [CrossRef]
- Johnson, C.B.; Zhang, J.; Lucas, D. The Role of the Bone Marrow Microenvironment in the Response to Infection. Front. Immunol. 2020, 11, 585402. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E.; Loscalzo, J. Platelet-Monocyte Aggregates: Bridging Thrombosis and Inflammation. Circulation 2002, 105, 2130–2132. [Google Scholar] [CrossRef]
- Hansen, I.S.; Baeten, D.L.P.; den Dunnen, J. The Inflammatory Function of Human IgA. Cell. Mol. Life Sci. 2019, 76, 1041–1055. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Henao-mejia, J.; Flavell, R.A. Review Regulation of the Antimicrobial Response by NLR Proteins. Immunity 2011, 34, 665–679. [Google Scholar] [CrossRef]
- Linz, B.M.L.; Neely, C.J.; Kartchner, L.B.; Mendoza, A.E.; Khoury, A.L.; Truax, A.; Sempowski, G.; Eitas, T.; Brickey, J.; Ting, J.P.Y.; et al. Innate Immune Cell Recovery Is Positively Regulated by NLRP12 during Emergency Hematopoiesis. J. Immunol. 2017, 198, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Garn, H.; Bahn, S.; Baune, B.T.; Binder, E.B.; Bisgaard, H.; Chatila, T.A.; Chavakis, T.; Culmsee, C.; Dannlowski, U.; Gay, S.; et al. Current Concepts in Chronic Inflammatory Diseases: Interactions between Microbes, Cellular Metabolism, and Inflammation. J. Allergy Clin. Immunol. 2016, 138, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Fuentes, F.; Vilahur, G.; Badimon, L.; Palomo, I. Mechanisms of Chronic State of Inflammation as Mediators That Link Obese Adipose Tissue and Metabolic Syndrome. Mediat. Inflamm. 2013, 2013, 136584. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of Monocytes, Macrophages, and Dendritic Cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Goto, K.; Morimoto, M.; Osaki, M.; Tanio, A.; Izutsu, R.; Fujiwara, Y.; Okada, F. The Impact of AMIGO2 on Prognosis and Hepatic Metastasis in Gastric Cancer Patients. BMC Cancer 2022, 22, 280. [Google Scholar] [CrossRef]
- Bauernfeind, A.L.; Babbitt, C.C. The Predictive Nature of Transcript Expression Levels on Protein Expression in Adult Human Brain. BMC Genom. 2017, 18, 322. [Google Scholar] [CrossRef]
- Jiang, A.; Zhou, Y.; Gong, W.; Pan, X.; Gan, X.; Wu, Z.; Liu, B.; Qu, L.; Wang, L. CCNA2 as an Immunological Biomarker Encompassing Tumor Microenvironment and Therapeutic Response in Multiple Cancer Types. Oxidative Med. Cell. Longev. 2022, 2022, 5910575. [Google Scholar] [CrossRef] [PubMed]
- Stangel, D.; Erkan, M.; Buchholz, M.; Gress, T.; Michalski, C.; Raulefs, S.; Friess, H.; Kleeff, J. Kif20a Inhibition Reduces Migration and Invasion of Pancreatic Cancer Cells. J. Surg. Res. 2015, 197, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lu, J.; Yang, Z.; Li, E.; Cao, Y.; Xie, L. Mitotic Functions and Characters of KIF11 in Cancers. Biomolecules 2024, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, J.Z.; Du, J.L.; Huang, Z.X.; Wei, L.X. Increased CDC20 Expression Is Associated with Development and Progression of Hepatocellular Carcinoma. Int. J. Oncol. 2014, 45, 1547–1555. [Google Scholar] [CrossRef]
- Lv, S.; Xu, W.; Zhang, Y.; Zhang, J.; Dong, X. NUF2 as an Anticancer Therapeutic Target and Prognostic Factor in Breast Cancer. Int. J. Oncol. 2020, 57, 1358–1367. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Luo, T.; Liu, H.; Chen, L.; Wang, J.; Zhao, Y.; Li, X.; Li, H.; Li, M.; Lu, L. Klrb1 Loss Promotes Chronic Hepatic Inflammation and Metabolic Dysregulation. Genes 2024, 15, 1444. https://doi.org/10.3390/genes15111444
Yang S, Luo T, Liu H, Chen L, Wang J, Zhao Y, Li X, Li H, Li M, Lu L. Klrb1 Loss Promotes Chronic Hepatic Inflammation and Metabolic Dysregulation. Genes. 2024; 15(11):1444. https://doi.org/10.3390/genes15111444
Chicago/Turabian StyleYang, Shuqi, Tingting Luo, Haoran Liu, Li Chen, Jinyong Wang, Yongju Zhao, Xuemin Li, Haohuan Li, Mingzhou Li, and Lu Lu. 2024. "Klrb1 Loss Promotes Chronic Hepatic Inflammation and Metabolic Dysregulation" Genes 15, no. 11: 1444. https://doi.org/10.3390/genes15111444
APA StyleYang, S., Luo, T., Liu, H., Chen, L., Wang, J., Zhao, Y., Li, X., Li, H., Li, M., & Lu, L. (2024). Klrb1 Loss Promotes Chronic Hepatic Inflammation and Metabolic Dysregulation. Genes, 15(11), 1444. https://doi.org/10.3390/genes15111444






