Evolutionary Analysis and Catalytic Function of LOG Proteins in Plants
Abstract
1. Introduction
2. Material and Methods
2.1. Data Collection
2.2. Phylogenetic and Motif Analysis
2.3. Plasmid Construction and Protein Expression
2.4. Enzymatic Assays
2.5. Prediction of Protein Structure
3. Results
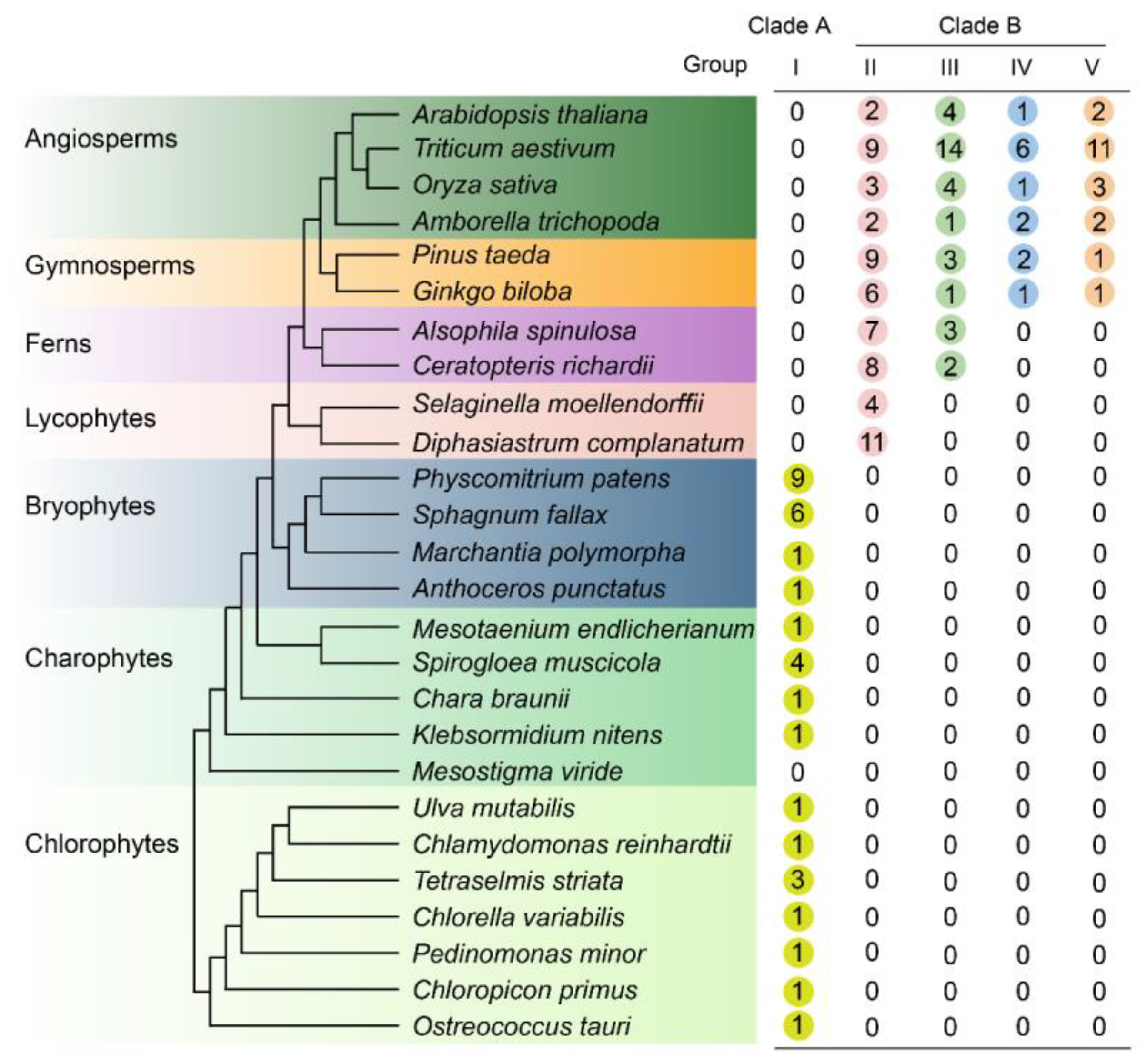
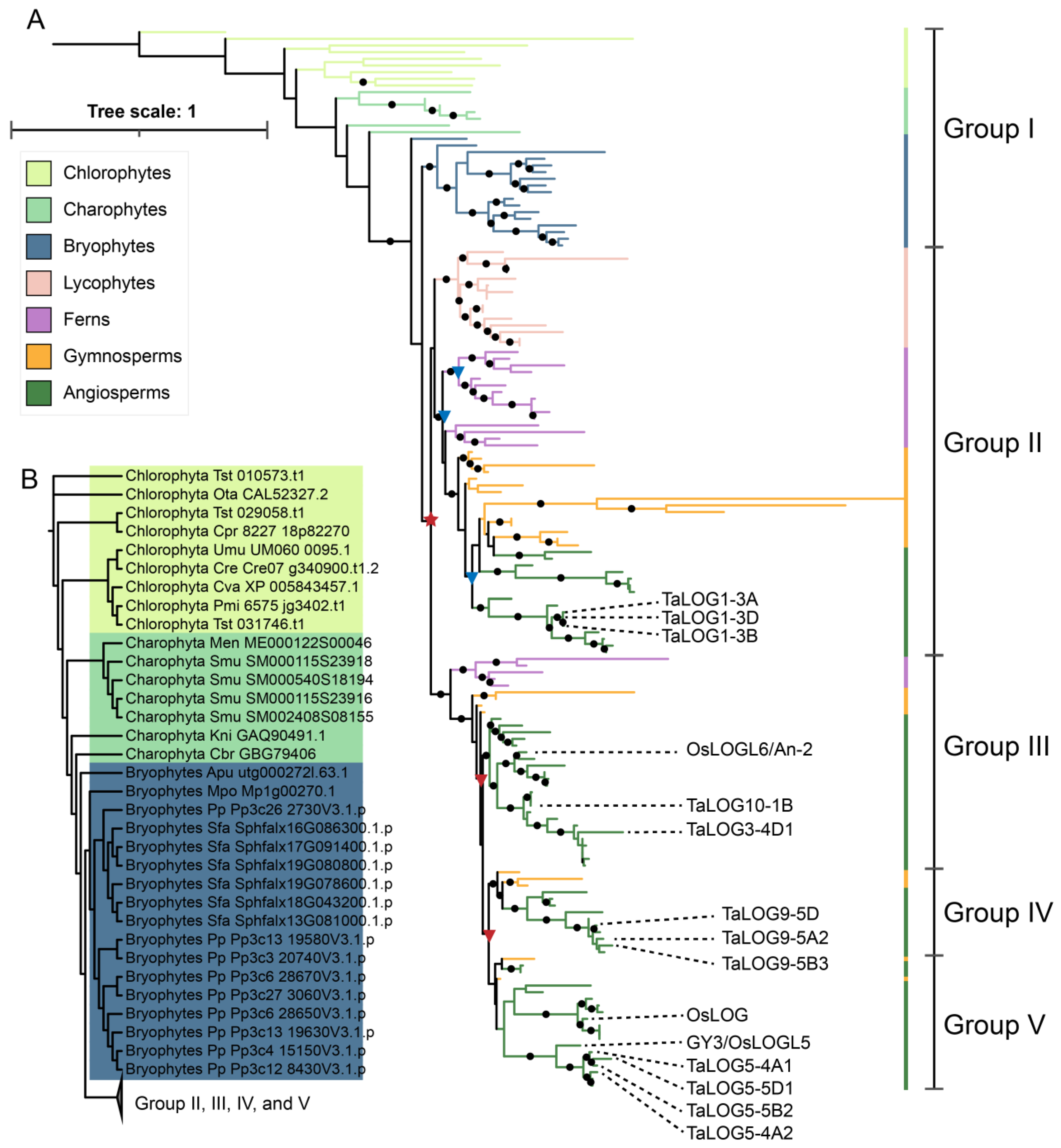
3.1. Phylogenetic Distribution Pattern of LONELY GUY (LOG) Gene in Green Plants
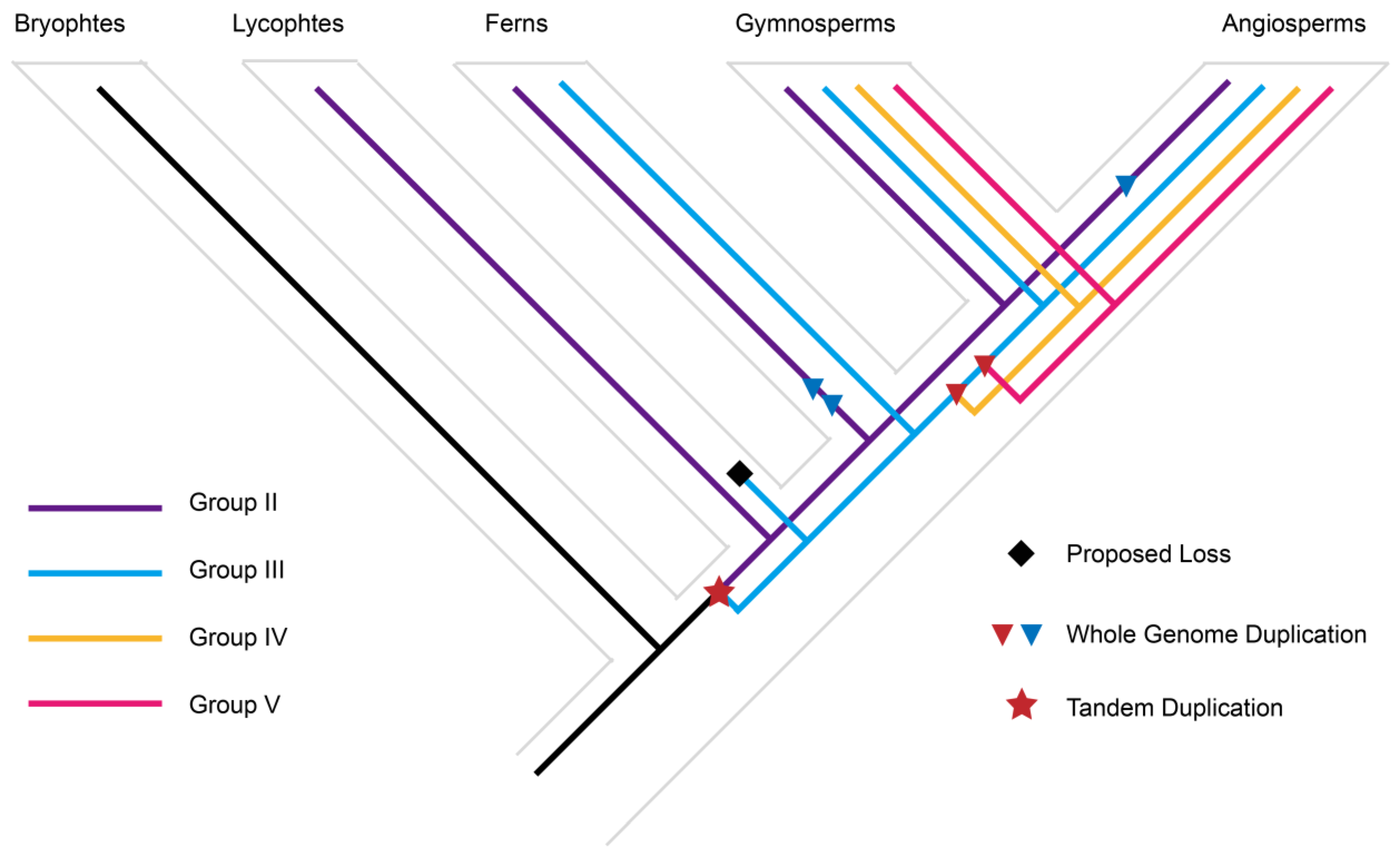
3.2. Origin and Expansion of LOG Family
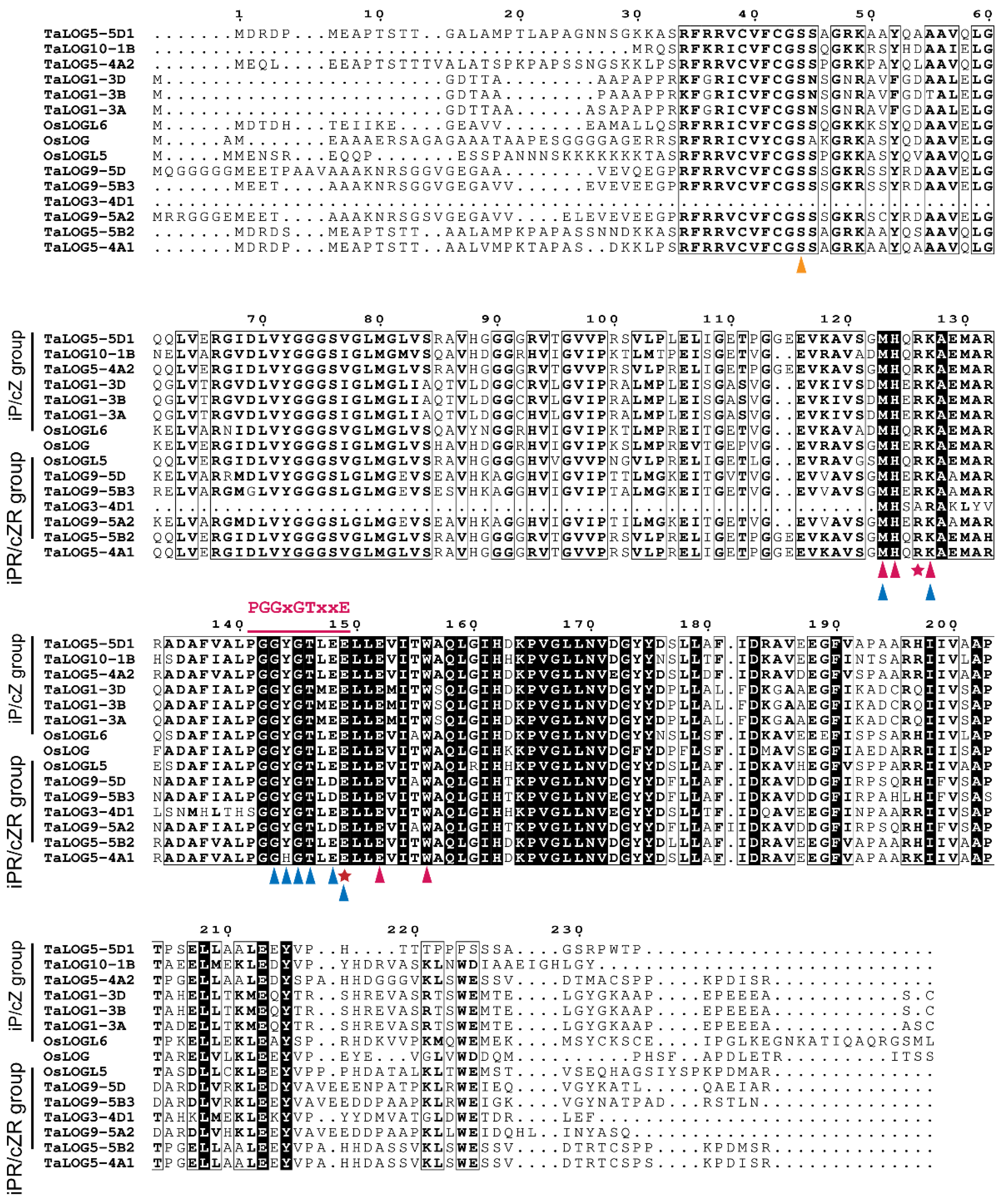
3.3. The Evolutionary Changes in Conserved Motifs in LOG Homologs
3.4. The Catalytic Activity of Different LOG Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Zhao, J.; Song, J.; Jameson, P.E. Cytokinin dehydrogenase: A genetic target for yield improvement in wheat. Plant Biotechnol. J. 2020, 18, 614–630. [Google Scholar] [CrossRef]
- Cortleven, A.; Leuendorf, J.E.; Frank, M.; Pezzetta, D.; Bolt, S.; Schmülling, T. Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ. 2019, 42, 998–1018. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin signaling networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H. Cytokinin biosynthesis and transport for systemic nitrogen signaling. Plant J. For. Cell Mol. Biol. 2021, 105, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Wybouw, B.; De Rybel, B. Cytokinin—A Developing Story. Trends Plant Sci. 2019, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhou, T.; Luo, J.; Liu, H.; Wang, Y.; Shangguan, Y.; Zhu, J.; Li, Y.; Sang, T.; Wang, Z.; et al. An-2 Encodes a Cytokinin Synthesis Enzyme that Regulates Awn Length and Grain Production in Rice. Mol. Plant 2015, 8, 1635–1650. [Google Scholar] [CrossRef]
- Wu, B.; Meng, J.; Liu, H.; Mao, D.; Yin, H.; Zhang, Z.; Zhou, X.; Zhang, B.; Sherif, A.; Liu, H.; et al. Suppressing a phosphohydrolase of cytokinin nucleotide enhances grain yield in rice. Nat. Genet. 2023, 55, 1381–1389. [Google Scholar] [CrossRef]
- Chen, L.; Jameson, G.B.; Guo, Y.; Song, J.; Jameson, P.E. The LONELY GUY gene family: From mosses to wheat, the key to the formation of active cytokinins in plants. Plant Biotechnol. J. 2022, 20, 625–645. [Google Scholar] [CrossRef]
- Svolacchia, N.; Sabatini, S. Cytokinins. Curr. Biol. 2023, 33, R10–R13. [Google Scholar] [CrossRef]
- Rashotte, A.M.; Carson, S.D.; To, J.P.; Kieber, J.J. Expression profiling of cytokinin action in Arabidopsis. Plant Physiol. 2003, 132, 1998–2011. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Liu, J.; Zhang, P.; Kudoyarova, G.; Liu, C.-J.; Zhang, K.J.P.C. Spatially distributed cytokinins: Metabolism, signaling, and transport. Plant Commun. 2024, 5, 100936. [Google Scholar] [CrossRef] [PubMed]
- Márquez-López, R.E.; Quintana-Escobar, A.O.; Loyola-Vargas, V.M. Cytokinins, the Cinderella of plant growth regulators. Phytochem. Rev. 2019, 18, 1387–1408. [Google Scholar] [CrossRef]
- Kakimoto, T. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol. 2001, 42, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef]
- Sakakibara, H. Five unaddressed questions about cytokinin biosynthesis. J. Exp. Bot. 2024, erae348. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef]
- Takei, K.; Yamaya, T.; Sakakibara, H. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-Zeatin. J. Biol. Chem. 2004, 279, 41866–41872. [Google Scholar] [CrossRef]
- Naseem, M.; Bencurova, E.; Dandekar, T. The Cytokinin-Activating LOG-Family Proteins Are Not Lysine Decarboxylases. Trends Biochem. Sci. 2018, 43, 232–236. [Google Scholar] [CrossRef]
- Kopečná, M.; Blaschke, H.; Kopečný, D.; Vigouroux, A.; Končitíková, R.; Novák, O.; Kotland, O.; Strnad, M.; Moréra, S.; von Schwartzenberg, K. Structure and function of nucleoside hydrolases from Physcomitrella patens and maize catalyzing the hydrolysis of purine, pyrimidine, and cytokinin ribosides. Plant Physiol. 2013, 163, 1568–1583. [Google Scholar] [CrossRef]
- Kojima, M.; Makita, N.; Miyata, K.; Yoshino, M.; Iwase, A.; Ohashi, M.; Surjana, A.; Kudo, T.; Takeda-Kamiya, N.; Toyooka, K.; et al. A cell wall–localized cytokinin/purine riboside nucleosidase is involved in apoplastic cytokinin metabolism in Oryza sativa. Proc. Natl. Acad. Sci. USA 2023, 120, e2217708120. [Google Scholar] [CrossRef]
- Lu, Y.; Tarkowská, D.; Turečková, V.; Luo, T.; Xin, Y.; Li, J.; Wang, Q.; Jiao, N.; Strnad, M.; Xu, J. Antagonistic roles of abscisic acid and cytokinin during response to nitrogen depletion in oleaginous microalga Nannochloropsis oceanica expand the evolutionary breadth of phytohormone function. Plant J. For. Cell Mol. Biol. 2014, 80, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S. Exploring the Role of a Cytokinin-Activating Enzyme LONELY GUY in Unicellular Microalga Chlorella variabilis. Front. Plant Sci. 2020, 11, 611871. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.i.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Takei, K.; Sakakibara, H.; Sugiyama, T. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 26405–26410. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chen, M.X.; Li, X.S.; Liang, Y.Q.; Zhang, D.Y.; Wood, A.J.; Oliver, M.J.; Zhang, J.H. Ancestral gene duplications in mosses characterized by integrated phylogenomic analyses. J. Syst. Evol. 2022, 60, 144–159. [Google Scholar] [CrossRef]
- Satterlee, J.W.; Alonso, D.; Gramazio, P.; Jenike, K.M.; He, J.; Arrones, A.; Villanueva, G.; Plazas, M.; Ramakrishnan, S.; Benoit, M.; et al. Convergent evolution of plant prickles by repeated gene co-option over deep time. Science 2024, 385, eado1663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, R.; Yan, P.; Zeng, L.; Zhao, W.; Feng, H.; Mu, R.; Hou, W. PE (Prickly Eggplant) encoding a cytokinin-activating enzyme responsible for the formation of prickles in eggplant. Hortic. Res. 2024, 11, uhae134. [Google Scholar] [CrossRef]
- Hammes, G.G. Multiple conformational changes in enzyme catalysis. Biochemistry 2002, 41, 8221–8228. [Google Scholar] [CrossRef]

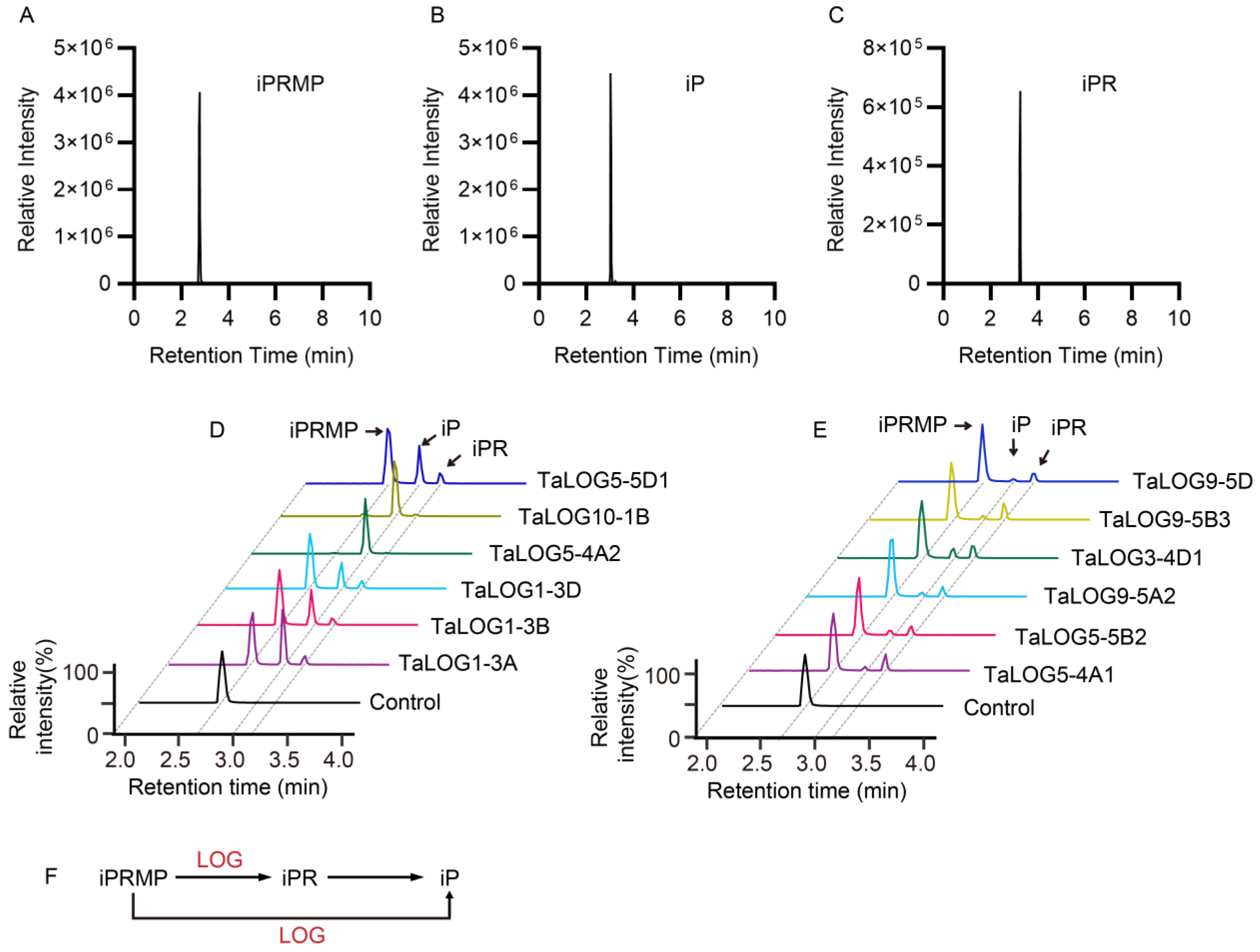
| Protein | Group | Primary Activities | Product Ratios of iPR/iP |
|---|---|---|---|
| TaLOG5-5D1 | V | iPRMP→iP | 0.27 |
| TaLOG10-1B | III | iPRMP→iP * | 0.02 |
| TaLOG5-4A2 | V | iPRMP→iP * | 0.01 |
| TaLOG1-3D | II | iPRMP→iP | 0.27 |
| TaLOG1-3B | II | iPRMP→iP | 0.19 |
| TaLOG1-3A | II | iPRMP→iP | 0.15 |
| TaLOG9-5D | IV | iPRMP→iPR | 3.42 |
| TaLOG9-5B3 | IV | iPRMP→iPR | 4.2 |
| TaLOG3-4D1 | III | iPRMP→iPR | 1.28 |
| TaLOG9-5A2 | IV | iPRMP→iPR | 2.02 |
| TaLOG5-5B2 | V | iPRMP→iPR | 1.68 |
| TaLOG5-4A1 | V | iPRMP→iPR | 3.98 |
| Compare Groups | Identity | Aligned Residues | RMSD | TM-Score |
|---|---|---|---|---|
| TaLOG5-4A1 vs. TaLOG5-4A2 | 0.91 | 206 | 1.72 | 0.84 |
| TaLOG5-4A1 vs. TaLOG5-5B2 | 0.86 | 224 | 2.06 | 0.91 |
| TaLOG5-4A1 vs. TaLOG5-5D1 | 0.91 | 195 | 1.79 | 0.79 |
| TaLOG5-4A2 vs. TaLOG5-5B2 | 0.87 | 203 | 1.85 | 0.82 |
| TaLOG5-4A2 vs. TaLOG5-5D1 | 0.86 | 195 | 1.71 | 0.79 |
| TaLOG5-5B2 vs. TaLOG5-5D1 | 0.91 | 193 | 1.51 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Yin, H.; Li, Y.; Zhou, J.; Bi, S.; Yan, W.; Li, Y. Evolutionary Analysis and Catalytic Function of LOG Proteins in Plants. Genes 2024, 15, 1420. https://doi.org/10.3390/genes15111420
Zhao C, Yin H, Li Y, Zhou J, Bi S, Yan W, Li Y. Evolutionary Analysis and Catalytic Function of LOG Proteins in Plants. Genes. 2024; 15(11):1420. https://doi.org/10.3390/genes15111420
Chicago/Turabian StyleZhao, Chunjie, Huanran Yin, Yuqi Li, Jiacheng Zhou, Siteng Bi, Wenhao Yan, and Yunzhen Li. 2024. "Evolutionary Analysis and Catalytic Function of LOG Proteins in Plants" Genes 15, no. 11: 1420. https://doi.org/10.3390/genes15111420
APA StyleZhao, C., Yin, H., Li, Y., Zhou, J., Bi, S., Yan, W., & Li, Y. (2024). Evolutionary Analysis and Catalytic Function of LOG Proteins in Plants. Genes, 15(11), 1420. https://doi.org/10.3390/genes15111420







