Dynamic Expression of Genes Encoding Ubiquitin Conjugating Enzymes (E2s) During Neuronal Differentiation and Maturation: Implications for Neurodevelopmental Disorders and Neurodegenerative Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Cultures of Hippocampal Neurons
2.2. Immunocytochemistry
2.3. RNA Isolation
2.4. Quantitative Real-Time RT-PCR
2.5. Promoter Analysis
3. Results
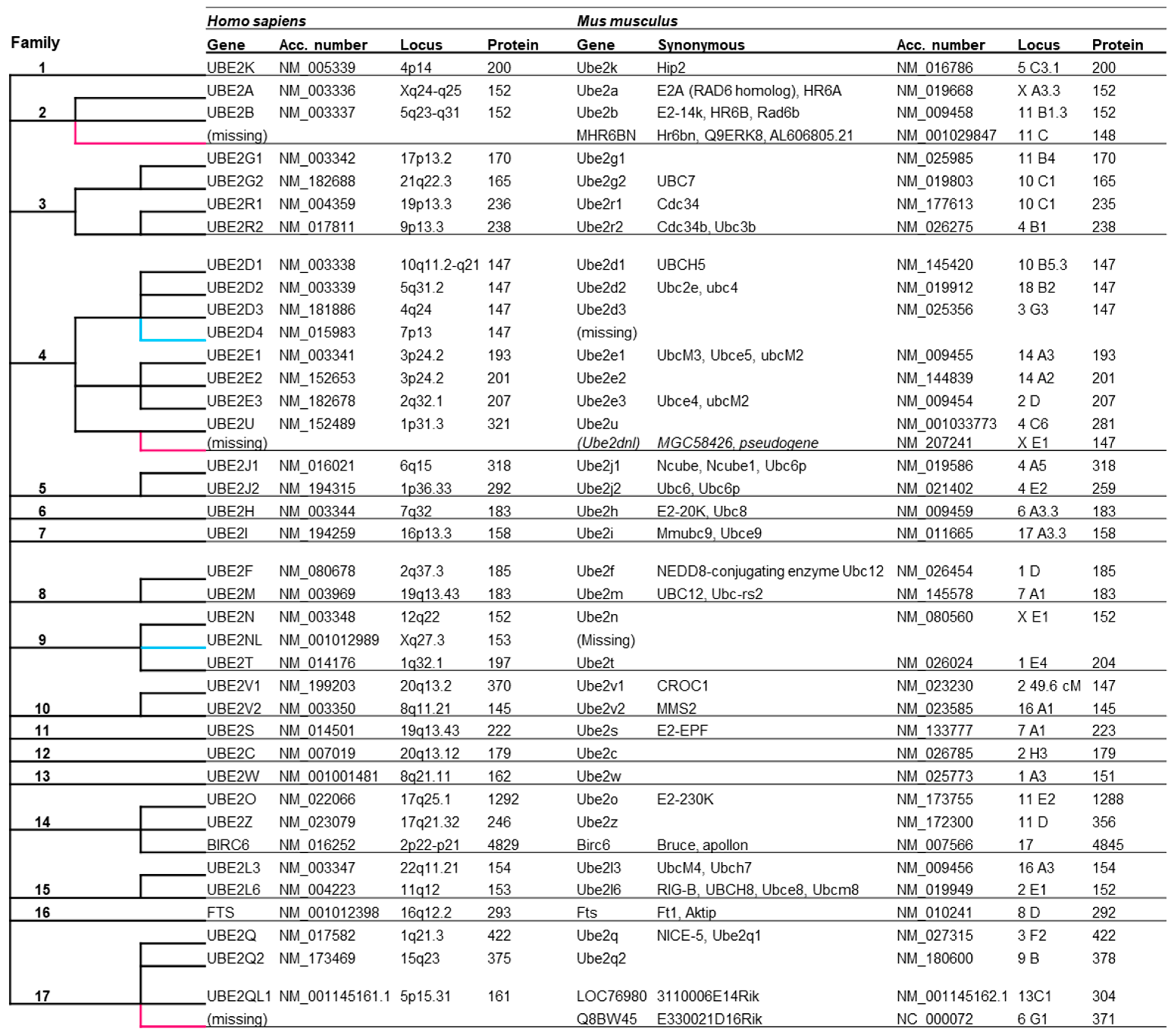
3.1. E2 Orthologs in Human and Mouse
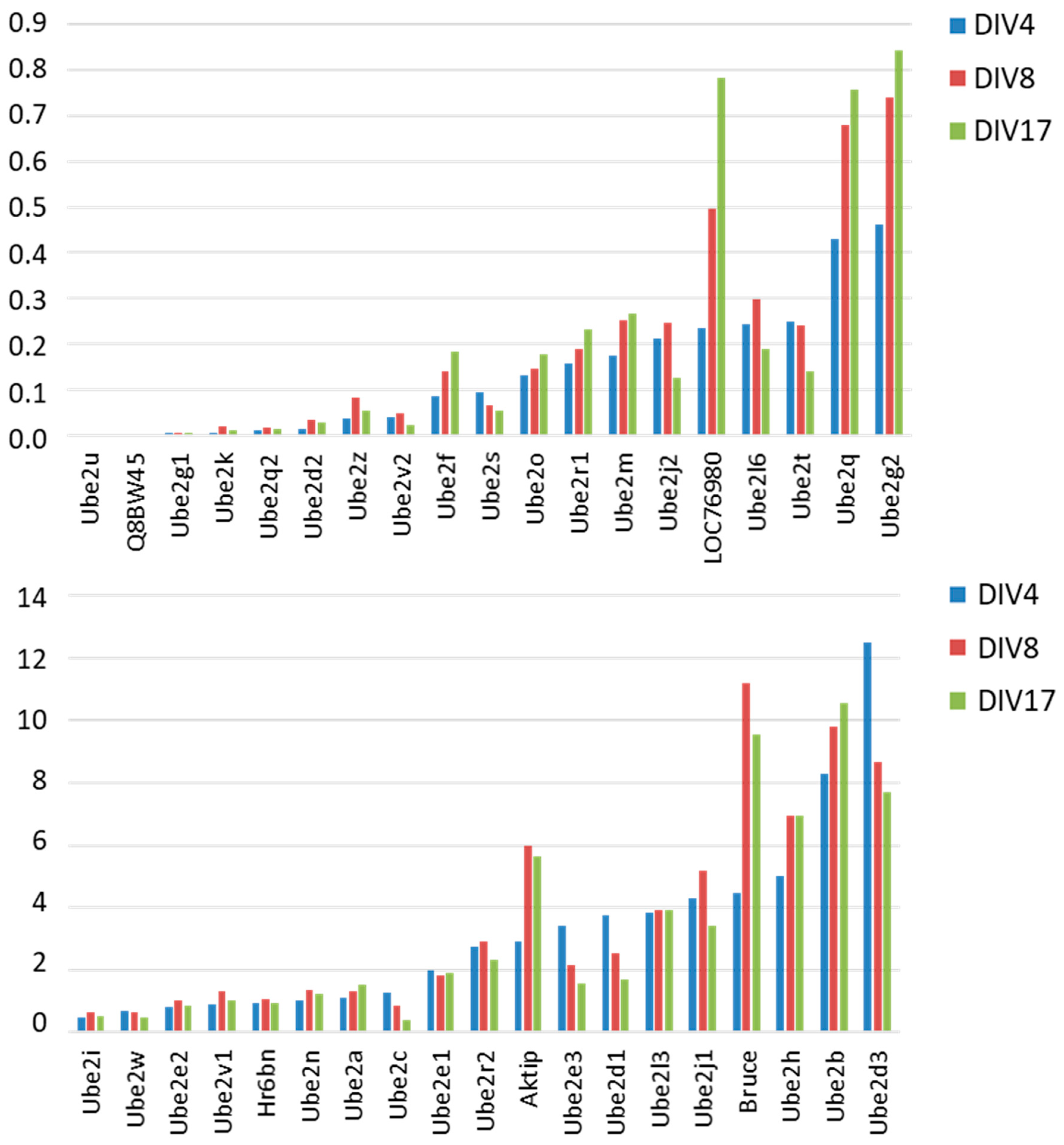
3.2. Differential Expression of E2 Genes in Young Hippocampal Neurons (DIV4) in Cultures
3.3. Differential Expression of E2 Genes During Neuronal Differentiation and Maturation
3.4. Variation in E2 Gene Expression During Short and Late-Responses to NMDA
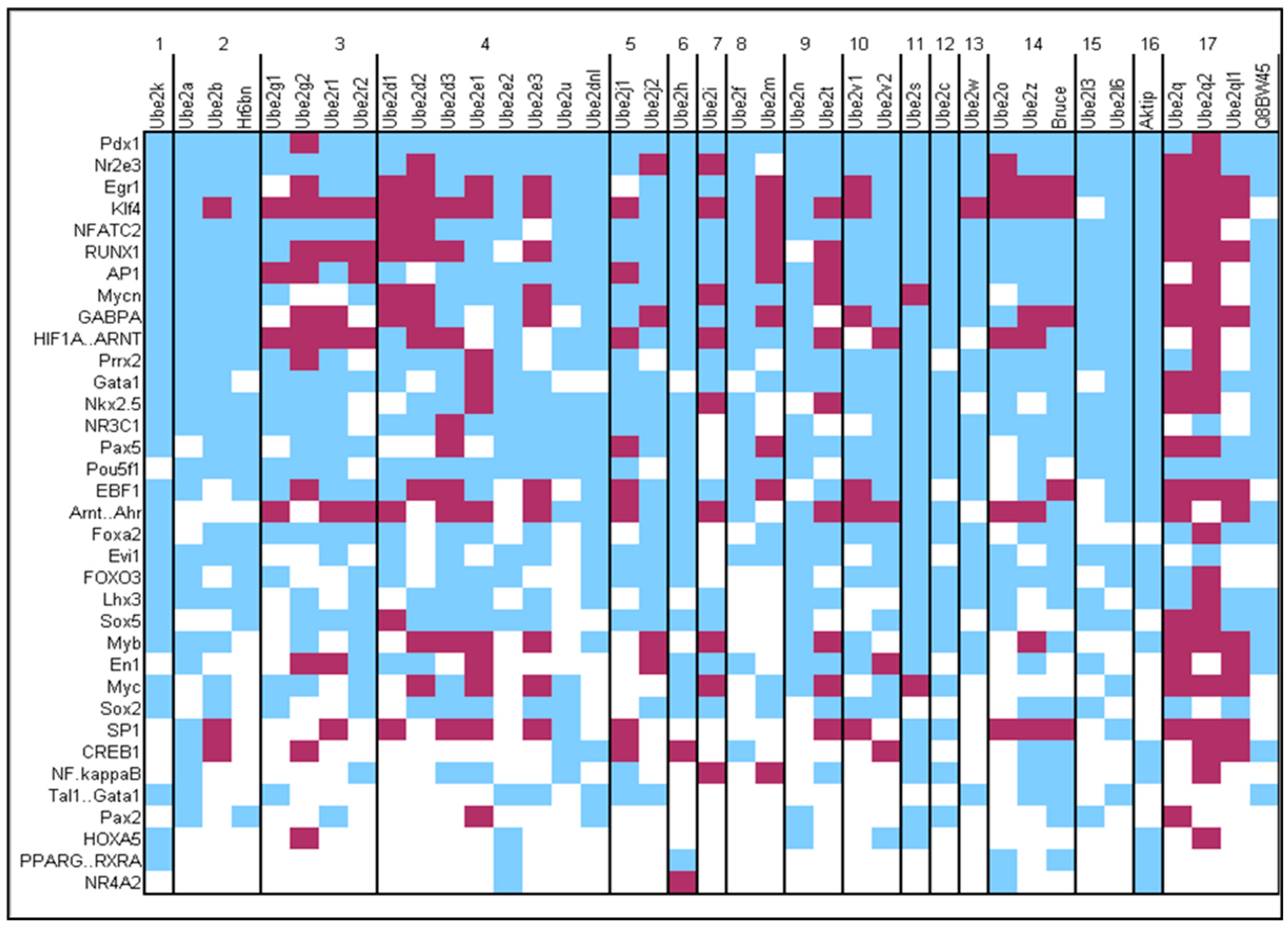
3.5. Analysis of the Regulation of Expression of Genes Encoding E2s
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dye, B.T.; Schulman, B.A. Structural Mechanisms Underlying Posttranslational Modification by Ubiquitin-Like Proteins. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Glickman, M.H.; Ciechanover, A. The Ubiquitin-Proteasome Proteolytic Pathway: Destruction for the Sake of Construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef] [PubMed]
- Matunis, M.J.; Coutavas, E.; Blobel, G. A Novel Ubiquitin-like Modification Modulates the Partitioning of the Ran-GTPase-Activating Protein RanGAP1 between the Cytosol and the Nuclear Pore Complex. J. Cell Biol. 1996, 135, 1457–1470. [Google Scholar] [CrossRef] [PubMed]
- Loriol, C.; Parisot, J.; Poupon, G.; Gwizdek, C.; Martin, S. Developmental Regulation and Spatiotemporal Redistribution of the Sumoylation Machinery in the Rat Central Nervous System. PLoS ONE 2012, 7, e33757. [Google Scholar] [CrossRef]
- Ji, Z.-S.; Liu, Q.-L.; Zhang, J.; Yang, Y.-H.; Li, J.; Zhang, G.-W.; Tan, M.-H.; Lin, H.-S.; Guo, G.-Q. SUMOylation of Spastin Promotes the Internalization of GluA1 and Regulates Dendritic Spine Morphology by Targeting Microtubule Dynamics. Neurobiol. Dis. 2020, 146, 105133. [Google Scholar] [CrossRef]
- Kawakami, T. NEDD8 Recruits E2-Ubiquitin to SCF E3 Ligase. EMBO J. 2001, 20, 4003–4012. [Google Scholar] [CrossRef]
- Vogl, A.M.; Brockmann, M.M.; Giusti, S.A.; Maccarrone, G.; Vercelli, C.A.; Bauder, C.A.; Richter, J.S.; Roselli, F.; Hafner, A.-S.; Dedic, N.; et al. Neddylation Inhibition Impairs Spine Development, Destabilizes Synapses and Deteriorates Cognition. Nat. Neurosci. 2015, 18, 239–251. [Google Scholar] [CrossRef]
- Staub, O. Ubiquitylation and Isgylation: Overlapping Enzymatic Cascades Do the Job. Sci. STKE 2004, 2004, pe43. [Google Scholar] [CrossRef]
- Nakka, V.P.; Lang, B.T.; Lenschow, D.J.; Zhang, D.-E.; Dempsey, R.J.; Vemuganti, R. Increased Cerebral Protein ISGylation after Focal Ischemia Is Neuroprotective. J. Cereb. Blood Flow Metab. 2011, 31, 2375–2384. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Gu, C.; Jiang, J.; Gao, Y. Kaempferol Alleviates Injury in Human Retinal Pigment Epithelial Cells via STAT1 Ubiquitination-Mediated Degradation of IRF7. Front. Biosci. (Landmark Ed.) 2024, 29, 247. [Google Scholar] [CrossRef]
- Bedja-Iacona, L.; Richard, E.; Marouillat, S.; Brulard, C.; Alouane, T.; Beltran, S.; Andres, C.R.; Blasco, H.; Corcia, P.; Veyrat-Durebex, C.; et al. Post-Translational Variants of Major Proteins in Amyotrophic Lateral Sclerosis Provide New Insights into the Pathophysiology of the Disease. Int. J. Mol. Sci. 2024, 25, 8664. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.-C.; Schuman, E.M. Ubiquitin, the Proteasome and Protein Degradation in Neuronal Function and Dysfunction. Nat. Rev. Neurosci. 2008, 9, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ambasta, R.K.; Kumar, P. Ubiquitin Biology in Neurodegenerative Disorders: From Impairment to Therapeutic Strategies. Ageing Res. Rev. 2020, 61, 101078. [Google Scholar] [CrossRef] [PubMed]
- Ebstein, F.; Küry, S.; Papendorf, J.J.; Krüger, E. Neurodevelopmental Disorders (NDD) Caused by Genomic Alterations of the Ubiquitin-Proteasome System (UPS): The Possible Contribution of Immune Dysregulation to Disease Pathogenesis. Front. Mol. Neurosci. 2021, 14, 733012. [Google Scholar] [CrossRef]
- Kishino, T.; Lalande, M.; Wagstaff, J. UBE3A/E6-AP Mutations Cause Angelman Syndrome. Nat. Genet. 1997, 15, 70–73. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Raymond, F.L.; O’Meara, S.; Edkins, S.; Teague, J.; Butler, A.; Dicks, E.; Stevens, C.; Tofts, C.; Avis, T.; et al. Mutations in CUL4B, Which Encodes a Ubiquitin E3 Ligase Subunit, Cause an X-Linked Mental Retardation Syndrome Associated with Aggressive Outbursts, Seizures, Relative Macrocephaly, Central Obesity, Hypogonadism, Pes Cavus, and Tremor. Am. J. Hum. Genet. 2007, 80, 345–352. [Google Scholar] [CrossRef]
- Nascimento, R.M.P.; Otto, P.A.; de Brouwer, A.P.M.; Vianna-Morgante, A.M. UBE2A, Which Encodes a Ubiquitin-Conjugating Enzyme, Is Mutated in a Novel X-Linked Mental Retardation Syndrome. Am. J. Hum. Genet. 2006, 79, 549–555. [Google Scholar] [CrossRef]
- Budny, B.; Badura-Stronka, M.; Materna-Kiryluk, A.; Tzschach, A.; Raynaud, M.; Latos-Bielenska, A.; Ropers, H.H. Novel Missense Mutations in the Ubiquitination-Related Gene UBE2A Cause a Recognizable X-Linked Mental Retardation Syndrome. Clin. Genet. 2010, 77, 541–551. [Google Scholar] [CrossRef]
- Froyen, G.; Corbett, M.; Vandewalle, J.; Jarvela, I.; Lawrence, O.; Meldrum, C.; Bauters, M.; Govaerts, K.; Vandeleur, L.; Van Esch, H.; et al. Submicroscopic Duplications of the Hydroxysteroid Dehydrogenase HSD17B10 and the E3 Ubiquitin Ligase HUWE1 Are Associated with Mental Retardation. Am. J. Hum. Genet. 2008, 82, 432–443. [Google Scholar] [CrossRef]
- Field, M.; Tarpey, P.S.; Smith, R.; Edkins, S.; O’Meara, S.; Stevens, C.; Tofts, C.; Teague, J.; Butler, A.; Dicks, E.; et al. Mutations in the BRWD3 Gene Cause X-Linked Mental Retardation Associated with Macrocephaly. Am. J. Hum. Genet. 2007, 81, 367–374. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Y.; Chen, Y.; Yuan, H.; Kuang, X.; Hou, D.; Li, X.; Pan, L.; Huang, G.; He, J.; et al. A Novel UBE2A Splice Site Variant Causing Intellectual Disability Type Nascimento. Clin. Case Rep. 2022, 10, e5990. [Google Scholar] [CrossRef] [PubMed]
- Vourc’h, P.; Martin, I.; Bonnet-Brilhault, F.; Marouillat, S.; Barthélémy, C.; Pierre Müh, J.; Andres, C. Mutation Screening and Association Study of the UBE2H Gene on Chromosome 7q32 in Autistic Disorder. Psychiatr. Genet. 2003, 13, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Glessner, J.T.; Wang, K.; Cai, G.; Korvatska, O.; Kim, C.E.; Wood, S.; Zhang, H.; Estes, A.; Brune, C.W.; Bradfield, J.P.; et al. Autism Genome-Wide Copy Number Variation Reveals Ubiquitin and Neuronal Genes. Nature 2009, 459, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Tsai, N.-P.; Wilkerson, J.R.; Guo, W.; Maksimova, M.A.; DeMartino, G.N.; Cowan, C.W.; Huber, K.M. Multiple Autism-Linked Genes Mediate Synapse Elimination via Proteasomal Degradation of a Synaptic Scaffold PSD-95. Cell 2012, 151, 1581–1594. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin Signalling in Neurodegeneration: Mechanisms and Therapeutic Opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, R. Aberrant Protein Aggregation in Amyotrophic Lateral Sclerosis. J. Neurol. 2024, 271, 4826–4851. [Google Scholar] [CrossRef]
- Kwiatkowski, T.J.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T.; et al. Mutations in the FUS/TLS Gene on Chromosome 16 Cause Familial Amyotrophic Lateral Sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef]
- Vance, C.; Rogelj, B.; Hortobágyi, T.; De Vos, K.J.; Nishimura, A.L.; Sreedharan, J.; Hu, X.; Smith, B.; Ruddy, D.; Wright, P.; et al. Mutations in FUS, an RNA Processing Protein, Cause Familial Amyotrophic Lateral Sclerosis Type 6. Science 2009, 323, 1208–1211. [Google Scholar] [CrossRef]
- Kawabe, H.; Brose, N. The Role of Ubiquitylation in Nerve Cell Development. Nat. Rev. Neurosci. 2011, 12, 251–268. [Google Scholar] [CrossRef]
- Ding, M.; Chao, D.; Wang, G.; Shen, K. Spatial Regulation of an E3 Ubiquitin Ligase Directs Selective Synapse Elimination. Science 2007, 317, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Craig, T.J.; Henley, J.M. Protein SUMOylation in Spine Structure and Function. Curr. Opin. Neurobiol. 2012, 22, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Lussier, M.P.; Herring, B.E.; Nasu-Nishimura, Y.; Neutzner, A.; Karbowski, M.; Youle, R.J.; Nicoll, R.A.; Roche, K.W. Ubiquitin Ligase RNF167 Regulates AMPA Receptor-Mediated Synaptic Transmission. Proc. Natl. Acad. Sci. USA 2012, 109, 19426–19431. [Google Scholar] [CrossRef] [PubMed]
- Loriol, C.; Khayachi, A.; Poupon, G.; Gwizdek, C.; Martin, S. Activity-Dependent Regulation of the Sumoylation Machinery in Rat Hippocampal Neurons. Biol. Cell 2013, 105, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.J.; Berrios, J.; Newbern, J.M.; Snider, W.D.; Philpot, B.D.; Hahn, K.M.; Zylka, M.J. An Autism-Linked Mutation Disables Phosphorylation Control of UBE3A. Cell 2015, 162, 795–807. [Google Scholar] [CrossRef]
- Watabe, K.; Kato, Y.; Sakuma, M.; Murata, M.; Niida-Kawaguchi, M.; Takemura, T.; Hanagata, N.; Tada, M.; Kakita, A.; Shibata, N. Praja1 RING-Finger E3 Ubiquitin Ligase Suppresses Neuronal Cytoplasmic TDP-43 Aggregate Formation. Neuropathology 2020, 40, 570–586. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, L.; Merillat, S.A.; Fischer, S.; Ochaba, J.; Thompson, L.M.; Barmada, S.J.; Scaglione, K.M.; Paulson, H.L. The Ubiquitin Conjugating Enzyme Ube2W Regulates Solubility of the Huntington’s Disease Protein, Huntingtin. Neurobiol. Dis. 2018, 109, 127–136. [Google Scholar] [CrossRef]
- Ying, M.; Zhan, Z.; Wang, W.; Chen, D. Origin and Evolution of Ubiquitin-Conjugating Enzymes from Guillardia Theta Nucleomorph to Hominoid. Gene 2009, 447, 72–85. [Google Scholar] [CrossRef]
- Michelle, C.; Vourc’h, P.; Mignon, L.; Andres, C.R. What Was the Set of Ubiquitin and Ubiquitin-like Conjugating Enzymes in the Eukaryote Common Ancestor? J. Mol. Evol. 2009, 68, 616–628. [Google Scholar] [CrossRef]
- van Wijk, S.J.L.; Timmers, H.T.M. The Family of Ubiquitin-Conjugating Enzymes (E2s): Deciding between Life and Death of Proteins. FASEB J. 2010, 24, 981–993. [Google Scholar] [CrossRef]
- Laumonnier, F.; Shoubridge, C.; Antar, C.; Nguyen, L.S.; Van Esch, H.; Kleefstra, T.; Briault, S.; Fryns, J.P.; Hamel, B.; Chelly, J.; et al. Mutations of the UPF3B Gene, Which Encodes a Protein Widely Expressed in Neurons, Are Associated with Nonspecific Mental Retardation with or without Autism. Mol. Psychiatry 2010, 15, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Sandelin, A.; Alkema, W.; Engström, P.; Wasserman, W.W.; Lenhard, B. JASPAR: An Open-Access Database for Eukaryotic Transcription Factor Binding Profiles. Nucleic Acids Res. 2004, 32, D91–D94. [Google Scholar] [CrossRef] [PubMed]
- Koerver, L.; Papadopoulos, C.; Liu, B.; Kravic, B.; Rota, G.; Brecht, L.; Veenendaal, T.; Polajnar, M.; Bluemke, A.; Ehrmann, M.; et al. The Ubiquitin-Conjugating Enzyme UBE2QL1 Coordinates Lysophagy in Response to Endolysosomal Damage. EMBO Rep. 2019, 20, e48014. [Google Scholar] [CrossRef] [PubMed]
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The Establishment of Polarity by Hippocampal Neurons in Culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Calderon de Anda, F.; Gärtner, A.; Tsai, L.-H.; Dotti, C.G. Pyramidal Neuron Polarity Axis Is Defined at the Bipolar Stage. J. Cell Sci. 2008, 121, 178–185. [Google Scholar] [CrossRef]
- Valor, L.M.; Grant, S.G.N. Integrating Synapse Proteomics with Transcriptional Regulation. Behav. Genet. 2007, 37, 18–30. [Google Scholar] [CrossRef]
- Kavakebi, P.; Hausott, B.; Tomasino, A.; Ingorokva, S.; Klimaschewski, L. The N-End Rule Ubiquitin-Conjugating Enzyme, HR6B, Is up-Regulated by Nerve Growth Factor and Required for Neurite Outgrowth. Mol. Cell Neurosci. 2005, 29, 559–568. [Google Scholar] [CrossRef]
- Oh, S.K.; Sarnow, P. Gene Regulation: Translational Initiation by Internal Ribosome Binding. Curr. Opin. Genet Dev. 1993, 3, 295–300. [Google Scholar] [CrossRef]
- Wheeler, T.C.; Chin, L.-S.; Li, Y.; Roudabush, F.L.; Li, L. Regulation of Synaptophysin Degradation by Mammalian Homologues of Seven in Absentia. J. Biol. Chem. 2002, 277, 10273–10282. [Google Scholar] [CrossRef]
- Gandini, M.A.; Henríquez, D.R.; Grimaldo, L.; Sandoval, A.; Altier, C.; Zamponi, G.W.; Felix, R.; González-Billault, C. CaV2.2 Channel Cell Surface Expression Is Regulated by the Light Chain 1 (LC1) of the Microtubule-Associated Protein B (MAP1B) via UBE2L3-Mediated Ubiquitination and Degradation. Pflug. Arch. 2014, 466, 2113–2126. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, X.; Treml, L.S.; Cancro, M.P.; Freedman, B.D. Mechanism and Regulatory Function of CpG Signaling via Scavenger Receptor B1 in Primary B Cells. J. Biol. Chem. 2009, 284, 22878–22887. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.D.; Ritterhoff, T.; Klevit, R.E.; Brzovic, P.S. E2 Enzymes: More than Just Middle Men. Cell Res. 2016, 26, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Magnati, S.; Alladio, E.; Bracco, E. A Survey on the Expression of the Ubiquitin Proteasome System Components HECT- and RBR-E3 Ubiquitin Ligases and E2 Ubiquitin-Conjugating and E1 Ubiquitin-Activating Enzymes during Human Brain Development. Int. J. Mol. Sci. 2024, 25, 2361. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T.; Fujita, S.; Kubota, H.; Inoue, D.; Mizuno, A.; Komatsu, T.; Yamaza, H.; Higami, Y.; Shimokawa, I. Identification of Fasting-Induced Genes in the Rat Hypothalamus: Relationship with Neuroprotection. Ann. N. Y. Acad. Sci. 2007, 1119, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.; Treccarichi, S.; Galati Rando, R.; Musumeci, A.; Todaro, V.; Federico, C.; Saccone, S.; Elia, M.; Calì, F. A de Novo ARIH2 Gene Mutation Was Detected in a Patient with Autism Spectrum Disorders and Intellectual Disability. Sci. Rep. 2024, 14, 15848. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, X.-O.; Pazour, G.J. Arih2 Regulates Hedgehog Signaling through Smoothened Ubiquitylation and ER-Associated Degradation. J. Cell Sci. 2022, 135, jcs260299. [Google Scholar] [CrossRef]
- Antonellis, P.J.; Engle, S.E.; Brewer, K.M.; Berbari, N.F. The Hedgehog Signaling Pathway Is Expressed in the Adult Mouse Hypothalamus and Modulated by Fasting. eNeuro 2021, 8, ENEURO.0276-21.2021. [Google Scholar] [CrossRef]
- Khayachi, A.; Gwizdek, C.; Poupon, G.; Alcor, D.; Chafai, M.; Cassé, F.; Maurin, T.; Prieto, M.; Folci, A.; De Graeve, F.; et al. Sumoylation Regulates FMRP-Mediated Dendritic Spine Elimination and Maturation. Nat. Commun. 2018, 9, 757. [Google Scholar] [CrossRef]
- Li, Y.-X.; Tan, Z.-N.; Li, X.-H.; Ma, B.; Adu Nti, F.; Lv, X.-Q.; Tian, Z.-J.; Yan, R.; Man, H.-Y.; Ma, X.-M. Increased Gene Dosage of RFWD2 Causes Autistic-like Behaviors and Aberrant Synaptic Formation and Function in Mice. Mol. Psychiatry 2024, 29, 2496–2509. [Google Scholar] [CrossRef]
- Bingol, B.; Sheng, M. Deconstruction for Reconstruction: The Role of Proteolysis in Neural Plasticity and Disease. Neuron 2011, 69, 22–32. [Google Scholar] [CrossRef]
- Montarolo, P.G.; Goelet, P.; Castellucci, V.F.; Morgan, J.; Kandel, E.R.; Schacher, S. A Critical Period for Macromolecular Synthesis in Long-Term Heterosynaptic Facilitation in Aplysia. Science 1986, 234, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Husi, H.; Ward, M.A.; Choudhary, J.S.; Blackstock, W.P.; Grant, S.G. Proteomic Analysis of NMDA Receptor-Adhesion Protein Signaling Complexes. Nat. Neurosci. 2000, 3, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Li, H.; Becker, K.G.; Dawson, V.L.; Dawson, T.M. Identification and Analysis of Plasticity-Induced Late-Response Genes. Proc. Natl. Acad. Sci. USA 2004, 101, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, D.F.; Valnegri, P.; Dearborn, J.T.; Fowler, S.C.; Bonni, A. Conditional Knockout of UBC13 Produces Disturbances in Gait and Spontaneous Locomotion and Exploration in Mice. Sci. Rep. 2019, 9, 4379. [Google Scholar] [CrossRef] [PubMed]
- Kodis, E.J.; Choi, S.; Swanson, E.; Ferreira, G.; Bloom, G.S. N-Methyl-D-Aspartate Receptor-Mediated Calcium Influx Connects Amyloid-β Oligomers to Ectopic Neuronal Cell Cycle Reentry in Alzheimer’s Disease. Alzheimers Dement. 2018, 14, 1302–1312. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, H.; Liu, C.; Jin, J.; Xing, Z.; Wu, Y. Age-Associated UBE2O Reduction Promotes Neuronal Death in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 93, 1083–1093. [Google Scholar] [CrossRef]
- Condos, T.E.; Dunkerley, K.M.; Freeman, E.A.; Barber, K.R.; Aguirre, J.D.; Chaugule, V.K.; Xiao, Y.; Konermann, L.; Walden, H.; Shaw, G.S. Synergistic Recruitment of UbcH7~Ub and Phosphorylated Ubl Domain Triggers Parkin Activation. EMBO J. 2018, 37, e100014. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, C.; Liu, Y.; Su, R.; Zhao, Y.; Li, Y. Mechanism and Disease Association with a Ubiquitin Conjugating E2 Enzyme: UBE2L3. Front. Immunol. 2022, 13, 793610. [Google Scholar] [CrossRef]
- Grünblatt, E.; Mandel, S.; Jacob-Hirsch, J.; Zeligson, S.; Amariglo, N.; Rechavi, G.; Li, J.; Ravid, R.; Roggendorf, W.; Riederer, P.; et al. Gene Expression Profiling of Parkinsonian Substantia Nigra Pars Compacta; Alterations in Ubiquitin-Proteasome, Heat Shock Protein, Iron and Oxidative Stress Regulated Proteins, Cell Adhesion/Cellular Matrix and Vesicle Trafficking Genes. J. Neural. Transm. 2004, 111, 1543–1573. [Google Scholar] [CrossRef]
- Kalchman, M.A.; Graham, R.K.; Xia, G.; Koide, H.B.; Hodgson, J.G.; Graham, K.C.; Goldberg, Y.P.; Gietz, R.D.; Pickart, C.M.; Hayden, M.R. Huntingtin Is Ubiquitinated and Interacts with a Specific Ubiquitin-Conjugating Enzyme. J. Biol. Chem. 1996, 271, 19385–19394. [Google Scholar] [CrossRef]
- Tak, Y.J.; Kang, S. The E2 Ubiquitin-Conjugating Enzyme HIP2 Is a Crucial Regulator of Quality Control against Mutant SOD1 Proteotoxicity. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166316. [Google Scholar] [CrossRef] [PubMed]
- Hans, F.; Fiesel, F.C.; Strong, J.C.; Jäckel, S.; Rasse, T.M.; Geisler, S.; Springer, W.; Schulz, J.B.; Voigt, A.; Kahle, P.J. UBE2E Ubiquitin-Conjugating Enzymes and Ubiquitin Isopeptidase Y Regulate TDP-43 Protein Ubiquitination. J. Biol. Chem. 2014, 289, 19164–19179. [Google Scholar] [CrossRef] [PubMed]
- Hergesheimer, R.C.; Chami, A.A.; de Assis, D.R.; Vourc’h, P.; Andres, C.R.; Corcia, P.; Lanznaster, D.; Blasco, H. The Debated Toxic Role of Aggregated TDP-43 in Amyotrophic Lateral Sclerosis: A Resolution in Sight? Brain 2019, 142, 1176–1194. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Pioro, E.P.; Goutman, S.A.; Kiernan, M.C. Nanoplastics and Neurodegeneration in ALS. Brain Sci. 2024, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.S.; Reale, L.A.; Lewis, K.E.; Walker, A.K.; Dickson, T.C.; Woodhouse, A.; Blizzard, C.A. Mislocalisation of TDP-43 to the Cytoplasm Causes Cortical Hyperexcitability and Reduced Excitatory Neurotransmission in the Motor Cortex. J. Neurochem. 2021, 157, 1300–1315. [Google Scholar] [CrossRef]
- Condorelli, D.F.; Dell’Albani, P.; Amico, C.; Lukasiuk, K.; Kaczmarek, L.; Giuffrida-Stella, A.M. Glutamate Receptor-Driven Activation of Transcription Factors in Primary Neuronal Cultures. Neurochem. Res. 1994, 19, 489–499. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paubel, A.; Marouillat, S.; Dangoumau, A.; Maurel, C.; Haouari, S.; Blasco, H.; Corcia, P.; Laumonnier, F.; Andres, C.R.; Vourc’h, P. Dynamic Expression of Genes Encoding Ubiquitin Conjugating Enzymes (E2s) During Neuronal Differentiation and Maturation: Implications for Neurodevelopmental Disorders and Neurodegenerative Diseases. Genes 2024, 15, 1381. https://doi.org/10.3390/genes15111381
Paubel A, Marouillat S, Dangoumau A, Maurel C, Haouari S, Blasco H, Corcia P, Laumonnier F, Andres CR, Vourc’h P. Dynamic Expression of Genes Encoding Ubiquitin Conjugating Enzymes (E2s) During Neuronal Differentiation and Maturation: Implications for Neurodevelopmental Disorders and Neurodegenerative Diseases. Genes. 2024; 15(11):1381. https://doi.org/10.3390/genes15111381
Chicago/Turabian StylePaubel, Agathe, Sylviane Marouillat, Audrey Dangoumau, Cindy Maurel, Shanez Haouari, Hélène Blasco, Philippe Corcia, Frédéric Laumonnier, Christian R. Andres, and Patrick Vourc’h. 2024. "Dynamic Expression of Genes Encoding Ubiquitin Conjugating Enzymes (E2s) During Neuronal Differentiation and Maturation: Implications for Neurodevelopmental Disorders and Neurodegenerative Diseases" Genes 15, no. 11: 1381. https://doi.org/10.3390/genes15111381
APA StylePaubel, A., Marouillat, S., Dangoumau, A., Maurel, C., Haouari, S., Blasco, H., Corcia, P., Laumonnier, F., Andres, C. R., & Vourc’h, P. (2024). Dynamic Expression of Genes Encoding Ubiquitin Conjugating Enzymes (E2s) During Neuronal Differentiation and Maturation: Implications for Neurodevelopmental Disorders and Neurodegenerative Diseases. Genes, 15(11), 1381. https://doi.org/10.3390/genes15111381







