Highlights
Distribution of the Pi-ta gene was explored in rice landraces in different regions in Yunnan Province. A total of 385 rice landraces collected from these regions were found to carry the Pi-ta gene.
The Pi-ta haplotypes in rice landraces in Yunnan Province were identified and discovered to encode 12 novel Pi-ta protein variations.
The evolutionary cluster and network of the Pi-ta haplotypes in rice landraces in Yunnan Province were analyzed. These results suggest that the Pi-ta haplotypes carrying R alleles evolved from S alleles in these rice landraces.
Abstract
Background: Rice blast, caused by Magnaporthe oryzae, seriously damages the yield and quality of rice worldwide. Pi-ta is a durable resistance gene that combats M. oryzae carrying AVR-Pita1. However, the distribution of the Pi-ta gene in rice germplasms in Yunnan Province has been inadequately studied. Methods: We analyzed the potential molecular evolution pattern of Pi-ta alleles by examining the diversity in the coding sequence (CDS) among rice varieties. Results: The results revealed that 95% of 405 rice landraces collected from different ecological regions in Yunnan Province carry Pi-ta alleles. We identified 17 nucleotide variation sites in the CDS regions of the Pi-ta gene across 385 rice landraces. These variations led to the identification of 28 Pi-ta haplotypes, encoding 12 novel variants. Among these, 5 Pi-ta haplotypes (62 rice landraces) carried R alleles. The evolutionary cluster and network of the Pi-ta haplotypes suggested that the Pi-ta S alleles were the ancestral alleles, which could potentially evolve into R variants through base substitution. Conclusions: This study suggests that Pi-ta alleles are diverse in the rice landraces in Yunnan, and the Pi-ta sites resistant to blast evolved from the susceptible plants of the rice landraces. These results provide the basis for breeding resistant varieties.
1. Introduction
Rice (Oryza sativa L.) is one of the most important food crops in many countries, including India, China, East Asia, Southeast Asia, and Africa, and plays a crucial role in catering to the nutritional requirements of 70% of the population in these regions [1]. This crop not only holds cultural significance but also forms the backbone of food security in these regions. There are several biological factors that influence the yield and quality of rice. Among them, fungi, bacteria, and viruses make the most significant contributions to disease-causing factors [2]. Rice blast, a devastating fungal disease caused by the pathogen M. oryzae (also known as Pyricularia oryzae), was initially reported in the United States in 1876 [3]. Strains of the fungus that have been isolated from Digitaria (e.g., crabgrass and fingergrass) have been designated as M. grisea. In contrast, those strains that have been isolated from rice and other hosts have been named M. oryzae [4]. The blast disease causes 10% to 30% of losses in rice yields, which can be enough to feed about 60 million people annually, and even no harvest under severe conditions [3,5]. In 2021, the Jeonbuk Province of Korea experienced a significant outbreak of rice blast. This region represents 27.7% of the country’s total rice cultivation area. Alarmingly, the extent of the outbreak was 23.7 times greater than that of 2019 and 2.6 times higher than 2020, respectively [6]. The average annual damage area of rice blast was about 75 million hm2 from 2013 to 2017 years in China [7]. Cultivating resistant varieties in the field has been demonstrated to be the most effective, economical, and sustainable way to control rice blast. The defensive mechanism in rice operates on the principles of the classical gene-for-gene theory. The resistance (R) gene in rice has the ability to recognize a corresponding avirulence (AVR) gene presented by the pathogen M. oryzae. Upon recognition, it triggers an immune response in the host plant and provides an immunoreaction against rice blast disease [8]. So far, more than 100 blast R genes have been identified in rice. Among these, 38 resistance genes have been successfully cloned and functionally verified [9,10]. For instance, certain rice lines that carry the Pi9 gene, a broad-spectrum blast resistance gene located on chromosome 6, have demonstrated resistance to 43 different blast isolates [9]. These isolates were collected from 13 different countries. The Pik-h gene found in a wide range of cultivars, such as the indica cvs. Tetep and Tadukan, showed resistance to 12 different isolates of blast fungus collected from India [11,12]. Most of these resistance genes, including Pi-ta, Pi-1, Pi25, Pigm, and Pia, are dominant and encode a nucleotide-binding site and leucine-rich domain, [9]. While the R gene-mediated immune response is highly effective, once attracted, a cultivated variety with a monogenic line becomes susceptible after 3–5 years in the rice field. This susceptibility is due to the rapid variations of specific races, which result in the functional loss of the AVR gene. However, the R gene is coevolving with the AVR gene in nature [9]. Therefore, analysis of the distribution and variation of the R gene in germplasm resources is beneficial to evaluate its effectiveness in the field and discover the novel evolutionary haplotypes for breeding new rice varieties.
Pi-ta is a potent and durable resistance gene against the rice blast disease caused by M. oryzae, which contains the AVR-Pita1 gene. The gene has been effectively deployed for the past 40 years to combat rice blast, and it continues to provide effective resistance not only in the United States but also worldwide [13]. The Pi-ta gene, known for its resistance properties, was first reported in the landrace rice variety of Taducan in the 1950. It was first cloned from the Yashiromochi rice cultivar against the blast fungus carrying the AVR-Pita1 gene, a member of the AVR-Pita gene family [14,15]. The Pi-ta gene was located in the centromere of chromosome 12. Its coding region consists of 2 distinct sequences: CDS1 and CDS2. These sequences encode a predicted cytoplasmic protein, which consists of 928 amino acids. This protein is characterized by a nucleotide binding site and leucine-rich domains [14]. The Pi-ta protein can specifically recognize AVR-Pita1 effectors and trigger a series of defense responses to prevent infection by races of M. oryzae that carry the AVR-Pita1 gene, which is known as effector-triggered immunity [15,16,17]. Immunoreaction mediated by Pi-ta must be assisted by a resistance gene Ptr(t) [18,19]. Pi-ta and AVR-Pita1 are the earliest cloned race interactional pairs in blast R and AVR genes, and the specific interaction between them is determined only by the replacement mutation of a single amino acid in the Pi-ta protein at position 918, which serine (Ser-918) instead of alanine (Ala-918) impaired interaction and led to susceptibility [16]. The high degree of Pi-ta site polymorphism in cultivated rice and its relatives could be contributed to the selection pressure exerted during domestication, and a long terminal repeat retrotransposon located near the Pi-ta promoter was found in all resistant cultivars carrying the resistant Pi-ta gene [13]. The translation of Pi-ta haplotype sequences across different rice cultivars has revealed multiple variations. For example, there were 64 Pi-ta haplotypes found to encode 47 distinct Pi-ta protein variants in rice germplasm derived from 48 Indica rice accessions and publicized Pi-ta haplotype variants from 220 rice accessions [20]. Yunnan is the origin of multi-cropping agriculture, including rice [21]. However, the distribution of the Pi-ta gene in rice germplasms in Yunnan Province has been poorly studied. Therefore, investigating the distribution and variation of the Pi-ta gene in this region is crucial to enhance gene development and to improve our understanding of the interactions between the resistant gene Pi-ta and the avirulence gene AVR-Pita1.
Investigating the variation of the R gene in cultivated rice contributes to the evaluation of their effectiveness and persistence for the corresponding AVR gene in the field. However, the variation in the coding region sequence (CDS) in the Pi-ta gene and its corresponding protein products in rice germplasms from different regions in Yunnan Province remains unclear. Furthermore, the impact of this variation on the evolution of the Pi-ta gene is yet to be understood. In this study, we analyzed the variation and evolution of the coding region of Pi-ta in 385 rice germplasms collected from different regions in Yunnan Province. We discovered 12 novel Pi-ta variations, of which 5 novel Pi-ta proteins contained resistance (Ala-918) to rice blast and were derived from susceptible plants that hold the Pi-ta gene (Ser-918). This is the first report on the distribution and evolution of the Pi-ta gene rice landraces in different rice-growing regions in Yunnan Province. These findings have provided the basic material for resistant breeding against blast and enhanced our understanding of the effectiveness of Pi-ta in China.
2. Materials and Methods
2.1. Collection of Rice Landraces
In this study, we collected 405 samples from the different rice regions, including 48, 3, 1, 3, 46, 82, 128, and 94 samples from center, eastern, northeastern, northwestern, southeastern, southern, southwestern, and western, respectively, in the Yunnan Province of China.
2.2. DNA Preparation of Rice Landraces
The genome DNA of 405 samples collected from the different rice regions in Yunnan was extracted by the CTAB method. Briefly, the leaves of rice landraces (about 0.5 g) were prepared for extraction of total nucleic acids. Chloroform and isoamyl alcohol (24:1) were used to separate the total nucleic acids and proteins of these samples. Then, the total nucleic acids were precipitated with isopropyl alcohol and finally dissolved with 50 μL ddH2O for PCR amplification.
2.3. PCR Amplification and DNA Sequencing
A total of 2 coding sequences (CDS) of the Pi-ta gene were detected by using its 3 sets of specific primers in these samples of rice landraces (Table 1). Each PCR reaction was amplified in a total reaction volume of 25 µL containing the following components: 22 µL of 1.1× Mix Ver.2 (Qingke Biotech, Beijing, China), 1 µL (10 µM) of each primer, and 1 µL of DNA template. Reactions were performed in a C1000 thermal cycler (Bio-Rad, Hercules, CA, USA) with the following program: 1 cycle at 98 °C for 2 min for initial denaturation; 35 cycles at 98 °C for 10 s, 57 or 59 °C for 15 s, 72 °C for 30 s; and a final denaturation of 72 °C for 5 min. Each reaction was repeated twice. The size of the amplified DNA fragment was estimated using the DL 2000 DNA ladder (Qingke Biotech). The PCR products were sequenced using the same primers as above for PCR amplification. The DNA was sequenced by Qingke Life Technologies Biotechnology Co., Ltd. (Qingke, Beijing, China). The amplicons from each isolate were sequenced in 3 separate PCR replicates.

Table 1.
Primer pairs used in this study.
2.4. Data Analysis
The CDS1 and CDS2 sequences and the corresponding amino acids of the Pi-ta were assembled by DNASTAR v. 7.10 software (https://www.dnastar.com/, accessed on 27 June 2023). DnaSP v5.10.01 [22] was used for calculation of polymorphic sites (π), the number of DNA haplotypes, and the sliding window. Haplotype network analysis was performed using TCS v. 1.21 [23] (https://www.softpedia.com/get/Science-CAD/Posada-TCS.shtml, accessed on 29 April 2024). Fontaine’s method was used to calculate the diversity index of the rice blast fungus population [24]: diversity index = (1 − ∑ni = 1Pi2), where Pi is the frequency of haplotype i in a population. Then, Tajima’s test of neutrality was performed using MEGA v. 5.10. A phylogenetic tree was constructed using MEGA v. 5.10 using the neighbor joining method [25].
3. Results
3.1. Distribution of Pi-ta Gene in Rice Landraces Collected from Different Regions in Yunnan Province
To analyze the distributions of the Pi-ta gene in the different rice regions in Yunnan Province, a total of 405 samples were collected from the center, northeastern, northwestern, southeastern, southern, southwestern, and western regions in Yunnan Province, in China. The CDS (CDS1 and CDS2) regions of Pi-ta were detected using Pi-ta-specific primers. There were 385 samples carrying both the CDS1 and CDS2 regions among the 405 samples, while 18 samples were carrying the CDS2 but not CDS1 regions, and 2 samples were carrying the CDS1 but not CDS2 regions (Table 2). To exclude possible contamination, each PCR was repeated 3 times. These results suggest that the Pi-ta gene is wildly distributed among rice landraces across different rice cultivation areas in Yunnan Province.

Table 2.
Distributions of the CDS of the Pi-ta gene in rice landraces collected from different rice-growing regions in Yunnan Province.
3.2. The Novel Haplotypes of Pi-ta Gene Discovered in Rice Landraces in Yunnan Province
The Pi-ta locus across different rice cultivars has revealed multiple variations [20]. To investigate the diversity of the Pi-ta locus in the rice landraces in Yunnan Province, CDS1 and CDS2 of the Pi-ta gene were amplified from the 385 rice samples by using the specific primers, and the amplified PCR products were sequenced and assembled. These gene sequences were compared with the 16 reference sequences (GenBank accession: AF207842.1, EU770206.1, EU770207.1, EU770208.1, EU770209.1, EU770210.1, EU770211.1, EU770212.1, EU770213.1, EU770214.1, EU770215.1, EU770216.1, EU770217.1, EU770218.1, EU770219.1, EU770220.1), and a total of 52 mutation loci, including 35 haplotypes, were identified in the CDS of Pi-ta (Table S1). A total of 17 mutation loci and 28 different haplotypes (H01–H28) of the Pi-ta gene were found in the 385 rice landraces in Yunnan, and H01, H05, and H03 were the major haplotypes with high frequencies of 31.2%, 26.0%, and 15.1%, respectively (Table 3). The H02, H04, H6, H07, H08, H09, H10, and H11 haplotypes detected frequencies from 1.6% to 5.7%, while the remaining haplotypes were found with low frequencies (less than 1%) (Table 3). Among 28 Pi-ta haplotypes, except H01 (same with EU770212.1 and EU770213.1), H02 (same with EU770207 and EU770208), H05 (same with EU770211), H06 (same with EU770215.1), H07 (same with EU770209.1 andEU770210.1), and H11 (same with EU770217.1), the remaining 22 novel Pi-ta haplotypes were identified compared to previous published alleles (Table S1). The Pi-ta site resistant to blast (base-pairs G at 2752th) was observed in 6 haplotypes, of which 5 haplotypes were from the rice landraces in Yunnan, while the remaining haplotypes were susceptible (Table S1). The variation analysis of the Pi-ta alleles in the CDS positions suggested that the level of variation was higher in CDS1 compared to CDS2 (Figure 1). These findings suggest that the coding sequence (CDS) of the Pi-ta locus exhibits nucleotide polypeptides in various rice landraces from Yunnan Province. Further, some haplotypes possess the resistance gene against M. oryzae that carries AVR-Pita1.

Table 3.
Variations of the Pi-ta locus haplotypes in the 385 rice landraces collected from different rice-growing regions in Yunnan Province, China.
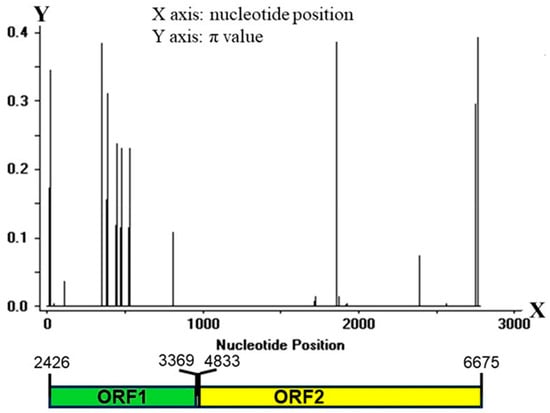
Figure 1.
Diversification of Pi-ta in rice landraces in Yunnan. Distribution of variation of the Pi-ta alleles was analyzed using sliding window. X-axis shows the distribution of variation within the full CDS regions. Lower pane indicates the corresponding schematic presentation of the two exons of Pi-ta. Window length: 10; step size: 1. π value corresponds with the level of variation at each site, because it is the sum of pair-wise differences divided by the number of pairs within the population.
3.3. Distribution of Pi-ta Haplotype in Rice Landraces of Different Rice-Growing Regions in Yunnan
To verify the distribution of the Pi-ta haplotype in different rice-growing regions in Yunnan, we further analyzed 28 Pi-ta haplotype dates based on the location collected from the rice simples. The results indicate that the Pi-ta haplotypes are distributed across the rice landraces in eight distinct rice-growing regions in Yunnan (Table 4). The distribution frequency of the Pi-ta haplotypes was 12.2%, 0.8%, 0.3%, 0.8%, 11.9%, 19.2%, 32.2%, and 22.6% in central, eastern, northeastern, northwestern, southeastern, southern, southwestern, and western Yunnan Province, respectively. In addition, analysis of the diversity indicated that this index was 0.79, 0.67, 0, 0.67, 0.77, 0.78, 0.80, and 0.75 for central, eastern, northeastern, southeastern, southern, southwestern and western Yunnan, respectively (Table 4). In summary, the diversity index of Pi-ta alleles was ordered in Yunnan Province as: southwestern > central > southern > southeastern > western > eastern or northwestern > northeastern. Among 28 haplotypes of the Pi-ta gene, 9 haplotypes were detected in 47 rice samples from central. A total of 3 haplotypes (H06, H07, and H12 or H01, H05, and H06) were detected in 3 rice samples from eastern or northwestern, only 1 (H07) haplotype was detected in 1 rice sample from northeastern, 8 haplotypes were detected in 46 rice samples from southeastern, 12 haplotypes were detected in 74 rice samples from southern, 20 haplotypes were detected in 124 rice samples from southwestern, and 13 haplotypes were detected in 87 rice sample from western Yunnan (Table 4).

Table 4.
Distribution of Pi-ta haplotypes in different rice-growing regions in Yunnan.
3.4. Variation Distributions of R/S Alleles of Pi-ta Locus Proteins in Rice Landraces in Yunnan Province
The variations of the nucleotide result in encoding the different protein products. To investigate the protein types (PT) of variants in 35 alleles of the Pi-ta locus, multiple amino acid alignments were performed. A total of 32 variants to amino acid sites were identified, and 22 protein products were found in the CDS regions of these Pi-ta loci. Of these the coding PT01, including the published haplotypes EU04, EU05, EU07, EU08, and EU09 and the haplotypes H01, H07, H09, H15, and H22 of rice landraces in Yunnan, had the highest ratio. A total of 5 Pi-ta haplotypes coded PT02 (EU02, EU03, H02, H10, and H20), PT04 (EU06, H04, H05, H12, and H19), and PT05(EU10, EU12, H06, H08, and H11), respectively. There were 2 Pi-ta coding PT16 (H25 and H28), while the remaining Pi-ta haplotypes coded a corresponding protein product, respectively (Table 5). Interestingly, we found 12 novel Pi-ta proteins in the 28 alleles of rice landraces in Yunnan compared to the published 16 alleles, and 5 out of 12 novel Pi-ta proteins (the haplotype H03, H13, H16, H18, and H21) were resistant to the rice blast AVR-Pita1 gene, as the amino acid (alanine) at the position 918 was known to be a key site for resistance to rice blast [16]. A total of 62 out of 385 rice landraces in Yunnan coded to the 5 novel Pi-ta-resistant proteins (Ala-918), and the frequency was 16.1%, while the remaining rice landraces in Yunnan holding the Pi-ta gene were susceptible (Table 6). In summary, these results show that the Pi-ta gene in the rice-growing regions in Yunnan is constantly varying, and the variation of the Pi-ta gene population is of great significance, because in some cases, the Pi-ta gene has evolved haplotype alleles that resist the rice blast AVR-Pita1 gene. Meanwhile, this evolution is enormously beneficial for the cultivation of the germplasm resources.

Table 5.
Variations of the Pi-ta loci proteins in rice landraces in Yunnan, China.

Table 6.
Distribution of functional Pi-ta alleles in the 385 samples collected from different rice-growing regions in Yunnan.
3.5. R Alleles of Pi-ta Derived from the S Alleles in Rice Landraces in Yunnan Province
To analyze the evolutionary relationships in the 28 Pi-ta haplotype populations and 16 Pi-ta variants from GenBank, a haplotype population genetic tree was constructed using the neighbor joining method. The result showed that the Pi-ta haplotype populations could be divided into 2 different clusters (Ⅰ and Ⅱ). Cluster Ⅰ contained 4 Pi-ta haplotypes (Figure 2A), including wild rice (Oryza barthii, EU770218.1), Oryza glaberrima (EU770219.1), Oryza sativa f. spontanea, and Oryza sativa Indica Group (EU770206.1), while Cluster Ⅱ contained wild rice (Oryza rufipogon, EU770209.1, EU770213.1, EU770214.1), Oryza glaberrima (EU770215.1), Oryza sativa (EU770217.1, EU770220.1, EU770207.1, EU770208.1, EU770210.1, EU770212.1, AF207842.1, EU770211.1, EU770216.1), and the remaining haplotypes (Figure 2A). These results show that the Pi-ta R/S alleles in Yunnan rice landraces were located in a same cluster of Oryza rufipogon. Interestingly, the Pi-ta R alleles of AF207842.1, H03, H13, H16, H18, and H21 were far from the S alleles (Figure 2B), suggesting that the Pi-ta R alleles were derived from S alleles.
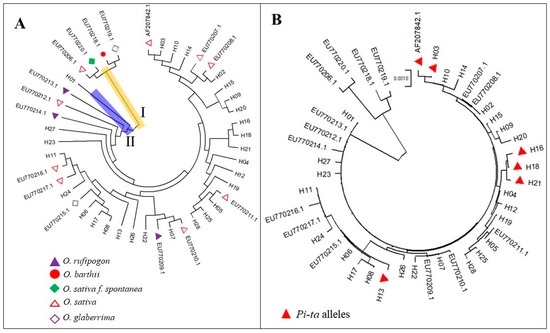
Figure 2.
Neighbor joining phylogenetic tree of Pi-ta resistance (R)/susceptibility (S) alleles. (A), systematical evolution of 44 Pi-ta alleles. R/S alleles of the Pi-ta can be divided into 2 different clusters. Cluster I contained 4 Pi-ta haplotypes (wild O. barthii, O. glaberrima, O. sativa f. spontanea, and O. sativa Indica Group), while Cluster II contained wild Oryza rufipogon and Oryza glaberrima, and all of Pi-ta haplotypes in rice landraces in Yunnan. (B), the phylogenetic relationship of Pi-ta R/S alleles. The Pi-ta R alleles were derived from S alleles in rice landraces in Yunnan. These Pi-ta haplotype alleles were obtained from rice landraces in Yunnan (28 Pi-ta haplotype alleles, H01–H28) and the published GenBank (16 Pi-ta haplotype alleles, accession number: AF207842.1, EU770206.1, EU770207.1, EU770208.1, EU770209.1, EU770210.1, EU770211.1, EU770212.1, EU770213.1, EU770214.1, EU770215.1, EU770216.1, EU770217.1, EU770218.1, EU770219.1, EU770220.1).
3.6. Stepwise Evolutionary Process of Pi-ta Haplotypes in Rice Landraces in Yunnan Province
Genetic variation is a result of long-term natural evolution. To further understand the stepwise evolutionary deductive relationship between Pi-ta haplotype populations, the haplotype network was developed through the TCS Network (http://darwin.uvigo.es/) based on polymorphisms nucleotide of Pi-ta coding for 28 alleles identified from rice landraces in Yunnan and 13 reference sequences (6 haplotype sequences from the same rice landraces in Yunnan) obtained from GenBank. A total of 5 major evolutionary clades (A, B, C, D, and E) were observed among 35 Pi-ta haplotype alleles (Figure 3). H01 (same with EU770212.1) was found to be the most original ancestor of the remaining Pi-ta haplotypes. Clade A contained 4 Pi-ta-susceptible orthologues (EU770206.1, EU770218.1, EU770219.1, and EU770220.1) and occurred because of the multiple mutational steps from H01. Clade B contained 7 Pi-ta orthologues, of which the haplotype H13 carried a resistant site (Ala-918) and evolved from a single mutational step in the Pi-ta-susceptible haplotype H06 (same with EU7770215.1). Clade C consisted of 2 Pi-ta-resistant alleles (H03 and AF207842.1). The haplotype H03 was generated from a single base mutational step in the Pi-ta-susceptible allelic H10. Clade D contained 12 Pi-ta-susceptible orthologues, while Clade E consisted of Pi-ta R (H16, H18, and H21) and S alleles (H04), in which H04 was the remaining origin of 3 Pi-ta R alleles. These results suggest that the B, C, and E clades of Pi-ta R alleles in 385 rice landraces in Yunnan evolved from S alleles.
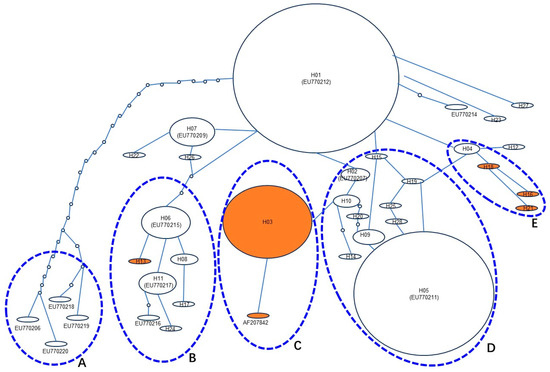
Figure 3.
The haplotype network for the 28 Pi-ta alleles and the 13 reference Pi-ta alleles in rice. Haplotype network analysis was performed using TCS1.21 (http://darwin.uvigo.es/). The Pi-ta haplotypes were major divided into 5 evolutionary clades. Clade A contained 4 Pi-ta orthologues and they derived from the published sequences in GenBank. Clade D possessed the most Pi-ta orthologues, but not contained its R allele. In contrast, clade B, C, and E included the Pi-ta R allele at least one and derived from the S orthologues. The original Pi-ta alleles were designated as the H01 haplotype in the network. Each Pi-ta haplotype was separated by mutational events. The node in the network represents an extinct or a missing haplotype not found among the samples. All haplotypes were displayed as circles. The size of the circles corresponded to the haplotype frequency. H01–H28 were obtained from 385 rice landraces in Yunnan. The AF207842.1, EU770206.1, EU770207.1 (same with H02), EU770209.1 (same with H07), EU770211.1 (same with H05), EU770212.1 (same with H01), EU770214.1, EU770215.1 (same with H06), EU770216.1, EU770217.1 (same with H11), EU770218.1, EU770219.1, and EU770220.1 (GenBank accession number) of the Pi-ta haplotypes were obtained from GenBank. White color indicates the susceptibility alleles of Pi-ta gene, and yellow color indicates the resistance alleles of Pita gene. A to E, 5 major haplotypes of Pi-ta in Yunnan Province of China, are shaded.
3.7. Pi-ta Gene Undergoing the Process of Contraction in Rice Landraces in Yunnan Province
The natural selection pressure on Pi-ta was calculated by Tajima’s neutrality test on 385 of the Pi-ta CDS sequences. Tajima’s D value was not significantly different from 0 (D = 0.55445; p > 0.1) (Table 7). This result suggests that Pi-ta may suffer from balancing selection, in which the frequency of Pi-ta R and S alleles occurs at a relatively high rate in the population of 385 rice landraces in Yunnan, indicating that the Pi-ta populations are undergoing the process of contraction.

Table 7.
Tajima’s neutrality test of Pi-ta in 385 rice landraces in Yunnan.
4. Discussion
Rice blast is one of the most serious diseases affecting rice crops. Disease resistance breeding is the most economically and ecological friendly way to control this disease. However, most resistance genes have a short life span in the field [9]. Therefore, it is mandatory to select germplasm resources with polygenic resistance for the sustainable control of rice blast. Currently, over 100 blast resistance genes have been identified, and among them the Pi-ta gene stands out as one of the most effective and durable resistance genes. In the United States, the Pi-ta gene has conferred resistance to the major pathogenic forms of M. oryzae [26]. A study has shown that the Pi-ta gene provides 14 years of durable resistance to the contemporary field populations of M. oryzae in rice-growing areas of the southern United States [27]. Some studies have reported the introduction of the Pi-ta gene into cultivated rice varieties from “Tetep” and “Taducan” [10,15,28]. Our results showed that approximately 95% (385 out of 405) of rice landraces collected from different regions in Yunnan Province carried the Pi-ta gene. These samples with the Pi-ta gene could be divided into 28 haplotypes based on the variation analysis of nucleotide sequences in the coding regions and found 12 novel Pi-ta protein products. The haplotype diversity of the Pi-ta gene may be associated with the difference in rice landraces in these different regions. Yunnan in China is known as the origin of multi-cropping agriculture, including rice [21]. This rich history and genetic diversity make it a valuable resource for ongoing research and development in disease resistance for crops such as rice. These results indicate that the haplotypes of the Pi-ta gene are diverse, the variation of nucleotides in its coding region is favorable, and the cultivated rice landraces in Yunnan Province have long held the Pi-ta gene. Thakur and his workers [20] analyzed the variation of Pi-ta alleles in 529 rice landraces in India and in 220 rice accessions, finding that there was a high degree of nucleotide variation in the Pi-ta gene in the intron region, and that 64 Pi-ta haplotypes and 47 Pi-ta protein variants were identified, according to the nucleotide polymorphism of the coding region and amino acid sequences of its locus, respectively. Wang et al. [13] showed that the Pi-ta alleles in rice accessions from 6 Oryza spp. (Oryza sativa, Oryza glaberrima, Oryza rufipogon, Oryza nivara, Oryza Glaberrima, Oryza Rufipogon, and Oryza Nivara) could be divided into 16 different haplotypes. The diversity of the Pi-ta gene haplotypes observed in this study aligns closely with the findings from two previously mentioned studies. The plant R gene polymorphism is an important part of plant innate immune resistance to pathogens, and most R genes are highly polymorphic and diverse [29]. Similarly, avirulence (AVR) genes corresponding to R genes are also in frequent variation. Studies have shown that the AVR-Pita1 gene, an AVR gene from M. oryzae and the interaction with Pi-ta triggering a downstream immune response, is also in a condition of frequent variation [30]. Thus, the diversity of the Pi-ta gene haplotype in different growing regions of rice in Yunnan may also be related to the continuous variation of AVR-Pita1, as the host resistance gene is inclined to continuous variation when the AVR gene form in the pathogen is in a situation of frequent mutation [31,32]. Furthermore, previous studies have shown that the frequency of avirulent gene AVR-Pita1 in 366 M. oryzae rice isolates from different rice regions in Yunnan fields is 46.7–72.4%, and among them, the sequenced 60 isolates code for 18 AVR-Pita1 haplotypes, of which 6 haplotypes are virulent to the Pi-ta R alleles, and the mutations of AVR-Pita1 are responsible for defeating race-specific resistance in nature [33]. Thus, the diversity of the Pi-ta gene haplotype in different growing regions of rice in Yunnan may also be related to the continuous variation of AVR-Pita1, as the host resistance gene is inclined to continuous variation when the AVR gene form in the pathogen is in a situation of frequent mutation [28,30,31]. This represents the coevolution of the AVR gene and the R gene with the host and pathogen interaction. In addition, the continuous variation of AVR-Pita1 may contribute to its unique location. Specifically, AVR-Pita1 is located on chromosome 3 of the M. oryzae genome and is in close proximity to a telomere [34]. The coevolution of the rice gene Pi-ta and M. oryzae gene AVR-Pita1 is still the focus of researchers [15,16,35], probably as the pair of genes strongly supports a hypothesis for gene-to-gene. For instance, Jia et al. [36] showed that rice expressing the Pi-ta gene is resistant to isolates of M. oryzae, expressing AVR-Pita1 in a gene-to-gene manner. Resistant reactions to blast were triggered by the direct interaction of Pi-ta with AVR-Pita1 products, and serine instead of alanine at position 918th in the Pi-ta protein resulted in the functional loss of resistance in plants or the replacement mutation of a single amino acid at position 178th in the AVR-Pita176 protein also disrupted to their physical interaction in vitro. In addition, an immunoreaction mediated by Pi-ta was required to be assisted by the resistance gene Ptr(t) [18,19]. These results suggest that the resistant reaction mediated by the Pi-ta gene is complex in rice.
Genetic evolution might be viewed as a form of biological adaptation to environmental conditions. Lee et al.’s [35] analyses of the genetic evolution of the Pi-ta gene in invasive weedy rice in the United States showed that Pi-ta in weed rice in the United States can be divided into 5 clusters, containing 8 different Pi-ta haplotypes. Only 1 subcluster (a haplotype) in these clusters, however, contained Pi-ta haplotype (Ala-918) resistance to rice blast. In the current study, 35 Pi-ta alleles can be divided into 2 different clusters. Cluster I contained 4 Pi-ta haplotypes (wild O. barthii, O. glaberrima, O. sativa f. spontanea, and O. sativa Indica Group), while Cluster II contained wild Oryza rufipogon and Oryza glaberrima. Furthermore, in 28 Pi-ta haplotypes in rice landraces collected from different regions of Yunnan, apart from 5 haplotypes carrying Pi-ta R alleles, the remaining haplotypes were derived from susceptible plants holding the Pi-ta gene. Analysis of the molecular evolution and functional adaptability of the Pi-ta gene in 36 wild Oryza rufipogon showed that haplotype H2 (Ser-918) is the ancestor of haplotype H1 (Ala-918), and most rice accessions containing the Pi-ta gene belong to H2 in the 26 haplotypes; it was rare that the haplotype H1 would emerge in the process of rice cultivation and domestication and nucleotide diversity of the Pi-ta gene in these rice accessions [35]. Our results showed that H01 (same with EU770212.1) (Ser-918) was the ancestor of the other haplotypes. In general, the variation frequency of nucleotides in the CDS1 and CDS2 regions of the Pi-ta gene was low, and the results are similar to those of previous studies. To investigate the diversity of the exon and intron regions of the Pi-ta gene in 51 rice samples from 6 O. spp., the Pi-ta alleles were classified into 2 main branches, consisting of 16 different sequences with a large number of insertions and deletions [13]. Despite this, the DNA sequence showed 16 variations of the Pi-ta allele in 51 rice samples, but only 9 corresponding protein products were predicted, of which only 1 Pi-ta resistance allele was identified [13]. Their results suggest that the Pi-ta gene has a certain proportion of amino acid synonymous substitution under natural selection, and only a few of them have evolved to carry gene sites resistant to rice blast. In this study, we found 12 novel Pi-ta protein products in 385 rice landraces in Yunnan, of which 5 novel protein products (Ala-918) possessed the ability to recognize AVR-Pita1, and a total of 62 rice landraces coded these proteins. Interestingly, all of the Pi-ta resistance alleles in the rice landraces in Yunnan were derived from the susceptible plants carrying Pi-ta. Furthermore, analysis of Tajima’s neutrality test showed that the Pi-ta alleles could suffer from balancing selection, indicating that the Pi-ta locus probably maintained the diversity of the sequences via the pattern. This information indicated that the Pi-ta gene of rice in Yunnan is constantly subjected to variations, and the Pi-ta gene is always under natural selection pressure; although, it had evolved to the resistance allele. The molecular characteristics of the plant R gene reveal the degree of structural variation that affects its ability to detect the corresponding AVR genes from pathogens [37,38], and the continuous evolution of the Pi-ta gene further indicates that AVR-Pita1 is also in a condition of constant variation. In conclusion, our findings indicate that the Pi-ta gene is present in rice landraces across different rice-growing regions in Yunnan. Furthermore, certain rice accessions with the Pi-ta gene have developed resistant alleles. This discovery significantly contributes to the rich genetic resources available for the investigation of pathogenicity and screening rice accessions for resistance to rice blast.
5. Conclusions
In this study, we first reported the distribution of the Pi-ta gene rice landraces across different rice-growing regions in Yunnan Province. We also identified the haplotypes of the Pi-ta gene locus within these regions and found 12 novel allelic haplotypes, including 5 sites resistant against M. oryzae. Initially, the rice landraces carrying the Pi-ta haplotypes in these regions were susceptible alleles; however, due to variations in the Pi-ta gene, some of these haplotypes evolved into resistant alleles against M. oryzae through mutation in a single amino acid at position 918.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes15101325/s1, Table S1: Distribution of the CDS of Pi-ta gene haplotypes in the 385 rice landraces collected from the different rice-growing regions in Yunnan Province, China.
Author Contributions
H.L.: conceptualization, software, validation, methodology, roles/writing—original draft, writing—review and editing. L.L. (Lin Lu): data curation, methodology, visualization, writing—review and editing. Q.W.: data curation, formal analysis, investigation, writing—review and editing. Z.G.: resources, visualization, supervision, writing—review and editing. L.L. (Lina Liu): formal analysis, methodology, roles/writing—original draft. C.H.: investigation, validation, writing—review and editing. J.S.: data curation, supervision, writing—review and editing. C.D.: methodology, supervision, writing—review and editing. Q.M.: methodology, supervision, writing—review and editing. J.L.: conceptualization, funding acquisition, project administration, writing—review and editing. All authors commented on the article before submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Projects of Yunnan Province (202301AS070003), the National Natural Science Foundation of China (31860481), the Key Laboratory of Green Prevention and Control of Agricultural Transboundary Pests of Yunnan Province (202305AG340007). The funding organizations had no role in the design, data collection, analysis, and interpretation of data or in writing the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request after approval from the Agricultural Environment and Resource Research Institute, Yunnan Academy of Agricultural Sciences, in China.
Acknowledgments
We would like to thank the workmates and students who assisted in conducting the study.
Conflicts of Interest
The authors declare that they have no competing interests.
Abbreviations
S: susceptibility; R: resistance; AVR: avirulence gene; N: number of sequences; S: number of segregating sites; k: number of nucleotide differences; π: indicates nucleotide diversity; D: the Tajima test statistic; PT: protein types; CDS: coding sequence.
References
- Kumar MK, P.; Gowda DK, S.; Moudgal, R.; Kumar, N.K.; Gowda KT, P.; Vishwanath, K. Impact of Fungicides on Rice Production in India; InTech: London, UK, 2013; Chapter 4; pp. 77–99. [Google Scholar] [CrossRef]
- Hargrove, T.R. Rice Improvement in China and Other Asian Countries; International Rice Research Institute and Chinese Academy of Sciences: Los Baños, Philippines, 1980; pp. 135–148. Available online: https://pdf.usaid.gov/pdf_docs/pnaaj141.pdf (accessed on 24 April 2024).
- Wang, X.; Lee, S.; Wang, J.; Ma, J.; Bianco, T.; Jia, T. Current Advances on Genetic Resistance to Rice Blast Disease. In Rice-Germplasm, Genetics and Improvement; Yan, W.G., Ed.; IntechOpen: London, UK, 2014; pp. 195–217. [Google Scholar] [CrossRef]
- Klaubauf, S.; Tharreau, D.; Fournier, E.; Groenewald, J.; Crous, P.; de Vries, R.; Lebrun, M.-H. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Stud. Mycol. 2014, 79, 85–120. [Google Scholar] [CrossRef]
- Kumar, V.; Jain, P.; Venkadesan, S.; Karkute, S.G.; Bhati, J.; Abdin, M.Z.; Sevanthi, A.M.; Mishra, D.C.; Chaturvedi, K.K.; Rai, A.; et al. Understanding rice-magnaporthe oryzae interaction in resistant and susceptible cultivars of rice under panicle blast infection using a time-course transcriptome analysis. Genes 2021, 12, 301. [Google Scholar] [CrossRef]
- Chung, H.; Lee, W.-I.; Choi, S.Y.; Choi, N.-J.; Kim, S.-M.; Yoon, J.-Y.; Lee, B.C. Outbreak of rice panicle blast in Jeonbuk province of Korea in 2021. Plant Pathol. J. 2023, 39, 136–140. [Google Scholar] [CrossRef]
- Cao, N.; Chen, Y.; Ji, Z.J.; Zeng, Y.X.; Yang, C.D.; Liang, Y. Recent progress in molecular mechanism of rice blast resistance. Chin. J. Rice Sci. 2019, 33, 489–498. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, L.; Wang, G.-L. Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome. Mol. Genet. Genom. 2002, 267, 472–480. [Google Scholar] [CrossRef]
- Devanna, B.N.; Jain, P.; Solanke, A.U.; Das, A.; Thakur, S.; Singh, P.K.; Kumari, M.; Dubey, H.; Jaswal, R.; Pawar, D.; et al. Understanding the dynamics of blast resistance in rice-magnaporthe oryzae interactions. J. Fungi 2022, 8, 584. [Google Scholar] [CrossRef]
- Khanna, A.; Sharma, V.; Ellur, R.K.; Shikari, A.B.; Krishnan, S.G.; Singh, U.D.; Prakash, G.; Sharma, T.R.; Rathour, R.; Variar, M.; et al. Marker assisted pyramiding of major blast resistance genes Pi9 and Pi-ta in the genetic background of an elite Basmati rice variety, Pusa Basmati 1. Indian J. Genet. Plant Breed. 2015, 75, 417. [Google Scholar] [CrossRef]
- Ramkumar, G.; Srinivasarao, K.; Mohan, K.M.; Sudarshan, I.; Sivaranjani, A.K.P.; Gopalakrishna, K.; Neeraja, C.N.; Balachandran, S.M.; Sundaram, R.M.; Prasad, M.S.; et al. Development and validation of functional marker targeting an InDel in the major rice blast disease resistance gene Pi54 (Pikh). Mol. Breed. 2010, 27, 129–135. [Google Scholar] [CrossRef]
- Xu, X.; Hayashi, N.; Wang, C.T.; Kato, H.; Fujimura, T.; Kawasaki, S. Efficient authentic fine mapping of the rice blast resistance gene Pik-h in the Pik cluster, using new Pik-h-differentiating isolates. Mol. Breed. 2008, 22, 289–299. [Google Scholar] [CrossRef]
- Shu, Q.Y.; Wu, D.; Wang, Y.J.X.; Chen, X.; Jia, Y.; Jia, M.H.; Pinson, S.R.M.; Wang, X.; Wu, B.M.; Singh, P.K.; et al. Haplotype diversity at the Pi-ta locus in cultivated rice and its wild relatives. Phytopathology 2008, 98, 1305–1311. [Google Scholar] [CrossRef][Green Version]
- Khang, C.H.; Park, S.-Y.; Lee, Y.-H.; Valent, B.; Kang, S. Genome organization and evolution of the AVR-Pita avirulence gene family in the Magnaporthe grisea species complex. Mol. Plant-Microbe Interact. 2008, 21, 658–670. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, E.; Lee, S.; Bianco, T. Coevolutionary dynamics of rice blast resistance gene Pi-ta and Magnaporthe oryzae avirulence Gene AVR-Pi-ta 1. Phytopathology 2016, 106, 676–683. [Google Scholar] [CrossRef]
- Bryan, G.T.; Wu, K.S.; Farrall, L.; Jia, Y.; Hershey, H.P.; McAdams, S.A.; Faulk, K.N.; Donaldson, G.K.; Tarchini, R.; Valent, B. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2033–2046. [Google Scholar] [CrossRef]
- He, N.; Huang, F.; Yu, M.; Zhu, Y.; Li, Q.Q.; Yang, D. Analysis of a rice blast resistance gene Pita-Fuhui2663 and development of selection marker. Sci. Rep. 2022, 12, 14917. [Google Scholar] [CrossRef]
- Jia, Y.; Martin, R. Identification of a new locus Ptr(t) required for rice blast resistance gene Pi-ta-mediated resistance. Mol. Plant-Microbe Interact. 2008, 21, 396–403. [Google Scholar] [CrossRef]
- Lee, S.; Costanzo, S.; Jia, Y.; Olsen, K.M.; Caicedo, A.L. Evolutionary dynamics of the genomic region around the blast resistance gene Pi-ta in AA genome Oryza species. Genetics 2009, 183, 1315–1325. [Google Scholar] [CrossRef]
- Thakur, S.; Gupta, Y.K.; Singh, P.K.; Rathour, R.; Variar, M.; Prashanthi, S.K.; Singh, A.K.; Singh, U.D.; Chand, D.; Rana, J.C.; et al. Molecular diversity in rice blast resistance gene Pi-ta makes it highly effective against dynamic population of Magnaporthe oryzae. Funct. Integr. Genom. 2013, 13, 309–322. [Google Scholar] [CrossRef]
- Martello, R.D. The origins of multi-cropping agriculture in Southwestern China: Archaeobotanical insights from third to first millennium B.C. Yunnan. Asian Archaeol. 2022, 6, 65–85. [Google Scholar] [CrossRef]
- Rozas, J.; Sánchez-Del, B.J.; Messeguer, X.; Rozas, R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003, 19, 2496–2497. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Fontaine, C.; Lovett, P.N.; Sanou, H.; Maley, J.; Bouvet, J.-M. Genetic diversity of the shea tree (Vitellaria paradoxa C.F. Gaertn), detected by RAPD and chloroplast microsatellite markers. Heredity 2004, 93, 639–648. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Z.; Fjellstrom, R.G.; Moldenhauer, K.A.K.; Azam, A.; Correll, J.; Lee, F.N.; Xia, Y.; Rutger, J.N. Rice Pi-ta gene confers resistance to the major pathotypes of the rice blast fungus in the United States. Phytopathology 2004, 94, 296–301. [Google Scholar] [CrossRef]
- Lee, F.N.; Cartwright, R.D.; Jia, Y.; Correll, J.C. Field Resistance Expressed When the Pi-ta Gene Is Compromised by Magnaporthe Oryzae; Springer: Dordrecht, The Netherlands, 2009; pp. 281–289. [Google Scholar] [CrossRef]
- Jia, Y. Artificial introgression of a large chromosome fragment around the rice blast resistance gene Pi-ta in backcross progeny and several elite rice cultivars. Heredity 2009, 103, 333–342. [Google Scholar] [CrossRef]
- Hulbert, S.H.; Webb, C.A.; Smith, S.M.; Sun, Q. RESISTANCEGENECOMPLEXES: Evolution and Utilization. Annu. Rev. Phytopathol. 2001, 39, 285–312. [Google Scholar] [CrossRef]
- Orbach, M.J.; Farrall, L.; Sweigard, J.A.; Chumley, F.G.; Valent, B. A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell 2000, 12, 2019–2032. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Zhang, X.; Zhang, Q.; Huang, J.; Chen, J.-Q.; Hartl, D.L.; Tian, D. Rapidly evolving R genes in diverse grass species confer resistance to rice blast disease. Proc. Natl. Acad. Sci. USA 2013, 110, 18572–18577. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Costanzo, S.; Bianco, T. Understanding the Co-Evolution of the Rice Blast Resistance Gene Pi-ta and Magnaporthe Oryzae Avirulence Gene AVR-Pita; Springer: Dordrecht, The Netherlands, 2009; Volume 106, pp. 137–147. [Google Scholar] [CrossRef]
- Li, J.; Lu, L.; Jia, Y.; Li, C. Effectiveness and durability of the rice Pi-ta gene in Yunnan province of China. Phytopathology 2014, 104, 762–768. [Google Scholar] [CrossRef]
- Huang, C.-L.; Hwang, S.-Y.; Chiang, Y.-C.; Lin, T.-P. Molecular evolution of the Pi-ta gene resistant to rice blast in wild rice (Oryza rufipogon). Genetics 2008, 179, 1527–1538. [Google Scholar] [CrossRef]
- Lee, S.; Jia, Y.; Jia, M.; Gealy, D.R.; Olsen, K.M.; Caicedo, A.L. Molecular evolution of the rice blast resistance gene Pi-ta in invasive weedy rice in the USA. PLoS ONE 2011, 6, e26260. [Google Scholar] [CrossRef]
- Jia, Y.; McAdams, S.A.; Bryan, G.T.; Hershey, H.P.; Valent, B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 2000, 19, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- E Rose, L.; Bittner-Eddy, P.D.; Langley, C.H.; Holub, E.B.; Michelmore, R.W.; Beynon, J.L.; LE, R. The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 2004, 166, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Mackill, D.J.; Bonman, J.M.; McCouch, S.R.; Guiderdoni, E.; Notteghem, J.L.; Tanksley, S.D. Molecular mapping of genes for resistance to rice blast (Pyricularia grisea Sacc.). Theor. Appl. Genet. 1996, 93, 859–863. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).